Wheat-Psathyrostachys huashanica 4Ns Additional Line Confers Resistance to Fusarium Head Blight
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
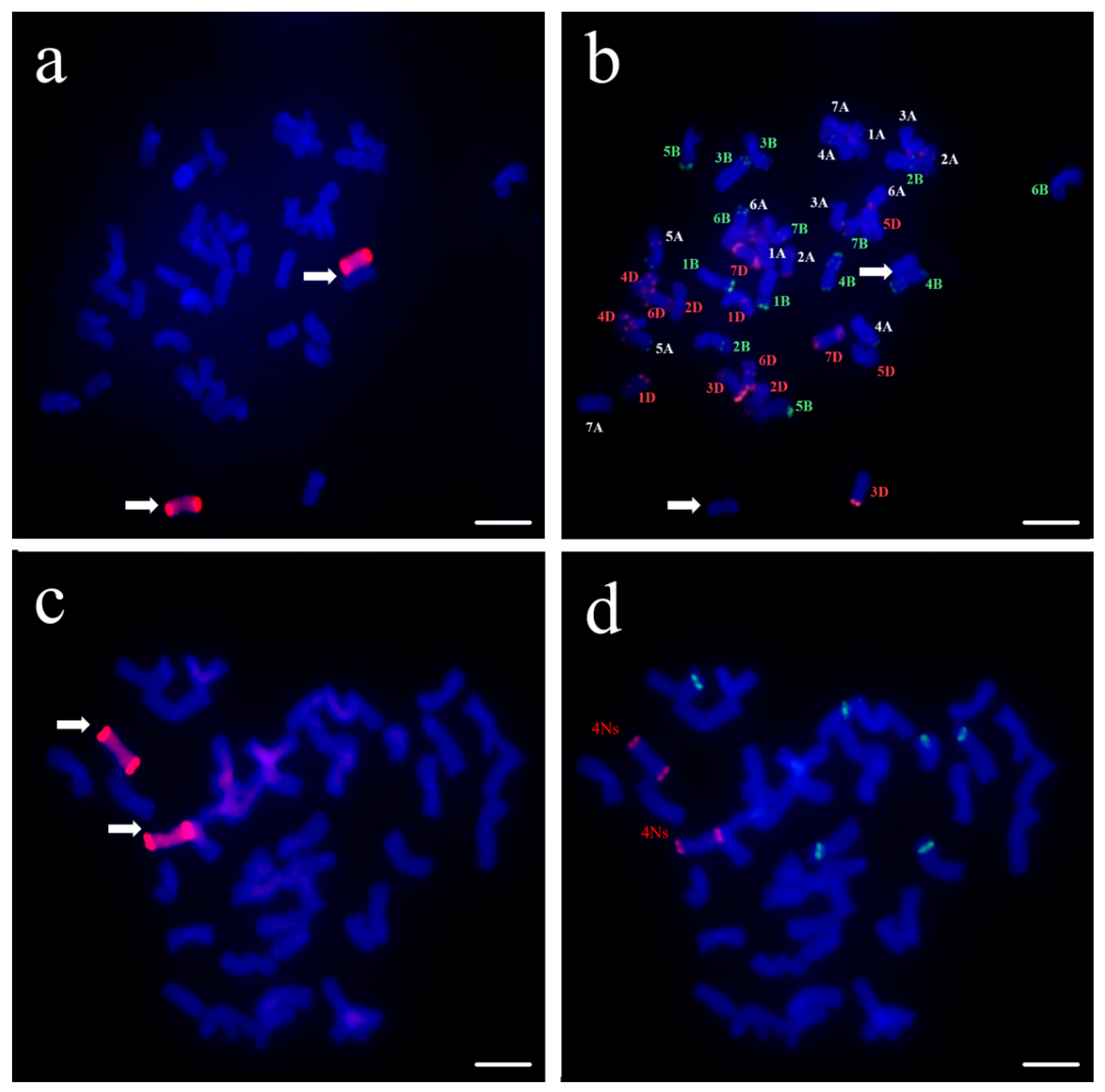
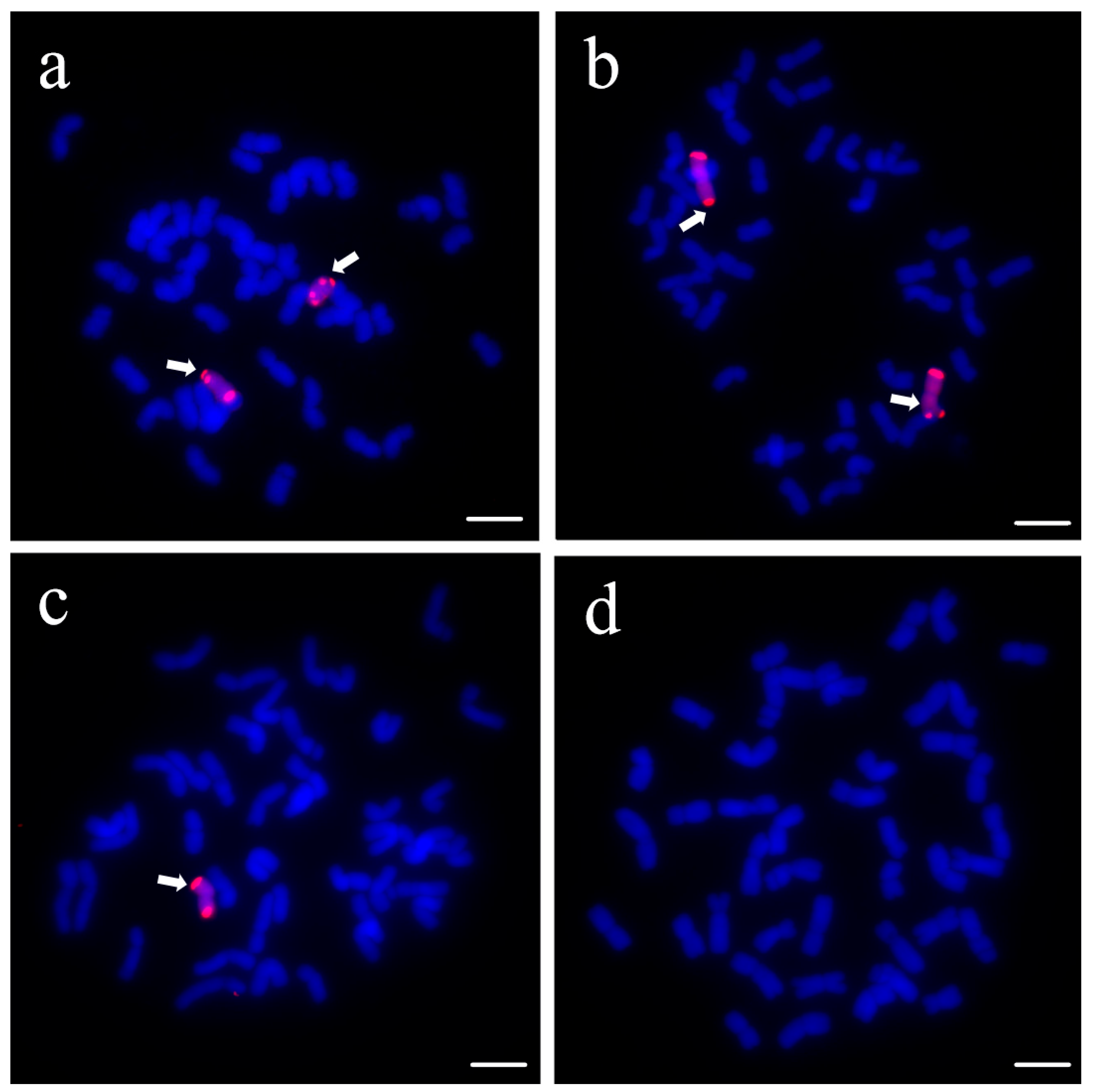
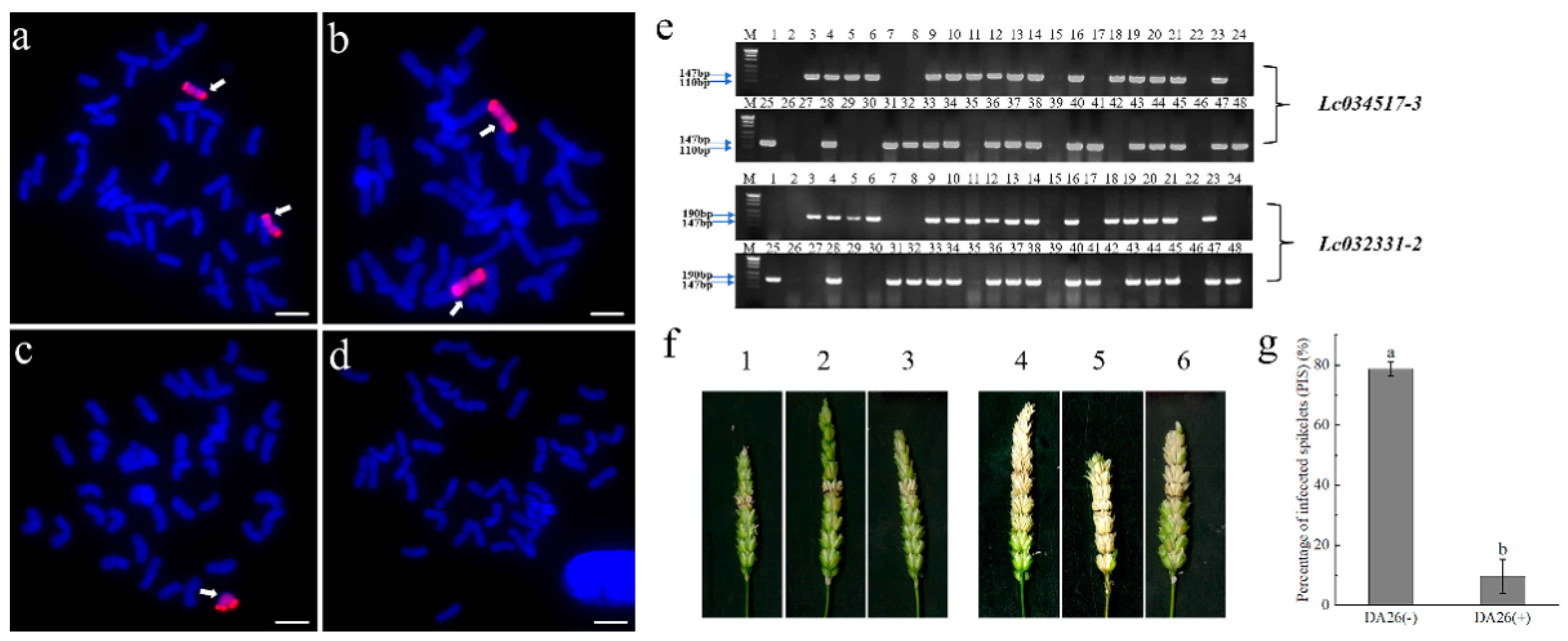
2.2. Cytogenetic Analysis
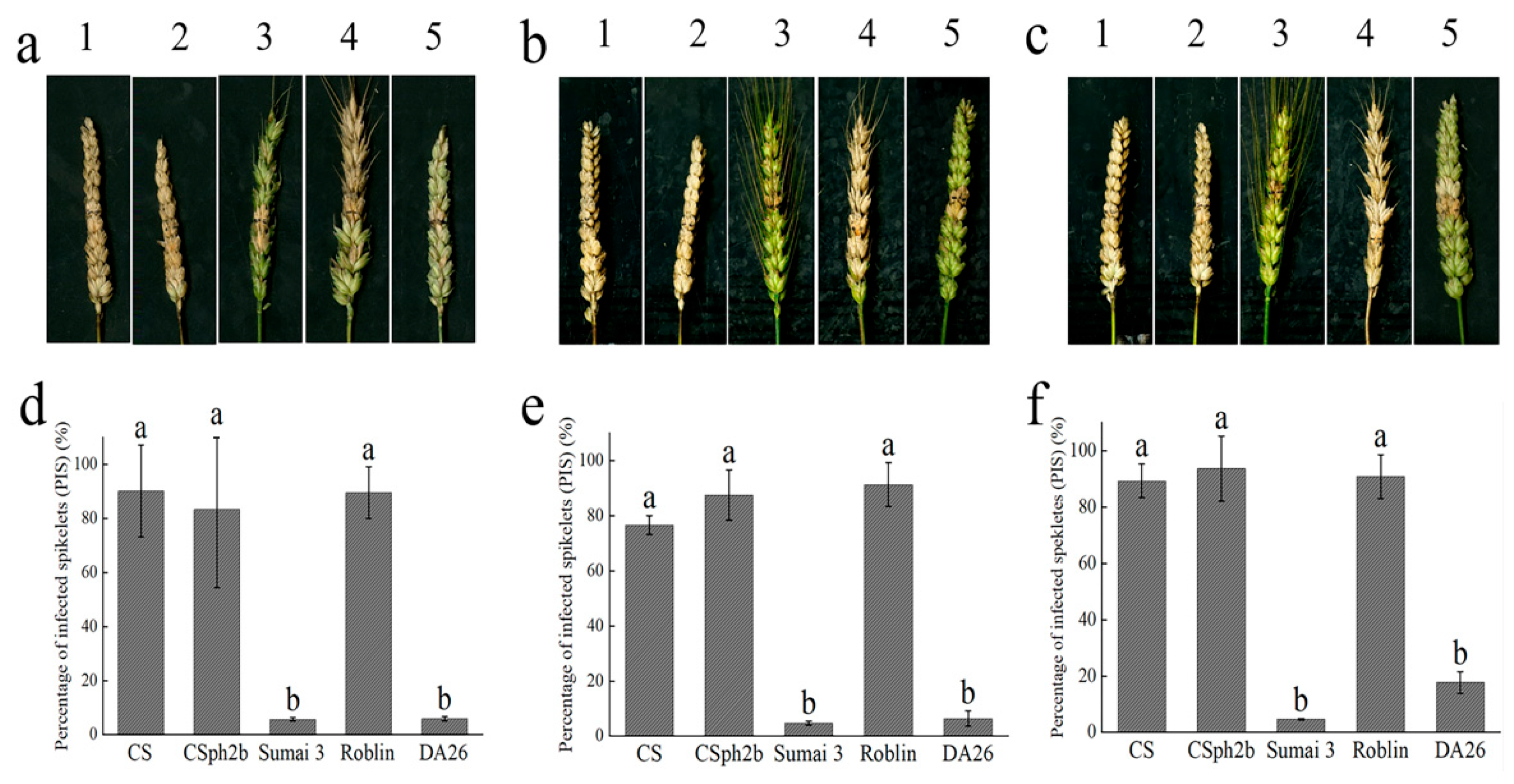
2.3. Evaluation and Statistical Analysis of FHB Resistance
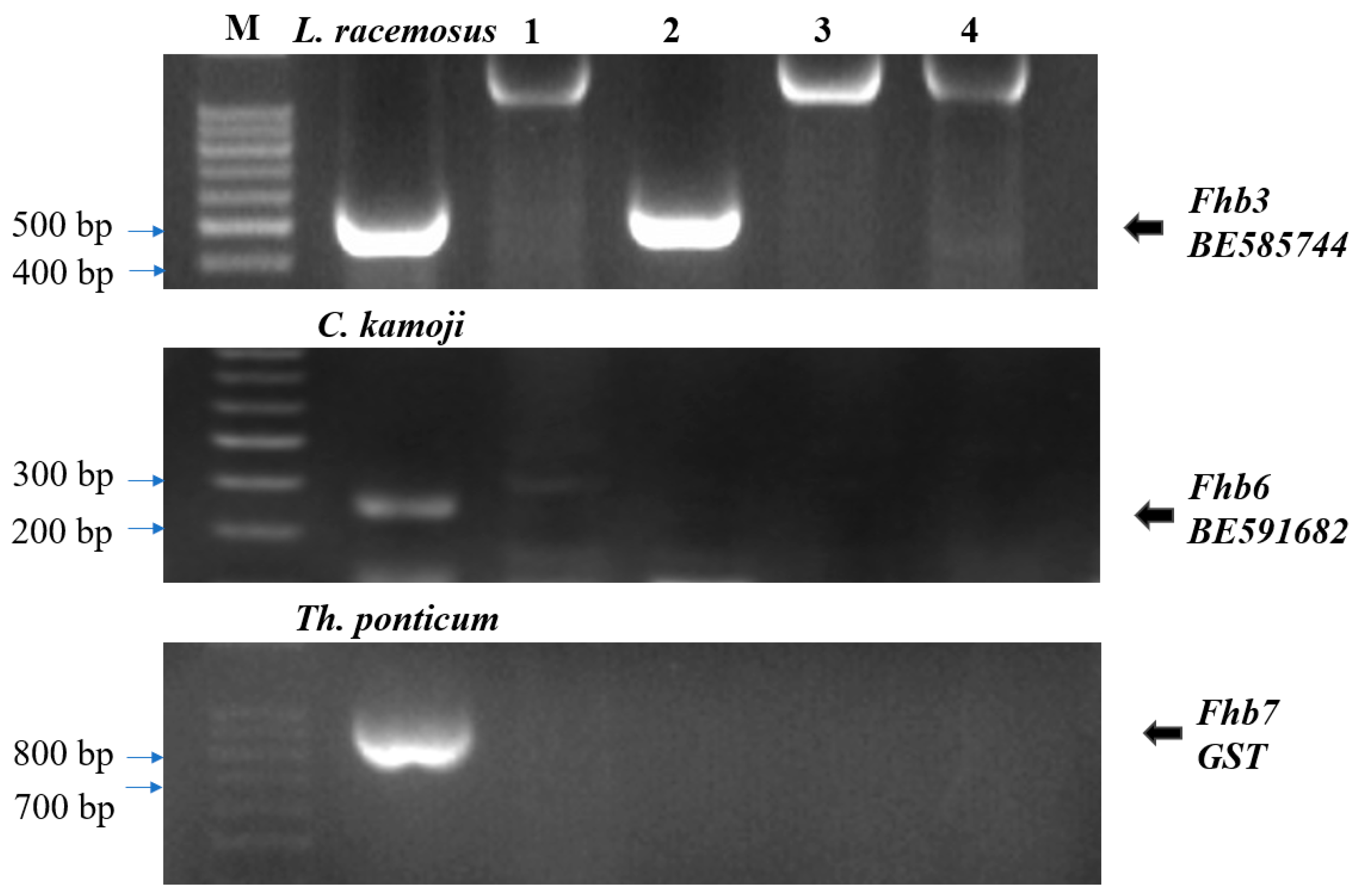
2.4. Detection of Known FHB Resistance Genes from Wild Relatives
2.5. Agronomic Trait Evaluation
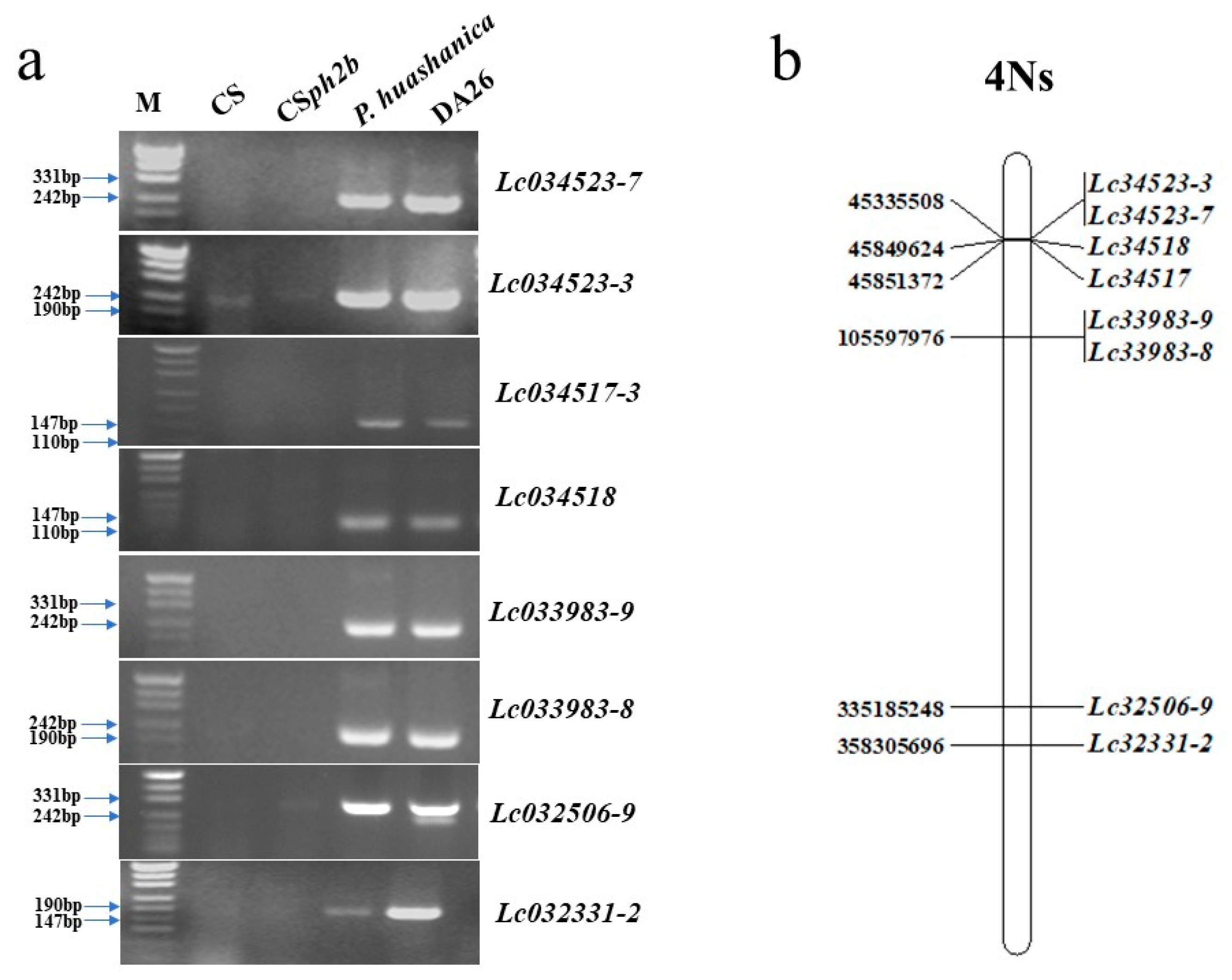
2.6. Development and Verification of PCR-Based Markers
3. Results
3.1. Identification of Wheat–Psathyrostachys huashanica 4Ns Additional Line
3.2. The 4Ns Additional Line Showed High Resistance to FHB
3.3. Detection of Exogenous Genes for FHB Resistance
3.4. The Specific Markers of Chromosome 4Ns for Wheat Breeding Selection
3.5. The FHB Resistance and Marker Linkage Analysis in DA26 Selfing Line
3.6. Agronomic Performance of 4Ns Additional Line
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Igrejas, G.; Branlard, G. The importance of wheat. In Wheat Quality for Improving Processing and Human Health; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–7. [Google Scholar] [CrossRef]
- McMullen, M.; Bergstrom, G.; De Wolf, E.; Dill-Macky, R.; Hershman, D.; Shaner, G.; Van Sanford, D. A unified effort to fight an enemy of wheat and barley: Fusarium head blight. Plant Dis. 2012, 96, 1712–1728. [Google Scholar] [CrossRef] [PubMed]
