Inhibitory Effects of Aqueous and Hydroalcoholic Extracts from Jatobá Coat (Hymenaea courbaril L.) on Pancreatic Amylase and Starch Absorption
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction Procedure
2.3. In Vitro Assay of Pancreatic α-Amylase
2.4. In Vivo Experiments
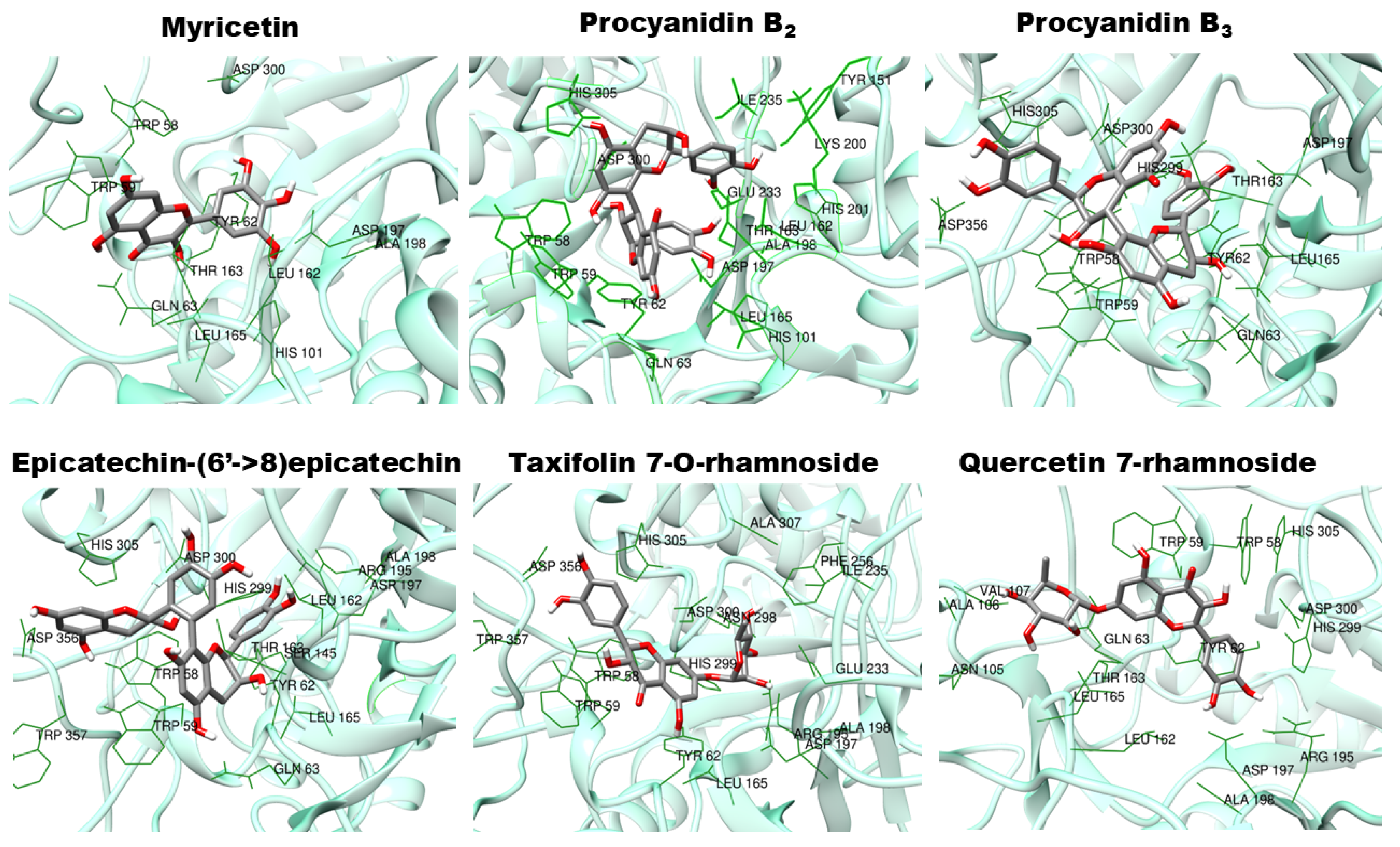
2.5. Binding Simulations (Docking)
2.6. Curve Fitting and Statistical Analysis
3. Results and Discussion
3.1. α-Amylase Activity as a Function of the Jatobá Extracts Concentrations
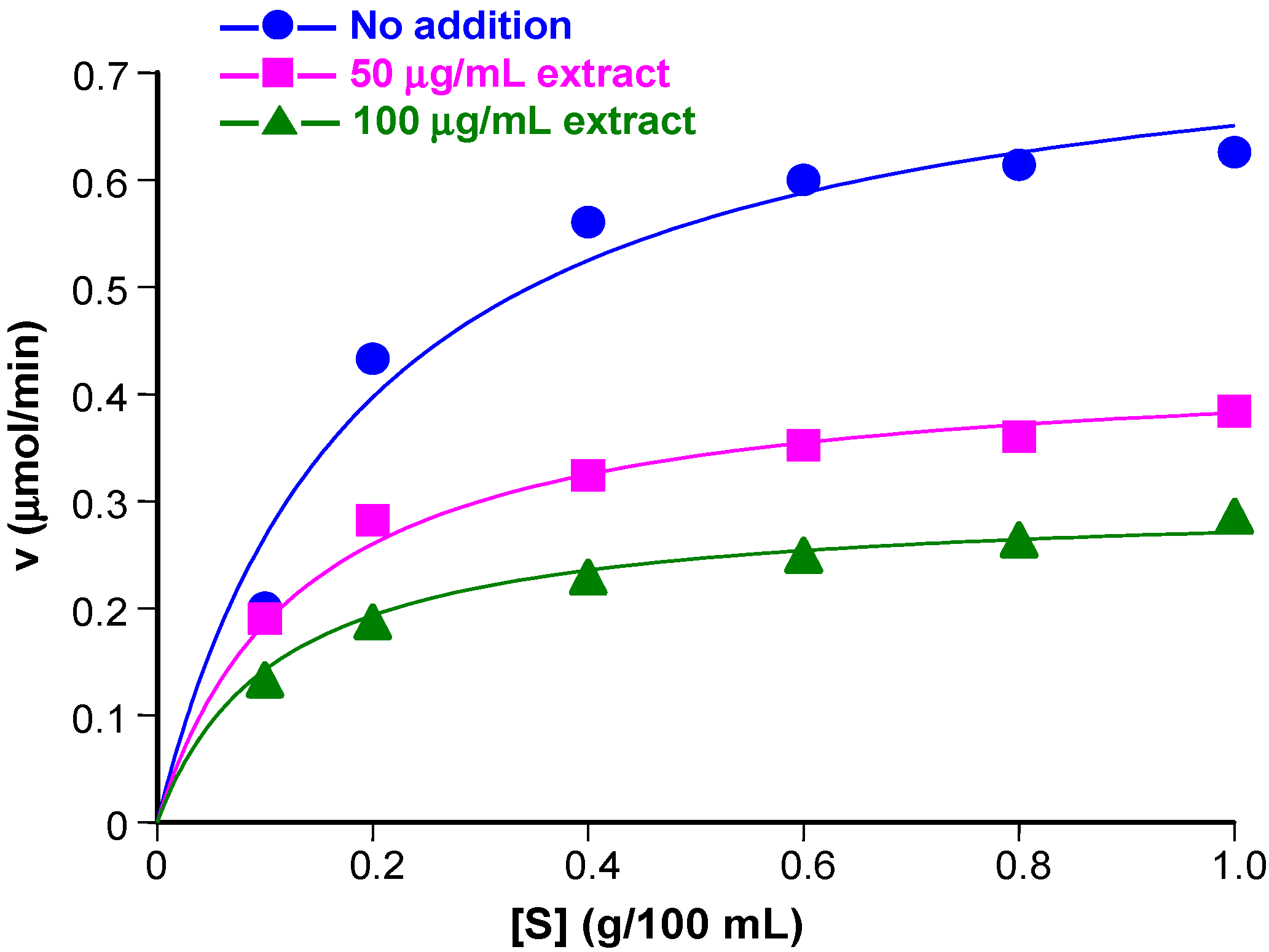
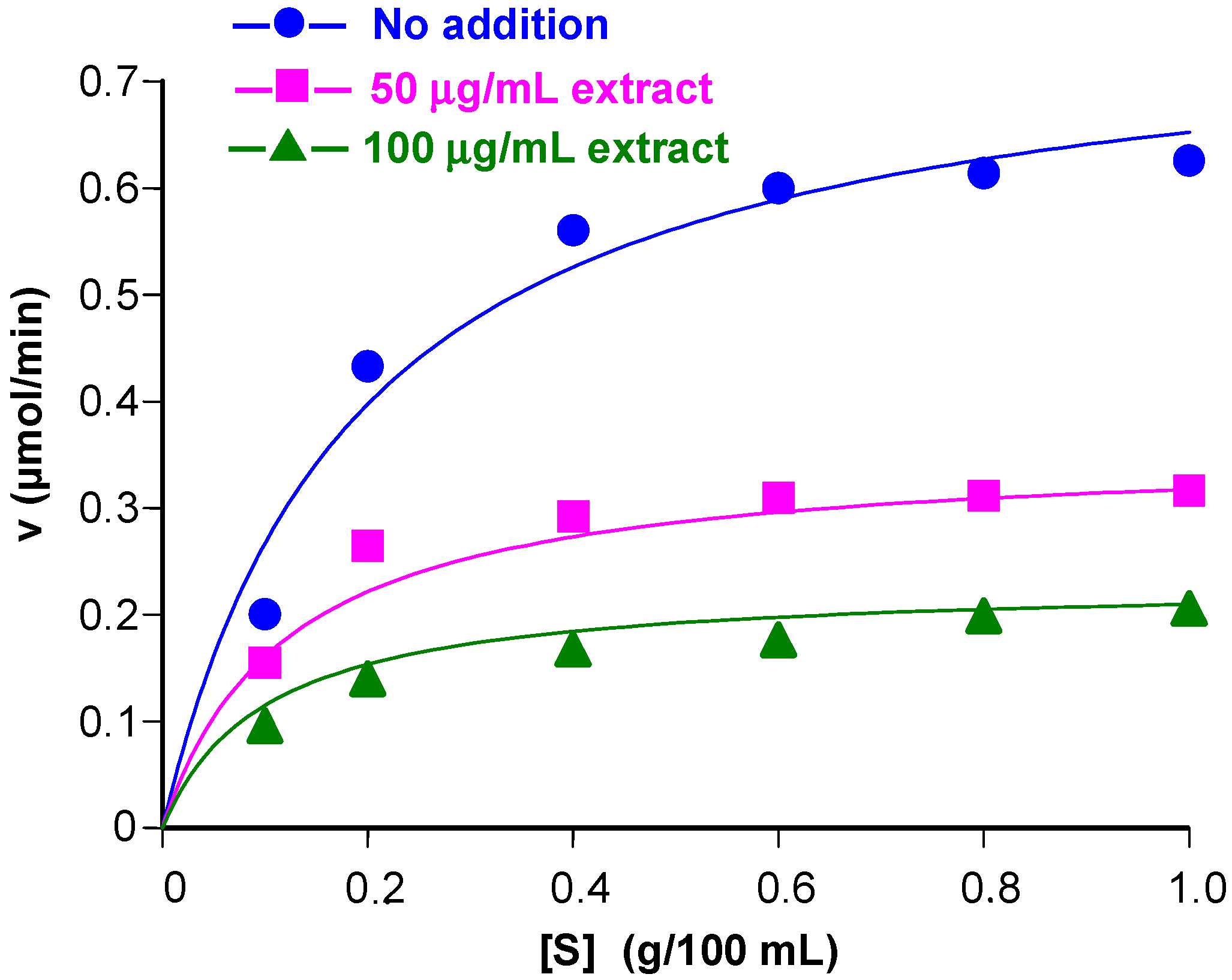
3.2. Inhibition of α-Amylase Activity at Various Substrate Concentrations
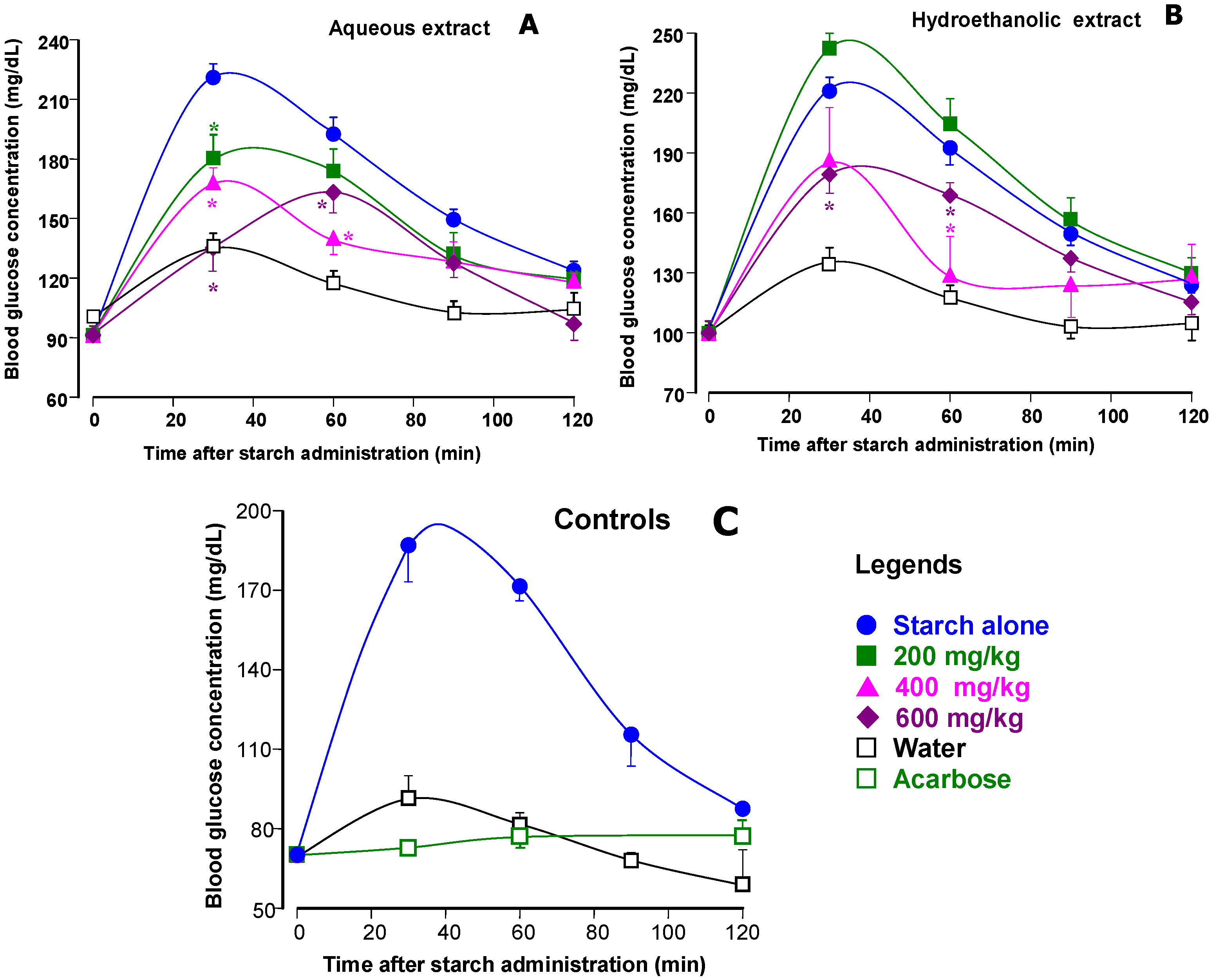
3.3. Starch Tolerance Tests in Mice
3.4. Comparison of the Actions of the Jatobá Extracts with Those of Other Plant Extracts
| Plant Species | Kind of Preparation | α-Amylase Inhibition In Vitro (IC50 in µg/mL) | Starch Digestion Inhibition In Vivo (ID50 in mg/kg) | Animal | References |
|---|---|---|---|---|---|
| Tamarindus indica (tamarind) | Seed hydroethanolic extract | 13.3 | 151.4 | Mouse | [49] |
| Cytinus hypocistis | Whole plant ultrasound extract | 14.0 | 349.8 | Mouse | [24] |
| Araucaria angustifolia | Seed coat extract (rich in tannins) | 45.0 | ≈308.6 | Rat | [28] |
| Hymenaea courbaril (jatobá) | Fruit coat hydroalcoholic extract | 51.1 | 385.7 and 436.3 * | Mouse | This work |
| Hymenaea courbaril (jatobá) | Fruit coat aqueous extract | 81.9 | 500.1 | Mouse | This work |
| Vitis vinifera (grape) | Grape pomace hydroethanolic extract | 145.0 | 131.6 | Rat | [27] |
| Camellia sinensis (purple tea) | Aqueous extract (tea preparation) | 630.0 | 96.8 | Mouse | [42] |
| Myrciaria jaboticaba (jabuticaba) | Fruit peel hydroethanolic extract | 1963.0 | 250.0 | Mouse | [29] |
3.5. Compounds Possibly Involved
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- da Silva Oliveira, F.G.; de Souza Araújo, C.; Rolim, L.A.; Barbosa-Filho, J.M.; da Silva Almeida, J.R.G. Chapter 12—The genus Hymenaea (Fabaceae): A chemical and pharmacological review. In Studies in Natural Products Chemistry; Rahman, A.-u., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 58, pp. 339–388. [Google Scholar]
- Grisi, P.U.; Dorneles, M.C.; Ranal, M.A.; Oliveira, A.P.; Mendes-Rodrigues, C.; Santana, D.G. Ex situ initial seedling development of Hymenaea courbaril L. (Fabaceae): A crucial phase to preserve the species. Hoehnea 2019, 46, e742018. [Google Scholar] [CrossRef]
