The Neuroprotective Potential of Betalains: A Focused Review
Abstract
1. Introduction
2. Betalains and Their Sources
2.1. Chemical Structure and Classification of Betalains

2.2. Occurrence in Nature and Role of Betalains in Plants
2.3. Sources of Betalains
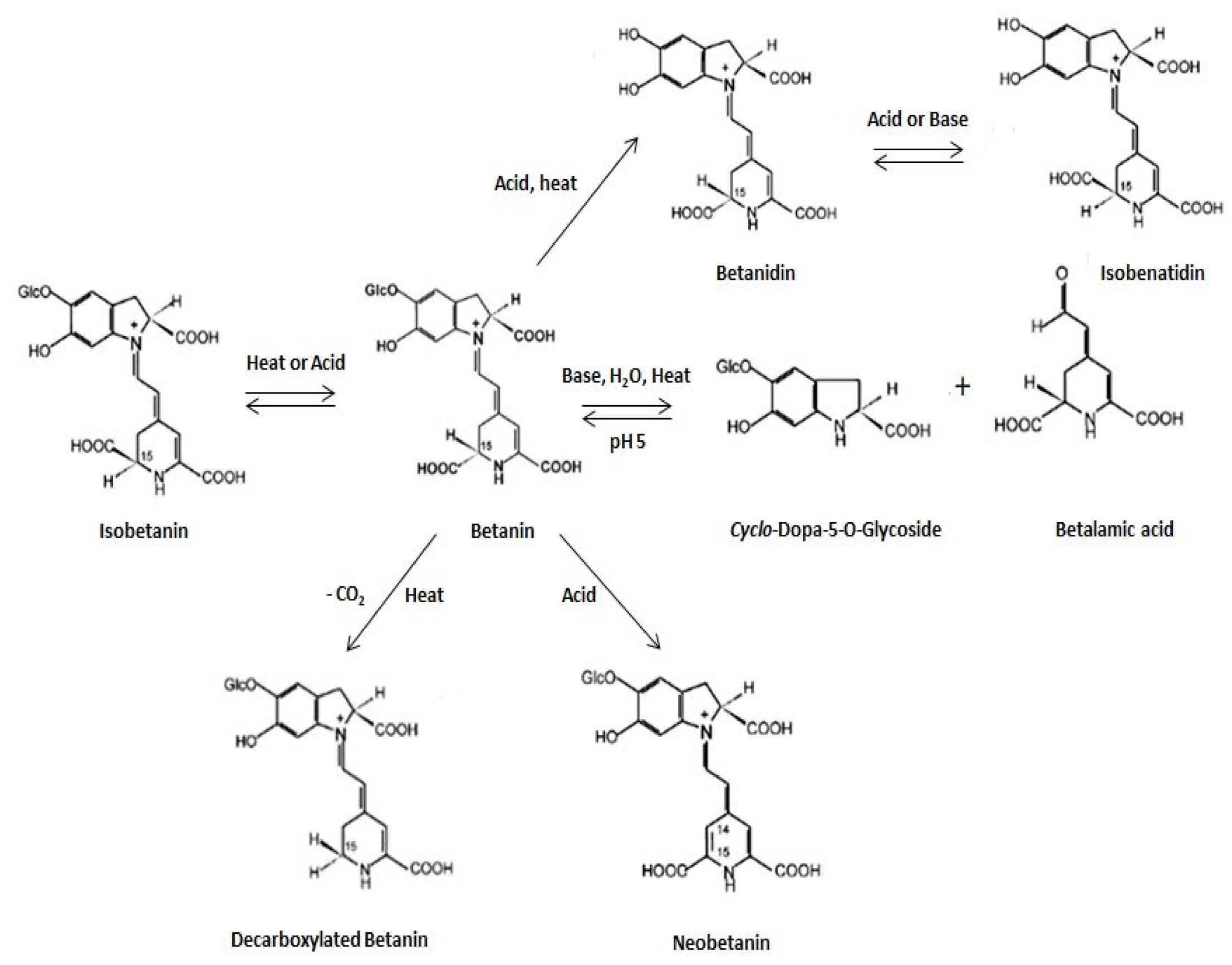
3. Chemical Stability and Bioavailability of Betalains
4. Neuroprotective Potential of Betalains
4.1. Preclinical Studies
| No. | Experimental Model | Substances and Doses/Route of Administration (In Vivo) | Main Findings | Reference |
|---|---|---|---|---|
| Models of Alzheimer’s disease | ||||
| 1. | Transgenic Caenorhabditis elegans | 50 µM betanin and isobetanin | Reduction in beta-amyloid-induced toxicity; reduction in amyloid-β42 aggregation | [55] |
| 2. | In vitro assessment of AchE | 12.5–400 µM betanin and glycine betaine | An 86.6% and 92.9% inhibition of acetylcholinesterase with IC50 of 1.271 and 1.203 µM | [56] |
| 3. | AlCl3-induced Alzheimer in SD rats | 10–20 mg/kg betalain/oral | Reduction in cognitive deficit; reduction in inflammatory cytokines in the brain | [57] |
| 4. | Scopolamine-induced Alzheimer symptoms in Wistar rats | 25–50 mg/kg betanin/oral | Reduction in learning deficit in passive avoidance tests | [58] |
| 5. | Trimethyltin-induced neurodegeneration in mice | 50–100 mg/kg betanin/oral | Prevention of hippocampal CA1 neural degeneration; increase in choline acetyltransferase activity; improvement of spatial learning and memory deficits | [59] |
| Models of Parkinson’s disease | ||||
| 6. | Rotenone-induced Parkinson in mice | 50–100 mg/kg betanins/s.c | Improvement of motor tests; reduction in substantia nigra degeneration in treated animals | [60] |
| 7. | Rotenone-induced Parkinson in mice | 100–200 mg/kg betanin/oral | Prevention of substantia nigra, striatum and motor cortex neural degeneration | [61] |
| 8. | In vitro assessment of 6-hydroxydopamine toxicity in PC12 cells | 1–200 µM betanin | Reduction in PC12 cells apoptosis; reduction in the progression of neural death | [62] |
| Other models of neurodegeneration and brain injuries | ||||
| 9. | High fat-diet fed mice | 0.86 mg/kg indicaxanthin/oral | Reduction in neuronal apoptosis by decreasing the expression of neuroinflammatory proteins and ROS and nitrogen species | [63] |
| 10 | Ischemia–reperfusion induced neural injuries in ICR mice | 50–100 mg/kg betanin/oral | Reduction in brain infarctions and hippocampal white matter lesions | [64] |
| 11. | Paracetamol and diclofenac induced neurotoxicity in rats | 25 mg/kg betanin/oral | Protection against drug-induced neurotoxicity; amelioration of biochemical and histopathological brain changes | [65] |
| 12. | D-galactose-induced neurotoxicity in mice | 50–100 mg/kg betanin/oral | Reversal of D-galactose-induced learning and memory impairments | [66] |
4.2. Studies in Humans
4.3. Molecular Mechanisms of Betalains Responsible for Neuroprotective Effects
4.3.1. Inhibition of β-Amyloid Aggregation
4.3.2. Cholinergic Mechanisms
4.3.3. Anti-Inflammatory Mechanisms
4.3.4. Reduction in Oxidative Stress
4.3.5. Reduction in Apoptosis
5. Safety Profile of Betalains
