Longer Internode with Same Cell Length: LcSOC1-b2 Gene Involved in Height to First Pod but Not Flowering in Lentil (Lens culinaris Medik.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Trait Evaluations
2.2. Scanning Electron Microscopy of Stem Epidepmal Cells
2.3. Identification of LcSOC1 Gene in Lentil
2.4. RNA Extraction, cDNA Synthesis and qPCR Gene Expression Analysis
2.5. DNA Extraction, PCR, Sequencing and SNP Identification
2.6. ASQ Plant Genotyping
2.7. Statistical Treatment
3. Results
3.1. Scanning Electron Microscopy (SEM) of Internode Epidermal Cells
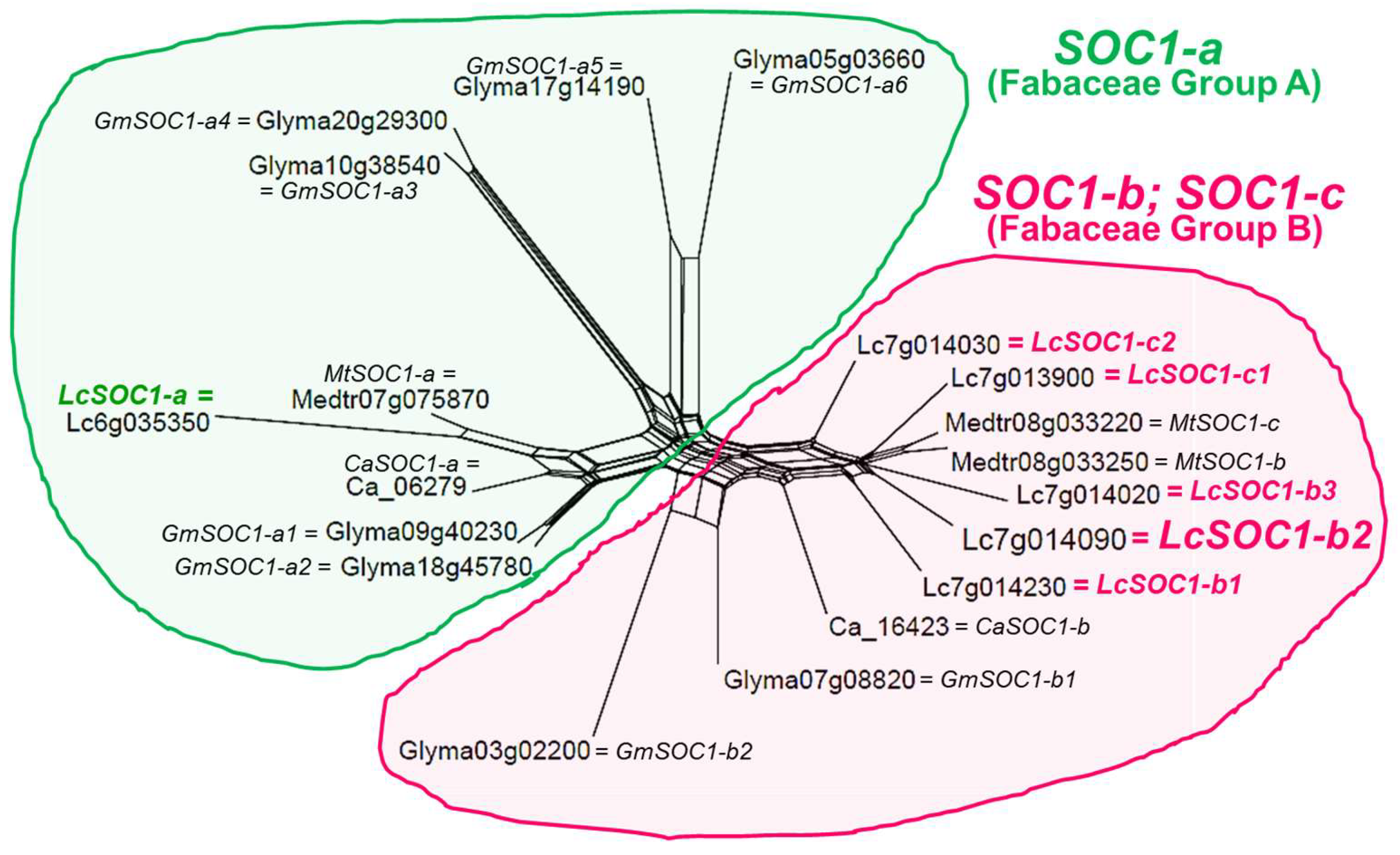
3.2. Molecular Phylogenetic Analysis of LcSOC1 Genes in Lentil and Other Legumes
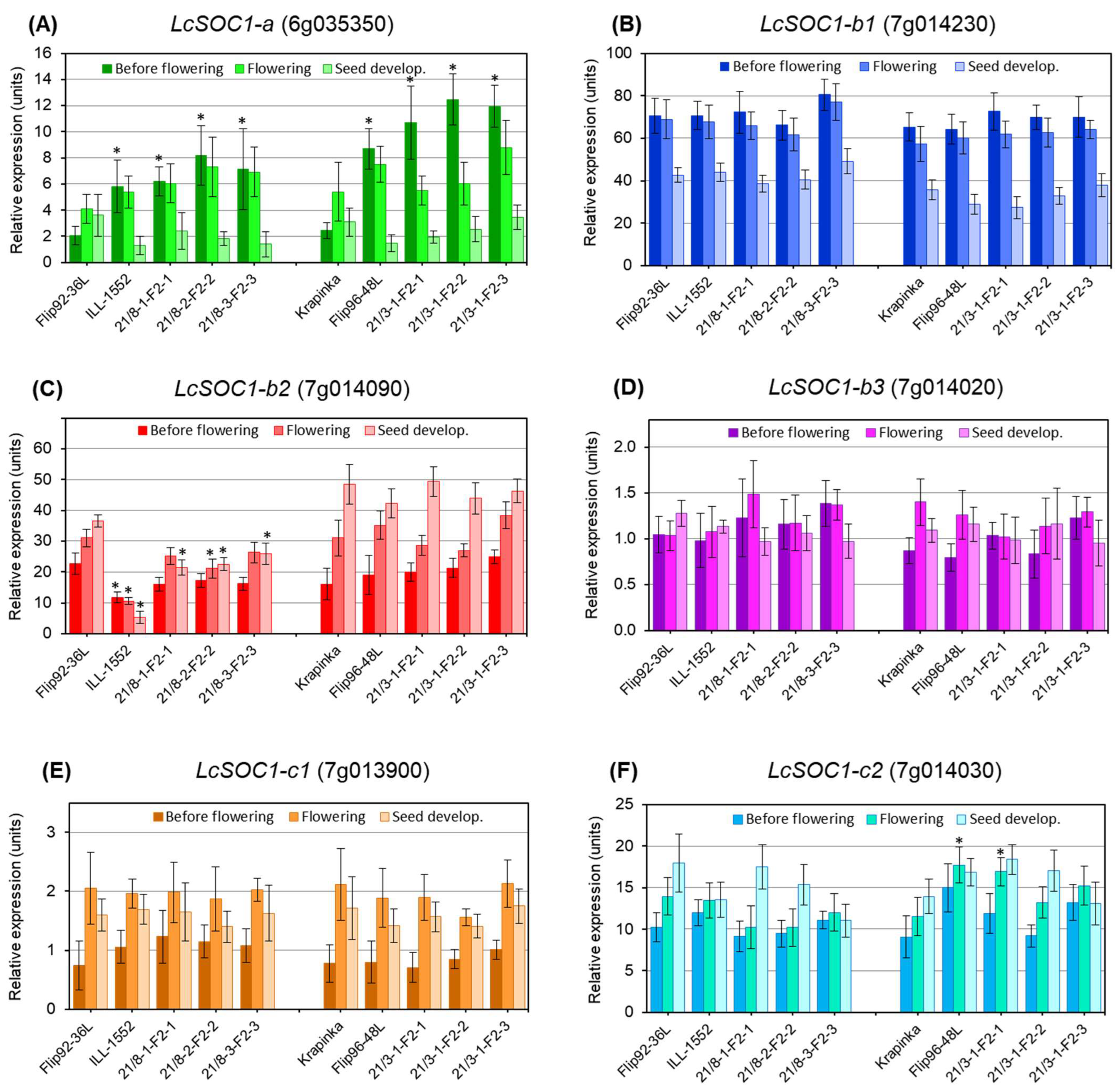
3.3. Differential Expression of Six LcSOC1 Genes During Lentil Plants Development
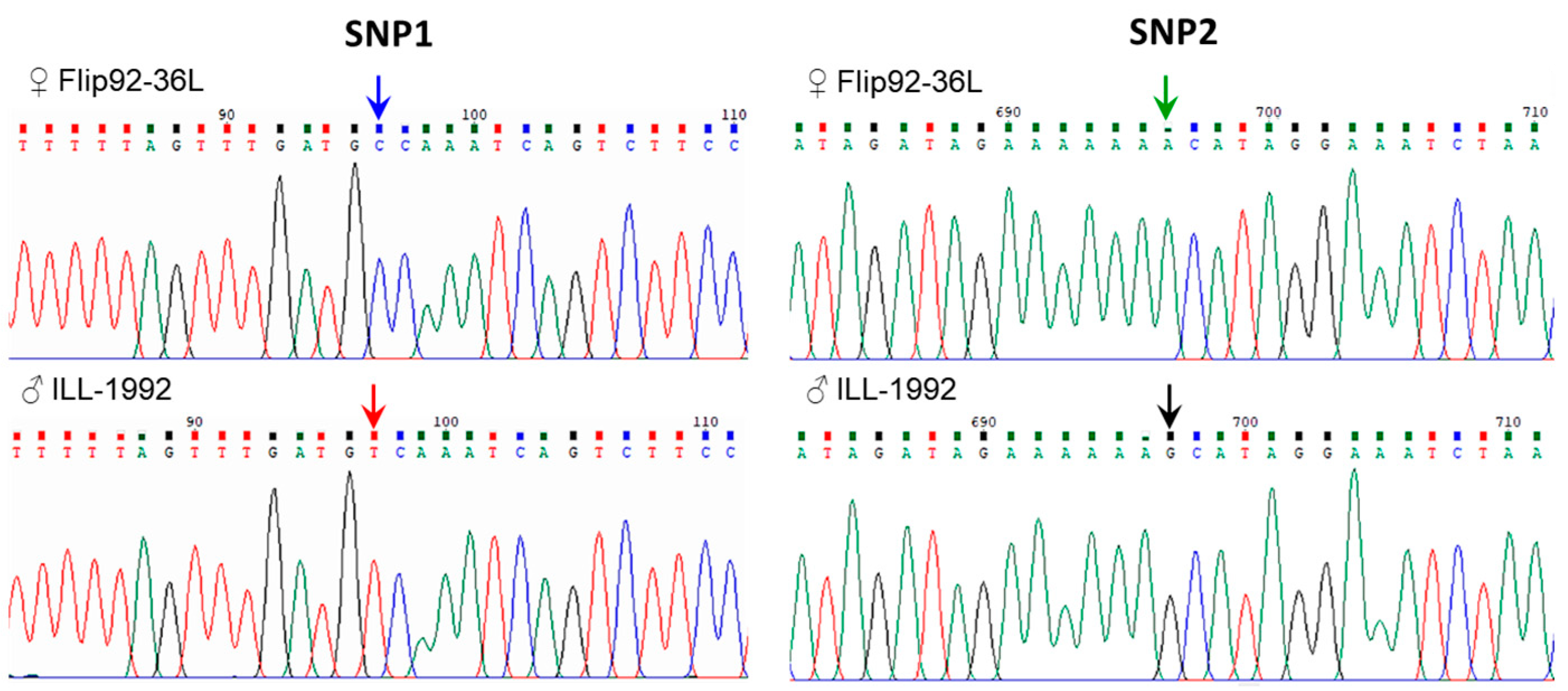
3.4. SNP Identification in LcSOC1-b2 Gene in Lentil Accessions
3.5. SNP Genotyping of Lentil Plants with ASQ Molecular Markers for the LcSOC1-b2 Gene and Marker-Trait Associations (MTA) with HFP
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuzbakova, M.; Khassanova, G.; Oshergina, I.; Ten, E.; Jatayev, S.; Yerzhebayeva, R.; Bulatova, K.; Khalbayeva, S.; Schramm, C.; Anderson, P.; et al. Height to first pod: A review of genetic and breeding approaches to improve combine harvesting in legume crops. Front. Plant Sci. 2022, 13, 948099. [Google Scholar] [CrossRef]
- Simon-Miquel, G.; Reckling, M.; Plaza-Bonilla, D. Maximising soybean productivity with late maturity groups in Mediterranean irrigated systems. Field Crops Res. 2024, 307, 109274. [Google Scholar] [CrossRef]
- Beagley, C.J.; Weller, J.L. Genetic control of stem elongation in legume crops and its potential relevance. Crop Sci. 2024, 64, 1971–1986. [Google Scholar] [CrossRef]
- Goufo, P.; Kluver, R.W.; Cerrudo, A.; Naeve, S.L. Insights into management and physiological determinants of lowest pod height in soybean. Agron. J. 2024, 116, 3191–3204. [Google Scholar] [CrossRef]