- Bezerra, G.P.; Góis, R.W.S.; Brito, T.S.; Lima, F.J.B.; Bandeira, M.A.M.; Romero, N.R.; Magalhães, P.J.C.; Santiago, G.M.P. Phytochemical study guided by the myorelaxant activity of the crude extract, fractions and constituent from stem bark of Hymenaea courbaril L. J. Ethnopharmacol. 2013, 149, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.; Costa, A.R.C.; Justino, M.N.; Camara, M.B.P.; Vasconcelos, O.L.S.; Marques, J.C.; Brandão, C.M.; Marques, G.E.C. Chemical prospection and antioxidant activity of Humiria balsamifera (Aubl.) A. St. Hil. and Hymenae acourbaril L. Nat. Prod. Res. 2023, 38, 614–618. [Google Scholar] [CrossRef]
- Veggi, P.C.; Prado, J.M.; Bataglion, G.A.; Eberlin, M.N.; Meireles, M.A.A. Obtaining phenolic compounds from jatoba (Hymenaea courbaril L.) bark by supercritical fluid extraction. J. Supercrit. Fluids 2014, 89, 68–77. [Google Scholar] [CrossRef]
- Soares, L.A.; Germano, A.T.; Vitali, L.; Mazzuti, S.; Santana, L.C.L.A. Solid-state fermentation as a pre-treatment method for the extraction of bioactive compounds from pomegranate (Punica granatum L.) and jatobá peel (Hymenaea courbaril L.). Food Biosci. 2025, 63, 105716. [Google Scholar] [CrossRef]
- Vardanega, R.; Ferreira, W.S.; Viganó, J.; Prado, J.M.; Morano, R.S.; Veggi, P.C. Exploring the economic viability of Jatobá (Hymenaea courbaril L.) bark extraction: A comparative study. Sep. Purif. Technol. 2024, 350, 127946. [Google Scholar] [CrossRef]
- Pal, P.; Singh, A.K.; Srivastava, R.K.; Rathore, S.S.; Sahoo, U.K.; Subudhi, S.; Sarangi, P.K.; Prus, P. Circular bioeconomy in action: Transforming food wastes into renewable food resources. Foods 2024, 13, 3007. [Google Scholar] [CrossRef]
- Sasaki, K.; Matsukura, Y.; Shijama, K.; Miyake, M.; Fujiwara, D.; Konishi, Y. High-performance liquid chromatographic purification of oligomeric procyanidins, trimers up to nonamers, derived from the bark of jatoba (Hymenaea courbaril). Biosci. Biotechnol. Biochem. 2009, 73, 1274–1279. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Khanashyam, A.C.; Mundanat, A.S.; Shah, K.; Babu, K.S.; Thorakkattu, P.; Al-Asmari, F.; Pandiselvam, R. Valorization of Fruit Waste for Bioactive Compounds and Their Applications in the Food Industry. Foods 2023, 12, 556. [Google Scholar] [CrossRef]