- Bottalico, A.; Perrone, G. Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. Eur. J. Plant Pathol. 2002, 108, 611–624. [Google Scholar] [CrossRef]
- Alisaac, E.; Mahlein, A.K. Fusarium head blight on wheat: Biology, modern detection and diagnosis and integrated disease management. Toxins 2023, 15, 192. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Zhang, X.; Yao, J.; Cheng, S. Breeding for the resistance to Fusarium head blight of wheat in China. Front. Agric. Sci. Eng. 2019, 6, 251. [Google Scholar] [CrossRef]
- Sobrova, P.; Adam, V.; Vasatkova, A.; Beklova, M.; Zeman, L.; Kizek, R. Deoxynivalenol and its toxicity. Interdiscip. Toxicol. 2010, 3, 94–99. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, H.; Liu, J.; Ren, X.; Ding, Y.; Sun, F.; Zhu, Z.; He, X.; Zhou, Y.; Bai, G.; et al. Fhb9, a major QTL for Fusarium head blight resistance improvement in wheat. J. Integr. Agric. 2024, in press. [Google Scholar] [CrossRef]
- Wang, H.; Sun, S.; Ge, W.; Zhao, L.; Hou, B.; Wang, K.; Lyu, Z.; Chen, L.; Xu, S.; Guo, J.; et al. Horizontal gene transfer of Fhb7 from fungus underlies Fusarium head blight resistance in wheat. Science 2020, 368, eaba5435. [Google Scholar] [CrossRef]
- Li, G.; Zhou, J.; Jia, H.; Gao, Z.; Fan, M.; Luo, Y.; Zhao, P.; Xue, S.; Li, N.; Yuan, Y.; et al. Mutation of a histidine-rich calcium-binding-protein gene in wheat confers resistance to Fusarium head blight. Nat. Genet. 2019, 51, 1106–1112. [Google Scholar] [CrossRef]
