Dated Phylogeny of Banisteriopsis (Malpighiaceae) Suggests an Ancient Colonization of the Cerrado and No Evidence of Human Manipulation in the Origin of B. caapi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Taxon Sampling
2.2. DNA Extraction, Amplification, and Sequencing
2.3. Phylogenetic Reconstruction and Estimation of Divergence Times
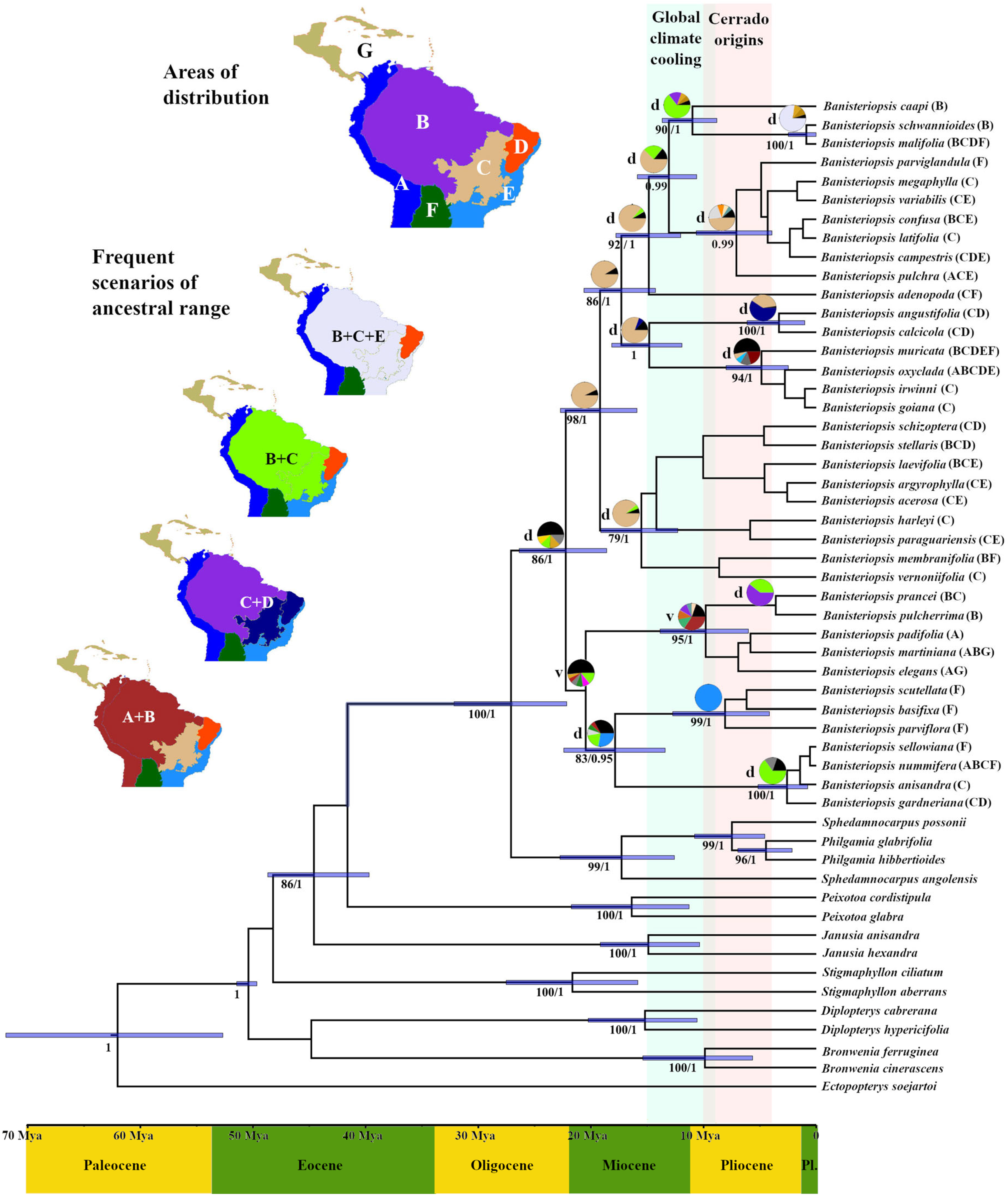
2.4. Biogeographic Analysis
3. Results
3.1. Phylogenetic Analysis
3.2. Divergence Times and Reconstruction of Ancestral Areas
4. Discussion
4.1. Origin of the Genus Banisteriopsis and Biome Transitions
4.2. Wide Ancient Forest as the Origin of B. caapi
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- De Assis, G.L.; Rodrigues, J.A. De quem é a Ayahuasca? Notas sobre a patrimonialização de uma “bebida sagrada” amazônica. Relig. E Soc. 2017, 37, 46–70. [Google Scholar] [CrossRef]
- De Frias, U.A.; Mendes-Costa, M.C.; Takahashi, J.A.; Oki, Y. Banisteriopsis species: A source of bioactive of potential medical application. Ind. J. Biotechnol. Wellness Ind. 2012, 1, 163–171. [Google Scholar] [CrossRef]
- Santos, B.W.L.; de Oliveira, R.C.; Sonsin-Oliveira, J.; Fagg, C.W.; Barbosa, J.B.F.; Caldas, E.D. Biodiversity of β-Carboline Profile of Banisteriopsis caapi and Ayahuasca, a Plant and a Brew with Neuropharmacological Potential. Plants 2020, 9, 870. [Google Scholar] [CrossRef]
- Tupper, K.W. Entheogenic healing: The spiritual effects and therapeutic potential of ceremonial ayahuasca use. In The Healing Power of Spirituality: How Religion Helps Humans Thrive; Ellens, J.H., Ed.; Praeger: Santa Barbara, CA, USA, 2009; pp. 269–282. [Google Scholar]