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 6-OHDA | 6-hydroxydopamine |
| AchE | acetylcholinesterase |
| APP | amyloid beta precursor protein |
| ARE | antioxidant responsive element |
| BACE1 | beta-site amyloid precursor protein cleaving enzyme 1 |
| COX2 | cyclooxygenase 2 |
| FGF | fibroblast growth factor |
| FRAP | ferric-reducing antioxidant power |
| IC50 | half-maximal inhibitory concentration |
| ICAM1 | intercellular adhesion molecule 1 |
| IL1 | interleukin 1 |
| IL6 | interleukin 6 |
| KEAP1 | Kelch-like ECH-associated protein 1 |
| LOX | lipooxygenase |
| NF-kB | nuclear factor-kB |
| Nrf2 | nuclear factor erythroid 2-related factor |
| PI3K | phosphatidylinositol 3 kinase |
| PARP | poly (ADP-ribose) polymerase |
| SAPK | stress-activated protein kinase |
| SIRT1 | silent information regulator 2-related protein 1 |
| VCAM | vascular cell adhesion molecule-1 |
| TNFα | tumor necrosis factor alpha |
References
- Tămaş, M.; Balica, G.; Ştefănescu, C. Anthocyanins, betacyanins and the systematics of Caryophyllids. Contrib. Bot. 2018, 53, 7–17. [Google Scholar] [CrossRef]
- Coy-Barrera, E. Analysis of betalains (betacyanins and betaxanthins). In Recent Advances in Natural Products Analysis; Sanches Silva, A., Nabavi, S.F., Saeedi, M., Nabavi, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 593–619. [Google Scholar]
- Corrêa, R.C.G.; Garcia, J.A.A.; Correa, V.G.; Vieira, T.F.; Bracht, A.; Peralta, R.M. Pigments and vitamins from plants as functional ingredients: Current trends and perspectives. Adv. Food Nutr. Res. 2019, 90, 259–303. [Google Scholar] [CrossRef] [PubMed]
- Birchfield, A.S.; McIntosh, C.A. Metabolic Engineering and Synthetic Biology of Plant Natural Products—A Minireview. Curr. Plant Biol. 2020, 24, 100163. [Google Scholar] [CrossRef]
- Cai, Y.-Z.; Sun, M.; Corke, H. Characterization and application of betalain pigments from plants of the Amaranthaceae. Trends Food Sci. Technol. 2005, 16, 370–376. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, P.; Guerrero-Rubio, M.A.; Henarejos-Escudero, P.; García-Carmona, F.; Gandía-Herrero, F. Health-promoting potential of betalains in vivo and their relevance as functional ingredients: A review. Trends Food Sci. Technol. 2022, 122, 66–82. [Google Scholar] [CrossRef]
- Tossi, V.E.; Martínez Tosar, L.; Pitta-Álvarez, S.I.; Causin, H.F. Casting light on the pathway to betalain biosynthesis: A review. Environ. Exp. Bot. 2021, 186, 104464. [Google Scholar] [CrossRef]
- Miguel, M.G. Betalains in Some Species of the Amaranthaceae Family: A Review. Antioxidants 2018, 7, 53. [Google Scholar] [CrossRef]
- Kusznierewicz, B.; Mróz, M.; Koss-Mikołajczyk, I.; Namieśnik, J. Comparative evaluation of different methods for determining phytochemicals and antioxidant activity in products containing betalains—Verification of beetroot samples. Food Chem. 2021, 362, 130132. [Google Scholar] [CrossRef]
- Lira, S.M.; Dionisio, A.P.; Holanda, M.O.; Marques, C.G.; Silva, G.S.D.; Correa, L.C. Metabolic profile of pitaya (Hylocereus polyrhizus (F.A.C. Weber) Britton & Rose) by UPLC-QTOF-MSE and assessment of its toxicity and anxiolytic-like effect in adult zebrafish. Food Res. Int. 2020, 127, 108701. [Google Scholar] [CrossRef]