- Moghaddam, P.R.; Wilman, D. Cell wall thickness and cell dimensions in plant parts of eight forage species. J. Agric. Sci. 1998, 131, 59–67. [Google Scholar] [CrossRef]
- Jones, R.L. The role of gibberellins in plant cell elongation. Crit. Rev. Plant Sci. 1983, 1, 23–47. [Google Scholar] [CrossRef]
- Fukazawa, J.; Sakai, T.; Ishida, S.; Yamaguchi, I.; Kamiya, Y.; Takahashi, Y. Repression of shoot growth, a bZIP transcriptional activator, regulates cell elongation by controlling the level of gibberellins. Plant Cell 2000, 12, 901–915. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, X.; Shan, J.; Li, Y.; Zhang, Y.; Wang, Y.; Li, W.; Zhao, L. Overexpression of GmGAMYB accelerates the transition to flowering and increases plant height in soybean. Front. Plant Sci. 2021, 12, 667242. [Google Scholar] [CrossRef]
- Timpte, C.S.; Wilson, A.K.; Estelle, M. Effects of the axr2 mutation of Arabidopsis on cell shape in hypocotyl and inflorescence. Planta 1992, 188, 271–278. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, K.; Zhu, H.; Zhang, X.; Yan, W.; Xu, N.; Liu, D.; Hu, J.; Wu, Y.; Weng, Y.; et al. Melon short internode (CmSi) encodes an ERECTA-like receptor kinase regulating stem elongation through auxin signaling. Hortic. Res. 2020, 7, 202. [Google Scholar] [CrossRef]
- Zhang, X.; Hou, X.; Liu, Y.; Zheng, L.; Yi, Q.; Zhang, H.; Huang, X.; Zhang, J.; Hu, Y.; Yu, G.; et al. Maize brachytic2 (br2) suppresses the elongation of lower internodes for excessive auxin accumulation in the intercalary meristem region. BMC Plant Biol. 2019, 19, 589. [Google Scholar] [CrossRef]
- Zhiponova, M.K.; Morohashi, K.; Vanhoutte, I.; Machemer-Noonan, K.; Revalska, M.; Van Montagu, M.; Grotewold, E.; Russinova, E. Helix-loop-helix/basic helix-loop-helix transcription factor network represses cell elongation in Arabidopsis through an apparent incoherent feed-forward loop. Proc. Natl. Acad. Sci. USA 2014, 111, 2824–2829. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Z.; Wang, Y.; Mu, S.; Yue, H.; Luo, Y.; Zhang, Z.; Li, Y.; Chen, P. A mutation in CsDWF7 gene encoding a delta7 sterol C-5(6) desaturase leads to the phenotype of super compact in cucumber (Cucumis sativus L.). Theor. Appl. Genet. 2024, 137, 20. [Google Scholar] [CrossRef]