- McKenna, T. Food of the Gods: The Search for the Original Tree of Knowledge: A Radical History of Plants, Drugs and Human Evolution; Random House Publishing Group, Bantam Books: New York, NY, USA, 1999. [Google Scholar]
- Narby, J. The Cosmic Serpent: DNA and the Origins of Knowledge; TarcherPerigee: New York, NY, USA, 1999. [Google Scholar]
- Naranjo, P. El ayahuasca en la arqueología ecuatoriana. América Indígena 1986, 46, 117–127. [Google Scholar]
- Naranjo, P. Archaeology and psychoactive plants. In Ethnobotany: Evolution of a Discipline; Schultes, R.E., Von Reis, S., Eds.; Dioscorides Press: Portland, OR, USA, 1995; pp. 393–399. [Google Scholar]
- Gow, P. River people: Shamanism and history in Western Amazonia. In Shamanism, History, and the State; Thomas, N., Humphrey, C., Eds.; University of Michigan Press: Ann Arbor, MI, USA, 1994; pp. 90–113. [Google Scholar]
- Brabec de Mori, B. Tracing hallucinations. Contributing to a critical ethnohistory of Ayahuasca usage in the Peruvian Amazon. In The Internationalization of Ayahuasca; Labate, B.C., Jungaberle, H., Eds.; LIT: Zurich, Switzerland, 2011; pp. 23–47. [Google Scholar]
- Brown, E.L. Investigating the Use of Coca and Other Psychoactive Plants in Pre-Columbian Mummies from Chile and Peru. Ph.D. Dissertation, University of Bradford, Bradford, UK, 2012. Available online: https://bradscholars.brad.ac.uk/entities/publication/49d56b5e-e4a8-4523-bf44-21e5e5acbe4a (accessed on 10 January 2024).
- Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. 2024. Available online: http://www.plantsoftheworldonline.org/ (accessed on 10 March 2024).
- Francener, A.; Almeida, R.F. Banisteriopsis in Flora e Funga do Brasil. Jardim Botânico do Rio de Janeiro. 2024. Available online: https://floradobrasil.jbrj.gov.br/FB8803 (accessed on 26 January 2024).
