Biodiversity Dynamics in a Ramsar Wetland: Assessing How Climate and Hydrology Shape the Distribution of Dominant Native and Alien Macrophytes
Abstract
1. Introduction
2. Results
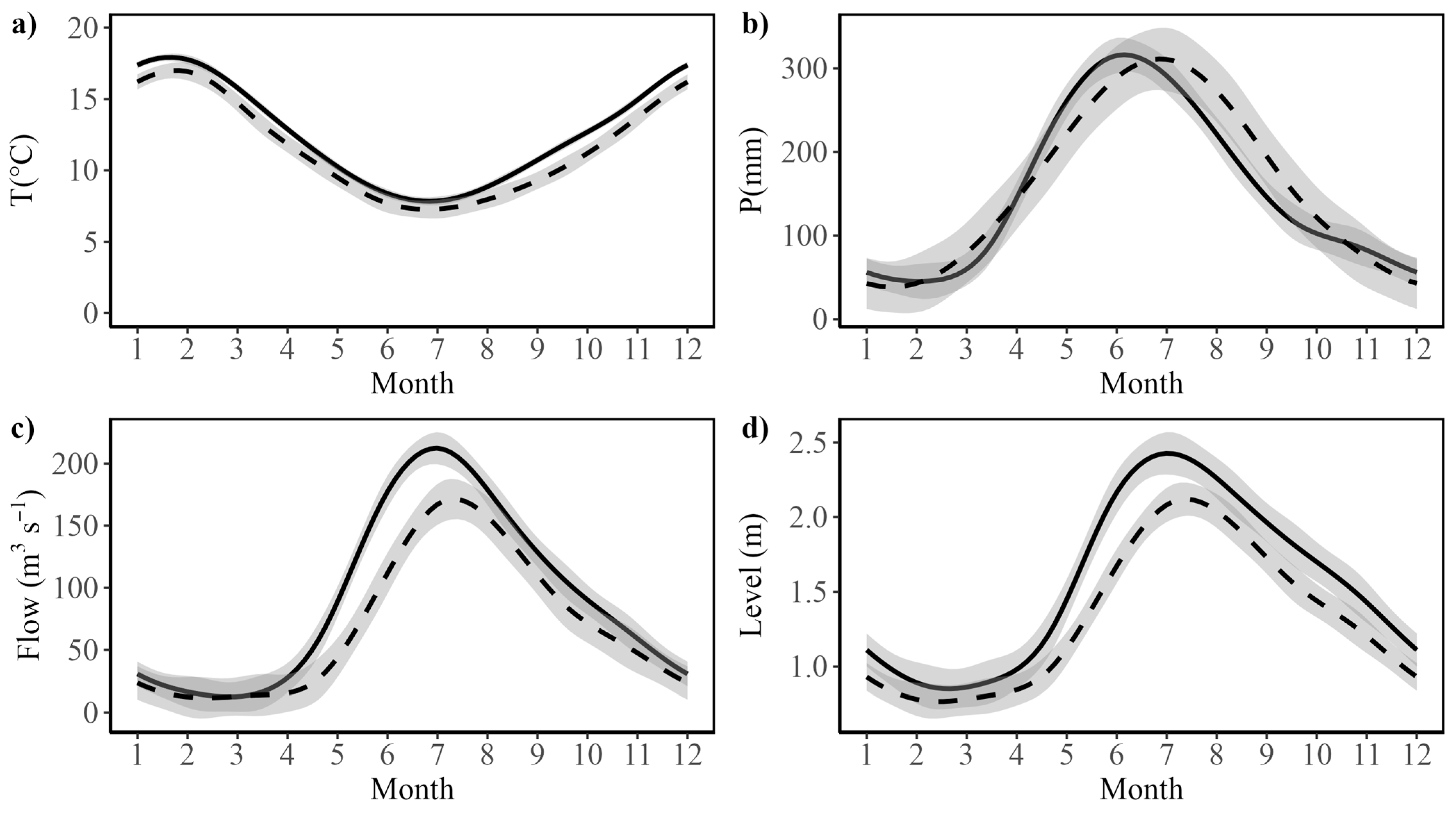
2.1. Environmental Variability
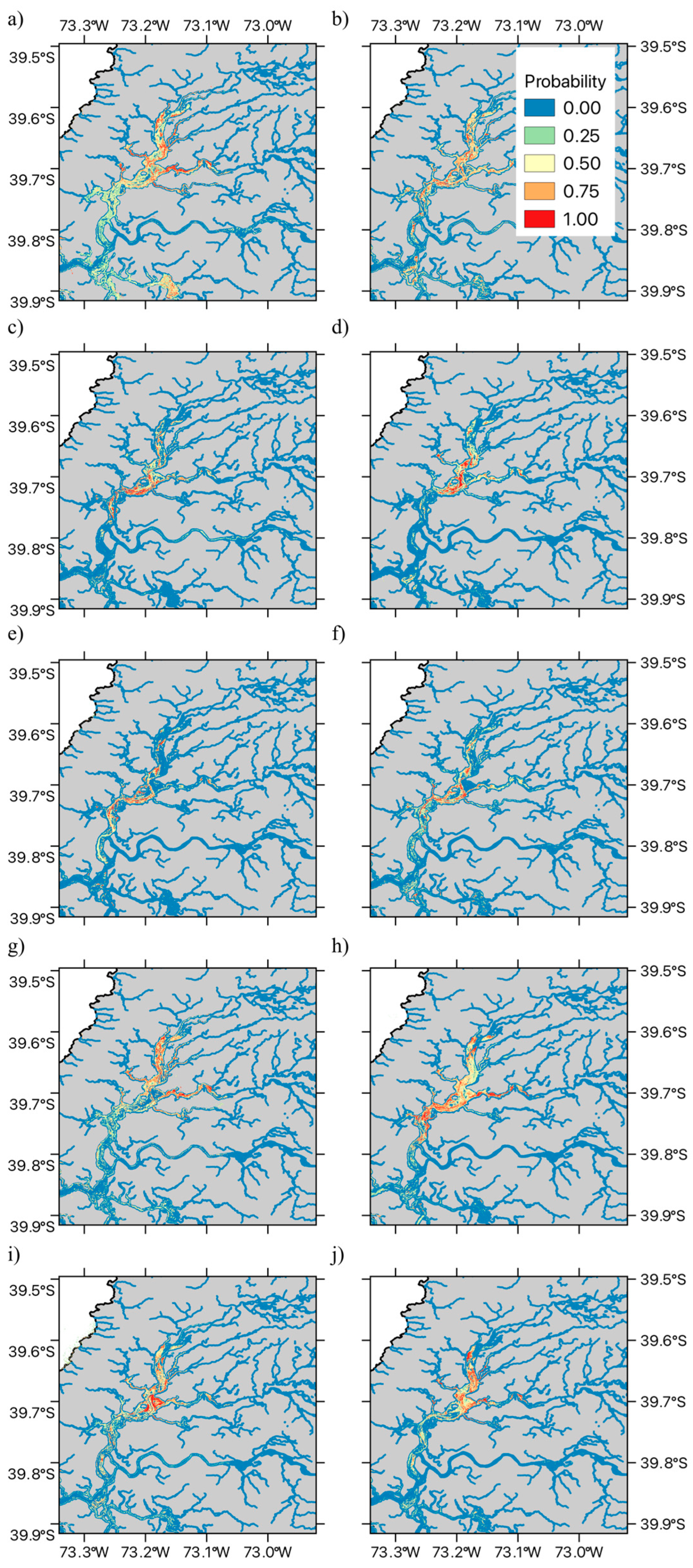
2.2. Species Distribution Modeling
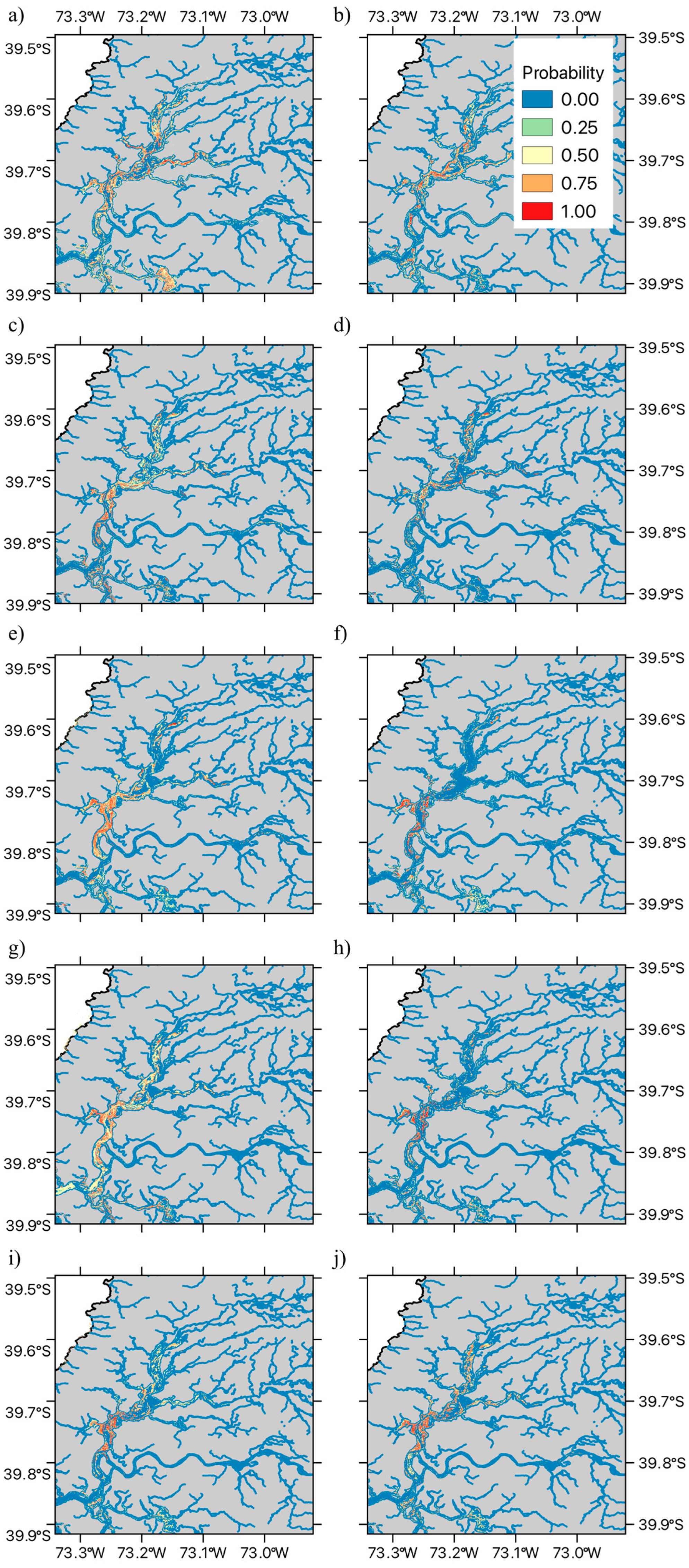
2.3. Assessing Interdecadal Variation in the Area of Suitable Habitat
3. Discussion
3.1. Climatic and Hydrological Variability in the Study Area
3.2. Species Distribution Modeling
3.3. Assessing Interdecadal Variation in the Area of Suitable Habitat
4. Materials and Methods
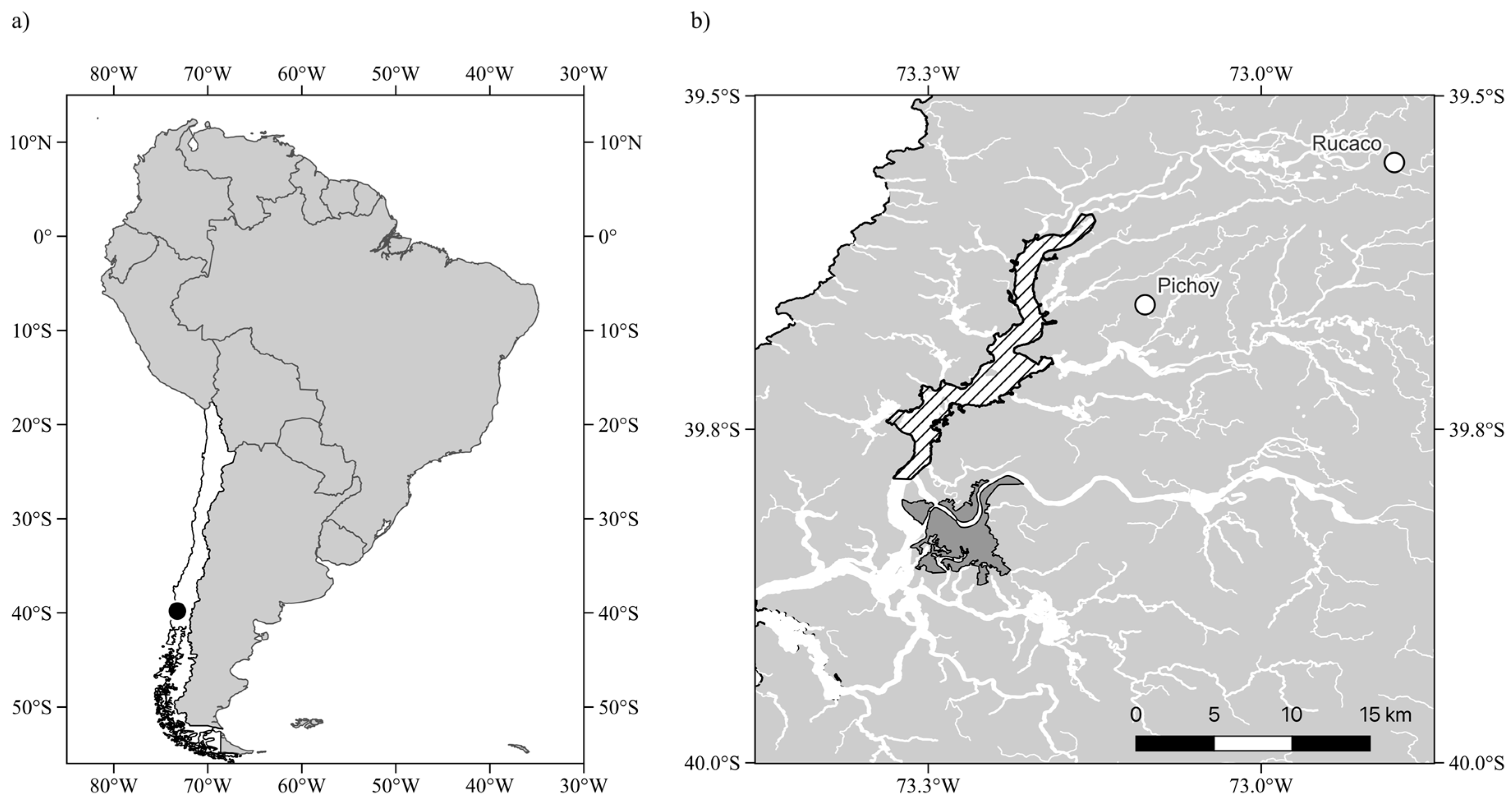
4.1. Study Area and Environmental Variability
4.1.1. Field Surveys
4.1.2. Environmental Variability
4.1.3. Assessing Interdecadal Variation
4.1.4. Assessing Recent Climatic and Hydrological Variability
4.2. Remote Sensing Image Acquisition and Processing
4.3. Species Distribution Modeling
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SEPF | Southeastern Pacific Flyway |
| RCW | Rio Cruces Wetland |
| IAS | Invasive Alien Species |
| ENSO | El Niño-Southern Oscillation |
| PDO | Pacific Decadal Oscillation |
| AMO | Atlantic Multidecadal Oscillation |
| SAM | Southern Annular Mode |
| AAO | Antarctic Oscillation |
| SDM | species distribution model |
| GAM | general additive model |
| T | average monthly air temperature |
| P | average monthly precipitation |
| Flow | river flow |
| Level | water level |
| df | degrees of freedom |
| MaxEnt | Maximum Entropy species distribution modeling software |
| N | number of monodominant macrophyte patches with diameter > 30 m |
| AUC | Area Under the Receiver Operating Characteristic Curve |
| MSS | Maximum test sensitivity plus specificity Cloglog threshold |
| ROC | Receiver Operating Characteristic Curve |
| OLS | Ordinary least Squares |
| CV | Coefficient of Variation |
| s.d. Level | standard deviation of water level |
| Thour | mean hourly temperature |
| sPhour | accumulated hourly precipitation |
| TYear | mean annual temperature |
| sPYear | accumulated annual precipitation |
| LevelYear | mean annual water level |
| AIC | Akaike Information Criterion |
| H2O2 | hydrogen peroxide |
| GPS | Global Positioning System |
| OLI | Operational Land Imager |
| WRS-2 | Worldwide Reference System 2 |
| GIS | Geographic Information System |
| RTOA | top-of-atmosphere reflectance |
| CHL | chlorophyll proxy |
| NDVI | normalized difference vegetation index |
| NIR | Near Infrared |
| HS | environmental habitat suitability |
References
- Dise, N.B. Peatland Response to Global Change. Science 2009, 326, 810–811. [Google Scholar] [CrossRef] [PubMed]
- Barbier, E.B.; Hacker, S.D.; Kennedy, C.; Koch, E.W.; Stier, A.C.; Silliman, B.R. The Value of Estuarine and Coastal Ecosystem Services. Ecol. Monogr. 2011, 81, 169–193. [Google Scholar] [CrossRef]
- Fariña, J.M.; Camaño, A. (Eds.) The Ecology and Natural History of Chilean Saltmarshes; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Marquet, P.A.; Abades, S.; Barría, I. Distribution and Conservation of Coastal Wetlands: A Geographic Perspective. In The Ecology and Natural History of Chilean Saltmarshes; Fariña, J.M., Camaño, A., Eds.; Springer: Cham, Switzerland, 2017; pp. 1–14. [Google Scholar]