- Yang, H.; Wu, Y.; Che, J.; Lyu, L.; Wu, W.; Cao, F.; Li, W. Effects of cadmium stress on the growth, physiology, mineral uptake, cadmium accumulation and fruit quality of ’Sharpblue’ blueberry. Sci. Hortic. 2024, 337, 113593. [Google Scholar] [CrossRef]
- Naveed, M.; Ghaffar, M.; Khan, Z.; Gul, N.; Ijaz, I.; Bibi, A.; Pervaiz, S.; Alharby, H.F.; Tariq, M.S.; Ahmed, S.R.; et al. Morphological and structural responses of Albizia lebbeck to different lead and nickel stress levels. Agriculture 2023, 13, 1302. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, W.; Lu, X.; Li, S.; Li, Y.; Shan, Y.; Wang, S.; Zhou, Y.; Chen, L. Effects of different light conditions on morphological, anatomical, photosynthetic and biochemical parameters of Cypripedium macranthos Sw. Photosynth. Res. 2024, 160, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.W.; Lee, J.G.; Yoo, J.H.; Lim, J.D.; Choi, I.Y.; Kim, M.J.; Yu, C.Y.; Seong, E.S. Cellular morphology and transcriptome comparative analysis of Astragalus membranaceus Bunge sprouts cultured in vitro under different LED light. Plants 2023, 12, 1914. [Google Scholar] [CrossRef]
- Narayan, S.; Sharma, R.K.; Kumar, V.; Sanyal, I.; Shirke, P.A. Alterations in plant anatomy and higher lignin synthesis provides drought tolerance in cluster bean [Cyamopsis tetragonoloba (L.) Taub.]. Plant Physiol. Biochem. 2023, 201, 107905. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Z.; Wang, X.; Qian, Y.; Wu, W. Preliminary analysis on the mechanism leading to difference in internode length between Venus Seedless grapevine and its shorter internode mutant Zijinzaosheng. J. Fruit Sci. 2021, 38, 1423–1434. [Google Scholar] [CrossRef]
- Jiu, S.; Xu, Y.; Wang, J.; Wang, L.; Wang, S.; Ma, C.; Guan, L.; Abdullah, M.; Zhao, M.; Xu, W.; et al. Genome-wide identification, characterization, and transcript analysis of the TCP transcription factors in Vitis vinifera. Front. Genet. 2019, 10, 1276. [Google Scholar] [CrossRef]