- Gates, B. Banisteriopsis, Diplopterys (Malpighiaceae). Flora Neotrop. 1982, 30, 1–237. [Google Scholar]
- Davis, C.C.; Anderson, W.R. A complete generic phylogeny of Malpighiaceae inferred from nucleotide sequence data and morphology. Am. J. Bot. 2010, 97, 2031–2048. [Google Scholar] [CrossRef]
- Crisp, M.D.; Arroyo, M.T.K.; Cook, L.G.; Gandolfo, M.A.; Jordan, G.J.; McGlone, M.S.; Weston, P.H.; Westoby, M.; Wilf, P.; Linder, H.P. Phylogenetic biome conservatism on a global scale. Nature 2009, 458, 754–756. [Google Scholar] [CrossRef]
- Antonelli, A.; Zizka, A.; Carvalho, F.A.; Scharn, R.; Bacon, C.D.; Silvestro, D.; Condamine, F.L. Amazonia is the primary source of Neotropical biodiversity. Proc. Natl. Acad. Sci. USA 2018, 115, 6034–6039. [Google Scholar] [CrossRef]
- Schultes, R.E. Hallucinogen distribution. J. Psychedelic Drugs 1977, 9, 247–263. [Google Scholar] [CrossRef]
- Ott, J. Pharmacoteon: Entheogenic Drugs, Their Plant Sources and History; Natural Products Co., Ltd.: Kennewick, WA, USA, 1996. [Google Scholar]
- de Oliveira, R.C.; Sonsin-Oliveira, J.; dos Santos, T.A.C.; Simas e Silva, M.; Anderson, C.; de Queiroz, L.P. Lectotypification of Banisteriopsis caapi and B. quitensis (Malpighiaceae), names associated with an important ingredient of Ayahuasca. TAXON 2021, 70, 185–188. [Google Scholar] [CrossRef]
- Cai, L.; Xi, Z.; Peterson, K.; Rushworth, C.; Beaulieu, J.; Davis, C.C. Phylogeny of Elatinaceae and the tropical Gondwanan origin of the Centroplacaceae (Malpighiaceae, Elatinaceae) Clade. PLoS ONE 2016, 9, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Benson, D.A.; Cavanaugh, M.; Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2013, 43, D30. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small amounts of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar] [CrossRef]
- Paithankar, K.R.; Prasad, K.S. Precipitation of DNA by polyethylene glycol and ethanol. Nucleic Acids Res. 1991, 19, 1346. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and USA analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Müller, J.; Müller, K.; Neinhuis, C.; Quandt, D. PhyDE®: Phylogenetic Data Editor. 2006. Available online: www.phyde.de (accessed on 10 January 2024).
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML-VI-HPC: Maximum Likelihood-based Phylogenetic Analyses with Thousands of Taxa and Mixed Models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef]
- Suchard, M.A.; Lemey, P.; Baele, G.; Ayres, D.L.; Drummond, A.J.; Rambaut, A. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol. 2018, 4, vey016. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. ModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Germeraad, J.H.; Hopping, C.A.; Muller, J. Palynology of Tertiary sediments from tropical areas. Rev. Palaeobot. Palynol. 1968, 6, 189–348. [Google Scholar] [CrossRef]
- Gernhard, T. The conditioned reconstructed process. J. Theor. Biol. 2008, 4, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Yule, G.U. A mathematical theory of evolution, based on the conclusions of dr. J. C. Willis, FRS. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1925, 213, 21–87. [Google Scholar]
- Drummond, A.J.; Bouckaert, R.R. Bayesian Evolutionary Analysis with BEAST, 1st ed.; Hardcover; Cambridge University Press: Cambridge, UK, 2015; p. 260. [Google Scholar]
- Nee, S.; Holmes, E.C.; May, R.M.; Harvey, P.H. Extinction rates can be estimated from molecular phylogenies. Philosophical. Trans. R. Soc. Lon. Ser. B Biol. Sci. 1994, 344, 77–82. [Google Scholar] [CrossRef]
- Magallon, S.; Sanderson, M.J. Absolute diversification rates in angiosperm clades. Evolution 2001, 55, 1762–1780. [Google Scholar]
- Drummond, A.J.; Rambaut, A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 2007, 7, 214. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. Proc. Gatew. Comput. Environ. Workshop (GCE) 2010. Available online: http://www.phylo.org/index.php/ (accessed on 10 January 2024).