- Stagg, C.L.; Osland, M.J.; Moon, J.A.; Hall, C.T.; Feher, L.C.; Jones, W.R.; Couvillion, B.R.; Hartley, S.B.; Vervaeke, W.C. Quantifying Hydrologic Controls on Local- and Landscape-Scale Indicators of Coastal Wetland Loss. Ann. Bot. 2019, 125, 365–376. [Google Scholar] [CrossRef]
- Navarro, N.; Abad, M.; Bonnail, E.; Izquierdo, T. The Arid Coastal Wetlands of Northern Chile: Towards an Integrated Management of Highly Threatened Systems. J. Mar. Sci. Eng. 2021, 9, 948. [Google Scholar] [CrossRef]
- Hidalgo-Corrotea, C.; Alaniz, A.J.; Vergara, P.M.; Moreira-Arce, D.; Carvajal, M.A.; Pacheco-Cancino, P.; Espinosa, A. High Vulnerability of Coastal Wetlands in Chile at Multiple Scales Derived from Climate Change, Urbanization, and Exotic Forest Plantations. Sci. Total Environ. 2023, 903, 166130. [Google Scholar] [CrossRef]
- Barbier, E.B. Coastal Wetlands; Elsevier: Amsterdam, The Netherlands, 2019; pp. 947–964. [Google Scholar] [CrossRef]
- Perillo; Wolanski, E.; Cahoon, D.R.; Hopkinson, C.S. Coastal Wetlands: An Integrated Ecosystem Approach; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9 78-0-444-63893-9. [Google Scholar]
- Hopkinson, C.S.; Wolanski, E.; Cahoon, D.R.; Perillo, G.M.E.; Brinson, M.M. Coastal Wetlands; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–75. [Google Scholar] [CrossRef]
- Estades, C.F.; Vukasovic, M.A.; Aguirre, J. Birds in Coastal Wetlands of Chile. In The Ecology and Natural History of Chilean Saltmarshes; Fariña, J.M., Camaño, A., Eds.; Springer: Cham, Switzerland, 2017; pp. 47–70. [Google Scholar]
- Ruiz, S.; Jimenez-Bluhm, P.; Pillo, F.D.; Baumberger, C.; Galdames, P.; Marambio, V.; Salazar, C.; Mattar, C.; Sanhueza, J.; Schultz-Cherry, S.; et al. Temporal Dynamics and the Influence of Environmental Variables on the Prevalence of Avian Influenza Virus in Main Wetlands in Central Chile. Transbound. Emerg. Dis. 2021, 68, 1601–1614. [Google Scholar] [CrossRef]
- Gherardi-Fuentes, C.; Ruiz, J.; Navedo, J.G. Insights into Migratory Connectivity and Conservation Concerns of Red Knots Calidris canutus in the Austral Pacific Coast of the Americas. Bird Conserv. Int. 2022, 32, 223–231. [Google Scholar] [CrossRef]
- Lagos, N.A.; Labra, F.A.; Jaramillo, E.; Marn, A.; Faria, J.M.; Camaño, A. Ecosystem Processes, Management and Human Dimension of Tectonically-Influenced Wetlands along the Coast of Central and Southern Chile. Gayana 2019, 83, 57–62. [Google Scholar] [CrossRef]
- Zedler, J.B.; Kercher, S. Wetland Resources: Status, Trends, Ecosystem Services, and Restorability. Annu. Rev. Environ. Resour. 2005, 30, 39–74. [Google Scholar] [CrossRef]
- Lopetegui, E.J.; Vollman, R.S.; Contreras, H.C.; Valenzuela, C.D.; Suarez, N.L.; Herbach, E.P.; Huepe, J.U.; Jaramillo, G.V.; Leischner, B.P.; Riveros, R.S. Emigration and Mortality of Black-Necked Swans (Cygnus melancoryphus) and Disappearance of the Macrophyte Egeria densa in a Ramsar Wetland Site of Southern Chile. AMBIO A J. Hum. Environ. 2007, 36, 607–610. [Google Scholar] [CrossRef]
- Lagos, N.A.; Paolini, P.; Jaramillo, E.; Lovengreen, C.; Duarte, C.; Contreras, H. Environmental Processes, Water Quality Degradation, and Decline of Waterbird Populations In the Rio Cruces Wetland, Chile. Wetlands 2008, 28, 938–950. [Google Scholar] [CrossRef]
- Winckler, P.; Contreras-López, M.; Garreaud, R.; Meza, F.; Larraguibel, C.; Esparza, C.; Gelcich, S.; Falvey, M.; Mora, J. Analysis of Climate-Related Risks for Chile’s Coastal Settlements in the ARClim Web Platform. Water 2022, 14, 3594. [Google Scholar] [CrossRef]