- Cacciatore, I.; Baldasare, L.; Fornasari, E.; Molica, A.; Pinnen, F. Recent advances in the treatment of neurodegenerative diseases based on GSH delivery systems. Oxidative Med. Cell. Longev. 2012, 2012, 240146. [Google Scholar] [CrossRef]
- Erkkinen, M.G.; Kim, M.-O.; Geschwind, M.D. Clinical neurology and epidemiology of the major neurodegenerative disorders. Cold Spring Harb. Perspect. Biol. 2018, 10, a033118. [Google Scholar] [CrossRef]
- Belhadj Slimen, I.; Najar, T.; Abderrabba, M. Chemical and Antioxidant Properties of Betalains. J. Agric. Food Chem. 2017, 65, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Giridhar, P. Plant betalains: Chemistry and biochemistry. Phytochemistry 2015, 117, 267–295. [Google Scholar] [CrossRef]
- Celli, G.B.; Brooks, M.S.-L. Impact of extraction and processing conditions on betalains and comparison of properties with anthocyanins-A current review. Food Res. Int. 2017, 100, 501–509. [Google Scholar] [CrossRef]
- Azeredo, H.M.C. Betalains: Properties, sources, applications, and stability—A review. Int. J. Food Sci. Technol. 2009, 44, 2365–2376. [Google Scholar] [CrossRef]
- Herbach, K.M.; Stintzing, F.C.; Carle, R. Betalain Stability and Degradation? Structural and Chromatic Aspects. J. Food Sci. 2006, 71, R41–R50. [Google Scholar] [CrossRef]
- Polturak, G.; Aharoni, A. “La vie en rose”: Biosynthesis, sources, and application of betalain pigments. Mol. Plant 2018, 11, 7–22. [Google Scholar] [CrossRef]
- Jain, G.; Gould, K.S. Are betalain pigments the functional homologues of anthocyanins in plants? Environ. Exp. Bot. 2015, 119, 48–53. [Google Scholar] [CrossRef]
- Strack, D.; Vogt, T.; Schliemann, W. Recent advances in betalain research. Phytochemistry 2003, 62, 247–269. [Google Scholar] [CrossRef]
- Pavoković, D.; Krsnik-Rasol, M. Complex Biochemistry and Biotechnological Production of Betalains. Food Technol. Biotechnol. 2011, 49, 145–155. [Google Scholar]
- Timoneda, A.; Feng, T.; Sheehan, H.; Walker-Hale, N.; Pucker, B.; Lopez-Nieves, S.; Brockington, S. The evolution of betalain biosynthesis in Caryophyllales. New Phytol. 2019, 224, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Llano, L.E.; Guerrero-Rubio, M.A.; Lozada-Ramírez, J.D.; García-Carmona, F.; Gandía-Herrero, F. First betalain producing bacteria break the exclusive presence of the pigments in the plant kingdom. Mol. Biol. Physiol. 2019, 10, e00345-19. [Google Scholar] [CrossRef] [PubMed]
- Harris, N.N.; Javellana, J.; Davies, K.M.; Lewis, D.H.; Jameson, P.E.; Deroles, S.C. Betalain production is possible in anthocyanin-producing plant species given the presence of DOPA-dioxygenase and L-DOPA. BMC Plant Biol. 2012, 12, 34. [Google Scholar] [CrossRef]
- Hernández-Ledesma, P.; Berendsohn, W.G.; Borsch, T.; Mering, S.V.; Akhani, H.; Arias, S.; Uotila, P. A taxonomic backbone for the global synthesis of species diversity in the angiosperm order Caryophyllales. Willdenowia 2015, 45, 281. [Google Scholar] [CrossRef]
- Stevens, P.F. (2001 Onwards). Angiosperm Phylogeny Website. Version 14. July 2017. Available online: http://www.mobot.org/MOBOT/research/APweb/ (accessed on 15 January 2025).