- Su, Z.; Bernardo, A.; Tian, B.; Chen, H.; Wang, S.; Ma, H.; Cai, S.; Liu, D.; Zhang, D.; Li, T.; et al. A deletion mutation in TaHRC confers Fhb1 resistance to Fusarium head blight in wheat. Nat. Genet. 2019, 51, 1099–1105. [Google Scholar] [CrossRef]
- Guo, X.; Shi, Q.; Liu, Y.; Su, H.; Zhang, J.; Wang, M.; Wang, C.; Wang, J.; Zhang, K.; Fu, S.; et al. Systemic development of wheat–Thinopyrum Elongatum translocation lines and their deployment in wheat breeding for Fusarium head blight resistance. Plant J. 2023, 114, 1475–1489. [Google Scholar] [CrossRef]
- Bainotti, C.T.; Lewis, S.; Campos, P.; Alberione, E.; Salines, N.; Gomez, D.; Fraschina, J.; Salines, J.; Formica, M.B.; Donaire, G.; et al. MS INTA 416: A new argentinean wheat cultivar carrying Fhb1 and Lr47 resistance genes. Crop Breed. Appl. Biotechnol. 2017, 17, 274–280. [Google Scholar] [CrossRef]
- Zhang, M.; Jiang, P.; Wu, Q.; Han, X.; Man, J.; Sun, J.; Liang, J.; Chen, J.; Zhao, Q.; Guo, Y.; et al. Identification of candidate genes for Fusarium head blight resistance from QTLs using RIL population in wheat. Plant Mol. Biol. 2024, 114, 62. [Google Scholar] [CrossRef]
- Zheng, N.; Li, G.; Zhang, K.; Zheng, H.; Yang, J.; Yan, K.; Shi, C.; Su, Z.; Chen, F.; Wang, D.; et al. Analysis of Fhb1 gene and resistance to Fusarium head blight in 3,177 diverse wheat accessions. J. Cereal Sci. 2022, 104, 103387. [Google Scholar] [CrossRef]