- Plafker, G.; Savage, J.C. Mechanism of the Chilean Earthquakes of May 21 and 22, 1960. GSA Bull. 1970, 81, 1001–1030. [Google Scholar] [CrossRef]
- DeMets, C.; Gordon, R.G.; Argus, D.; Stein, S. Current Plate Motions. Geophys. J. Int. 1990, 101, 425–478. [Google Scholar] [CrossRef]
- Moreno, M.S.; Bolte, J.; Klotz, J.; Melnick, D. Impact of Megathrust Geometry on Inversion of Coseismic Slip from Geodetic Data: Application to the 1960 Chile Earthquake. Geophys. Res. Lett. 2009, 36. [Google Scholar] [CrossRef]
- Manzano, C.; Jaramilo, L.; Pino, Q.M. Tidal Flats of Recent Origin: Distribution and Sedimentological Characterization in the Estuarine Cruces River Wetland, Chile. Lat. Am. J. Aquat. Res. 2020, 48, 662–673. [Google Scholar] [CrossRef]
- Garcés-Vargas, J.; Schneider, W.; Pinochet, A.; Piñones, A.; Olguin, F.; Brieva, D.; Wan, Y. Tidally Forced Saltwater Intrusions Might Impact the Quality of Drinking Water, the Valdivia River (40° S), Chile Estuary Case. Water 2020, 12, 2387. [Google Scholar] [CrossRef]
- Ramírez, C.; Martín, C.S.; Medina, R.; Contreras, D. Estudio de La Flora Hidrófila Del Santuario de La Naturaleza “Río Cruces”(Valdivia, Chile). Gayana Bot. 1991, 48, 64–80. [Google Scholar]
- Rubilar, P.; Jaramillo, E.; Salamanca, M.; Chandia, C.; Figueroa, C.; Franyola, G. Programa de Monitoreo Ambiental Actualizado Del Humedal Del Río Cruces y Sus Ríos Tributarios; 2015–2020; Informe Final Consolidado; Universidad Austral de Chile: Valdivia, Chile, 2020. [Google Scholar]
- Saenz-Agudelo, P.; Delrieu-Trottin, E.; DiBattista, J.D.; Martínez-Rincon, D.; Morales-González, S.; Pontigo, F.; Ramírez, P.; Silva, A.; Soto, M.; Correa, C. Monitoring Vertebrate Biodiversity of a Protected Coastal Wetland Using EDNA Metabarcoding. Environ. DNA 2022, 4, 77–92. [Google Scholar] [CrossRef]
- Escaida, J. Crisis Socioambiental: El Humedal Del Río Cruces y El Cisne de Cuello Negro; Ediciones Universidad Austral de Chile: Valdivia, Chile, 2014; Volume 1. [Google Scholar]
- Jaramillo, E.; Lagos, N.A.; Labra, F.A.; Paredes, E.; Acuña, E.; Melnick, D.; Manzano, M.; Velásquez, C.; Duarte, C. Recovery of Black-Necked Swans, Macrophytes and Water Quality in a Ramsar Wetland of Southern Chile: Assessing Resilience Following Sudden Anthropogenic Disturbances. Sci. Total Environ. 2018, 628, 291–301. [Google Scholar] [CrossRef]
- Jaramillo, E.; Duarte, C.; Labra, F.A.; Lagos, N.A.; Peruzzo, B.; Silva, R.; Velasquez, C.; Manzano, M.; Melnick, D. Resilience of an Aquatic Macrophyte to an Anthropogenically Induced Environmental Stressor in a Ramsar Wetland of Southern Chile. Ambio 2019, 48, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Velásquez, C.; Jaramillo, E.; Camus, P.; Labra, F.; Martín, C.S. Dietary Habits of the Black-Necked Swan Cygnus melancoryphus (Birds: Anatidae) and Variability of the Aquatic Macrophyte Cover in the Río Cruces Wetland, Southern Chile. PLoS ONE 2019, 14, e0226331. [Google Scholar] [CrossRef]
- Yarrow, M.; Marin, V.H.; Finlayson, M.; Tironi, A.; Delgado, L.E.; Fischer, F. The Ecology of Egeria densa Planchon (Liliopsida: Alismatales): A Wetland Ecosystem Engineer? Rev. Chil. Hist. Nat. 2009, 82, 299–313. [Google Scholar] [CrossRef]
- Fariña, J.M.; Silliman, B.R.; Bertness, M.D. Can Conservation Biologists Rely on Established Community Structure Rules to Manage Novel Systems? … Not in Salt Marshes. Ecol. Appl. 2009, 19, 413–422. [Google Scholar] [CrossRef]