- Solovchenko, A.; Yahia, E.M.; Chen, C. Pigments in Postharvest Physiology and Biochemistry of Fruits and Vegetables; Yahia, E.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 225–252. [Google Scholar]
- Rahimi, P.; Abedimanesh, S.; Mesbah Namin, S.A.; Ostadrahimi, A. Betalains, the nature-inspired pigments, in health and diseases. Crit. Rev. Food Sci. Nutr. 2018, 59, 2949–2978. [Google Scholar] [CrossRef]
- Gengatharan, A.; Dykes, G.A.; Choo, W.S. Betalains: Natural plant pigments with potential application in functional foods. LWT Food Sci. Technol. 2015, 64, 645–649. [Google Scholar] [CrossRef]
- Carreón-Hidalgo, J.P.; Franco-Vásque, D.C.; Gómez-Linton, D.R.; Pérez-Flores, L.J. Betalain plant sources, biosynthesis, extraction, stability enhancement methods, bioactivity, and applications. Food Res. Int. 2022, 151, 110821. [Google Scholar] [CrossRef]
- Melgar, B.; Pereira, E.; Oliveira, M.B.P.P.; Garcia-Castello, E.M.; Rodriguez-Lopez, A.D.; Sokovic, M.; Barros, L.; Ferreira, I.C.F.R. Extensive profiling of three varieties of Opuntia spp. fruit for innovative food ingredients. Food Res. Int. 2017, 101, 259–265. [Google Scholar] [CrossRef]
- Agostini-Costa, T.S. Bioactive compounds and health benefits of Pereskioideae and Cactoideae: A review. Food Chem. 2020, 327, 126961. [Google Scholar] [CrossRef]
- Escribano, J.; Cabanes, J.; Jiménez-Atiénzar, M.; Ibañez-Tremolada, M.; Gómez-Pando, L.R.; García-Carmona, F.; Gandía-Herrero, F. Characterization of betalains, saponins and antioxidant power in differently colored quinoa (Chenopodium quinoa) varieties. Food Chem. 2017, 234, 285–294. [Google Scholar] [CrossRef]
- Miranda, P.H.S.; dos Santos, A.C.; de Freitas, B.C.; de Souza Martins, G.A.; de Vilas Boas, E.V.; Damiani, C. A scientific approach to extraction methods and stability of pigments from Amazonian fruits. Trends Food Sci. Technol. 2021, 113, 335–345. [Google Scholar]
- Grewall, P.S.; Modavi, C.; Russ, Z.N.; Harris, N.C.; Dueber, J.E. Bioproduction of a betalain color palette in Saccharomyces cerevisiae. Metab. Eng. 2018, 45, 180–188. [Google Scholar]
- Pátkai, G.; Barta, J. Decomposition of betacyanins and betaxanthins by heat and pH changes. Nahr. Food 1996, 40, 267–270. [Google Scholar] [CrossRef]
- Guneser, O. Kinetic Modelling of Betalain Stability and Color Changes in Yogurt During Storage. Pol. J. Food Nutr. Sci. 2021, 71, 135–145. [Google Scholar]
- Schwartz, S.J.; von Elbe, J.H. Identification of betanin degradation products. Z. Leb. Forsch. 1983, 176, 448–453. [Google Scholar]
- Skalicky, M.; Kubes, J.; Shokoofeh, H.; Tahjib-Ul-Arif, M.; Pavla, V.; Hejnak, V. Betacyanins and Betaxanthins in Cultivated Varieties of Beta vulgaris L. Compared to Weed Beets. Molecules 2020, 25, 5395. [Google Scholar] [CrossRef]
- Von Elbe, J.H. Stability of betalains as food colors. Food Technol. 1975, 5, 42–44. [Google Scholar]
- Koop, B.L.; da Silva, M.N.; da Silva, F.D.; Lima, K.T.D.S.; Soares, L.S.; de Andrade, C.J.; Valencia, G.A.; Monteiro, A.R. Flavonoids, anthocyanins, betalains, curcumin, and carotenoids: Sources, classification and enhanced stabilization by encapsulation and adsorption. Food Res. Int. 2022, 153, 110929. [Google Scholar] [CrossRef]
- Kumar, S.S.; Chauhan, A.S.; Giridhar, P. Nanoliposomal encapsulation mediated enhancement of betalain stability: Characterisation, storage stability and antioxidant activity of Basella rubra L. fruits for its applications in vegan gummy candies. Food Chem. 2020, 333, 127442. [Google Scholar]
- Matencio, A.; Guerrero-Rubio, M.A.; Gandia-Herrero, F.; Garcia-Carmona, F.; Lopez-Nicolas, J.M. Nanoparticles of betalamic acid derivatives with cyclodextrins. Physicochemistry, production characterization and stability. Food Hydrocoll. 2021, 110, 106176. [Google Scholar]
- Khan, M.I. Plant betalains: Safety, antioxidant activity, clinical efficacy and bioavailability. Compr. Rev. Food Sci. Food Saf. 2016, 15, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Tesoriere, L.; Gentile, C.; Angileri, F.; Attanzio, A.; Tutone, M.; Allegra, M.; Livrea, M.A. Trans-epithelial transport of the betalain pigments indicaxanthin and betanin across Caco-2 cell monolayers and influence of food matrix. Eur. J. Nutr. 2013, 52, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Desseva, I.; Stoyanova, M.; Petkova, N.; Mihaylova, D. Red beetroot juice phytochemicals bioaccesibility: An in vitro approach. Pol. J. Food Nutr. Sci. 2020, 70, 45–53. [Google Scholar] [CrossRef]