- Cosme, F.; Aires, A.; Pinto, T.; Oliveira, I.; Vilela, A.; Gonçalves, B. A Comprehensive Review of Bioactive Tannins in Foods and Beverages: Functional Properties, Health Benefits, and Sensory Qualities. Molecules 2025, 30, 800. [Google Scholar] [CrossRef]
- Rangel-Galván, M.; Pacheco-Hernández, Y.; Lozoya-Gloria, E.; Villa-Ruano, N. Dietary natural products as inhibitors of α-amylase and α-glucosidase: An updated review of ligand-receptor correlations validated by docking studies. Food Biosci. 2024, 62, 105456. [Google Scholar] [CrossRef]
- Garg, P.; Duggal, N. Type 2 diabetes mellitus, its impact on quality of life and how the disease can be managed-a review. Obes. Med. 2022, 35, 100459. [Google Scholar] [CrossRef]
- Del Angelo, G.L.; Oliveira, I.S.; Albuquerque, B.R.; Kagueyama, S.S.; Silva, T.B.V.; Santos Filho, J.R.; Dias, M.I.; Pereira, C.; Calhelha, R.C.; Alves, M.J.; et al. Jatoba (Hymenaea courbaril L.) pod residue: A source of phenolic compounds as valuable biomolecules. Plants 2024, 13, 3207. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R. Insulin Resistance and Type 2 Diabetes. Diabetes 2012, 61, 778–779. [Google Scholar] [CrossRef]
- Tomic, D.; Shaw, J.E.; Magliano, D.J. The burden and risks of emerging complications of diabetes mellitus. Nat. Rev. Endocrinol. 2022, 18, 525–539. [Google Scholar] [CrossRef]
- Petroni, M.L.; Brodosi, L.; Marchignoli, F.; Sasdelli, A.S.; Caraceni, P.; Marchesini, G.; Ravaioli, F. Nutrition in Patients with Type 2 Diabetes: Present Knowledge and Remaining Challenges. Nutrients 2021, 13, 2748. [Google Scholar] [CrossRef]
- Adebayo, J.; Koketsu, M.; Aljarba, N.; Alkahtani, S.; Batiha, G.E.; Olaiya, C.O. Inhibition mechanism of alpha-amylase, a diabetes target, by a steroidal pregnane and pregnane glycosides derived from Gongronema latifolium Benth. Front. Mol. Biosci. 2022, 10, 866719. [Google Scholar] [CrossRef]
- Sun, L.; Warren, F.J.; Gidley, M.J. Natural products for glycaemic control: Polyphenols as inhibitors of alpha-amylase. Trends Food Sci. Technol. 2019, 91, 262–273. [Google Scholar] [CrossRef]
- Farias, D.P.; Araújo, F.F.; Neri-Numa, I.A.; Pastore, G.M. Antidiabetic potential of dietary polyphenols: A mechanistic review. Food Res. Int. 2021, 145, 110383. [Google Scholar] [CrossRef]
- Cisneros-Yupanqui, M.; Lant, A.; Mihaylova, D.; Krastanov, A.I.; Rizzi, C. The α-amylase and α-glucosidase inhibition capacity of grape pomace: A review. Food Bioprocess Technol. 2023, 16, 691–703. [Google Scholar] [CrossRef]