- Velasquez, C.; Jaramillo, E.; Camus, P.A.; Martín, C.S. Consumption of Aquatic Macrophytes by the Red-Gartered Coot Fulica Armillata (Aves: Rallidae) in a Coastal Wetland of North Central Chile. Gayana 2019, 83, 68–72. [Google Scholar] [CrossRef]
- Ramirez, C.; Álvarez, M. Hydrophilic Flora and Vegetation of the Coastal Wetlands of Chile. In The Ecology and Natural History of Chilean Saltmarshes; Springer: Cham, Switzerland, 2017; pp. 71–103. [Google Scholar]
- Bouma, T.J.; Vries, M.B.D.; Low, E.; Peralta, G.; Tánczos, I.C.; Koppel, J.; van de Herman, P.M.J. Trade-offs related to ecosystem engineering: A case study on stiffness of emerging macrophytes. Ecology 2005, 86, 2187–2199. [Google Scholar] [CrossRef]
- Leonard, L.A.; Croft, A.L. The Effect of Standing Biomass on Flow Velocity and Turbulence in Spartina Alterniflora Canopies. Estuar. Coast. Shelf Sci. 2006, 69, 325–336. [Google Scholar] [CrossRef]
- Anthony, E.J. Shore Processes and Their Palaeoenvironmental Applications; Elsevier: Amsterdam, The Netherlands, 2008; Volume 4. [Google Scholar]
- Ma, G.; Han, Y.; Niroomandi, A.; Lou, S.; Liu, S. Numerical Study of Sediment Transport on a Tidal Flat with a Patch of Vegetation. Ocean Dyn. 2015, 65, 203–222. [Google Scholar] [CrossRef]
- Gillard, M.; Thiébaut, G.; Deleu, C.; Leroy, B. Present and Future Distribution of Three Aquatic Plants Taxa across the World: Decrease in Native and Increase in Invasive Ranges. Biol. Invasions 2017, 19, 2159–2170. [Google Scholar] [CrossRef]
- Les, D.H.; Mehrhoff, L.J. Introduction of Nonindigenous Aquatic Vascular Plants in Southern New England: A Historical Perspective. Biol. Invasions 1999, 1, 281–300. [Google Scholar] [CrossRef]
- Roberts, D.E.; Church, A.; Cummins, S. Invasion of Egeria into the Hawkesbury-Nepean River, Australia. J. Aquat. Plant Manag. 1999, 37, 31–34. [Google Scholar]
- Coetzee, J.A.; Bownes, A.; Martin, G.D. Prospects for the Biological Control of Submerged Macrophytes in South Africa. Afr. Èntomol. 2011, 19, 469–487. [Google Scholar] [CrossRef]
- Hussner, A. Alien Aquatic Plant Species in European Countries. Weed Res. 2012, 52, 297–306. [Google Scholar] [CrossRef]
- Haramoto, T.; Ikusima, I. Life Cycle of Egeria densa Planch., an Aquatic Plant Naturalized in Japan. Aquat. Bot. 1988, 30, 389–403. [Google Scholar] [CrossRef]
- Asaeda, T.; Jayasanka, S.M.D.H.; Xia, L.-P.; Barnuevo, A. Application of Hydrogen Peroxide as an Environmental Stress Indicator for Vegetation Management. Engineering 2018, 4, 610–616. [Google Scholar] [CrossRef]
- Asaeda, T.; Senavirathna, M.D.H.J.; Krishna, L.V. Evaluation of Habitat Preferences of Invasive Macrophyte Egeria densa in Different Channel Slopes Using Hydrogen Peroxide as an Indicator. Front. Plant Sci. 2020, 11, 422. [Google Scholar] [CrossRef]
- Asaeda, T.; Rahman, M.; Liping, X.; Schoelynck, J. Hydrogen Peroxide Variation Patterns as Abiotic Stress Responses of Egeria densa. Front. Plant Sci. 2022, 13, 855477. [Google Scholar] [CrossRef]
- Xiong, W.; Xie, D.; Wang, Q.; Li, Y.; Shao, H.; Guo, Q.; Xu, K.; Wang, Y.; Xiao, K.; Tang, W.; et al. Large-Flowered Waterweed Egeria densa Planchon, 1849: A Highly Invasive Aquatic Species in China. BioInvasions Rec. 2024, 13, 787–798. [Google Scholar] [CrossRef]