- Korobkova, V.A.; Bespalova, L.A.; Yanovsky, A.S.; Chernook, A.G.; Kroupin, P.Y.; Arkhipov, A.V.; Yurkina, A.I.; Nazarova, L.A.; Mudrova, A.A.; Voropaeva, A.D.; et al. Permanent spreading of 1RS.1AL and 1RS.1BL translocations in modern wheat breeding. Plants 2023, 12, 1205. [Google Scholar] [CrossRef]
- Xing, L.; Yuan, L.; Lv, Z.; Wang, Q.; Yin, C.; Huang, Z.; Liu, J.; Cao, S.; Zhang, R.; Chen, P.; et al. Long-range assembly of sequences helps to unravel the genome structure and small variation of the wheat–Haynaldia Villosa translocated chromosome 6VS.6AL. Plant Biotechnol. J. 2021, 19, 1567–1578. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, X.; Yu, X.; Zhu, W.; Xu, L.; Cheng, Y.; Zhang, Y.; Wang, Y.; Zeng, J.; Fan, X.; et al. Identification and transfer of resistance to Fusarium head blight from Elymus Repens chromosome arm 7StL into wheat. J. Integr. Agric. 2024, in press. [Google Scholar] [CrossRef]
- Song, R.; Zhang, D.; Yang, J.; Cheng, Y.; Song, X.; Zhao, W.; Xia, M.; Zhang, Y.; Wei, L.; Cheng, M.; et al. Identification and transferring of a new Fusarium head blight resistance gene FhbRc2 from Roegneria ciliaris 3ScL chromosome arm into common wheat. Crop J. 2024, 12, 1718–1726. [Google Scholar] [CrossRef]
- Wu, D.; Wang, F.; Chen, L.; Mao, Y.; Li, Y.; Zhu, W.; Xu, L.; Zhang, Y.; Wang, Y.; Zeng, J.; et al. Characterization of the wheat-Tetraploid Thinopyrum Elongatum 7E(7D) substitution line with Fusarium head blight resistance. BMC Plant Biol. 2024, 24, 1006. [Google Scholar] [CrossRef]