- Aleixandre, A.; Gil, J.V.; Sineiro, J.; Rosell, C.M. Understanding phenolic acids inhibition of α-amylase and α-glucosidase and influence of reaction conditions. Food Chem. 2022, 372, 131231. [Google Scholar] [CrossRef] [PubMed]
- Visvanathan, R.; Houghton, M.J.; Barber, E.; Williamson, G. Structure-function relationships in (poly)phenol-enzyme binding: Direct inhibition of human salivary and pancreatic α-amylases. Food Res. Int. 2024, 188, 114504. [Google Scholar] [CrossRef] [PubMed]
- Correa, V.G.; Garcia-Manieri, J.A.A.; Silva, A.R.; Backes, E.; Correa, E.; Barros, L.; Bracht, A.; Peralta, R.M. Exploring the α-amylase-inhibitory properties of tannin-rich extracts of Cytinus hypocistis on starch digestion. Food Res. Int. 2023, 173, 113260. [Google Scholar] [CrossRef]
- Gopal, A.; Muralikrishna, G. α-Amylases: Structure and function relationship. Trends Carbohydr. Res. 2009, 1, 1–11. [Google Scholar]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Kato-Schwartz, C.G.; Correa, R.C.G.; Lima, D.S.; Sá-Nakanishi, A.B.; Gonçalves, G.d.A.; Seixas, F.A.V.; Haminiuk, C.W.I.; Barros, L.; Ferreira, I.C.R.; Bracht, A.; et al. Potential anti-diabetic properties of Merlot grape pomace extract: An in vitro, in silico and in vivo study of α-amylase and α-glucosidase inhibition. Food Res. Int. 2020, 137, 109462. [Google Scholar] [CrossRef]
- da Silva, S.M.; Koehnlein, E.A.; Bracht, A.; Castoldi, R.; Morais, G.R.; Baesso, M.L.; Peralta, R.A.; Souza, C.G.M.; Sá-Nakanishi, A.B.; Peralta, R.M. Inhibition of salivary and pancreatic α-amylases by a pinhão coat (Araucaria angustifolia) extract rich in condensed tannin. Food Res. Int. 2014, 56, 1–8. [Google Scholar] [CrossRef]
- Castilho, P.A.; Bracht, L.; Barros, L.; Albuquerque, B.R.; Dias, M.I.; Ferreira, I.C.F.R.; Comar, J.F.; da Silva, T.B.V.; Peralta, R.M.; Sá-Nakanishi, A.B.; et al. Effects of a Myrciaria jaboticaba peel extract on starch and triglyceride absorption and the role of cyanidin-3-O-glucoside. Food Funct. 2021, 12, 2644–2659. [Google Scholar] [CrossRef]