- Shi, L.; Zheng, X.; Shan, H.; Hua, Z.; Wen, Z.; Yin, J.; Chou, Q.; Zhang, X.; Ni, L.; Cao, T. Niche Difference Prevents Competitive Exclusion between the Invasive Submerged Macrophyte Elodea densa (Planch.) Casp. and Native Hydrilla verticillata (Lf) Royle in a Large Plateau Lake. Freshw. Biol. 2025, 70, e14381. [Google Scholar] [CrossRef]
- Rodriguez, R.; Fica, B. Guía Campo Plantas Vasculares Acuáticas En Chile; Corporación Chilena de la Madera: Concepción, Chile, 2020. [Google Scholar]
- Yáñez-Cuadra, V.; Moreno, M.; Ortega-Culaciati, F.; Donoso, F.; Báez, J.C.; Tassara, A. Mosaicking Andean Morphostructure and Seismic Cycle Crustal Deformation Patterns Using GNSS Velocities and Machine Learning. Front. Earth Sci. 2023, 11, 1096238. [Google Scholar] [CrossRef]
- González-Reyes, A.; Muñoz, A.A. Cambios En La Precipitación de La Ciudad de Valdivia (Chile) Durante Los Ltimos 150 Aos. Bosque 2013, 34, 200–213. [Google Scholar] [CrossRef]
- Hernandez, D.; Mendoza, P.A.; Boisier, J.P.; Ricchetti, F. Hydrologic Sensitivities and ENSO Variability Across Hydrological Regimes in Central Chile (28°–41°S). Water Resour. Res. 2022, 58, e2021WR031860. [Google Scholar] [CrossRef]
- Valdés-Pineda, R.; Cañón, J.; Valdés, J.B. Multi-Decadal 40- to 60-Year Cycles of Precipitation Variability in Chile (South America) and Their Relationship to the AMO and PDO Signals. J. Hydrol. 2018, 556, 1153–1170. [Google Scholar] [CrossRef]
- Garreaud, R.D.; Boisier, J.P.; Rondanelli, R.; Montecinos, A.; Sepúlveda, H.H.; Veloso-Aguila, D. The Central Chile Mega Drought (2010–2018): A Climate Dynamics Perspective. Int. J. Clim. 2020, 40, 421–439. [Google Scholar] [CrossRef]
- Oñate-Valdivieso, F.; Uchuari, V.; Oñate-Paladines, A. Large-Scale Climate Variability Patterns and Drought: A Case of Study in South—America. Water Resour. Manag. 2020, 34, 2061–2079. [Google Scholar] [CrossRef]
- Boisier, J.P.; Rondanelli, R.; Garreaud, R.D.; Muñoz, F. Anthropogenic and Natural Contributions to the Southeast Pacific Precipitation Decline and Recent Megadrought in Central Chile. Geophys. Res. Lett. 2016, 43, 413–421. [Google Scholar] [CrossRef]
- Boisier, J.P.; Alvarez-Garreton, C.; Cordero, R.R.; Damiani, A.; Gallardo, L.; Garreaud, R.D.; Lambert, F.; Ramallo, C.; Rojas, M.; Rondanelli, R. Anthropogenic Drying in Central-Southern Chile Evidenced by Long-Term Observations and Climate Model Simulations. Elem. Sci. Anth 2018, 6, 74. [Google Scholar] [CrossRef]
- González-Reyes, Á.; Jacques-Coper, M.; Muñoz, A.A. Seasonal Precipitation in South Central Chile: Trends in Extreme Events since 1900. Atmósfera 2020, 34, 371–384. [Google Scholar] [CrossRef]
- Mandrekar, J.N. Receiver Operating Characteristic Curve in Diagnostic Test Assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R.; Huyvaert, K.P. AIC Model Selection and Multimodel Inference in Behavioral Ecology: Some Background, Observations, and Comparisons. Behav. Ecol. Sociobiol. 2011, 65, 23–35. [Google Scholar] [CrossRef]
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Pean, C.; Berger, S.; Caud, N.; Chen, Y.; Goldfarb, L.; Gomis, M.; et al. Contribution of working group I to the sixth assessment report of the intergovernmental panel on climate change. Clim. Change 2021 Phys. Sci. Basis 2021, 2, 2391. [Google Scholar]
- Viale, M.; Valenzuela, R.; Garreaud, R.D.; Ralph, F.M. Impacts of Atmospheric Rivers on Precipitation in Southern South America Impacts of Atmospheric Rivers on Precipitation in Southern South America. J. Hydrometeorol. 2018, 19, 1671–1687. [Google Scholar] [CrossRef]