- Kieffer, M.; Master, V.; Waites, R.; Davies, B. TCP14 and TCP15 affect internode length and leaf shape in Arabidopsis. Plant J. 2011, 68, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Li, L.; Ma, T.; Pei, J.; Zhao, Z.; Lu, M.; Wu, A.; Lin, X. The SOC1-like gene BoMADS50 is associated with the flowering of Bambusa oldhamii. Hortic. Res. 2021, 8, 133. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.E.; Manzoor, M.A.; Kim, M.J.; Youn, Y.; Nam, J.; Hyung, N.; Kim, G.T. Molecular cloning and functional characterization of CmSOC1 gene and its promoter region from Chrysanthemum morifolium. Sci. Hortic. 2024, 329, 112991. [Google Scholar] [CrossRef]
- Lee, J.; Lee, I. Regulation and function of SOC1, a flowering pathway integrator. J. Exp. Bot. 2010, 61, 2247–2254. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastava, H.; Das, A.; Tribhuvan, K.U.; Durgesh, K.; Joshi, R.; Sevanthi, A.M.; Jain, P.K.; Singh, N.K.; Gaikwad, K. Identification and characterization of MADS box gene family in pigeonpea for their role during floral transition. 3 Biotech 2021, 11, 108. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, Q.; Yan, B.; Zhang, J.; Xue, C.; Chen, J.; Lin, Y.; Zhang, X.; Shen, W.; Chen, X. Single-molecule real-time and Illumina-based RNA sequencing data identified vernalization-responsive candidate genes in faba bean (Vicia faba L.). Front. Genet. 2021, 12, 656137. [Google Scholar] [CrossRef]
- Luo, X.; Liu, B.; Xie, L.; Wang, K.; Xu, D.; Tian, X.; Xie, L.; Li, L.; Ye, X.; He, Z.; et al. The TaSOC1-TaVRN1 module integrates photoperiod and vernalization signals to regulate wheat flowering. Plant Biotechnol. J. 2024, 22, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Song, G.Q.; Han, X.; Ryner, J.T.; Thompson, A.; Wang, K. Utilizing MIKC-type MADS-box protein SOC1 for yield potential enhancement in maize. Plant Cell Rep. 2021, 40, 1679–1693. [Google Scholar] [CrossRef]