- Williams, L.K.; Li, C.; Withers, S.G.; Brayer, G.D. Order and disorder: Differential structural impacts of myricetin and ethyl caffeate on human amylase, an antidiabetic target. J. Med. Chem. 2012, 55, 10177–10186. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Phillips, J.C.; Hardy, D.J.; Maia, J.D.D.; Stone, J.E.; Ribeiro, J.V.; Bernardi, R.C.; Buch, R.; Fiorin, G.; Hénin, J.; Jiang, W.; et al. Scalable molecular dynamics on CPU and GPU architectures with NAMD. J. Chem. Phys. 2020, 153, 044130. [Google Scholar] [CrossRef] [PubMed]
- Bugnon, M.; Goullieux, M.; Röhrig, U.F.; Perez, M.A.S.; Dina, A.; Michielin, O.; Zoete, V. SwissParam 2023: A modern web-based tool for efficient small molecule parametrization. J. Chem. Inf. Model. 2023, 63, 6469–6475. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New docking methods, expanded force field, and python bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Dallakyan, S.; Olson, A.J. Small-molecule library screening by docking with PyRx. Methods Mol. Biol. 2015, 1263, 243–250. [Google Scholar] [CrossRef]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Plowman, K.M. Enzyme Kinetics; McGraw-Hill Book Company: New York, NY, USA, 1972. [Google Scholar]
- Kalinovskii, A.P.; Sintsova, O.V.; Gladkikh, I.N.; Leychenko, E.V. Natural inhibitors of mammalian α-amylases as promising drugs for the treatment of metabolic diseases. Int. J. Mol. Sci. 2023, 24, 16514. [Google Scholar] [CrossRef]
- Cleland, W.W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. II. Inhibition: Nomenclature and Theory. Biochim. Biophys. Acta 1963, 7, 173–187. [Google Scholar] [CrossRef]
- Chou, T.C.; Talalay, P.A. simple generalized equation for the analysis of multiple inhibitions of Michaelis-Menten kinetic systems. J. Biol. Chem. 1977, 252, 6438–6442. [Google Scholar] [CrossRef]
- Beejmohun, V.; Peytavy-Izard, M.; Mignon, C.; Muscente-Paque, D.; Deplanque, X.; Ripoll, C.; Chapal1, N. Acute effect of Ceylon cinnamon extract on postprandial glycemia: Alpha-amylase inhibition, starch tolerance test in rats, and randomized crossover clinical trial in healthy volunteers. BMC Complement. Altern. Med. 2014, 14, 351. [Google Scholar] [CrossRef]
- Silva, T.B.V.; Castilho, P.A.; Sá-Nakanishi, A.B.; Seixas, F.A.V.; Dias, M.I.; Barros, L.; Ferreira, I.C.R.; Bracht, A.; Peralta, R.M. The inhibitory action of purple tea on in vivo starch digestion compared to other Camellia sinensis teas. Food Res. Int. 2021, 150, 110781. [Google Scholar] [CrossRef]
- Yu, M.; Zhu, S.; Huang, D.; Tao, X.; Li, Y. Inhibition of starch digestion by phenolic acids with a cinnamic acid backbone: Structural requirements for the inhibition of α-amylase and α-glucosidase. Food Chem. 2024, 435, 137499. [Google Scholar] [CrossRef] [PubMed]