- Kanner, J.; Harel, S.; Granit, R. Betalains A New Class of Dietary Cationized Antioxidants. J. Agric. Food Chem. 2001, 49, 5178–5185. [Google Scholar] [CrossRef]
- Tesoriere, L.; Allegra, M.; Butera, D.; Livrea, M.A. Absorption, excretion, and distribution of dietary antioxidant betalains in LDLs: Potential health effects of betalains in humans. Am. J. Clin. Nutr. 2004, 80, 941–945. [Google Scholar] [CrossRef]
- Wang, Y.; Adekolurejo, O.O.; Wang, B.; McDermott, K.; Do, T.; Marshall, L.J.; Boesch, C. Bioavailability and exretion profile of betacyanins-variability and correlations between different excretion routes. Food Chem. 2024, 437, 137663. [Google Scholar] [CrossRef]
- Sawicki, T.; Topolska, J.; Romaszko, E.; Wiczowski, W. Profile and content of betalains in plasma and urine of volunteers after long-term exposure of fermented red beet juice. J. Agric. Food Chem. 2018, 66, 4155–4163. [Google Scholar] [CrossRef]
- Allegra, M.; Carletti, F.; Gambino, G.; Tutone, M.; Attanzio, A.; Tesoriere, L.; Ferraro, G.; Sardo, P.; Almerico, A.M.; Livrea, M.A. Indicaxanthin from Opuntia ficus-indica crosses the blood-brain barrier and modulates neuronal bioelectric activity in rat hippocampus at dietary-consistent amounts. J. Agric. Food Chem. 2015, 63, 7353–7360. [Google Scholar] [CrossRef]
- Gambino, G.; Allegra, M.; Sardo, P.; Attanzio, A.; Tesoriere, L.; Livrea, M.A.; Ferraro, G.; Carletti, F. Brain distribution and modulation of neuronal excitability by indicaxanthin from Opuntia fuci indica administered at nutritionally relevant amounts. Front. Aging Neurosci. 2018, 10, 133. [Google Scholar] [CrossRef]
- Amjadi, S.; Mesgari Abbasi, M.; Shokouhi, B.; Ghorbani, M.; Hamishehkar, H. Enhancement of therapeutic efficacy of betanin for diabetes treatment by liposomal nanocarriers. J. Funct. Foods 2019, 59, 119–128. [Google Scholar] [CrossRef]
- Saghari, Y.; Movahedi, M.; Tebianian, M.; Entezari, M. The neuroprotective effect of betanin nanoparticles on brain ischemia-reperfusion injury. Anim. Gene 2023, 27, 200145. [Google Scholar] [CrossRef]
- Imamura, T.; Isozumi, N.; Higashimura, Y.; Koga, H.; Segawa, T.; Desaka, N.; Takagi, H.; Matsumoto, K.; Ohki, S.; Mori, M. Red-beet betalain pigments inhibit amyloid-β aggregation and toxicity in amyloid-β expressing Caenorhabditis elegans. Plant Foods Human Nutr. 2022, 77, 90–97. [Google Scholar] [CrossRef]
- Rehman, S.; Ashfaq, U.A.; Sufyan, M.; Shahid, I.; Ijaz, B.; Hussain, M. The insight of in silico and in vitro evaluation of Beta vulgaris phytochemicals against Alzheimer’s disease targeting acetylcholinesterase. PLoS ONE 2022, 17, e0264074. [Google Scholar] [CrossRef]
- Shunan, D.; Yu, M.; Guan, H.; Zhou, Y. Neuroprotective effect of betalain against AlCl3-induced Alzheimer disease in Sprague Dawley rats via putative modulation of oxidative stress and nuclear factor kappa B (NF-kB) signaling pathway. Biomed. Pharmacother. 2021, 137, 111369. [Google Scholar] [CrossRef]
- Salimi, A.; Sabur, M.; Dadkhah, M.; Shabani, M. Inhibition of scopolamine-induced memory and mitochondrial impairment by betanin. J. Biochem. Mol. Toxicol. 2022, 36, e23076. [Google Scholar] [CrossRef]
- Thong-Asa, W.; Prasartsri, S.; Klomkleaw, N.; Thongwan, N. The neuroprotective effect of betanin in trimethyltin-induced neurodegeneration in mice. Metab. Brain Dis. 2020, 35, 1395–1405. [Google Scholar] [CrossRef]
- ElSayed, M.H.; Atif, H.M.; Eladl, M.A.; Elaidy, S.M.; Helaly, A.M.N.; Hisham, F.A.; Farag, N.E.; Osman, N.M.S.; Ibrahiem, A.T.; Khella, H.W.Z.; et al. Betanin improves motor function and alleviates experimental Parkinsonism via downregulation of TLR4/MyD88/NF-kB pathway: Molecular docking and biological investigation. Biomed. Pharmacother. 2023, 164, 114917. [Google Scholar] [CrossRef]
- Thong-Asa, W.; Jedsadavitayakol, S.; Jutarattananon, S. Benefits of betanin in rotenone-induced Parkinson mice. Metab. Brain Dis. 2021, 36, 2567–2577. [Google Scholar] [CrossRef]