- Morrone, J.J. Biogeographical regionalisation of the Neotropical region. Zootaxa 2014, 3782, 1–110. [Google Scholar] [CrossRef]
- Yu, Y.; Christopher, B.; Xingjin, H. RASP 4: Ancestral State Reconstruction Tool for Multiple Genes and Characters. Mol. Biol. Evol. 2020, 37, 604–606. [Google Scholar] [CrossRef]
- Yu, Y.; Harris, A.J.; He, X.J. S-DIVA (statistical dispersal-vicariance analysis): A tool for inferring biogeographic histories. Mol. Phylogenet. Evol. 2010, 56, 848–850. [Google Scholar] [CrossRef]
- Beaulieu, J.M.; Tank, D.C.; Donoghue, M.J. A Southern Hemisphere origin for campanulid angiosperms, with traces of the break-up of Gondwana. BMC Evol. Biol. 2013, 13, 80. [Google Scholar] [CrossRef]
- Landis, M.J.; Matzke, N.J.; Moore, B.R.; Huelsenbeck, J.P. Bayesian analysis of biogeography when the number of areas is large. Syst. Biol. 2013, 62, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Paradis, E.; Schliep, K. Ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 2019, 35, 526–528. [Google Scholar] [CrossRef] [PubMed]
- Fiaschi, G.; Amorim, A.M.; Jardim, J.G.; Gomes, A.S.; Coelho, M.A.N. Biogeografia da flora da América do Sul. In Biogeografia da América do Sul; Carvalho, C.J.B., Almeida, E.A.B., Eds.; Roca: São Paulo, Brazil, 2016; pp. 215–226. [Google Scholar]
- Antonelli, A.; Verola, C.F.; Parisod, C.; Gustafsson, A.L.S. Climate cooling promoted the expansion of a threatened group of South American orchids (Epidendroideae: Laeliinae). Biol. J. Linn. Soc. 2010, 100, 597–607. [Google Scholar] [CrossRef]
- Simon, M.F.; Grether, R.; de Queiroz, L.P.; Skema, C.; Pennington, R.T.; Hughes, C.E. Recent assembly of the Cerrado, a neotropical plant diversity hotspot, by in situ evolution of adaptations to fire. Proc. Natl. Acad. Sci. USA 2009, 106, 20359–20364. [Google Scholar] [CrossRef]
- Zanella, F.C.V. Evolução da biota da diagonal de formações abertas secas da América do Sul. In Biogeografia da América do Sul: Padrões e Processos; Carvalho, C.J.B., Almeida, E.A.B., Eds.; Roca: São Paulo, Brazil, 2011; pp. 198–220. [Google Scholar]
- Simon, M.F.; Pennington, T. Evidence for adaptation to fire regimes in the tropical savannas of the Brazilian Cerrado. Int. J. Plant Sci. 2012, 6, 711–723. [Google Scholar] [CrossRef]
- Mendes, T.P.; De Souza, A.O.; Silva, M.J. Molecular phylogeny and diversification timing of the Chamaecrista sect. Absus subsect. Absus ser. Paniculatae, a newly circumscribed and predominantly endemic of the Cerrado Biome group. Phytotaxa 2020, 446, 159–182. [Google Scholar] [CrossRef]
- Cabral, F.N.; Trad, R.J.; Bittrich, V.; Amaral, M.C.E.; Martins, A.C.; Goldenberg, R.; Coelho, M.A.N.; Lucas, E.J. Phylogeny, divergence times, and diversification in Calophyllaceae: Linking key characters and habitat changes to the evolution of Neotropical Calophylleae. Mol. Phylogenet. Evol. 2021, 157, 107041. [Google Scholar] [CrossRef]
- Duarte, M.C.; Esteves, G.L.; Salatino, M.L.F.; Walsh, K.C.; Baum, D.A. Phylogenetic analyses of Eriotheca and related genera (Bombacoideae, Malvaceae). Syst. Bot. 2011, 36, 690–701. [Google Scholar] [CrossRef]
- De Oliveira Bünger, M.; Sobral-Souza, T.; da Silva, M.N.F.; de Lemos-Filho, J.P.; Lovato, M.B. The evolutionary history of Eugenia sect. Phyllocalyx (Myrtaceae) corroborates historically stable areas in the southern Atlantic Forests. Ann. Bot. 2016, 118, 1209–1223. [Google Scholar] [CrossRef]