- Nasir, K.; Rubab, S.L.; Fatima, H.; Shamim, F.; Tawab, A.; Arshad, I.; Iqbal, R.; Parveen, S.; Saadia, M.; Abbasi, B.B.K.; et al. Exploring antidiabetic potential of Nigella sativa and Silybum marianum seed extracts through bioassay-guided alpha-amylase inhibition activity and characterization of bioactive fractions. Results Chem. 2025, 15, 102183. [Google Scholar] [CrossRef]
- Omoboyowa, D.A.; Aribigbola, T.C.; Akinsulure, S.T.; Bodun, D.S.; Olugbogi, E.A.; Oni, E.A. In vitro and in silico anti-diabetes mechanism of phytochemicals from Curculigo pilosa and its pharmacokinetic profiling via α-amylase inhibition. Asp. Mol. Med. 2025, 5, 100064. [Google Scholar] [CrossRef]
- Huang, X.; Lin, K.; Liu, S.; Yang, J.; Zhao, H.; Zheng, X.-H.; Tsai, M.-J.; Chang, C.-S.; Huang, L.; Weng, C.-F. Combination of plant metabolites hinders starch digestion and glucose absorption while facilitating insulin sensitivity to diabetes. Front. Pharmacol. 2024, 15, 1362150. [Google Scholar] [CrossRef]
- Subramanian, R.; Asmawi, M.Z.; Sadikum, A. In vitro α-glucosidase and α-amylase inhibitory effects of Andrographis paniculata extract and andrographolide. Acta Biochim. Pol. 2008, 55, 391–398. [Google Scholar]
- Benrahou, K.; Doudach, L.; El Guourrami, O.; Mrabti, H.N.; Zengin, G.; Bouyahya, A.; Cherrah, Y.; El Abbes Faouzi, M. Acute toxicity, phenol conent, antioxidant and postprandial anti-diabetic activity of Echinops spinosus extracts. Int. J. Second. Metab. 2022, 9, 91–102. [Google Scholar]
- Souza, G.H.; Silva, B.P.; Viana Neto, G.A.; Finimundy, T.C.; Santos, T.F.D.; Bueno, P.S.A.; Souza, F.O.; Pilau, E.J.; Barros, L.; Comar, J.F.; et al. Tamarindus indica sub-products as potential tools for simultaneous management of diabetes and obesity. Food Funct. 2025, 16, 1969–1991. [Google Scholar] [CrossRef]
- Dave, L.A.; Montoya, C.A.; Rutherfurd, S.M.; Moughan, P. Gastrointestinal endogenous proteins as a source of bioactive peptides- an in silico study. PLoS ONE 2014, 9, e98922. [Google Scholar] [CrossRef]
- Herczeg, B. Normal serum albumin transfer from blood to small intestine and its role in albumin breakdown. Acta Chir. Acad. Sci. Hung 1975, 16, 13–25. [Google Scholar]