- Tan, B.; Zhao, L.; Li, L.; Zhang, H.; Zhu, W.; Xu, L.; Wang, Y.; Zeng, J.; Fan, X.; Sha, L.; et al. Identification of a wheat- Psathyrostachys huashanica 7Ns ditelosomic addition line conferring early maturation by cytological analysis and newly developed molecular and FISH markers. Front. Plant Sci. 2021, 12, 784001. [Google Scholar] [CrossRef]
- Kang, H.Y.; Tang, L.; Li, D.Y.; Diao, C.D.; Zhu, W.; Tang, Y.; Wang, Y.; Fan, X.; Xu, L.L.; Zeng, J.; et al. Cytogenetic study and stripe rust response of the derivatives from a wheat–Thinopyrum Intermedium–Psathyrostachys huashanica trigeneric hybrid. Genome 2016, 60, 393–401. [Google Scholar] [CrossRef]
- Zhang, H.; Zeng, C.; Li, L.; Zhu, W.; Xu, L.; Wang, Y.; Zeng, J.; Fan, X.; Sha, L.; Wu, D.; et al. RNA-Seq analysis revealed considerable genetic diversity and enabled the development of specific KASP markers for Psathyrostachys huashanica. Front. Plant Sci. 2023, 14, 1166710. [Google Scholar] [CrossRef]
- Pang, J.; Huang, C.; Wang, Y.; Wen, X.; Deng, P.; Li, T.; Wang, C.; Liu, X.; Chen, C.; Zhao, J.; et al. Molecular cytological analysis and specific marker development in wheat- Psathyrostachys huashanica Keng 3Ns additional line with elongated glume. Int. J. Mol. Sci. 2023, 24, 6726. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.Y.; Zhang, Z.J.; Xu, L.L.; Qi, W.L.; Tang, Y.; Wang, H.; Zhu, W.; Li, D.Y.; Zeng, J.; Wang, Y.; et al. Characterization of wheat-Psathyrostachys huashanica small segment translocation