- Santos, M.F.; Lucas, E.; Sano, P.T.; Buerki, S.; Staggemeier, V.G.; Forest, F. Biogeographical patterns of Myrcia s.l. (Myrtaceae) and their correlation with geological and climatic history in the Neotropics. Mol. Phylogenet. Evol. 2017, 108, 34–48. [Google Scholar] [CrossRef]
- Almeida, R.F.; Van den Berg, C. Biogeography of Stigmaphyllon (Malpighiaceae) and a meta-analysis of vascular plant lineages diversified in the Brazilian Atlantic Rainforests point to the Late Eocene origins of this megadiverse biome. Plants 2020, 9, 1569. [Google Scholar] [CrossRef] [PubMed]
- Lima, D.F.; Goldenberg, R.; Forest, F.; Cowan, R.S.; Lucas, E.J. Phylogeny and biogeography of Myrcia sect. Aguava (Myrtaceae, Myrteae) based on phylogenomic and Sanger data provide evidence for a Cerrado origin and geographically structured clades. Mol. Phylogenet. Evol. 2021, 157, 107043. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, D.J.P.; Shimizu, G.H.; Ortiz, E.M.; Jansen, R.K.; Simpson, B.B. Historical biogeography of Vochysiaceae reveals an unexpected perspective of plant evolution in the Neotropics. Am. J. Bot. 2020, 107, 1004–1020. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, A.J.C.; Melo, G.A.R.; Vasconcelos, T.N.C.; Gonçalves, R.B.; Giugliano, L.; Martins, A.C. Biogeography and early diversification of Tapinotaspidini oil-bees support presence of Paleocene savannas in South America. Mol. Phylogenet. Evol. 2020, 143, 106692. [Google Scholar] [CrossRef]
- Gagnon, E.; Ringelberg, J.J.; Bruneau, A.; Lewis, G.P.; Hughes, C.E. Global succulent biome phylogenetic conservatism across the pantropical Caesalpinia Group (Leguminosae). New Phytol. 2019, 222, 1994–2008. [Google Scholar] [CrossRef]
- Antonelli, A.; Ariza, M.; Albert, J.; Andermann, T.; Azevedo, J.; Bacon, C.; Faurby, S.; Guedes, T.; Hoorn, C.; Lohmann, L.G.; et al. Conceptual and empirical advances in Neotropical biodiversity research. PeerJ 2018, 6, e5644. [Google Scholar] [CrossRef]
- Strassburg, B.B.N.; Brooks, T.; Feltran-Barbieri, R.; Iribarrem, A.; Crouzeilles, R.; Loyola, R.; Latawiec, A.E.; Oliveira Filho, F.J.B.; Scaramuzza, C.A.M.; Scarano, F.R.; et al. Moment of truth for the Cerrado hotspot. Nat. Ecol. Evol. 2017, 1, 1–3. [Google Scholar] [CrossRef]
- Oliveira-Filho, A.T.; Ratter, J.A. A study of the origin of central Brazilian forests by the analysis of plant species distribution patterns. Edinb. J. Bot. 1995, 52, 141–194. [Google Scholar] [CrossRef]
- Machado, R.B.; Ramos Neto, M.B.; Pereira, P.G.P.; Caldas, E.F.; Gonçalves, D.A.; Santos, N.S.; Tabor, K.; Steininger, M. Estimativas de perda da área do Cerrado brasileiro. In Relatório Técnico não Publicado; Conservação Internacional: Brasília, Brazil, 2004; p. 26. [Google Scholar]
- Zachos, J.C.; Dickens, G.R.; Zeebe, R.E. An Early Cenozoic Perspective on Greenhouse Warming and Carbon-Cycle Dynamics. Nature 2008, 451, 279–283. [Google Scholar] [CrossRef]
- Micheels, A.; Bruch, A.A.; Uhl, D.; Utescher, T.; Mosbrugger, V. A Late Miocene climate model simulation with ECHAM4/ML and its quantitative validation with terrestrial proxy data. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007, 253, 251–270. [Google Scholar] [CrossRef]
