Interspecific Hybridization Barrier Between Paeonia ostii and P. ludlowii
Abstract
:1. Introduction
2. Results
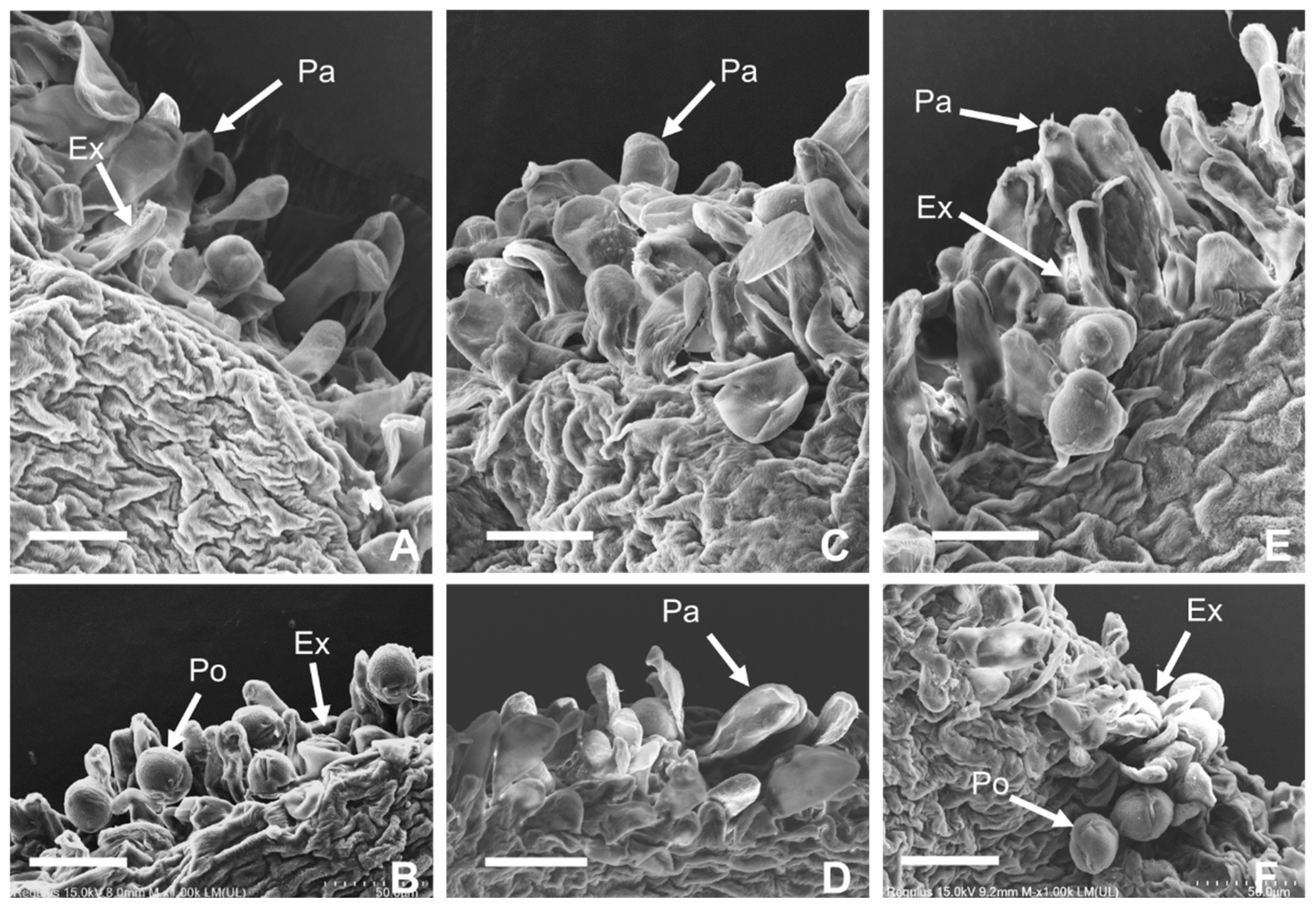
2.1. Morphology of Papilla Cell in Stigma and Pollen Germination
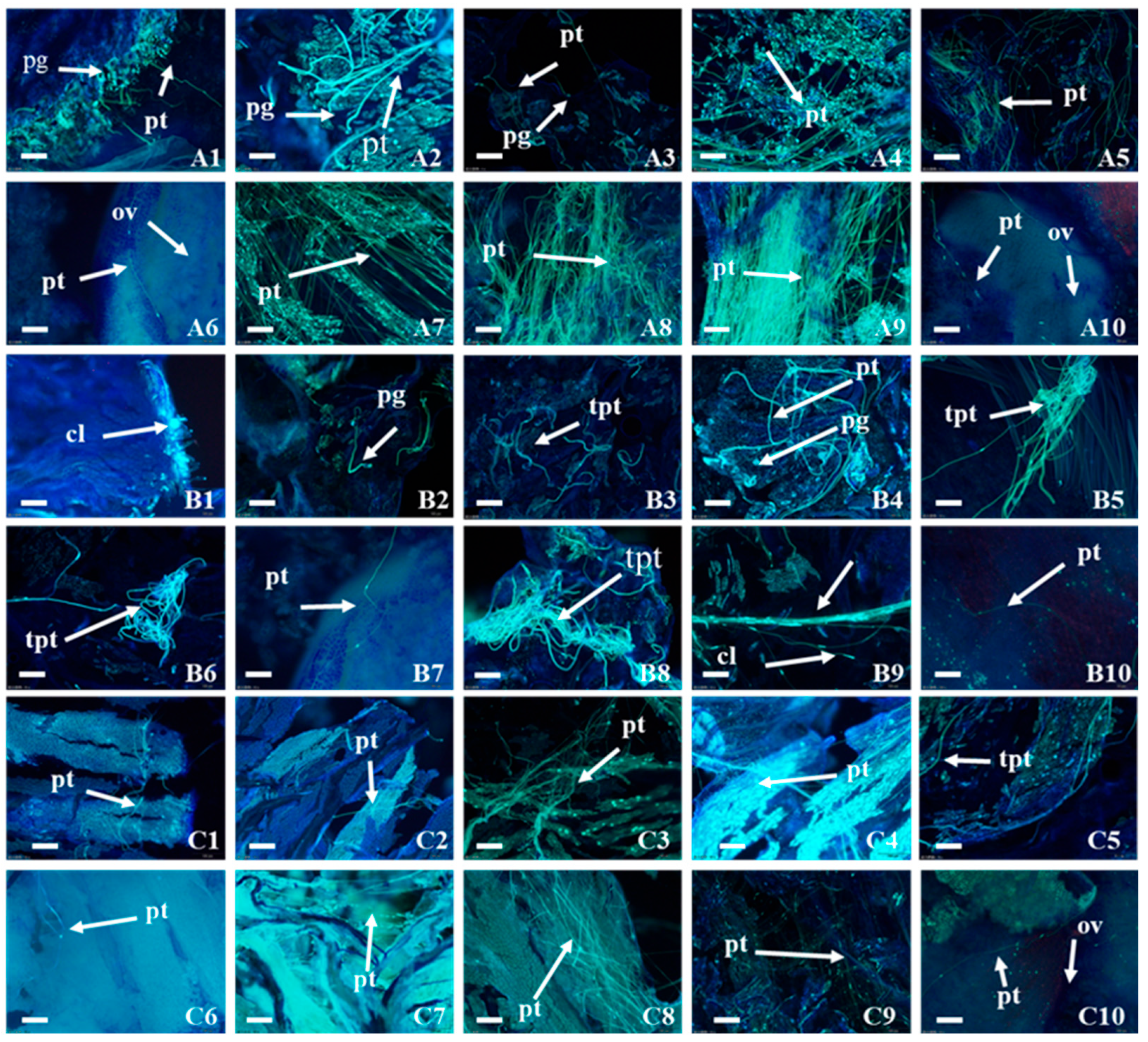
2.2. Pollen Germination and Growth in Pistil
2.3. Seed Setting Rate
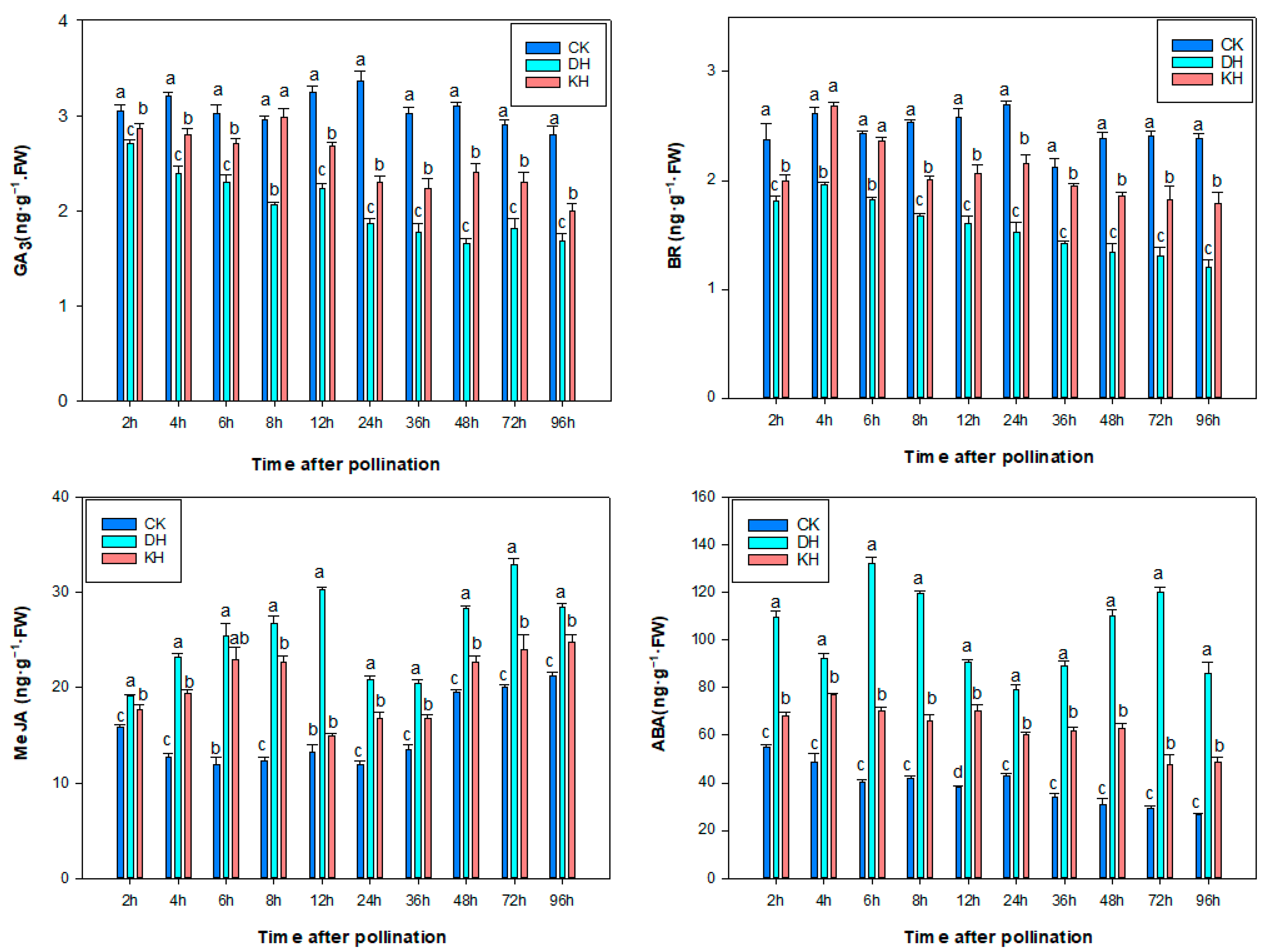
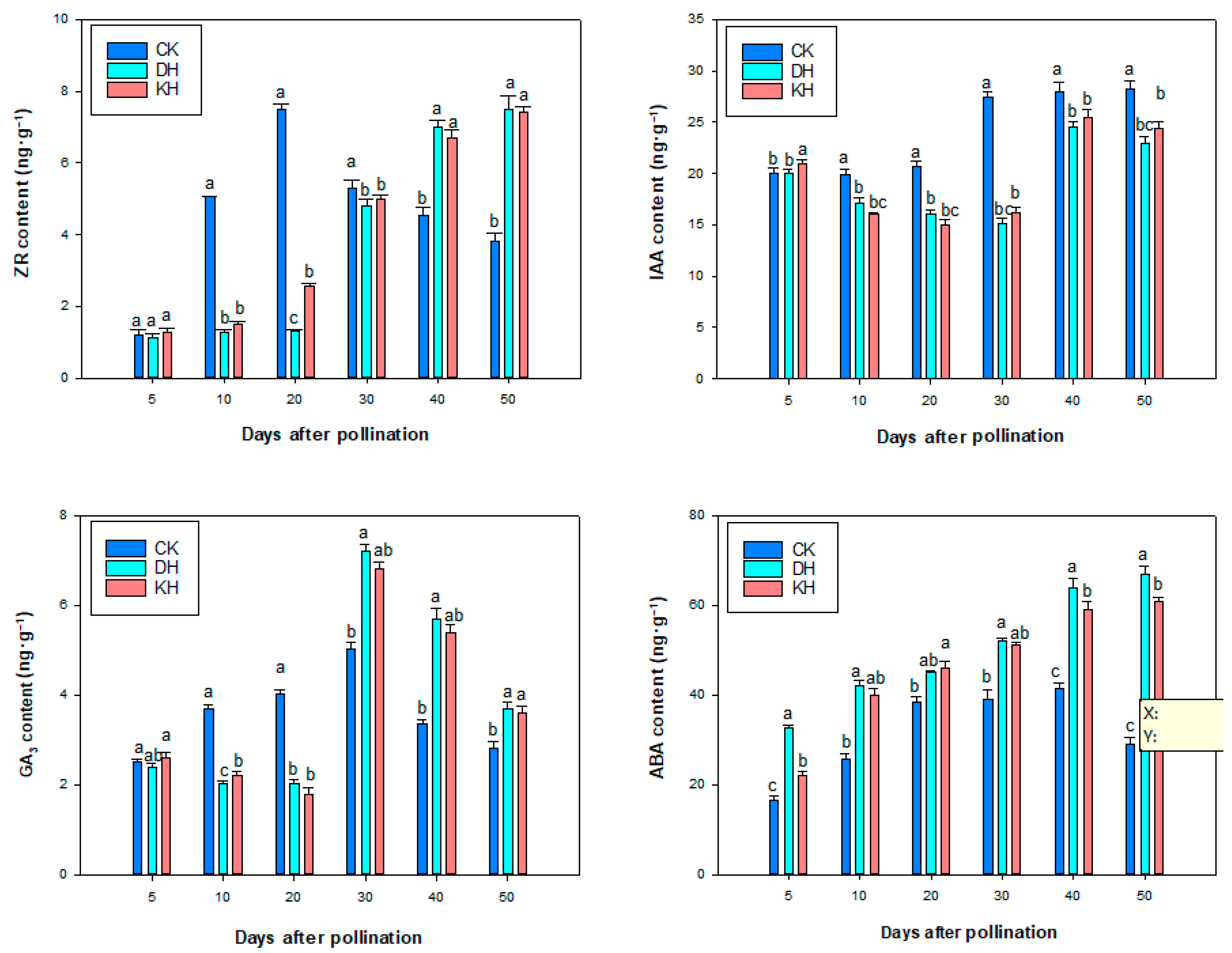
2.4. Hormone Contents
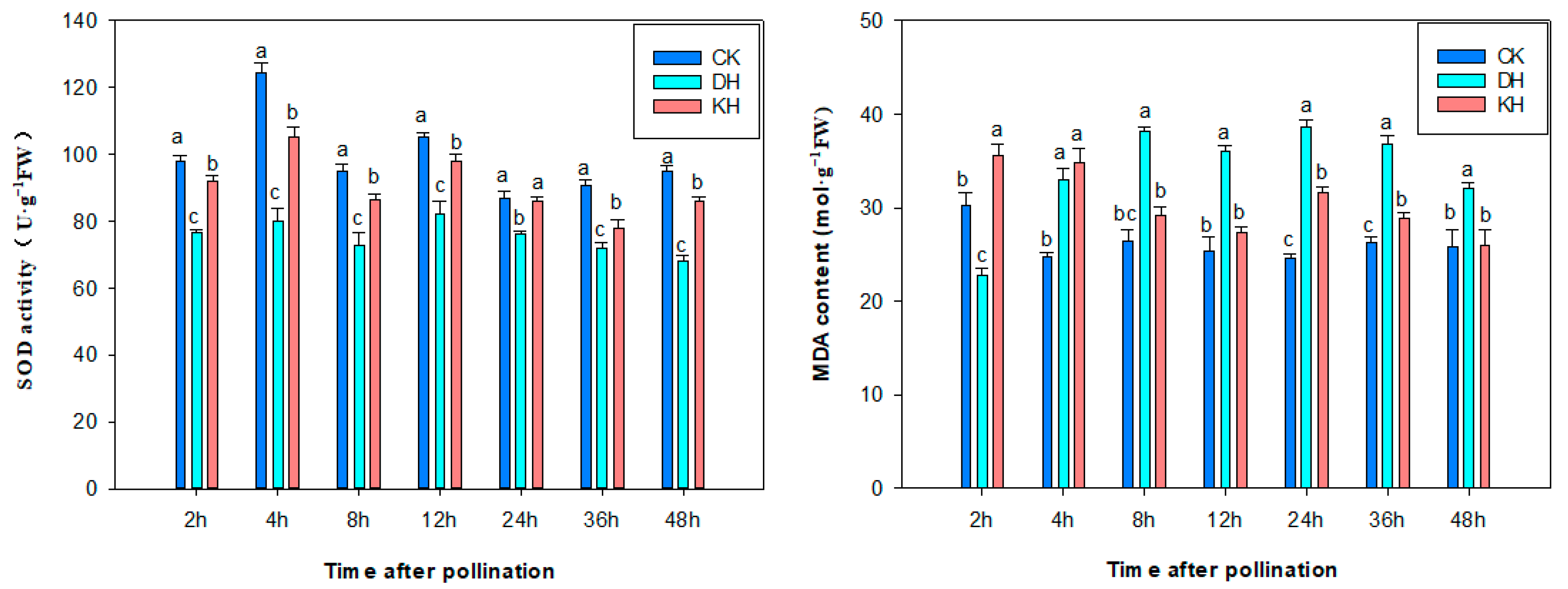
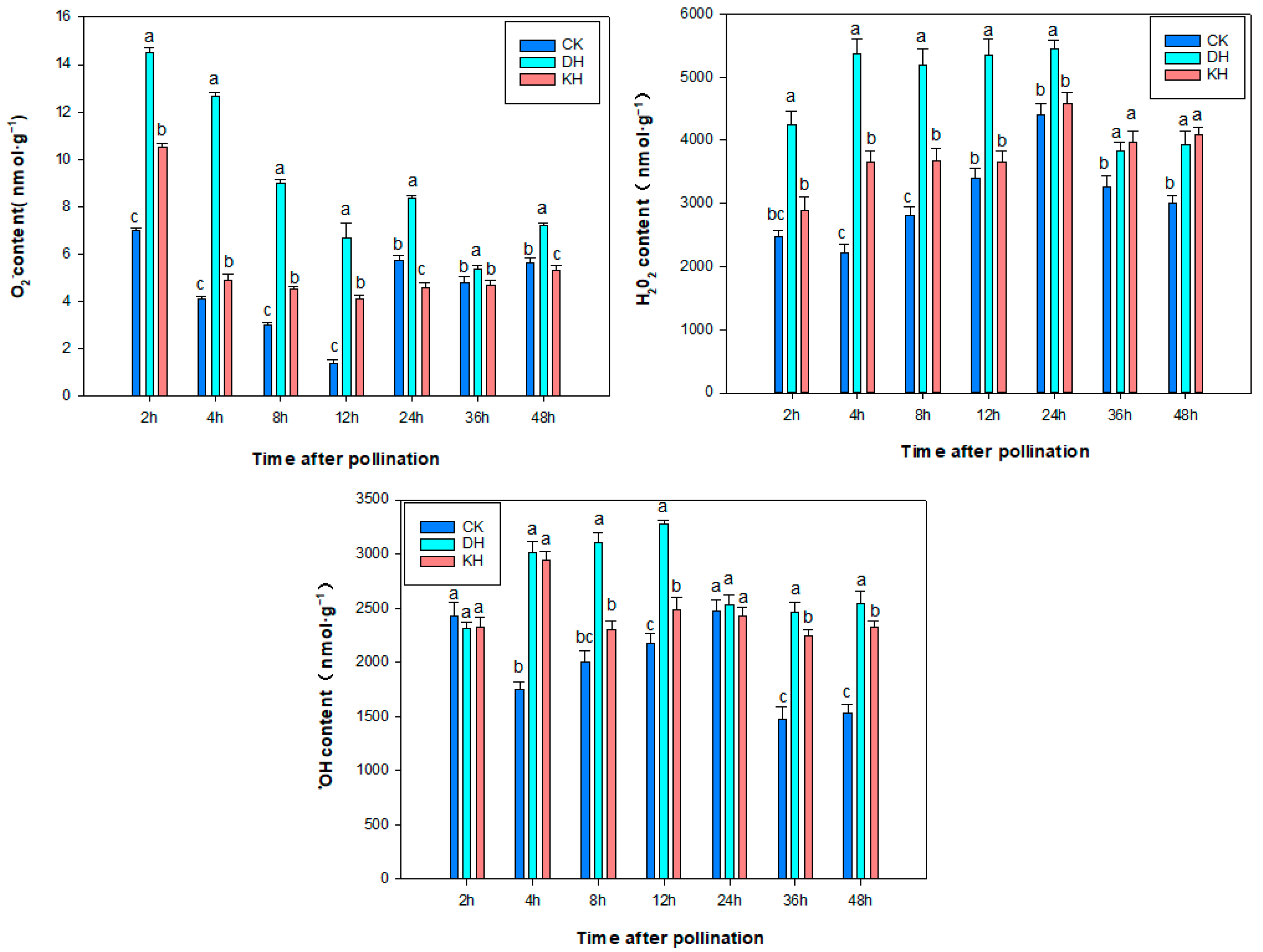
2.5. SOD Activity, ROS and MDA Content
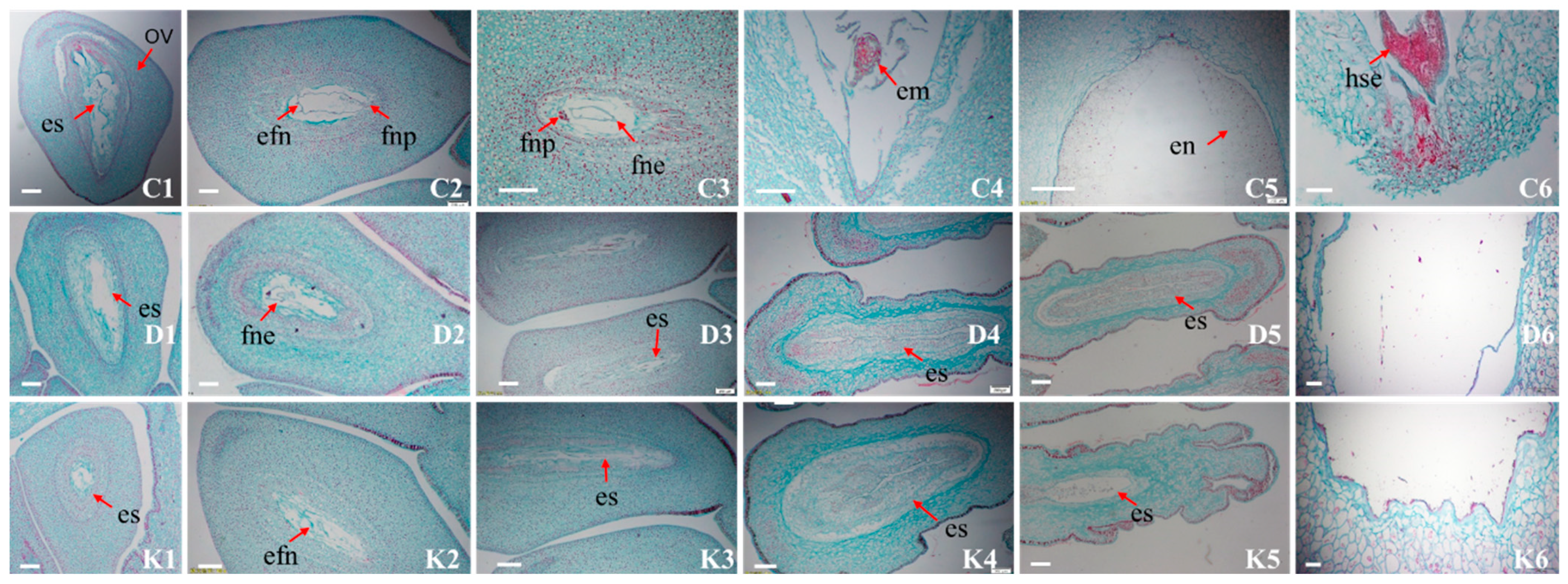
2.6. Embryo Development Observation of Anatomy During Embryo Development
2.7. Endogenous Hormone Contents
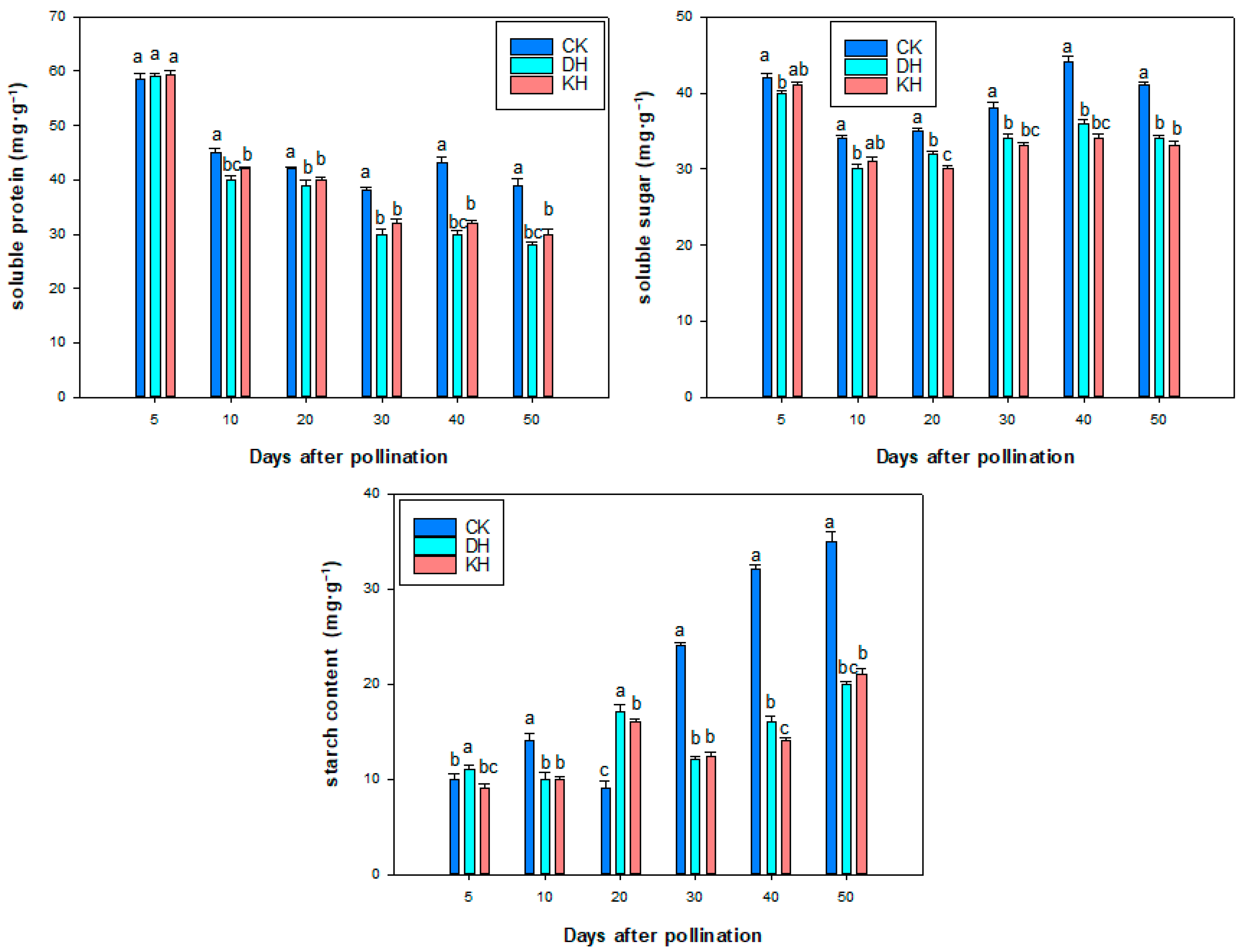
2.8. Nutrient Contents
3. Discussion
4. Material and Methods
4.1. Plant Materials and Pollen Collection
4.2. Sexual Cross Using Different Pollination Method
4.3. Paeonia ostii ‘Fengdan’ Stigma Sampling and SEM Observation
4.4. Observation of Pollen Germination, Growth and Embryo Development
4.5. O2−, H2O2, •OH, SOD and MDA Level Analyses
4.6. Endogenous Hormone Content and Nutrient Content Analyses
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Perrino, E.V.; Perrino, P. Crop Wild Relatives: Know How Past and Present to Improve Future Research, Conservation and Utilization Strategies, Especially in Italy: A Review. Genet. Resour. Crop Evol. 2020, 67, 1067–1105. [Google Scholar] [CrossRef]
- Zhang, F.; Batley, J. Exploring the Application of Wild Species for Crop Improvement in a Changing Climate. Curr. Opin. Plant Biol. 2020, 56, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.; Sears, E.R. The Wild Gene Resources of Wheat. Sci. Am. 1981, 244, 102–113. [Google Scholar] [CrossRef]
- Goulet, B.E.; Roda, F.; Hopkins, R. Hybridization in Plants: Old Ideas, New Techniques. Plant Physiol. 2017, 173, 65–78. [Google Scholar] [CrossRef]
- Zheng, T.; Zhang, X.; Yin, G.; Wang, L.; Han, Y.; Chen, L.; Huang, F.; Tang, J.; Xia, X.; He, Z. Genetic Gains in Grain Yield, Net Photosynthesis and Stomatal Conductance Achieved in Henan Province of China between 1981 and 2008. Field Crop Res. 2011, 122, 225–233. [Google Scholar] [CrossRef]
- Xing, G.; Qu, L.; Zhang, W.; Zhang, Y.; Yuan, X.; Lei, J. Study on Interspecific Hybridization between Tulip Cultivars and Wild Species Native to China. Euphytica 2020, 216, 66. [Google Scholar] [CrossRef]
- Maryenti, T.; Ishii, T.; Okamoto, T. Development and Regeneration of Wheat–Rice Hybrid Zygotes Produced by in vitro Fertilization System. New Phytol. 2021, 232, 2369–2383. [Google Scholar] [CrossRef]
- Katche, E.; Quezada-Martinez, D.; Katche, E.I.; Vasquez-Teuber, P.; Mason, A.S. Interspecific Hybridization for Brassica Crop Improvement. Crop Breed. Genet. Genom. 2019, 1, e190007. [Google Scholar] [CrossRef]