line with enhanced kernels per spike and stripe rust resistance. Genome 2016, 59, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.R.C. Synthetic and natural hybrids of Psathyrostachys huashanica. Genome 1987, 29, 811–816. [Google Scholar] [CrossRef]
- Kang, H.Y.; Zhang, H.Q.; Fan, X.; Zhou, Y.H. Morphological and cytogenetic studies on the hybrid between bread wheat and Psathyrostachys huashanica Keng ex Kuo. Euphytica 2008, 162, 441–448. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, F.; Zeng, C.; Zhu, W.; Xu, L.; Wang, Y.; Zeng, J.; Fan, X.; Sha, L.; Wu, D.; et al. Development and application of specific FISH probes for karyotyping Psathyrostachys huashanica chromosomes. BMC Genom. 2022, 23, 309. [Google Scholar] [CrossRef]
- Tang, Z.; Yang, Z.; Fu, S. Oligonucleotides replacing the roles of repetitive sequences pAs1, pSc119.2, pTa-535, pTa71, CCS1, and pAWRC.1 for FISH analysis. J. Appl. Genet. 2014, 55, 313–318. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Li, Z.; Man, J.; Xu, D.; Wen, L.; Yang, C.; Xu, Q.; Jiang, Q.-T.; Chen, G.-Y.; Deng, M.; et al. Genetic diversity of field Fusarium asiaticum and Fusarium graminearum isolates increases the risk of fungicide resistance. Phytopathol. Res. 2023, 5, 51. [Google Scholar] [CrossRef]
- NY/T 2954-2016; Technical Regulations for Resistance Evaluation of Wheat for Trials to Fusarium Head Blight Caused by Fusarium graminearum Schw. The Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2016.