- Antonelli, A.; Nylander, J.A.A.; Persson, C.; Sanmartín, I. Tracing the impact of the Andean uplift on Neotropical plant evolution. Proc. Natl. Acad. Sci. USA 2009, 105, 9749–9754. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Sanmartín, I. Why are there so many plant species in the Neotropics? Taxon 2011, 60, 403–414. [Google Scholar] [CrossRef]
- Colli-Silva, M.; Richardson, J.E.; Neves, E.G.; Watling, J.; Figueira, A.; Pirani, J.R. Domestication of the Amazonian fruit tree cupuaçu may have stretched over the past 8000 years. Commun. Earth Environ. 2023, 4, 401. [Google Scholar] [CrossRef]
- Serbin, G.M.; Pinangé, D.S.B.; Machado, R.M.; Vasconcelos, S.; Amorim, B.S.; Clement, C.R. Relationship between fruit phenotypes and domestication in hexaploid populations of biribá (Annona mucosa) in Brazilian Amazonia. PeerJ 2023, 11, e14659. [Google Scholar] [CrossRef]
- Vargas, O.M.; Dick, C.W. Diversification History of Neotropical Lecythidaceae, an Ecologically Dominant Tree Family of Amazon Rain Forest. In Neotropical Diversification: Patterns and Processes; Rull, V., Carnaval, A.C., Eds.; Springer: Cham, Switzerland, 2020; pp. 791–809. [Google Scholar] [CrossRef]
- Arévalo-Marín, E.; Casas, A.; Landrum, L.; Shock, M.P.; Alvarado-Sizzo, H.; Ruiz-Sanchez, E.; Clement, C.R. The Taming of Psidium guajava: Natural and Cultural History of a Neotropical Fruit. Front. Plant Sci. 2021, 12, 714763. [Google Scholar] [CrossRef]
- Piperno, D.R.; Pearsall, D.M. The Origins of Agriculture in the Lowland Neotropics; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Uhle, M. Los principios de las antiguas civilizaciones peruanas. In Boletín de la Sociedad Ecuatoriana de Estudios Históricos Americanos; Imprenta y Encuadernación Salesianas: Quito, Ecuador, 1920; Volume 4, pp. 448–458. [Google Scholar]
- Dillehay, T.D.; Rossen, J.; Ugent, D.; Karathanasis, A.; Vásquez, V.; Netherly, P.J. Early Holocene coca chewing in northern Peru. Antiquity 2010, 84, 939–953. [Google Scholar] [CrossRef]
- Ogalde, J.P.; Arriaza, B.T.; Soto, E.C. Identification of psychoactive alkaloids in ancient Andean human hair by gas chromatography/mass spectrometry. J. Archaeol. Sci. 2009, 36, 467–472. [Google Scholar] [CrossRef]
- Ogalde, J.P.; Arriaza, B.T.; Soto, E.C. Uso de plantas psicoactivas en el norte de Chile: Evidencia química del consumo de ayahuasca durante el Periodo Medio (500–1000 d.C.). Lat. Am. Antiq. 2010, 21, 441–450. [Google Scholar] [CrossRef]
- Ogalde, J.P.; Arriaza, B.T.; Santoro, C.M.; Capriles, J.M.; Puddu, G.; Ugalde, P.C.; Rothhammer, F. Pre-hispanic consumption of psychoactive substances in Northern Chile suggests early exchange networks with the Central Altiplano and the Amazon Region. Interciencia 2017, 42, 459–463. [Google Scholar]
- Miller, M.J.; Albarracín-Jordán, J.; Moore, C.; Capriles, J.M. Chemical evidence for the use of multiple psychotropic plants in a 1000-year-old ritual bundle from South America. Proc. Natl. Acad. Sci. USA 2019, 116, 11207–11212. [Google Scholar] [CrossRef]
- Albuquerque, M.B.B. Modalidades de usos e saberes do cipó Cabi. Saec.—Rev. História 2012, 27, 195–213. Available online: https://periodicos.ufpb.br/ojs2/index.php/srh/article/view/16438 (accessed on 21 February 2025).