- Payne, A.E.; Demory, M.-E.; Leung, L.R.; Ramos, A.M.; Shields, C.A.; Rutz, J.J.; Siler, N.; Villarini, G.; Hall, A.; Ralph, F.M. Responses and Impacts of Atmospheric Rivers to Climate Change. Nat. Rev. Earth Environ. 2020, 1, 143–157. [Google Scholar] [CrossRef]
- Garreaud, R.D.; Jacques-Coper, M.; Marín, J.C.; Narváez, D.A. Atmospheric Rivers in South-Central Chile: Zonal and Tilted Events. Atmosphere 2024, 15, 406. [Google Scholar] [CrossRef]
- Alvarez-Garreton, C.; Mendoza, P.A.; Boisier, J.P.; Addor, N.; Galleguillos, M.; Zambrano-Bigiarini, M.; Lara, A.; Puelma, C.; Cortes, G.; Garreaud, R.; et al. The CAMELS-CL Dataset: Catchment Attributes and Meteorology for Large Sample Studies—Chile Dataset. Hydrol. Earth Syst. Sci. 2018, 22, 5817–5846. [Google Scholar] [CrossRef]
- He, K.S.; Bradley, B.A.; Cord, A.F.; Rocchini, D.; Tuanmu, M.; Schmidtlein, S.; Turner, W.; Wegmann, M.; Pettorelli, N. Will Remote Sensing Shape the next Generation of Species Distribution Models? Remote Sens. Ecol. Conserv. 2015, 1, 4–18. [Google Scholar] [CrossRef]
- Pinto-Ledezma, J.N.; Cavender-Bares, J. Using Remote Sensing for Modeling and Monitoring Species Distributions. In Remote Sensing of Plant Biodiversity; Springer: Cham, Switzerland, 2020; pp. 199–223. [Google Scholar]
- Randin, C.F.; Ashcroft, M.B.; Bolliger, J.; Cavender-Bares, J.; Coops, N.C.; Dullinger, S.; Dirnböck, T.; Eckert, S.; Ellis, E.; Fernández, N.; et al. Monitoring Biodiversity in the Anthropocene Using Remote Sensing in Species Distribution Models. Remote Sens. Environ. 2020, 239, 111626. [Google Scholar] [CrossRef]
- Phillips, S.J. Transferability, Sample Selection Bias and Background Data in Presence-only Modelling: A Response to Peterson et al. (2007). Ecography 2008, 31, 272–278. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A Statistical Explanation of MaxEnt for Ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Valavi, R.; Guillera-Arroita, G.; Lahoz-Monfort, J.J.; Elith, J. Predictive Performance of Presence-only Species Distribution Models: A Benchmark Study with Reproducible Code. Ecol. Monogr. 2022, 92, e1486. [Google Scholar] [CrossRef]
- Fitzpatrick, M.C.; Gotelli, N.J.; Ellison, A.M. MaxEnt versus MaxLike: Empirical Comparisons with Ant Species Distributions. Ecosphere 2013, 4, 1–15. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel Methods Improve Prediction of Species’ Distributions from Occurrence Data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Sloey, T.M.; Willis, J.M.; Hester, M.W. Hydrologic and Edaphic Constraints on Schoenoplectus acutus, Schoenoplectus californicus, and Typha latifolia in Tidal Marsh Restoration. Restor. Ecol. 2015, 23, 430–438. [Google Scholar] [CrossRef]
- Sloey, T.M.; Howard, R.J.; Hester, M.W. Response of Schoenoplectus acutus and Schoenoplectus californicus at Different Life-History Stages to Hydrologic Regime. Wetlands 2016, 36, 37–46. [Google Scholar] [CrossRef]
- Alvarez-Garreton, C.; Boisier, J.P.; Garreaud, R.; Seibert, J.; Vis, M. Progressive Water Deficits during Multiyear Droughts in Basins with Long Hydrological Memory in Chile. Hydrol. Earth Syst. Sci. 2020, 25, 429–446. [Google Scholar] [CrossRef]
- Luebert, F.; Pliscoff, P. Sinopsis Bioclimática y Vegetacional de Chile; Editorial Universitaria: Santiago, Chile, 2006. [Google Scholar]
- 79. EB, CONC & ULS. 2025 Digital Herbarium. Available online: https://www.herbariodigital.cl (accessed on 10 February 2025).