- Gilson, M.K.; Liu, T.; Baitaluk, M.; Nicola, G.; Hwang, L.; Chong, J.; Binding, D.B. A public database for medicinal chemistry, computational chemistry and systems pharmacology. Nucleic Acids Res. 2015, 44, D1045–D1053. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissTarget Prediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef] [PubMed]
- Lo Piparo, E.; Scheib, H.; Frei, N.; Williamson, G.; Grigorov, M.; Chou, C.J. Flavonoids for controlling starch digestion: Structural requirements for inhibiting human alpha-amylase. J. Med. Chem. 2008, 51, 3555–3561. [Google Scholar] [CrossRef] [PubMed]


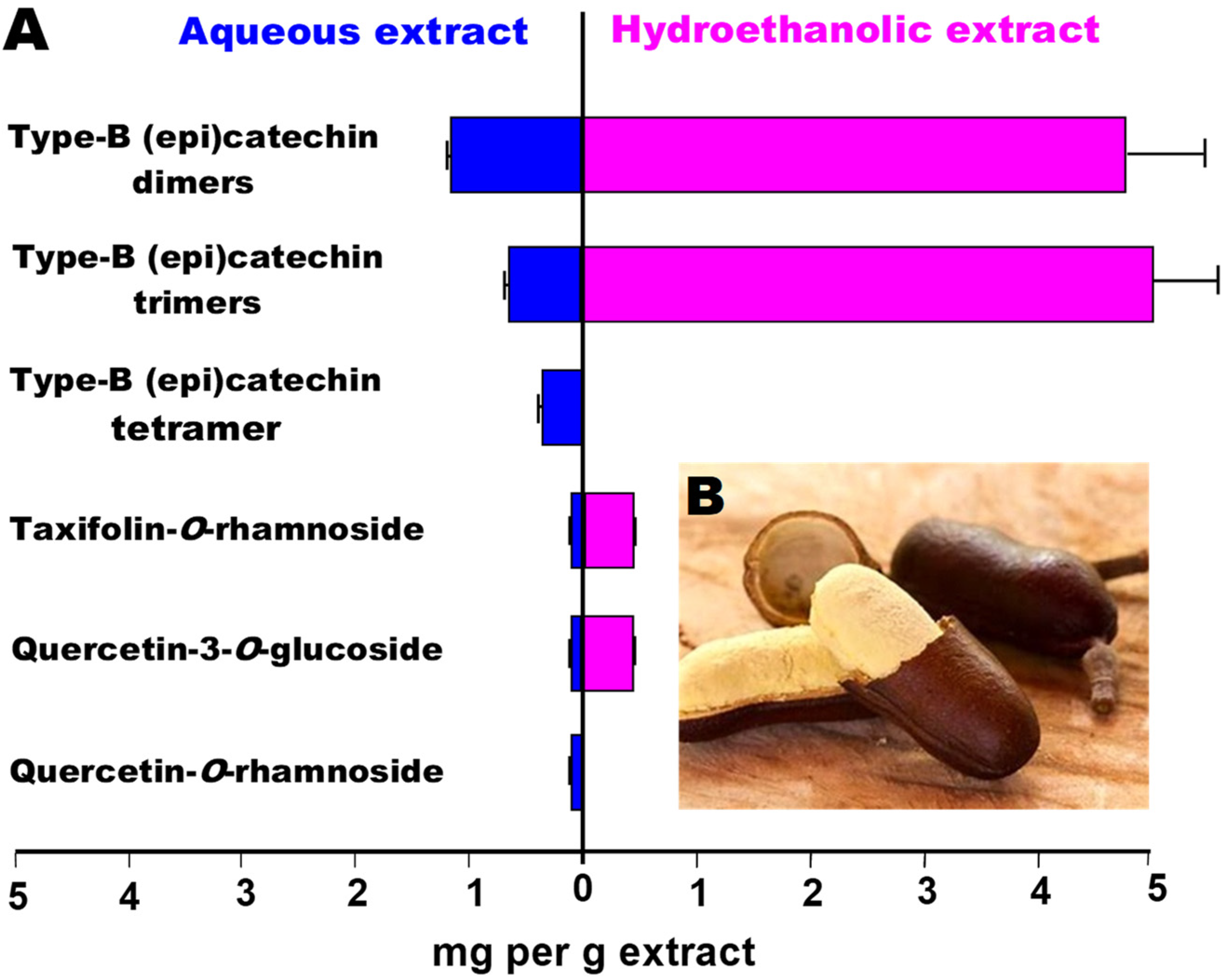
| Data from Del Angelo et al. [14] | Data Obtained at the PubChem Database (https://pubchem.ncbi.nlm.nih.gov, accessed on 10 December 2024) | |||||||
|---|---|---|---|---|---|---|---|---|
| UHPLC Peak | Rt (min) | Tentative Preliminary Identification by ESI-MS | Compound Name | CID | MW (g/mol) | log P | m/z Precursor [M-H]- | m/z Product ion |
| 1 | 4.86 | Type-B (epi)catechin dimer | Epicatechin (4b->6) catechin | 131752345 | 578.5 | 1.5 | dna | dna |
| 2 * | 5.09 | Type-B (epi)catechin dimer | Procyanidin B1 | 11250133 | 578.5 | 2.4 | 577.14 | 407.07 |
| 2 * | 5.09 | Type-B (epi)catechin dimer | Procyanidin B2 | 122738 | 578.5 | 2.4 | 577.14 | 407.07 |
| 3 | 6.17 | Type-B (epi)catechin trimer | Procyanidin C1 | 169853 | 866.8 | 3.3 | 865.23 | 407.09 |
| 4 | 6.95 | Type-B (epi)catechin dimer | Epicatechin-(6′->8)-epicatechin | 56941146 | 578.5 | 2.5 | dna | dna |
| 5 | 9.83 | Type-B (epi)catechin trimer | [Epicatechin-(4beta->6)]2-epicatechin | 163184612 | 866.8 | 3.3 | dna | dna |
| 6 | 10.49 | Type-B (epi)catechin trimer | Epicatechin-(4.beta.->8)epicatechin-(4.beta.->8)epicatechin | 10234623 | 866.8 | 3.3 | 865.19 | 695.10 |
| 7 ** | 11.11 | Type-B (epi)catechin tetramer | Cinnamtannin B2 | 16130973 | 1153.0 | 4.3 | dna | dna |
| 7 ** | 11.11 | Type-B (epi)catechin tetramer | Pavetannin C1 | 16165472 | 1153.0 | 4.3 | dna | dna |
| 8 | 17.55 | Taxifolin-O-rhamoside | Taxifolin 7-O-rhamnoside | 24721355 | 450.4 | 0.2 | 449.11 | 285.04 |
| 9 | 17.91 | Quercetin-3-O-glucoside | Isoquercetin | 5280804 | 464.4 | 0.4 | 463.41 | 301.25 |
| 10 *** | 21.34 | Quercetin-O-rhamnoside | Quercetin 3-O-rhamnoside | 5353915 | 448.4 | 0.9 | 445.08 | 301.04 |
| 10 *** | 21.34 | Quercetin-O-rhamnoside | Quercetin 7-rhamnoside | 14130919 | 448.4 | 0.9 | 447.09 | 301.03 |
| Reference | Myricetin | 5281672 | 318.2 | 1.2 | 315.01 | 317.02 | ||