- Fan, C.M.; Wang, X.; Wang, Y.W.; Hu, R.B.; Zhang, X.M.; Chen, J.X.; Fu, Y.F. Genome-wide expression analysis of soybean MADS genes showing potential function in the seed development. PLoS ONE 2013, 8, e62288. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.; Han, J.J.; Han, M.J.; An, G. Functional analyses of the flowering time gene OsMADS50, the putative Suppressor of Overexpression of CO 1/Agamous-Like 20 (SOC1/AGL20) ortholog in rice. Plant J. 2004, 38, 754–764. [Google Scholar] [CrossRef]
- Jaudal, M.; Zhang, L.; Che, C.; Li, G.; Tang, Y.; Wen, J.; Mysore, K.S.; Putterill, J. A SOC1-like gene MtSOC1a promotes flowering and primary stem elongation in Medicago. J. Exp. Bot. 2018, 69, 4867–4880. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Long, H.; Qiu, F.; Wang, Y.; Zhang, M.; Chao, Y.; Chen, L. MADS-box protein MtSOC1c regulates flowering and seed development in Medicago truncatula. Ind. Crops Prod. 2023, 193, 116125. [Google Scholar] [CrossRef]
- Fudge, J.B.; Lee, R.H.; Laurie, R.E.; Mysore, K.S.; Wen, J.; Weller, J.L.; Macknight, R.C. Medicago truncatula SOC1 genes are up-regulated by environmental cues that promote flowering. Front. Plant Sci. 2018, 9, 496. [Google Scholar] [CrossRef] [PubMed]
- Poulet, A.; Zhao, M.; Peng, Y.; Tham, F.F.; Jaudal, M.; Zhang, L.; van Wolfswinkel, J.C.; Putterill, J. Gene-edited Mtsoc1 triple mutant Medicago plants do not flower. Front. Plant Sci. 2024, 15, 1357924. [Google Scholar] [CrossRef]
- Yuan, H.Y.; Caron, C.T.; Ramsay, L.; Fratini, R.; de la Vega, M.P.; Vandenberg, A.; Weller, J.L.; Bett, K.E. Genetic and gene expression analysis of flowering time regulation by light quality in lentil. Ann. Bot. 2021, 128, 481–496. [Google Scholar] [CrossRef]
- Lou, S.; Teng, C.; Hou, W.; Zhou, X.; Zhang, H.; Liu, Y. Quantitative trait loci mapping of first pod height in faba bean based on Genotyping-by-sequencing (GBS). Agronomy 2024, 14, 2013. [Google Scholar] [CrossRef]
- Kalendar, R.; Baidyussen, A.; Serikbay, D.; Zotova, L.; Khassanova, G.; Kuzbakova, M.; Jatayev, S.; Hu, Y.G.; Schramm, C.; Anderson, P.A.; et al. Modified ‘Allele-specific qPCR’ method for SNP genotyping based on FRET. Front. Plant Sci. 2022, 12, 747886. [Google Scholar] [CrossRef]