- Qi, L.L.; Pumphrey, M.O.; Friebe, B.; Chen, P.D.; Gill, B.S. Molecular cytogenetic characterization of alien introgressions with gene Fhb3 for resistance to Fusarium head blight disease of wheat. Theor. Appl. Genet. 2008, 117, 1155–1166. [Google Scholar] [CrossRef]
- Cainong, J.C.; Bockus, W.W.; Feng, Y.; Chen, P.; Qi, L.; Sehgal, S.K.; Danilova, T.V.; Koo, D.H.; Friebe, B.; Gill, B.S. Chromosome engineering, mapping, and transferring of resistance to Fusarium head blight disease from Elymus tsukushiensis into wheat. Theor. Appl. Genet. 2015, 128, 1019–1027. [Google Scholar] [CrossRef]
- Li, T.; Tang, S.; Li, W.; Zhang, S.; Wang, J.; Pan, D.; Lin, Z.; Ma, X.; Chang, Y.; Liu, B.; et al. Genome Evolution and Initial Breeding of the triticeae grass Leymus Chinensis dominating the eurasian steppe. Proc. Natl. Acad. Sci. USA 2023, 120, e2308984120. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Liu, Y.; Hou, C.; Li, J.; Wang, J.; Zhang, Q.; Yang, Q.; Chen, X.; Wu, J. A 1Ns disomic addition from Psathyrostachys huashanica Keng confers resistance to powdery mildew in wheat. Agronomy 2020, 10, 312. [Google Scholar] [CrossRef]
- Du, W.; Wang, J.; Pang, Y.; Wu, J.; Zhao, J.; Liu, S.; Chen, X.; Yang, Q. Development and application of PCR markers specific to the 1Ns chromosome of Psathyrostachys huashanica Keng with leaf rust resistance. Euphytica 2014, 200, 207–220. [Google Scholar] [CrossRef]
- Du, W.; Wang, J.; Wang, L.; Wu, J.; Zhao, J.; Liu, S.; Chen, X.; Yang, Q. Molecular characterization of a wheat–Psathyrostachys huashanica Keng 2Ns disomic addition line with resistance to stripe rust. Mol. Genet. Genom. 2014, 289, 735–743. [Google Scholar] [CrossRef]
- Bai, S.; Yuan, F.; Zhang, H.; Zhang, Z.; Zhao, J.; Yang, Q.; Chen, X.; Wu, J. Characterization of the Wheat-Psathyrostachys huashanica Keng 2Ns/2D Substitution Line H139: A novel germplasm with enhanced resistance to wheat take-all. Front. Plant Sci. 2020, 11, 233. [Google Scholar] [CrossRef]
- Du, W.; Wang, J.; Pang, Y.; Wang, L.; Wu, J.; Zhao, J.; Chen, X.; Yang, Q. Isolation and characterization of a wheat–Psathyrostachys huashanica Keng 3Ns disomic addition line with resistance to stripe rust. Genome 2014, 57, 37–44. [Google Scholar] [CrossRef]
- Du, W.; Wang, J.; Lu, M.; Sun, S.; Chen, X.; Zhao, J.; Wu, J.; Yang, Q. Characterization of a wheat-Psathyrostachys huashanica Keng 4Ns disomic addition line for enhanced tiller numbers and stripe rust resistance. Planta 2014, 239, 97–105. [Google Scholar] [CrossRef]
- Du, W.; Wang, J.; Lu, M.; Sun, S.; Chen, X.; Zhao, J.; Wu, J.; Yang, Q. Molecular cytogenetic identification of a wheat–Psathyrostachys huashanica Keng 5Ns disomic addition line with stripe rust resistance. Mol. Breeding 2013, 31, 879–888. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Cheng, X.; Yang, Z.; Pang, Y.; Wang, C.; Wu, J.; Ji, W.; Chen, X.; Zhao, J. The addition of Psathyrostachys huashanica Keng 6Ns large segment chromosomes has positive impact on stripe rust resistance and plant spikelet number of common wheat. BMC Plant Biol. 2024, 24, 685. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Du, W.; Jing, F.; Wang, Z.; Wu, J.; Chen, X. Anatomy and cytogenetic identification of a wheat-Psathyrostachys huashanica Keng line with early maturation. PLoS ONE 2015, 10, e0131841. [Google Scholar] [CrossRef]
- Tan, B.; Wang, M.; Cai, L.; Li, S.; Zhu, W.; Xu, L.; Wang, Y.; Zeng, J.; Fan, X.; Sha, L.; et al. Cytogenetic and molecular marker analyses of a novel wheat–Psathyrostachys huashanica 7Ns Disomic addition line with powdery mildew resistance. Int. J. Mol. Sci. 2022, 23, 10285. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Wang, J.; Wang, L.; Zhang, J.; Chen, X.; Zhao, J.; Yang, Q.; Wu, J. Development and characterization of a Psathyrostachys huashanica Keng 7Ns chromosome addition line with leaf rust resistance. PLoS ONE 2013, 8, e70879. [Google Scholar] [CrossRef]