- Chaudhary, H.K.; Kaila, V.; Rather, S.A.; Tayeng, T. Distant Hybridization and Doubled-Haploidy Breeding. In Alien Gene Transfer in Crop Plants; Pratap, A., Kumar, J., Eds.; Springer: New York, NY, USA, 2014; Volume 1, pp. 143–164. [Google Scholar]
- Qu, L.J.; Li, L.; Lan, Z.; Dresselhaus, T. Peptide Signalling during the Pollen Tube Journey and Double Fertilization. J. Exp. Bot. 2015, 66, 5139–5150. [Google Scholar] [CrossRef]
- Hao, Q.; Xu, L.; Wang, H.; Liu, Q.; Wang, K. Evaluation of Pollen Viability, Stigma Receptivity, and the Cross Barrier between Tropical and Hardy Water Lily Cultivars. Flora 2022, 290, 152046. [Google Scholar] [CrossRef]
- Kaur, K.; Gupta, M.; Vikal, Y.; Singh, K.; Neelam, K. Callose Depositions Underlie the Incompatible Reaction in Intergeneric Crosses of Rice. Plant Genet. Resour. 2021, 19, 447–452. [Google Scholar] [CrossRef]
- Zinta, G.; Khan, A.; AbdElgawad, H.; Verma, V.; Srivastava, A.K. Unveiling the Redox Control of Plant Reproductive Development during Abiotic Stress. Front. Plant Sci. 2016, 7, 700. [Google Scholar] [CrossRef]
- Breygina, M.; Klimenko, E. ROS and Ions in Cell Signaling during Sexual Plant Reproduction. Int. J. Mol. Sci. 2020, 21, 9476. [Google Scholar] [CrossRef]
- Zhang, M.J.; Zhang, X.S.; Gao, X.Q. ROS in the Male–Female Interactions During Pollination: Function and Regulation. Front. Plant Sci. 2020, 11, 177. [Google Scholar] [CrossRef]
- Zhou, L.Z.; Dresselhaus, T. Multiple Roles of ROS in Flowering Plant Reproduction. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2023; Volume 105, pp. 139–176. [Google Scholar]
- Gong, W.; Oubounyt, M.; Baumbach, J.; Dresselhaus, T. Heat-Stress-Induced ROS in Maize Silks Cause Late Pollen Tube Growth Arrest and Sterility. iScience 2024, 27, 110081. [Google Scholar] [CrossRef]
- Hu, W.; Huang, Y.; Bai, H.; Liu, Y.; Wang, S.; Zhou, Z. Influence of Drought Stress on Pistil Physiology and Reproductive Success of Two Gossypium hirsutum Cultivars Differing in Drought Tolerance. Physiol. Plant 2020, 168, 909–920. [Google Scholar] [CrossRef]
- Yue, F.; Zheng, F.; Li, Q.; Mei, J.; Shu, C.; Qian, W. Comparative Transcriptome Analysis Points to the Biological Processes of Hybridization Incompatibility between Brassica napus and B. oleracea. Plant 2023, 12, 2622. [Google Scholar] [CrossRef]
- Ren, R.; Jiang, X.; Di, W.; Li, Z.; Li, B.; Xu, J.; Liu, Y. HSP70 Improves the Viability of Cryopreserved Paeonia lactiflora Pollen by Regulating Oxidative Stress and Apoptosis-like Programmed Cell Death Events. Plant Cell Tissue Organ Cult. 2019, 139, 53–64. [Google Scholar] [CrossRef]