| Molecule | PubChem CID | Vina Score | |
|---|---|---|---|
| Mean | SD | ||
| Myricetin | 5281672 | −7.70 | 0.00 |
| Epicatechin (4b->6) catechin | 131752345 | −8.63 | 0.05 |
| Procyanidin B1 | 11250133 | −9.30 | 0.00 |
| Procyanidin B2 | 122738 | −9.40 | 0.00 |
| Procyanidin C1 | 169853 | −7.60 | 0.00 |
| Epicatechin-(6′->8)-epicatechin | 56941146 | −9.00 | 0.00 |
| [Epicatechin-(4beta->6)]2-epicatechin | 163184612 | −7.88 | 0.72 |
| Epicatechin-(4.beta.->8)epicatechin-(4.beta.->8)epicatechin | 10234623 | −8.00 | 0.00 |
| Cinnamtannin B2 | 16130973 | −4.90 | 0.59 |
| Pavetannin C1 | 16165472 | −2.25 | 0.29 |
| Taxifolin 7-O-rhamnoside | 24721355 | −8.93 | 0.05 |
| Isoquercetin | 5280804 | −7.43 | 0.10 |
| Quercetin 3-O-rhamnoside | 5353915 | −7.80 | 0.00 |
| Quercetin 7-rhamnoside | 14130919 | −8.80 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polo, A.C.; Uber, T.M.; Souza, G.H.; Corrêa, R.C.G.; dos Santos Filho, J.R.; de Sá-Nakanishi, A.B.; Seixas, F.A.V.; Bracht, A.; Peralta, R.M. Inhibitory Effects of Aqueous and Hydroalcoholic Extracts from Jatobá Coat (Hymenaea courbaril L.) on Pancreatic Amylase and Starch Absorption. Plants 2025, 14, 1133. https://doi.org/10.3390/plants14071133
Polo AC, Uber TM, Souza GH, Corrêa RCG, dos Santos Filho JR, de Sá-Nakanishi AB, Seixas FAV, Bracht A, Peralta RM. Inhibitory Effects of Aqueous and Hydroalcoholic Extracts from Jatobá Coat (Hymenaea courbaril L.) on Pancreatic Amylase and Starch Absorption. Plants. 2025; 14(7):1133. https://doi.org/10.3390/plants14071133
Chicago/Turabian StylePolo, Ana Caroline, Thaís Marques Uber, Gustavo Henrique Souza, Rúbia Carvalho Gomes Corrêa, José Rivaldo dos Santos Filho, Anacharis Babeto de Sá-Nakanishi, Flávio Augusto Vicente Seixas, Adelar Bracht, and Rosane Marina Peralta. 2025. "Inhibitory Effects of Aqueous and Hydroalcoholic Extracts from Jatobá Coat (Hymenaea courbaril L.) on Pancreatic Amylase and Starch Absorption" Plants 14, no. 7: 1133. https://doi.org/10.3390/plants14071133
APA StylePolo, A. C., Uber, T. M., Souza, G. H., Corrêa, R. C. G., dos Santos Filho, J. R., de Sá-Nakanishi, A. B., Seixas, F. A. V., Bracht, A., & Peralta, R. M. (2025). Inhibitory Effects of Aqueous and Hydroalcoholic Extracts from Jatobá Coat (Hymenaea courbaril L.) on Pancreatic Amylase and Starch Absorption. Plants, 14(7), 1133. https://doi.org/10.3390/plants14071133