- Hou, C.; Han, J.; Zhang, L.; Geng, Q.; Zhao, L.; Liu, S.; Yang, Q.; Chen, X.; Wu, J. Identification of resistance to FHB and molecular cytogenetics of interspecific derivatives between wheat and Psathyrostachys huashanica. Breed Sci. 2022, 72, 21089. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, S.; Lang, T.; Wang, Y.; Long, H.; Deng, G.; Chen, Q.; Guo, Y.; Xuan, P.; Xiao, J.; et al. Transmission of Dasypyrum villosum 3V Chromosome in Different Wheat Genetic Backgrounds. J. Plant Genet. Resour. 2021, 22, 1355–1364. [Google Scholar] [CrossRef]
- Li, H.; Liu, H.; Dai, Y.; Huang, S.; Zhang, J.; Gao, Y.; Chen, J. Transmission Characteristics of Thinopyrum elongatum E Chromosomes in Tetraploid Wheat Background. Yi Chuan 2016, 38, 1019–1028. [Google Scholar] [CrossRef]
- Kong, L.; Song, X.; Xiao, J.; Sun, H.; Dai, K.; Lan, C.; Singh, P.; Yuan, C.; Zhang, S.; Singh, R.; et al. Development and characterization of a complete set of Triticum aestivum–Roegneria ciliaris disomic addition lines. Theor. Appl. Genet. 2018, 131, 1793–1806. [Google Scholar] [CrossRef]
- Song, R.; Cheng, Y.; Wen, M.; Song, X.; Wang, T. Transferring a new Fusarium head blight resistance locus FhbRc1 from Roegneria ciliaris into wheat by developing alien translocation lines. Theor. Appl. Genet. 2023, 136, 36. [Google Scholar] [CrossRef]
- Liu, L.; Luo, Q.; Li, H.; Li, B.; Li, Z.; Zheng, Q. Physical mapping of the blue-grained gene from Thinopyrum ponticum chromosome 4Ag and development of blue-grain-related molecular markers and a FISH probe based on SLAF-Seq technology. Theor. Appl. Genet. 2018, 131, 2359–2370. [Google Scholar] [CrossRef]
- Wang, J.; Lu, M.; Du, W.L.; Zhang, J.; Dong, X.Y.; Wu, J.; Zhao, J.X.; Yang, Q.H.; Chen, X.H. A novel PCR-based marker for identifying Ns chromosomes in wheat-Psathyrostachys huashanica Keng derivative lines. Span J. Agric. Res. 2013, 11, 1094–1100. [Google Scholar] [CrossRef]
- Du, W.L.; Wang, J.; Wang, L.M.; Pang, Y.H.; Wu, J.; Zhao, J.X.; Yang, Q.H.; Chen, X.H. A novel SCAR marker for detecting Psathyrostachys huashanica Keng chromatin introduced in wheat. Genet. Mol. Res. 2013, 12, 4797–4806. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.M.; Du, W.L.; Chen, L.G.; Liu, S.H.; Wu, J.; Zhao, J.X.; Yang, Q.H.; Chen, X.H. Development of 5Ns chromosome-specific SCAR markers for utilization in future wheat breeding programs. Russ. J. Genet. 2014, 50, 606–612. [Google Scholar] [CrossRef]

| Materials | Plant Height (cm) | Tiller | Spike Length (cm) | The Number of Spikelets | Grains per Spike | 1000 Grain Weight (g) |
|---|---|---|---|---|---|---|
| CS | 144.17 ± 1.26 a | 10.79 ±1.17 a | 9.86 ± 0.29 a | 24.79 ± 0.44 a | 57.21 ± 1.20 a | 23.05 ± 0.05 a |
| CSph2b | 141.76 ± 0.97 ab | 10.21 ±0.33 a | 9.56 ± 0.18 a | 24.07 ± 0.49 a | 54.43 ± 1.90 a | 22.69 ± 0.13 a |
| DA26 | 138.30 ± 2.33 b | 11.57 ±1.29 a | 9.34 ± 0.27 a | 23.07 ± 0.68 a | 55.07 ± 2.45 a | 22.50 ± 0.71 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Peng, H.; Zhang, H.; Li, L.; Saqlain, M.; Wu, D.; Zhu, W.; Xu, L.; Cheng, Y.; Wang, Y.; et al. Wheat-Psathyrostachys huashanica 4Ns Additional Line Confers Resistance to Fusarium Head Blight. Plants 2025, 14, 1104. https://doi.org/10.3390/plants14071104
Li Y, Peng H, Zhang H, Li L, Saqlain M, Wu D, Zhu W, Xu L, Cheng Y, Wang Y, et al. Wheat-Psathyrostachys huashanica 4Ns Additional Line Confers Resistance to Fusarium Head Blight. Plants. 2025; 14(7):1104. https://doi.org/10.3390/plants14071104
Chicago/Turabian StyleLi, Yinghui, Hang Peng, Hao Zhang, Liangxi Li, Muhammad Saqlain, Dandan Wu, Wei Zhu, Lili Xu, Yiran Cheng, Yi Wang, and et al. 2025. "Wheat-Psathyrostachys huashanica 4Ns Additional Line Confers Resistance to Fusarium Head Blight" Plants 14, no. 7: 1104. https://doi.org/10.3390/plants14071104
APA StyleLi, Y., Peng, H., Zhang, H., Li, L., Saqlain, M., Wu, D., Zhu, W., Xu, L., Cheng, Y., Wang, Y., Zeng, J., Sha, L., Zhang, H., Fan, X., Zhou, Y., & Kang, H. (2025). Wheat-Psathyrostachys huashanica 4Ns Additional Line Confers Resistance to Fusarium Head Blight. Plants, 14(7), 1104. https://doi.org/10.3390/plants14071104