- Schultes, R.E. Recognition of variability in wild plants by Indians of the Northwest Amazon: An enigma. J. Ethnobiol. 1986, 6, 229–238. [Google Scholar]
- Oliveira, R.C.; Pinheiro, N.; Maciel, J.R.; Welker, C.A.D.; Sonsin-Oliveira, J.; de Oliveira, M.L.S. Ethnobotany and Wood Anatomy of Banisteriopsis caapi Ethnotaxa and Diplopterys cf. pubipetala, Components of Ayahuasca in Brazilian Rituals. Econ. Bot. 2023, 77, 18–47. [Google Scholar] [CrossRef]
- Luz, T.Z.; Cunha-Machado, A.S.; Batista, J.S. First DNA barcode efficiency assessment for an important ingredient in the Amazonian ayahuasca tea: Mariri/jagube, Banisteriopsis caapi (Malpighiaceae). Genet. Resour. Crop Evol. 2023, 70, 1605–1616. [Google Scholar] [CrossRef]
- Clement, C.R.; Denevan, W.M.; Heckenberger, M.J.; Junqueira, A.B.; Neves, E.G.; Teixeira, W.G.; Woods, W.I. The domestication of Amazonia before European conquest. Proc. R. Soc. B 2015, 282, 1–9. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, T.A.C.; Amorim, B.S.; Maciel, J.R.; Welker, C.A.D.; Biazatti, S.C.; Oliveira, R.C. Dated Phylogeny of Banisteriopsis (Malpighiaceae) Suggests an Ancient Colonization of the Cerrado and No Evidence of Human Manipulation in the Origin of B. caapi. Plants 2025, 14, 1149. https://doi.org/10.3390/plants14071149
Santos TAC, Amorim BS, Maciel JR, Welker CAD, Biazatti SC, Oliveira RC. Dated Phylogeny of Banisteriopsis (Malpighiaceae) Suggests an Ancient Colonization of the Cerrado and No Evidence of Human Manipulation in the Origin of B. caapi. Plants. 2025; 14(7):1149. https://doi.org/10.3390/plants14071149
Chicago/Turabian StyleSantos, Thais A. C., Bruno S. Amorim, Jefferson R. Maciel, Cassiano A. D. Welker, Scheila Cristina Biazatti, and Regina C. Oliveira. 2025. "Dated Phylogeny of Banisteriopsis (Malpighiaceae) Suggests an Ancient Colonization of the Cerrado and No Evidence of Human Manipulation in the Origin of B. caapi" Plants 14, no. 7: 1149. https://doi.org/10.3390/plants14071149
APA StyleSantos, T. A. C., Amorim, B. S., Maciel, J. R., Welker, C. A. D., Biazatti, S. C., & Oliveira, R. C. (2025). Dated Phylogeny of Banisteriopsis (Malpighiaceae) Suggests an Ancient Colonization of the Cerrado and No Evidence of Human Manipulation in the Origin of B. caapi. Plants, 14(7), 1149. https://doi.org/10.3390/plants14071149