- Salgotra, R.K.; Gupta, B.B.; Stewart, J.C.N. From genomics to functional markers in the era of next-generation sequencing. Biotechnol. Lett. 2014, 36, 417–426. [Google Scholar] [CrossRef]
- Cheng, A.; Chai, H.H.; Ho, W.K.; Bamba, A.S.A.; Feldman, A.; Kendabie, P.; Halim, R.A.; Tanzi, A.; Mayes, S.; Massawe, F. Molecular marker technology for genetic improvement of underutilised crops. In Crop Improvement; Abdullah, S.N.A., Chai-Ling, H., Wagstaff, C., Eds.; Springer: Cham, Switzerland, 2017; pp. 47–70. [Google Scholar] [CrossRef]
- Rahman, M.A.; Woo, J.H.; Song, Y.; Lee, S.H.; Hasan, M.M.; Azad, M.A.K.; Lee, K.W. Heat shock proteins and antioxidant genes involved in heat combined with drought stress responses in perennial rye grass. Life 2022, 12, 1426. [Google Scholar] [CrossRef]
- Kyu, K.L.; Taylor, C.M.; Douglas, C.A.; Malik, A.I.; Colmer, T.D.; Siddique, K.H.M.; Erskine, W. Genetic diversity and candidate genes for transient waterlogging tolerance in mungbean at the germination and seedling stages. Front. Plant Sci. 2024, 15, 1297096. [Google Scholar] [CrossRef]
- Pandurangaiah, S.; Ravishankar, K.V.; Shivashankar, K.S.; Sadashiva, A.T.; Pillakenchappa, K.; Narayanan, S.K. Differential expression of carotenoid biosynthetic pathway genes in two contrasting tomato genotypes for lycopene content. J. Biosci. 2016, 41, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Khassanova, G.; Jatayev, S.; Gabdola, A.; Kuzbakova, M.; Zailasheva, A.; Kylyshbayeva, G.; Schramm, C.; Schleyer, K.; Philp-Dutton, L.; Sweetman, C.; et al. Haplotypes of ATP-binding cassette CaABCC6 in chickpea from Kazakhstan are associated with salinity tolerance and leaf necrosis via oxidative stress. Biomolecules 2024, 14, 823. [Google Scholar] [CrossRef]
- Vavilov Research Institute of Plant Genetic Resources. Germplasm Collection. Available online: https://www.vir.nw.ru/unu-kollektsiya-vir/ (accessed on 24 July 2024).
- Kazakhstan Reestr, cv. Krapinka. Available online: https://sortcom.kz/КРАПИНКА/ (accessed on 24 July 2024).