- Flora of Chile. Available online: http://www.efloras.org/flora_page.aspx?flora_id=60 (accessed on 12 March 2025).
- Rodriguez, R.; Marticorena, C.; Alarcón, D.; Baeza, C.; Cavieres, L.; Finot, V.L.; Fuentes, N.; Kiessling, A.; Mihoc, M.; Pauchard, A.; et al. Catálogo de las plantas vasculares de Chile. Gayana. Bot. 2018, 75, 1–430. [Google Scholar]
- Thiers, B. Index Herbariorum: A global Directory of Public Herbaria and Associated Staff. New York Botanical Garden’s Virtual Herbarium. 2025. Available online: http://sweetgum.nybg.org/ih/ (accessed on 12 March 2025).
- POWO. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. 2025. Available online: https://powo.science.kew.org/ (accessed on 12 March 2025).
- Moritz, S.; Bartz-Beielstein, T. ImputeTS: Time Series Missing Value Imputation in R. R J. 2017, 9, 207. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Tusell, F. Kalman Filtering in R. J. Stat. Softw. 2011, 39, 1–27. [Google Scholar] [CrossRef]
- Wood, S.N. Generalized Additive Models: An Introduction with R; Chapman and Hall/CRC: Boca Raton, FL, USA, 2017; ISBN 9781315370279. [Google Scholar]
- Wood, S.N. Fast Stable Restricted Maximum Likelihood and Marginal Likelihood Estimation of Semiparametric Generalized Linear Models. J. R. Stat. Soc. Ser. B 2011, 73, 3–36. [Google Scholar] [CrossRef]
- Wood, S.N. Generalized Additive Models. Annu. Rev. Stat. Appl. 2024, 12, 497–526. [Google Scholar] [CrossRef]
- Wood, S.; Wood, M.S. Package ‘Mgcv’. R Package Version 2015, 1, 729. [Google Scholar]
- Wickham, H.; Wickham, H. Toolbox. In Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; pp. 33–74. [Google Scholar]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A Review of Methods to Deal with It and a Simulation Study Evaluating Their Performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Naimi, B.; Hamm, N.A.S.; Groen, T.A.; Skidmore, A.K.; Toxopeus, A.G. Where Is Positional Uncertainty a Problem for Species Distribution Modelling? Ecography 2014, 37, 191–203. [Google Scholar] [CrossRef]
- Chander, G.; Markham, B.L.; Barsi, J.A. Revised Landsat-5 Thematic Mapper Radiometric Calibration. IEEE Geosci. Remote Sens. Lett. 2007, 4, 490–494. [Google Scholar] [CrossRef]
- Ahn, Y.H.; Shanmugam, P.; Ryu, J.H. Atmospheric Correction of the Landsat Satellite Imagery for Turbid Waters. Gayana 2004, 68, 1–8. [Google Scholar]
- Masek, J.G.; Vermote, E.F.; Saleous, N.E.; Wolfe, R.; Hall, F.G.; Huemmrich, K.F.; Gao, F.; Kutler, J.; Lim, T.-K. A Landsat Surface Reflectance Dataset for North America, 1990–2000. IEEE Geosci. Remote Sens. Lett. 2006, 3, 68–72. [Google Scholar] [CrossRef]
- Pekel, J.-F.; Cottam, A.; Gorelick, N.; Belward, A.S. High-Resolution Mapping of Global Surface Water and Its Long-Term Changes. Nature 2016, 540, 418–422. [Google Scholar] [CrossRef]
- Pickens, A.H.; Hansen, M.C.; Hancher, M.; Stehman, S.V.; Tyukavina, A.; Potapov, P.; Marroquin, B.; Sherani, Z. Mapping and Sampling to Characterize Global Inland Water Dynamics from 1999 to 2018 with Full Landsat Time-Series. Remote Sens. Environ. 2020, 243, 111792. [Google Scholar] [CrossRef]
- Pickens, A.H.; Hansen, M.C.; Stehman, S.V.; Tyukavina, A.; Potapov, P.; Zalles, V.; Higgins, J. Global Seasonal Dynamics of Inland Open Water and Ice. Remote Sens. Environ. 2022, 272, 112963. [Google Scholar] [CrossRef]
- Hijmans, R.J. Terra: Spatial Data Analysis. In CRAN: Contributed Packages; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- Wickham, H. dplyr: A Grammar of Data Manipulation; R Package Version 04; R Core Team: Vienna, Austria, 2015. [Google Scholar]
- QGIS. QGIS Geographic Information System; QGIS Association: Grüt, Switzerland, 2022. [Google Scholar]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum Entropy Modeling of Species Geographic Distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of Species Distributions with Maxent: New Extensions and a Comprehensive Evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the Black Box: An Open-source Release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annu. Rev. Ecol., Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Ortega-Huerta; Peterson, A. T. Ecological Niches and Geographic Distributions. Rev. Mex. Biod. 2008, 79, 205–216. [Google Scholar]
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Peterson, A.T. ORIGINAL ARTICLE: Predicting Species Distributions from Small Numbers of Occurrence Records: A Test Case Using Cryptic Geckos in Madagascar. J. Biogeogr. 2007, 34, 102–117. [Google Scholar] [CrossRef]
- Papeş, M.; Gaubert, P. Modelling Ecological Niches from Low Numbers of Occurrences: Assessment of the Conservation Status of Poorly Known Viverrids (Mammalia, Carnivora) across Two Continents. Divers. Distrib. 2007, 13, 890–902. [Google Scholar] [CrossRef]
- Wisz, M.S.; Hijmans, R.J.; Li, J.; Peterson, A.T.; Graham, C.H.; Guisan, A.; NCEAS Predicting Species Distributions Working Group. Effects of Sample Size on the Performance of Species Distribution Models. Divers. Distrib. 2008, 14, 763–773. [Google Scholar] [CrossRef]