- Zhou, P.; Li, J.; Jiang, H.; Yang, Z.; Sun, C.; Wang, H.; Su, Q.; Jin, Q.; Wang, Y.; Xu, Y. Hormonal and Transcriptomic Analysis Reveals the Role of ABA and BR in Breaking the Barrier of Inter-Subgeneric Hybridization in Water Lily (Nymphaea). bioRxiv 2023, 5, 556322. [Google Scholar] [CrossRef]
- Xie, D.L.; Zheng, X.L.; Zhou, C.Y.; Kanwar, M.K.; Zhou, J. Functions of Redox Signaling in Pollen Development and Stress Response. Antioxidants 2022, 11, 287. [Google Scholar] [CrossRef]
- Aybeke, M. Saliciylic Acid Is Also Effective Along with Abscisic Acid and Gibberellic Acid in the Orchid Post-Pollination Process. Osman. Korkut Üniversitesi Bilim. Enstitüsü Derg. 2024, 7, 1600–1616. [Google Scholar] [CrossRef]
- Önder, S.; Erbaş, S.; Önder, D.; Tonguç, M.; Mutlucan, M. Seed Filling. In Seed Biology Updates; Jimenez-Lopez, J.C., Ed.; IntechOpen: Rijeka, Croatia, 2022; pp. 1–39. [Google Scholar]
- Zhao, X.; Zhang, X.; Wu, Y.; Yu, F.; Su, B.; Li, X.; Huang, D. Cross Compatibility and Endogenous Phytohormone Profiles in Interspecific Hybridization between Iris tectorum and Iris germanica. Sci. Hortic. 2024, 327, 112837. [Google Scholar] [CrossRef]
- Shi, D.; Tang, C.; Wang, R.; Gu, C.; Wu, X.; Hu, S.; Jiao, J.; Zhang, S. Transcriptome and Phytohormone Analysis Reveals a Comprehensive Phytohormone and Pathogen Defence Response in Pear Self-/Cross-Pollination. Plant Cell Rep. 2017, 36, 1785–1799. [Google Scholar] [CrossRef]
- Mesejo, C.; Yuste, R.; Martínez-Fuentes, A.; Reig, C.; Iglesias, D.J.; Primo-Millo, E.; Agustí, M. Self-pollination and Parthenocarpic Ability in Developing Ovaries of Self-incompatible Clementine Mandarins (Citrus clementina). Physiol. Plant. 2013, 148, 87–96. [Google Scholar] [CrossRef]
- Breygina, M.; Voronkov, A.; Galin, I.; Akhiyarova, G.; Polevova, S.; Klimenko, E.; Ivanov, I.; Kudoyarova, G. Dynamics of Endogenous Levels and Subcellular Localization of ABA and Cytokinins during Pollen Germination in Spruce and Tobacco. Protoplasma 2023, 260, 237–248. [Google Scholar] [CrossRef]
- Rauf, S.; Al-Khayri, J.M.; Zaharieva, M.; Monneveux, P.; Khalil, F. Breeding Strategies to Enhance Drought Tolerance in Crops. In Advances in Plant Breeding Strategies: Agronomic, Abiotic and Biotic Stress Traits; Al-Khayri, J.M., Jain, S.M., Johnson, D.V., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 397–445. [Google Scholar]
- Li, S.; Liu, K.; Yu, S.; Jia, S.; Chen, S.; Fu, Y.; Sun, F.; Luo, Q.; Wang, Y. The Process of Embryo Abortion of Stenospermocarpic Grape and It Develops into Plantlet in vitro Using Embryo Rescue. Plant Cell Tissue Organ Cult. 2020, 143, 389–409. [Google Scholar] [CrossRef]
- Amiteye, S. In Vitro Embryo Rescue Techniques and Applications in Hybrid Plant Development. In Advanced Crop Improvement; Volume 2, Raina, A., Wani, M.R., Laskar, R.A., Tomlekova, N., Khan, S., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 419–456. [Google Scholar]