- Aljowaie, R.M.; Zengin, G.; Jaradat, N.; Mammadova, A.O.; Azad, A.K.; Beylerli, O.; Beilerli, A.; Valioglu, F.; Biturku, J.; Kiren, I. Lamiales epidermal dynamics: Unveiling antimicrobial resilience in a structural symphony. Microsc. Res. Tech. 2024, 87, 2134–2142. [Google Scholar] [CrossRef]
- Chavan, R.R.; Singh, A.P. The structural organization of the outer tissues in the gametophytic stem of the umbrella moss Hypnodendron menziesii optimizes load bearing. Planta 2024, 260, 73. [Google Scholar] [CrossRef] [PubMed]
- Shavrukov, Y.N.; Dry, I.B.; Thomas, M.R. Inflorescence and bunch architecture development in Vitis vinifera L. Aust. J. Grape Wine Res. 2004, 10, 116–124. [Google Scholar] [CrossRef]
- Şenel, G.; Akbulut, M.K.; Şeker, Ş.S. Comparative anatomical properties of some Epidendroideae and Orchidoideae species distributed in NE Turkey. Protoplasma 2019, 256, 655–668. [Google Scholar] [CrossRef]
- Almalki, W.H. Opuntioideae cactus stem Bioimaging analysis: Bridging taxonomy and antimicrobial research. Microsc. Res. Tech. 2024, 87, 1173–1182. [Google Scholar] [CrossRef]
- Olonova, M.V.; Shiposha, V.D.; Romanets, R.S. Phenotypic plasticity of the stem epidermis in the bluegrasses (Poa L.) of section Stenopoa Dumort. (Poaceae). I. Mesomorphic species. Acta Biol. Sib. 2022, 8, 655–672. [Google Scholar] [CrossRef]
- GenomeNet Database. Available online: https://www.genome.jp/tools/blast (accessed on 24 July 2024).
- PCD, Pulse Crops Database. Available online: https://www.pulsedb.org/organism/640 (accessed on 24 July 2024).
- LIS, Legume Information System Database. Available online: https://www.legumeinfo.org/ (accessed on 24 July 2024).
- SplitTree4. University of Tübingen (Germany). Available online: https://uni-tuebingen.de/en/fakultaeten/mathematisch-naturwissenschaftliche-fakultaet/fachbereiche/informatik/lehrstuehle/algorithms-in-bioinformatics/software/splitstree (accessed on 24 July 2024).
- Sweetman, C.; Khassanova, G.; Miller, T.K.; Booth, N.J.; Kurishbayev, A.; Jatayev, S.; Gupta, N.K.; Langridge, P.; Jenkins, C.L.D.; Soole, K.L.; et al. Salt-induced expression of intracellular vesicle trafficking genes, CaRab-GTP, and their association with Na+ accumulation in leaves of chickpea (Cicer arietinum L.). BMC Plant Biol. 2020, 20, 183. [Google Scholar] [CrossRef]
- Khassanova, G.; Kurishbayev, A.; Jatayev, S.; Zhubatkanov, A.; Zhumalin, A.; Turbekova, A.; Amantaev, B.; Lopato, S.; Schramm, C.; Jenkins, C.; et al. Intracellular vesicle trafficking genes, RabC-GTP, are highly expressed under salinity and rapid dehydration but down-regulated by drought in leaves of chickpea (Cicer arietinum L.). Front. Genet. 2019, 10, 40. [Google Scholar] [CrossRef]
- Vandesompele, J.; Preter, D.K.; Pattyn, F.; Poppe, B.; Roy, V.N.; Paepe, A.D.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034. [Google Scholar] [CrossRef]
- Gutierrez, L.; Mauriat, M.; Guénin, S.; Pelloux, J.; Lefebvre, J.F.; Louvet, R.; Rusterucci, C.; Moritz, T.; Guerineau, F.; Bellini, C.; et al. The lack of a systematic validation of reference genes: A serious pitfall undervalued in reverse transcription-polymerase chain reaction (RT-PCR) analysis in plants. Plant Biotechnol. J. 2008, 6, 609–618. [Google Scholar] [CrossRef] [PubMed]
- ABI Guide. Available online: https://assets.thermofisher.com/TFS-Assets/LSG/manuals/cms_042380.pdf (accessed on 24 July 2024).