- Hadipour, E.; Fereidoni, M.; Tayarani-Najaran, Z. Betanin attenuates oxidative stress induced by 6-OHDA in PC12 cells via SAPK/JNK and PI3K pathways. Neurochem. Res. 2020, 45, 395–403. [Google Scholar] [CrossRef]
- Terzo, S.; Amato, A.; Calvi, P.; Giardina, M.; Nuzzo, D.; Picone, P.; Palumbo-Piccionello, A.; Amata, S.; Giardina, I.C.; Massaro, A.; et al. Positive impact of indicaxanthin from Opuntia ficus-indica fruit on high-fat diet-induced neuronal damage and gut microbial dysbiosis. Neural Regen. Res. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Thong-asa, W.; Puenpha, K.; Lairaksa, T.; Saengjinda, S. Neuroprotective effects of betanin in mice with cerebral ischemia-reperfusion injury. Exp. Anim. 2023, 72, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Motawi, T.K.; Ahmed, S.A.; El-Boghdady, N.A.; Metwally, N.S.; Nasr, N.N. Protective effects of betanin against paracetamol and diclofenac induced neurotoxicity and endocrine disruption in rats. Biomarkers 2019, 24, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Q.; Yang, G.-Q. Betacyanins from Portulaca oleracea L. ameliorate cognition deficits induced by D-galactose in the brains of senescent mice. Phytomedicine 2010, 17, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Link, C.D. Expression of human beta-amyloid peptide in transgenic Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 1995, 92, 9368–9372. [Google Scholar] [CrossRef]
- Geloso, M.C.; Corvino, V.; Michetti, F. Trimethyltin-induced hippocampal degeneration as a tool to investigate neurodegenerative processes. Neurochem. Int. 2011, 58, 729–738. [Google Scholar] [CrossRef]
- Power, A.E.; Vazdarjanova, A.; McGaugh, J.L. Muscarinic cholinergic influences in memory consolidation. Neurobiol. Learn. Mem. 2003, 80, 178–193. [Google Scholar] [CrossRef]
- Von Wrangel, C.; Schwabe, K.; John, N.; Krauss, J.K.; Alam, M. The rotenone-induced rat model of Parkinson’s disease: Behavioral and electrophysiological findings. Behav. Brain Res. 2015, 279, 52–61. [Google Scholar] [CrossRef]
- Marzi, S.J.; Schilder, B.M.; Nott, A.; Sala Frigerio, C.; Willaime-Morawek, S.; Bucholc, M.; Hanger, D.P.; James, C.; Lewis, P.A.; Lourida, I.; et al. Artificial intelligence for neurodegenerative experimental models. Alzheimer’s Dement. 2023, 19, 5970–5987. [Google Scholar] [CrossRef]
- Petrie, M.; Rejeski, J.; Basu, S.; Laurienti, P.J.; Marsh, A.P.; Norris, J.L.; Kim-Shapiro, D.-B.; Burdette, J.H. Beetroot juice: An ergogenic aid for exercise and the aging brain. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2017, 72, 1284–1289. [Google Scholar] [CrossRef]
- Presley, T.D.; Morgan, A.R.; Bechtold, E.; Clodfelter, W.; Dove, R.W.; Jennings, J.M.; Burdette, J.H. Acute effect of a high nitrate diet on brain perfusion in older adults. Nitric Oxide 2011, 24, 34–42. [Google Scholar]
- Vanhatalo, A.; L’Heureux, J.E.; Kelly, J.; Blackwell, J.R.; Wylie, L.J.; Fulford, J.; Winyard, P.G.; Williams, D.W.; Van der Giezen, M.; Jones, A.M. Network analysis of nitrate-sensitive oral microbiome reveals interactions with cognitive functions and cardiovascular health across dietary interventions. Redox Biol. 2021, 41, 101933. [Google Scholar] [CrossRef]
- Worley, M.L.; Reed, E.L.; Chapman, C.L.; Kueck, P.; Seymour, L.; Fitts, T.; Zazulak, H.; Schlader, Z.; Johnson, B.D. Acute beetroot juice consumption does not alter cerebral autoregulation or cardiovagal baroreflex sensitivity during lower-body negative pressure in healthy adults. Front. Hum. Neurosci. 2023, 17, 1115355. [Google Scholar] [CrossRef]
- Aliahmadi, M.; Amiri, F.; Bahrami, L.S.; Hosseini, A.F.; Abiri, B.; Vafa, M. Effects of raw red beetroot consumption on metabolic markers and cognitive function in type 2 diabetes patients. J. Diabetes Metab. Dis. 2021, 20, 673–682. [Google Scholar] [CrossRef]
- Vaccaro, M.G.; Innocenti, B.; Cione, E.; Gallelli, L.; De Sarro, G.; Bonilla, D.A.; Cannataro, R. Acute effects of a chewable beetroot-based supplement on cognitive performance: A double-blind randomized placebo-controlled crossover clinical trial. Eur. J. Nutr. 2024, 63, 303–321. [Google Scholar] [CrossRef]