- Ahmad, F.; Slinkard, A.E. The Extent of Embryo and Endosperm Growth Following Interspecific Hybridization between Cicer arietinum L. and Related Annual Wild Species. Genet. Resour. Crop Evol. 2004, 51, 765–772. [Google Scholar] [CrossRef]
- Jia, Z.; Gao, P.; Yin, F.; Quilichini, T.D.; Sheng, H.; Song, J.; Yang, H.; Gao, J.; Chen, T.; Yang, B.; et al. Asymmetric Gene Expression in Grain Development of Reciprocal Crosses between Tetraploid and Hexaploid Wheats. Commun. Biol. 2022, 5, 1412. [Google Scholar] [CrossRef]
- He, D.; Guo, H.; He, S.; Zhang, M.; Chang, Y.; Wang, Z.; Liu, Y. Transcriptome Analysis Reveals the Role of Phytohormones in the Distant Hybridization of Peony Embryo Abortion. Horticulturae 2023, 9, 694. [Google Scholar] [CrossRef]
- Shen, S.; Zhang, L.; Liang, X.G.; Zhao, X.; Lin, S.; Qu, L.H.; Liu, Y.P.; Gao, Z.; Ruan, Y.L.; Zhou, S.L. Delayed Pollination and Low Availability of Assimilates Are Major Factors Causing Maize Kernel Abortion. J. Exp. Bot. 2018, 69, 1599–1613. [Google Scholar] [CrossRef]
- Waniale, A.; Mukasa, S.B.; Tugume, A.K.; Kubiriba, J.; Tushemereirwe, W.K.; Tumuhimbise, R. Early Withering of Enlarged Ovules in Pollinated Fruits of Bananas (Musa spp.) Suggest Abortion after Fertilization. Horticulturae 2022, 8, 426. [Google Scholar] [CrossRef]
- Hong, D.Y. Peonies of the World: Discussion on the Basic Principles of Taxonomy; Science Press: Beijing, China, 2024; pp. 1–171. [Google Scholar]
- Jia, W.Q.; Wang, Y.L.; Guo, Y.Z.; Wang, Z.; Qi, Q.; Yan, S.N.; Liu, H.C.; He, S.L. Characterization of Pollen Germination and Storage of Paeonia ludlowii. Sci. Silvae Sin. 2021, 57, 82–92. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, M.; Li, S.; Chen, Q.; Teixeira da Silva, J.A.; Wang, A.; Yu, X.; Wang, L. Germplasm Resources and Genetic Breeding of Paeonia: A Systematic Review. Hortic. Res. 2020, 7, 107. [Google Scholar] [CrossRef]
- Çetinbaş-Genç, A. Adverse Effects of Heat Stress in Relation to Actin Cytoskeleton on Pollen Performance of Echinopsis chamaecereus (Cactaceae). Braz. J. Bot. 2020, 43, 29–34. [Google Scholar] [CrossRef]
- Kapoor, I.; Chahal, G.K.; Sharma, M.; Ghai, N.; Dhatt, A.S. Barriers to Interspecific Hybridization Between Cucurbita pepo L. and Cucurbita moschata Duch. J. Plant Growth Regul. 2024, 43, 2599–2614. [Google Scholar] [CrossRef]
- Qin, P.; Ting, D.; Shieh, A.; McCormick, S. Callose Plug Deposition Patterns Vary in Pollen Tubes of Arabidopsis thaliana ecotypes and Tomato Species. BMC Plant Biol. 2012, 12, 178. [Google Scholar] [CrossRef]
- Williams, E.G.; Knox, R.B.; Rouse, J.L. Pollination Sub-Systems Distinguished by Pollen Tube Arrest after Incompatible Interspecific Crosses in Rhododendron (Ericaceae). J. Cell Sci. 1982, 53, 255–277. [Google Scholar] [CrossRef]