- Aarts, G.; Fieberg, J.; Matthiopoulos, J. Comparative Interpretation of Count, Presence–Absence and Point Methods for Species Distribution Models. Methods Ecol. Evol. 2012, 3, 177–187. [Google Scholar] [CrossRef]
- Hastie, T.; Fithian, W. Inference from Presence-only Data; the Ongoing Controversy. Ecography 2013, 36, 864–867. [Google Scholar] [CrossRef]
- Renner, I.W.; Warton, D.I. Equivalence of MAXENT and Poisson Point Process Models for Species Distribution Modeling in Ecology. Biometrics 2013, 69, 274–281. [Google Scholar] [CrossRef]
- Freeman, E.A.; Moisen, G.G. A Comparison of the Performance of Threshold Criteria for Binary Classification in Terms of Predicted Prevalence and Kappa. Ecol. Model. 2008, 217, 48–58. [Google Scholar] [CrossRef]
- Koopmans, L.H.; Owen, D.B.; Rosenblatt, J.I. Confidence Intervals for the Coefficient of Variation for the Normal and Log Normal Distributions. Biometrika 1964, 51, 25–32. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S. In Statistics and Computing; Springer: New York, NY, USA, 2002; pp. 1–12. [Google Scholar] [CrossRef]
- Portet, S. A Primer on Model Selection Using the Akaike Information Criterion. Infect. Dis. Model. 2020, 5, 111–128. [Google Scholar] [CrossRef] [PubMed]

| Variable | T (°C) | P (mm) | Flow (m3s−1) | Level (m) |
|---|---|---|---|---|
| Intercept | 13.00 ± 0.06 *** | 153.06 ± 7.40 *** | 88.25 ± 2.11 *** | 1.53 ± 0.02 *** |
| 2013–2023 | −1.01 ± 0.13 *** | 4.36 ± 8.31 ns | −21.69 ± 4.64 *** | −0.23 ± 0.03 *** |
| s(Group) | 6.85 | 5.40 | 6.292 | 6.415 |
| F(df) | 647.7 (8) *** | 100.1 (8) ns | 149.9 (8) *** | 114.5 (8) *** |
| GCV | 1.69 | 7425.90 | 2178.90 | 0.070294 |
| R2adj | 0.89 | 0.55 | 0.70 | 0.80 |
| Year | L8 Scene Date 1 | N | AUC Train | AUC Test | MSS |
|---|---|---|---|---|---|
| (a) Elodea densa | |||||
| 2015 | 28/01/2015 | 26 | 0.93 ± 0.005 | 0.89 ± 0.027 | 0.36 ± 0.098 |
| 2016 | 30/12/2015 | 353 | 0.93 ± 0.001 | 0.92 ± 0.003 | 0.35 ± 0.02 |
| 2017 | 30/11/2016 | 46 | 0.97 ± 0.001 | 0.95 ± 0.008 | 0.19 ± 0.029 |
| 2018 | 05/02/2018 | 72 | 0.95 ± 0.002 | 0.94 ± 0.012 | 0.19 ± 0.03 |
| 2019 | 14/01/2019 | 94 | 0.97 ± 0.001 | 0.97 ± 0.003 | 0.23 ± 0.063 |
| 2020 | 11/02/2020 | 37 | 0.96 ± 0.001 | 0.93 ± 0.015 | 0.37 ± 0.071 |
| 2021 | 08/03/2021 | 352 | 0.89 ± 0.001 | 0.88 ± 0.005 | 0.38 ± 0.038 |
| 2022 | 21/12/2021 | 64 | 0.9 ± 0.002 | 0.87 ± 0.016 | 0.3 ± 0.058 |
| 2023 | 03/02/2023 | 37 | 0.93 ± 0.003 | 0.9 ± 0.015 | 0.35 ± 0.022 |
| 2024 | 21/01/2024 | 69 | 0.95 ± 0.001 | 0.93 ± 0.012 | 0.21 ± 0.034 |
| (b) Schoenoplectus californicus | |||||
| 2015 | 28/01/2015 | 28 | 0.92 ± 0.002 | 0.89 ± 0.007 | 0.23 ± 0.068 |
| 2016 | 30/12/2015 | 204 | 0.93 ± 0.002 | 0.92 ± 0.007 | 0.3 ± 0.059 |
| 2017 | 30/11/2016 | 18 | 0.95 ± 0.003 | 0.93 ± 0.017 | 0.46 ± 0.11 |
| 2018 | 05/02/2018 | 38 | 0.96 ± 0.001 | 0.94 ± 0.009 | 0.24 ± 0.1 |
| 2019 | 14/01/2019 | 40 | 0.95 ± 0.001 | 0.93 ± 0.008 | 0.35 ± 0.035 |
| 2020 | 11/02/2020 | 50 | 0.96 ± 0.001 | 0.95 ± 0.004 | 0.45 ± 0.099 |
| 2021 | 08/03/2021 | 132 | 0.9 ± 0.002 | 0.89 ± 0.006 | 0.38 ± 0.068 |
| 2022 | 21/12/2021 | 29 | 0.96 ± 0.003 | 0.94 ± 0.012 | 0.44 ± 0.127 |
| 2023 | 03/02/2023 | 309 | 0.92 ± 0.001 | 0.91 ± 0.005 | 0.31 ± 0.029 |
| 2024 | 21/01/2024 | 300 | 0.94 ± 0.001 | 0.93 ± 0.005 | 0.28 ± 0.07 |
| Species and Variables | β ± SE | t | p-Value |
|---|---|---|---|
| (a) Elodea densa | |||
| Intercept | −3733.32 ± 1157.59 | −3.23 | 0.018 *** |
| Year | 1.83 ± 0.57 | 3.22 | 0.018 *** |
| TYear (°C) | 6.93 ± 1.72 | 4.02 | 0.007 *** |
| s.d. Level (m) | −32.94 ± 11.76 | −2.80 | 0.031 ** |
| (b) Schoenoplectus californicus | |||
| Intercept | 3072 ± 1395 | 2.20 | 0.093 ns |
| Year | −1.46 ± 0.68 | −2.15 | 0.098 ns |
| TYear (°C) | −6.19 ± 1.85 | −3.35 | 0.029 ** |
| sPYear (mm) | 0.02 ± 0.003 | 5.13 | 0.007 *** |
| Level (m) | −46.47 ± 9.99 | −4.65 | 0.010 ** |
| s.d. Level (m) | 14.95 ± 12.38 | 1.20 | 0.294 ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labra, F.A.; Jaramillo, E. Biodiversity Dynamics in a Ramsar Wetland: Assessing How Climate and Hydrology Shape the Distribution of Dominant Native and Alien Macrophytes. Plants 2025, 14, 1116. https://doi.org/10.3390/plants14071116
Labra FA, Jaramillo E. Biodiversity Dynamics in a Ramsar Wetland: Assessing How Climate and Hydrology Shape the Distribution of Dominant Native and Alien Macrophytes. Plants. 2025; 14(7):1116. https://doi.org/10.3390/plants14071116
Chicago/Turabian StyleLabra, Fabio A., and Eduardo Jaramillo. 2025. "Biodiversity Dynamics in a Ramsar Wetland: Assessing How Climate and Hydrology Shape the Distribution of Dominant Native and Alien Macrophytes" Plants 14, no. 7: 1116. https://doi.org/10.3390/plants14071116
APA StyleLabra, F. A., & Jaramillo, E. (2025). Biodiversity Dynamics in a Ramsar Wetland: Assessing How Climate and Hydrology Shape the Distribution of Dominant Native and Alien Macrophytes. Plants, 14(7), 1116. https://doi.org/10.3390/plants14071116