- Doyle, J. DNA protocols for plants. In Molecular Techniques in Taxonomy; NATO ASI Series; Hewitt, G.M., Johnston, A.W.B., Young, J.P.W., Eds.; Springer: Berlin/Heidelberg, Germany, 1991; Volume 57, pp. 283–293. [Google Scholar] [CrossRef]
- McKim, S.M. Moving on up—Controlling internode growth. New Phytol. 2020, 226, 672–678. [Google Scholar] [CrossRef]
- Hedden, P.; Sponsel, V. A century of gibberellin research. J. Plant Growth Regul. 2015, 34, 740–760. [Google Scholar] [CrossRef]
- Srivastava, L.M.; Sawhney, V.K.; Taylor, I.E.P. Gibberellic acid induced cell elongation in lettuce hypocotyls. Proc. Natl. Acad. Sci. USA 1975, 72, 1107–1111. [Google Scholar] [CrossRef]
- Derbyshire, P.; Findlay, K.; McCann, M.C.; Roberts, K. Cell elongation in Arabidopsis hypocotyls involves dynamic changes in cell wall thickness. J. Exp. Bot. 2007, 58, 2079–2089. [Google Scholar] [CrossRef] [PubMed]
- Rood, S.B.; Zanewich, K.P.; Bray, D.F. Growth and development of Brassica genotypes differing in endogenous gibberellin content. II. Gibberellin content, growth analyses and cell size. Physiol. Plant. 1990, 79, 679–685. [Google Scholar] [CrossRef]
- Fujino, K.; Koda, Y.; Kikuta, Y. Reorientation of cortical microtubules in the sub-apical region during tuberization in single-node stem segments of potato in culture. Plant Cell Physiol. 1995, 36, 891–895. [Google Scholar] [CrossRef]



| Accessions | Cell Length (µm) | Cell Width (µm) | ||
|---|---|---|---|---|
| Average | SE 1 | Average | SE | |
| Flip92-36L | 270.8 | 24.7 | 47.1 | 3.7 |
| ILL-1552 | 267.4 | 18.1 | 27.2 | 1.7 |
| Significance | n.s. 2 | *** p < 0.001 | ||
| Genotype | Fluoro- Phore | n | HFP (cm) | HFP MWU-Test with Total | FT (Days) | FT MWU-Test with Total | ||
|---|---|---|---|---|---|---|---|---|
| Average | SE 1 | Average | SE | |||||
| aa | FAM | 22 | 11.73 | 0.76 | p = 0.0222, ** p < 0.1 | 39.68 | 0.33 | p = 0.3087, n.s. |
| ab | FAM/VIC | 11 | 14.18 | 0.96 | p = 0.8267, n.s. 2 | 40.18 | 0.49 | p = 0.7910, n.s. |
| bb | VIC | 27 | 15.58 | 0.65 | p = 0.0546, ** p < 0.1 | 40.27 | 0.29 | p = 0.4499, n.s. |
| Total | 60 | 13.88 | 0.48 | - | 40.03 | 0.20 | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuzbakova, M.; Khassanova, G.; Jatayev, S.; Daniyeva, N.; Sweetman, C.; Jenkins, C.L.D.; Soole, K.L.; Shavrukov, Y. Longer Internode with Same Cell Length: LcSOC1-b2 Gene Involved in Height to First Pod but Not Flowering in Lentil (Lens culinaris Medik.). Plants 2025, 14, 1157. https://doi.org/10.3390/plants14081157
Kuzbakova M, Khassanova G, Jatayev S, Daniyeva N, Sweetman C, Jenkins CLD, Soole KL, Shavrukov Y. Longer Internode with Same Cell Length: LcSOC1-b2 Gene Involved in Height to First Pod but Not Flowering in Lentil (Lens culinaris Medik.). Plants. 2025; 14(8):1157. https://doi.org/10.3390/plants14081157
Chicago/Turabian StyleKuzbakova, Marzhan, Gulmira Khassanova, Satyvaldy Jatayev, Nurgul Daniyeva, Crystal Sweetman, Colin L. D. Jenkins, Kathleen L. Soole, and Yuri Shavrukov. 2025. "Longer Internode with Same Cell Length: LcSOC1-b2 Gene Involved in Height to First Pod but Not Flowering in Lentil (Lens culinaris Medik.)" Plants 14, no. 8: 1157. https://doi.org/10.3390/plants14081157
APA StyleKuzbakova, M., Khassanova, G., Jatayev, S., Daniyeva, N., Sweetman, C., Jenkins, C. L. D., Soole, K. L., & Shavrukov, Y. (2025). Longer Internode with Same Cell Length: LcSOC1-b2 Gene Involved in Height to First Pod but Not Flowering in Lentil (Lens culinaris Medik.). Plants, 14(8), 1157. https://doi.org/10.3390/plants14081157