- Read, S.M.; Clarke, A.E.; Bacic, A. Stimulation of Growth of cultured Nicotiana tabacum W 38 Pollen Tubes by Poly (Ethylene Glycol) and Cu(II) Salts. Protoplasma 1993, 177, 1–14. [Google Scholar] [CrossRef]
- Kaufmane, E.; Rumpunen, K. Pollination, Pollen Tube Growth and Fertilization in Chaenomeles japonica (Japanese Quince). Sci. Hortic. 2002, 94, 257–271. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, F.; Qi, X.; Xu, W.; Yang, L. Salt Solution Treatment Plays an Important Role in Overcoming Pre-Fertilization Barriers during Asiatic and Oriental Lily Crossbreeding. Sci. Hortic. 2021, 288, 110343. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.Q.; Chen, F.D.; Teng, N.J.; Yao, Y.M.; Shan, X.; Dai, Z.L. Transcriptomic and Proteomic Analysis Reveals Mechanisms of Low Pollen-Pistil Compatibility during Water Lily Cross Breeding. BMC Plant Biol. 2019, 19, 542. [Google Scholar] [CrossRef]
- Sun, C.-Q.; Cao, J.; Wang, J.H.; Zhou, P.; Xu, Y.C.; Chen, F.D. Pectin Methylesterase Regulates Pollen Germination on Stigma after Pollination in Water Lily. Sci. Hortic. 2023, 320, 112207. [Google Scholar] [CrossRef]
- Kao, Y.-T.; Gonzalez, K.L.; Bartel, B. Peroxisome Function, Biogenesis, and Dynamics in Plants. Plant Physiol. 2018, 176, 162–177. [Google Scholar] [CrossRef]
- Díaz, J.; Bernal, A.; Pomar, F.; Merino, F. Induction of Shikimate Dehydrogenase and Peroxidase in Pepper (Capsicum annuum L.) Seedlings in Response to Copper Stress and Its Relation to Lignification. Plant Sci. 2001, 161, 179–188. [Google Scholar] [CrossRef]
- Pickersgill, B. Interspecific Hybridization by Sexual Means. In Plant Breeding: Principles and Prospects; Springer: Berlin/Heidelberg, Germany, 1993; pp. 63–78. [Google Scholar] [CrossRef]
- Kovaleva, L.; Zakharova, E.; Minkina, Y.V.; Voronkov, A. Effects of Flavonols and Phytohormones on Germination and Growth of Petunia Male Gametophyte. Allelopath. J. 2009, 23, 51–62. [Google Scholar] [CrossRef]
- Jia, W.Q.; Guo, Y.Z.; Wang, Y.L.; Zhu, X.P.; Wang, Z.; Liu, G.X.; Liu, H.C.; He, S.L.; Zhang, X.Y. Effects of storage conditions on pollen longevity of Paeonia qiui. Trans. CSAE 2020, 36, 307–315. [Google Scholar] [CrossRef]
- Çetinbaş-Genç, A.; Vardar, F. Effect of Methyl Jasmonate on In-Vitro Pollen Germination and Tube Elongation of Pinus nigra. Protoplasma 2020, 257, 1655–1665. [Google Scholar] [CrossRef]
- Vogler, F.; Schmalzl, C.; Englhart, M.; Bircheneder, M.; Sprunck, S. Brassinosteroids promote Arabidopsis pollen germination and growth. Plant Reprod. 2014, 27, 153–167. [Google Scholar] [CrossRef]
- Yang, H.H. Study on Intergeneric Distant Hybridization Between Prunus and Armeniaca and New Germplasm Creation. Master’s Thesis, Shandong Agricultural University, Tai’an, China, 2004. [Google Scholar]