- Bahn, G.; Park, J.S.; Yun, U.J.; Lee, Y.J.; Choi, Y.; Park, J.S.; Baek, S.H.; Choi, B.Y.; Cho, Y.S.; Kim, H.K.; et al. NRF2/ARE pathway negatively regulates BACE1 expression and ameliorates cognitive deficits in mouse Alzheimer’s models. Proc. Natl. Acad. Sci. USA 2019, 116, 12516–12523. [Google Scholar] [CrossRef]
- Minh Hung, H.; Nguyen, M.T.; Tran, P.T.; Truong, V.K.; Chapman, J.; Quynh Anh, L.H.; Derreumaux, P.; Vu, V.V.; Ngo, S.T. Impact of the Astaxanthin, Betanin, and EGCG Compounds on Small Oligomers of Amyloid Aβ40Peptide. J. Chem. Inf. Model. 2020, 60, 1399–1408. [Google Scholar]
- Martínez-Rodríguez, P.; Henarejos-Escudero, P.; Hernandez-Garcia, S.; Sanchez-Ferrer, A.; Gandía-Herrero, F. In vitro, in vivo and in silico evidence for the use of plant pigments betalains as potential nutraceuticals against Alzheimer’s disease. Food Front. 2024, 5, 2137–2154. [Google Scholar] [CrossRef]
- Tayarani-Najaran, Z.; Dehganpour Farashah, M.; Emami, S.A.; Ramazani, E.; Shahraki, N.; Hadipour, E. Protective effects of betanin, a novel acetylcholinesterase inhibitor, against H2O2-induced apoptosis in PC12 cells. Mol. Biol. Rep. 2024, 51, 986. [Google Scholar] [CrossRef]
- Giridharan, S.; Srinivasan, M. Mechanisms of NF-κB p65 and strategies for therapeutic manipulation. J. Inflamm. Res. 2018, 11, 407–419. [Google Scholar] [CrossRef]
- Pavel, M.; Vostinaru, O.; Mogosan, C.; Ghibu, S. Phytochemical and pharmacological research on some extracts obtained from Serpylli herba. Farmacia 2011, 59, 77–84. [Google Scholar]
- Ramanan, M.; Sinha, S.; Sudarshan, K.; Singh Aiden, I.; Doble, M. Inhibition of the enzymes in the leukotriene and prostaglandin pathways in inflammation by 3-aryl isocoumarins. Eur. J. Med. Chem. 2016, 124, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Sudarshan, K.; Boda, A.; Dogra, S.; Bose, I.; Yadav, P.N.; Singh Aiden, I. Discovery of an isocoumarin analogue that modulates neuronal functions via neurotrophin receptor TrkB. Bioorganic Med. Chem. Lett. 2019, 29, 585–590. [Google Scholar] [CrossRef]
- Reddy, M.K.; Alexander-Lindo, R.L.; Nair, M.G. Relative inhibition of lipid peroxidation, cyclooxygenase enzymes, and human tumor cell proliferation by natural food colors. J. Agric. Food Chem. 2005, 53, 9268–9273. [Google Scholar] [PubMed]
- Vidal, P.J.; Lopez-Nicolas, J.M.; Gandıa-Herrero, F.; Garcıa-Carmona, F. Inactivation of lipoxygenase and cyclooxygenase by natural betalains and semi-synthetic analogues. Food Chem. 2014, 154, 246–254. [Google Scholar] [CrossRef]
- Gentile, C.; Tesoriere, L.; Allegra, M.; Livrea, M.A.; D’Alessio, P. Antioxidant betalains from cactus pear (Opuntia ficus-indica) inhibit endothelial ICAM-1 expression. Ann. N. Y. Acad. Sci. 2004, 1028, 481–486. [Google Scholar]
- Allegra, M.; Tutone, M.; Tesoriere, L.; Attanzio, A.; Culletta, G.; Almerico, A.M. Evaluation of the IKK Binding of Indicaxanthin by Induced-Fit Docking, Binding Pose Metadynamics, and Molecular Dynamics. Front. Pharmacol. 2021, 12, 701568. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef]
- Czapski, J.; Mikolajczyk, K.; Kaczmarek, M. Relationship between antioxidant capacity of red beet juice and contents of its betalain pigments. Pol. J. Food Nutr. Sci. 2009, 59, 119–122. [Google Scholar]
- Clifford, T.; Howatson, G.; West, D.J.; Stevenson, E.J. The potential benefits of red beetroot supplementation in health and disease. Nutrients 2015, 7, 2801–2822. [Google Scholar] [CrossRef]
- Gliszczynska-Swigło, A.; Szymusiak, H.; Malinowska, P. Betanin, the main pigment of red beet: Molecular origin of its exceptionally high free radical-scavenging activity. Food Addit. Contam. 2006, 23, 1079–1087. [Google Scholar] [CrossRef]
- Phukan, B.C.; Roy, R.; Paul, R.; Mazumder, M.K.; Nath, J.; Bhattacharya, P.; Borah, A. Traversing through the cell signaling pathways of neuroprotection by betanin: Therapeutic relevance to Alzheimer’s and Parkinson’s disease. Metab. Brain Dis. 2023, 38, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Krajka-Kuzniak, V.; Paluszczak, J.; Szaefer, H.; Baer-Dubowska, W. Betanin, a beetroot component, induces nuclear factor erythroid-2-related factor 2-mediated expression of detoxifying/antioxidant enzymes in human liver cell lines. Br. J. Nutr. 2013, 110, 2138–2149. [Google Scholar] [CrossRef] [PubMed]
