Impact of Land-Use Change on Vascular Epiphytes: A Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Identifying the Main Aspects of Research
3. Results and Discussion
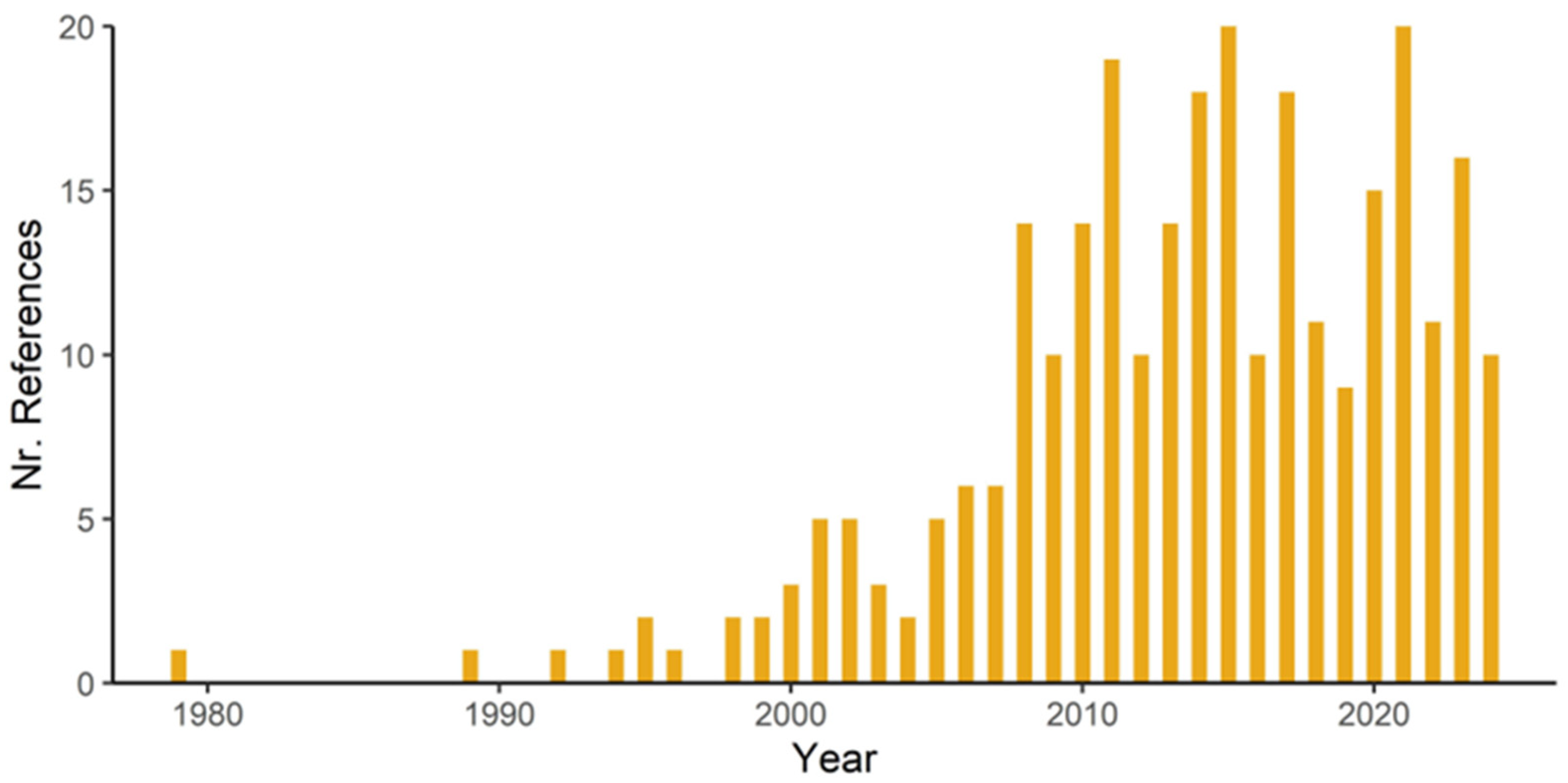
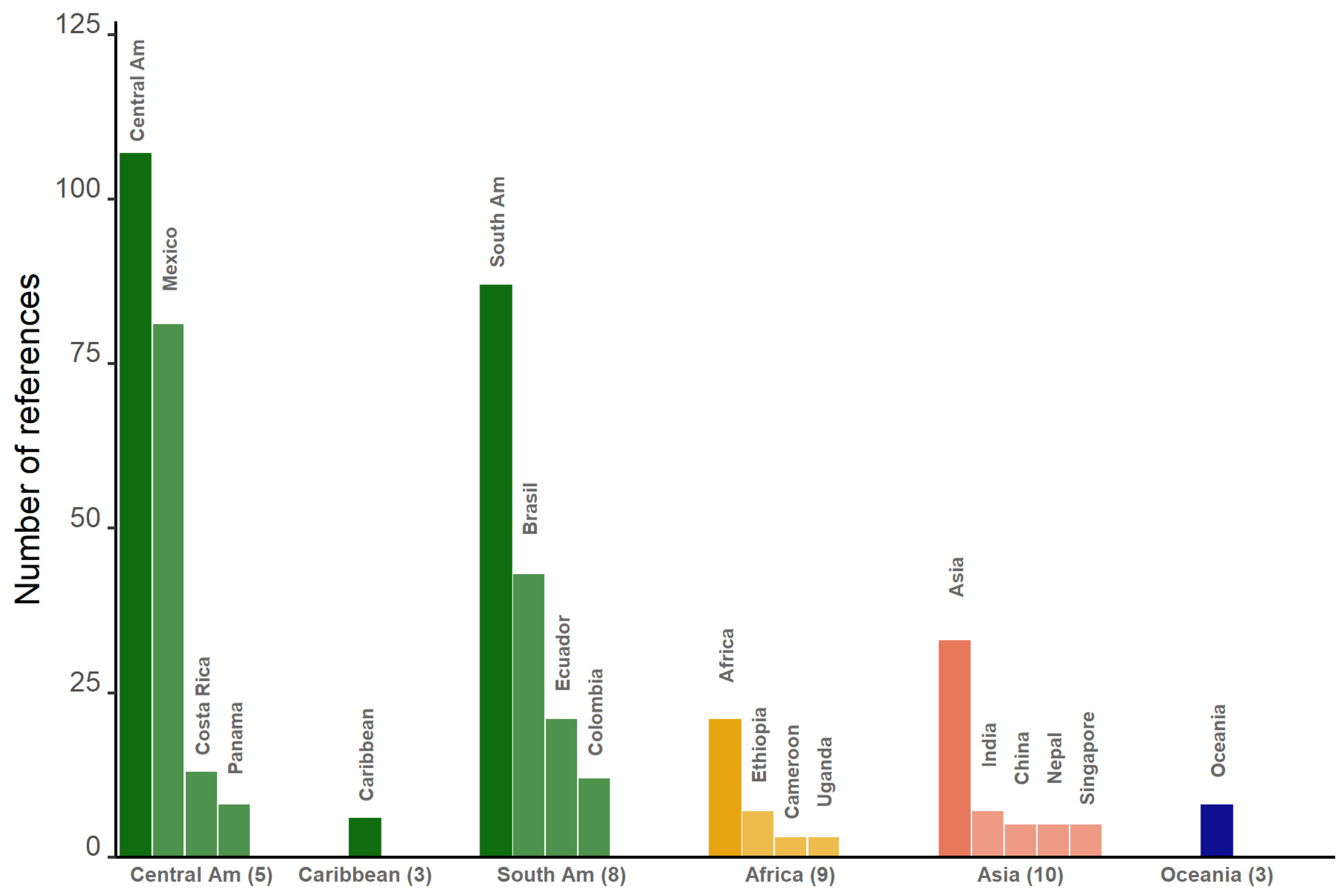
3.1. General Results