- Zhang, P. Cytological and Biochemical Basis of Cross Incompatibility in Poplars. Master’s Thesis, Beijing Forest University, Beijing, China, 2013. [Google Scholar]
- Xu, X.; Tian, Z.; Xing, A.; Wu, Z.; Li, X.; Dai, L.; Yang, Y.; Yin, J.; Wang, Y. Nitric Oxide Participates in Aluminum-Stress-Induced Pollen Tube Growth Inhibition in Tea (Camellia sinensis) by Regulating CsALMTs. Plants 2022, 11, 2233. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, X.; Li, S.; Zhang, L.; Song, X. Oxidative Stress and Aberrant Programmed Cell Death Are Associated With Pollen Abortion in Isonuclear Alloplasmic Male-Sterile Wheat. Front. Plant Sci. 2018, 9, 595. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, H.; Chen, J.; Jiang, X.; Tao, S.; Wu, J.; Zhang, S. Mitochondrial Dysfunction Mediated by Cytoplasmic Acidification Results in Pollen Tube Growth Cessation in Pyrus pyrifolia. Physiol. Plant. 2015, 153, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Mendler-Drienyovszki, N.; Cal, A.J.; Dobránszki, J. Progress and Prospects for Interspecific Hybridization in Buckwheat and the Genus Fagopyrum. Biotechnol. Adv. 2013, 31, 1768–1775. [Google Scholar] [CrossRef] [PubMed]
- Mondo, J.M.; Agre, P.A.; Edemodu, A.; Asiedu, R.; Akoroda, M.O.; Asfaw, A. Cross Compatibility in Intraspecific and Interspecific Hybridization in Yam (Dioscorea spp.). Sci. Rep. 2022, 12, 3432. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, Z.; Chen, F.; Zhu, Y.; Guo, X.; Fu, M.; Chen, J.; Wu, J.; Zhu, Z. Production and Identification of × Brassicoraphanus Distant Hybrids between Radish (Raphanus sativus L.) and Kohlrabi (Brassica oleracea L. Var. Caulorapa DC.). N. Z. J. Crop Hortic. 2023, 51, 341–354. [Google Scholar] [CrossRef]
- Chen, T.; Xie, M.; Jiang, Y.; Yuan, T. Abortion Occurs during Double Fertilization and Ovule Development in Paeonia ludlowii. J. Plant Res. 2022, 135, 295–310. [Google Scholar] [CrossRef]
- Chen, T.; Sun, Y.; Yuan, T. Transcriptome Sequencing and Gene Expression Analysis Revealed Early Ovule Abortion of Paeonia ludlowii. BMC Genom. 2023, 24, 78. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.Y.; Liu, Y.; Zhao, S.W. Anatomical Study on Seed Abortion of Syringa microphylla in Cultivated Condition. Acta Bot. Boreali-Occident. Sin. 2012, 32, 1997–2003. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Hu, L.; Zhang, J.; Xiao, F.; Zhang, S.; Shao, F.; Huang, L. Embryological Observations on Seed Abortion in Hibiscus syriacus L. and Physiological Studies on Nutrients, Enzyme Activity and Endogenous Hormones. BMC Plant Biol. 2023, 23, 665. [Google Scholar] [CrossRef]