- Sutariya, B.; Taneja, N.; Badgujar, L.; Saraf, M. Modulatory effect of betanin on high glucose induced epithelial to mesenchymal transition in renal proximal tubular cells. Biomed. Pharmacother. 2017, 89, 18–28. [Google Scholar] [CrossRef] [PubMed]
- El Shaffei, I.; Abdel-Latif, G.A.; Farag, D.B.; Schaalan, M.; Salama, R.M. Ameliorative effect of betanin on experimental cisplatin-induced liver injury; the novel impact of miRNA-34a on the SIRT1/PGC-1α signaling pathway. J. Biochem. Mol. Toxicol. 2021, 35, 1–14. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Additives and Nutrient Sources Added to Food. Scientific opinion on the re-evaluation of beetroot red (E162) as a food additive. EFSA J. 2015, 13, 4318. [Google Scholar]
- Reynoso, R.C.; Giner, T.V.; González de Mejia, E. Safety of a filtrate of fermented garambullo fruit: Biotransformation and toxicity studies. Food Chem. Toxicol. 1999, 37, 825–830. [Google Scholar]
- Von Elbe, J.H.; Schwartz, S.J. Absence of mutagenic activity and a short-term toxicity study of beet pigments as food colorants. Arch. Toxicol. 1981, 49, 93–98. [Google Scholar] [CrossRef]
- Kujawska, M.; Ignatowicz, E.; Murias, M.; Ewertowska, M.; Mikołajczyk, K.; Jodynis-Liebert, J. Protective Effect of Red Beetroot against Carbon Tetrachloride- and N-Nitrosodiethylamine-Induced Oxidative Stress in Rats. J. Agric. Food Chem. 2009, 57, 2570–2575. [Google Scholar]
- Conforti-Froes, N.; Varella-Garcia, M.; Manzanto, A.J. In vivo and in vitro evaluation of betanin mutagenicity. Cytologia 1990, 55, 203–220. [Google Scholar]
- Martinez, R.M.; Melo, C.P.B.; Pinto, I.C.; Mendes-Pierotti, S.; Vignoli, J.A.; Verri, W.A.; Casagrande, R. Betalains: A narrative review on pharmacological mechanisms supporting the nutraceutical potential towards health benefits. Foods 2024, 13, 3909. [Google Scholar] [CrossRef]
- Lim, S.H.; Bae, S.; Lee, H.S.; Han, H.-K.; Choi, C.-I. Effect of betanin, the major pigment of red beetroot (Beta vulgaris L.) on the activity of recombinant cytochrome P450 enzymes. Pharmaceuticals 2023, 16, 1224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Z.; Wang, Y.; Jin, W.; Zhang, Z.; Jin, L.; Qian, J.; Zheng, L. CYP3A4 and CYP3A5: The crucial roles in clinical drug metabolism and the significant implications of genetic polymorphism. Peer J. 2024, 12, e18636. [Google Scholar] [CrossRef]

| Betacyanins |
|---|
| Betanin group: Betanin (betanidin 5-O-glucoside); Phyllocactin (6′-O-malonyl-betanin); 2′-Apiosyl-phyllocactin; 2′-[5″-O-(E)-Feruloylapiosyl]-betanin; Hylocerenin (6′-O-(3″-hydroxy-3″-methyl)-betanin) |
| Amaranthin group: Amaranthin (betanidin 5-O-glucuronosylglucoside); Iresinin I (hydroxymethylglutaryl-amaranthin); Celosianin I, II (coumaroyl and feruloyl-amaranthin) |
| Gomphrenin group: Gomphrenin I (betanidin 6-O-glucoside); Gomphrenin II (coumaroyl derivative of gomphrenin I); Gomphrenin III (feruloyl derivative of gomphrenin I); Betanidin 6-O-(6-O-hydroxycinnamoyl)-sophoroside derivatives |
| 2-Descarboxy-betanin group: 2-Descarboxy-betanidin; 2-Descarboxy-betanin; 6′-O-malonyl-2-descarboxy-betanin |
| Betaxanthins |
| Amino acid-derived conjugate group: Portulacaxanthin II (L-tyrosine-betaxanthin); Portulacaxanthin III (glycine-betaxanthin); Tyrosine-betaxanthin; Tryptophan-betaxanthin; Dopaxanthin |
| Amine-derived conjugate group: 3-Methoxytyramine-betaxanthin; γ-aminobutyric acid-betaxanthin; Indicaxanthin;Vulgaxanthin I |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ştefănescu, C.; Voştinaru, O.; Mogoşan, C.; Crişan, G.; Balica, G. The Neuroprotective Potential of Betalains: A Focused Review. Plants 2025, 14, 994. https://doi.org/10.3390/plants14070994
Ştefănescu C, Voştinaru O, Mogoşan C, Crişan G, Balica G. The Neuroprotective Potential of Betalains: A Focused Review. Plants. 2025; 14(7):994. https://doi.org/10.3390/plants14070994
Chicago/Turabian StyleŞtefănescu, Cristina, Oliviu Voştinaru, Cristina Mogoşan, Gianina Crişan, and Georgeta Balica. 2025. "The Neuroprotective Potential of Betalains: A Focused Review" Plants 14, no. 7: 994. https://doi.org/10.3390/plants14070994
APA StyleŞtefănescu, C., Voştinaru, O., Mogoşan, C., Crişan, G., & Balica, G. (2025). The Neuroprotective Potential of Betalains: A Focused Review. Plants, 14(7), 994. https://doi.org/10.3390/plants14070994