- Li, W.X.; Chen, L.Y.; Lei, D.Y. Study on Correlation of Setting Rates between Male Varieties and Medium Hybrid Rice Combinations. J. Hunan Agric. Univ. 2006, 32, 5–7. [Google Scholar] [CrossRef]
- Du, C.; Yu, X.; Mou, Y.P.; Li, J.; Zhang, P.Y.; Yuan, X.L.; Wang, J. Comparison of Fruit Characteristics of 11 Populations in main Distribution Areas of Paeonia delavayi. Acta Agric. Univ. Jiangxiensis 2023, 45, 619–630. [Google Scholar] [CrossRef]
- Chen, T. Research on the Mechanism of Seeds Abortion in Paeonia ludlowii. Docter’s Thesis, Beijing Forestry University, Beijing, China, 2022. [Google Scholar]
- Yang, Y.; Chen, Y. Study on Relation between Endogenous Phytohormones and Ovule Abortion in Tetraploid Robinia pseudoacacia. Agric. Sci. Technol. 2016, 17, 1773–1776. [Google Scholar] [CrossRef]
- Ji, W.; Guo, R.; Wang, J.; Jiao, X.; Yan, Z.; Chang, Q.; Dong, Z.; Wang, Y. Grey Correlation Analysis of Physiological and Biochemical Factors in Embryo Abortion of Seedless Grape. Acta Hortic. Sin. 2019, 46, 1473–1485. [Google Scholar] [CrossRef]
- Zhou, S.L.; Zou, X.H.; Zhou, Z.Q.; Liu, J.; Xu, C.; Yu, J.; Wang, Q.; Zhang, D.M.; Wang, X.Q.; Ge, S. Multiple Species of Wild Tree Peonies Gave Rise to the ‘King of Flowers’, Paeonia suffruticosa Andrews. Proc. R. Soc. B Biol. Sci. 2014, 281, 20141687. [Google Scholar] [CrossRef]
- Guo, L.; Guo, S.; Xu, J.; He, L.; Carlson, J.E.; Hou, X. Phylogenetic Analysis Based on Chloroplast Genome Uncover Evolutionary Relationship of All the Nine Species and Six Cultivars of Tree Peony. Ind. Crop Prod. 2020, 153, 112567. [Google Scholar] [CrossRef]
- Xiao, P.X.; Li, Y.; Lu, J.; Zuo, H.; Pingcuo, G.; Ying, H.; Zhao, F.; Xu, Q.; Zeng, X.; Jiao, W.B. High-Quality Assembly and Methylome of a Tibetan Wild Tree Peony Genome (Paeonia ludlowii) Reveal the Evolution of Giant Genome Architecture. Hortic. Res. 2023, 10, uhad241. [Google Scholar] [CrossRef]
- Yuan, J.; Jiang, S.; Jian, J.; Liu, M.; Yue, Z.; Xu, J.; Li, J.; Xu, C.; Lin, L.; Jing, Y. Genomic Basis of the Giga-Chromosomes and Giga-Genome of Tree Peony Paeonia ostii. Nat. Commun. 2022, 13, 7328. [Google Scholar] [CrossRef]
- Jia, W.; Wang, Y.; Qi, Q.; He, S.; Mi, Z.; Zhu, X. Leaf Epidermal Morphology of Ten Wild Tree Peonies in China and Its Taxonomic Significance. Horticulturae 2022, 8, 502. [Google Scholar] [CrossRef]
- Zhang, K.; Cao, W.; Baskin, J.M.; Baskin, C.C.; Sun, J.; Yao, L.; Tao, J. Seed Development in Paeonia ostii (Paeoniaceae), with Particular Reference to Embryogeny. BMC Plant Biol. 2021, 21, 603. [Google Scholar] [CrossRef]
- Gajewska, E.; Skłodowska, M. Effect of Nickel on ROS Content and Antioxidative Enzyme Activities in Wheat Leaves. Biometals 2007, 20, 27–36. [Google Scholar] [CrossRef]
- Gokce, A.; Sekmen Cetinel, A.H.; Turkan, I. Involvement of GLR-Mediated Nitric Oxide Effects on ROS Metabolism in Arabidopsis Plants under Salt Stress. J. Plant Res. 2024, 137, 485–503. [Google Scholar] [CrossRef] [PubMed]
- Cheeseman, J.M. Hydrogen Peroxide Concentrations in Leaves under Natural Conditions. J. Exp. Bot. 2006, 57, 2435–2444. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Suriyakumar, P.; Vanchinathan, S.; Krishnan, V.; Lal, M.K.; Jha, P.K.; Chinnusamy, V.; Anand, A.; Prasad, P.V.V. Hydrogen Peroxide and GA3 Levels Regulate the High Night Temperature Response in Pistils of Wheat (Triticum aestivum L.). Antioxidants 2023, 12, 342. [Google Scholar] [CrossRef] [PubMed]
- Priyanka, N.; Geetha, N.; Manish, T.; Sahi, S.V.; Venkatachalam, P. Zinc Oxide Nanocatalyst Mediates Cadmium and Lead Toxicity Tolerance Mechanism by Differential Regulation of Photosynthetic Machinery and Antioxidant Enzymes Level in Cotton Seedlings. Toxicol. Rep. 2021, 8, 295–302. [Google Scholar] [CrossRef]
- Costa, H.; Gallego, S.M.; Tomaro, M.L. Effect of UV-B Radiation on Antioxidant Defense System in Sunflower Cotyledons. Plant Sci. 2002, 162, 939–945. [Google Scholar] [CrossRef]
- Delatorre, C.; Rodríguez, A.; Rodríguez, L.; Majada, J.P.; Ordás, R.J.; Feito, I. Hormonal Profiling: Development of a Simple Method to Extract and Quantify Phytohormones in Complex Matrices by UHPLC–MS/MS. J. Chromatogr. B 2017, 1040, 239–249. [Google Scholar] [CrossRef]
- Li, H.; Sun, Q.; Zhao, S.; Zhang, W. Principles and Techniques of Plant Physiological Biochemical Experiment; Higher Education Press: Beijing, China, 2000; pp. 195–197. [Google Scholar]


| Pollination Methods | Seed Number per Fruit | Numbers of Plump Seeds per Fruit | Signal Follicle Weight |
|---|---|---|---|
| CK | 20.90 ± 0.63 a | 20.71 ± 1.20 a | 9.80 ± 0.73 a |
| DH | 11.89 ± 0.77 c | 0.15 ± 0.10 c | 1.82 ± 0.15 c |
| KH | 18.52 ± 0.55 b | 3.53 ± 0.35 b | 3.68 ± 0.42 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Zhang, Y.; Wang, Y.; Zhao, G.; Jia, W.; He, S. Interspecific Hybridization Barrier Between Paeonia ostii and P. ludlowii. Plants 2025, 14, 1120. https://doi.org/10.3390/plants14071120
Guo Y, Zhang Y, Wang Y, Zhao G, Jia W, He S. Interspecific Hybridization Barrier Between Paeonia ostii and P. ludlowii. Plants. 2025; 14(7):1120. https://doi.org/10.3390/plants14071120
Chicago/Turabian StyleGuo, Yingzi, Yan Zhang, Yanli Wang, Guodong Zhao, Wenqing Jia, and Songlin He. 2025. "Interspecific Hybridization Barrier Between Paeonia ostii and P. ludlowii" Plants 14, no. 7: 1120. https://doi.org/10.3390/plants14071120
APA StyleGuo, Y., Zhang, Y., Wang, Y., Zhao, G., Jia, W., & He, S. (2025). Interspecific Hybridization Barrier Between Paeonia ostii and P. ludlowii. Plants, 14(7), 1120. https://doi.org/10.3390/plants14071120