3.2. Identifying the Main Aspects of Research
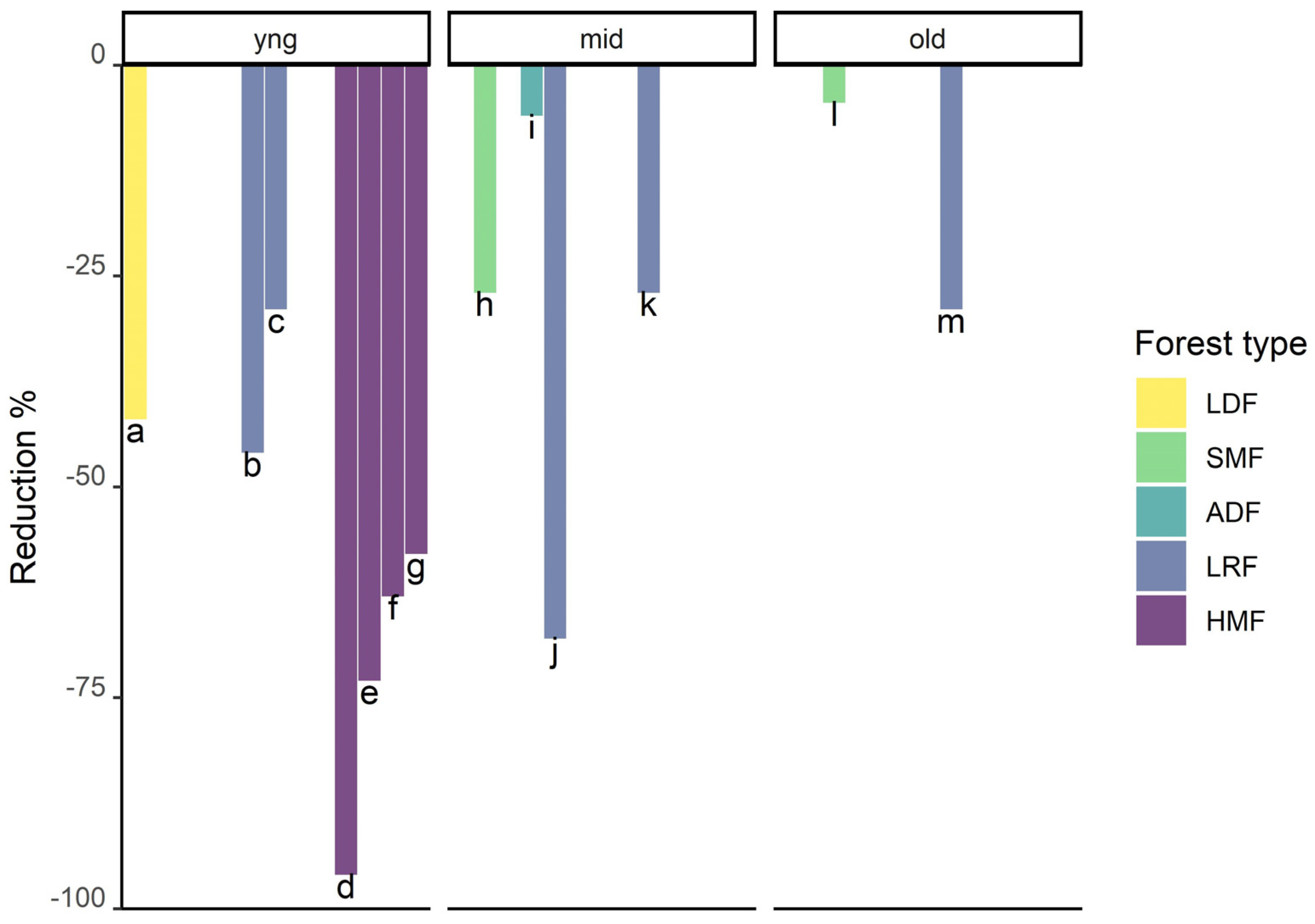
3.3. Tropical Forest Conversion and Epiphytes
3.4. Secondary Forests
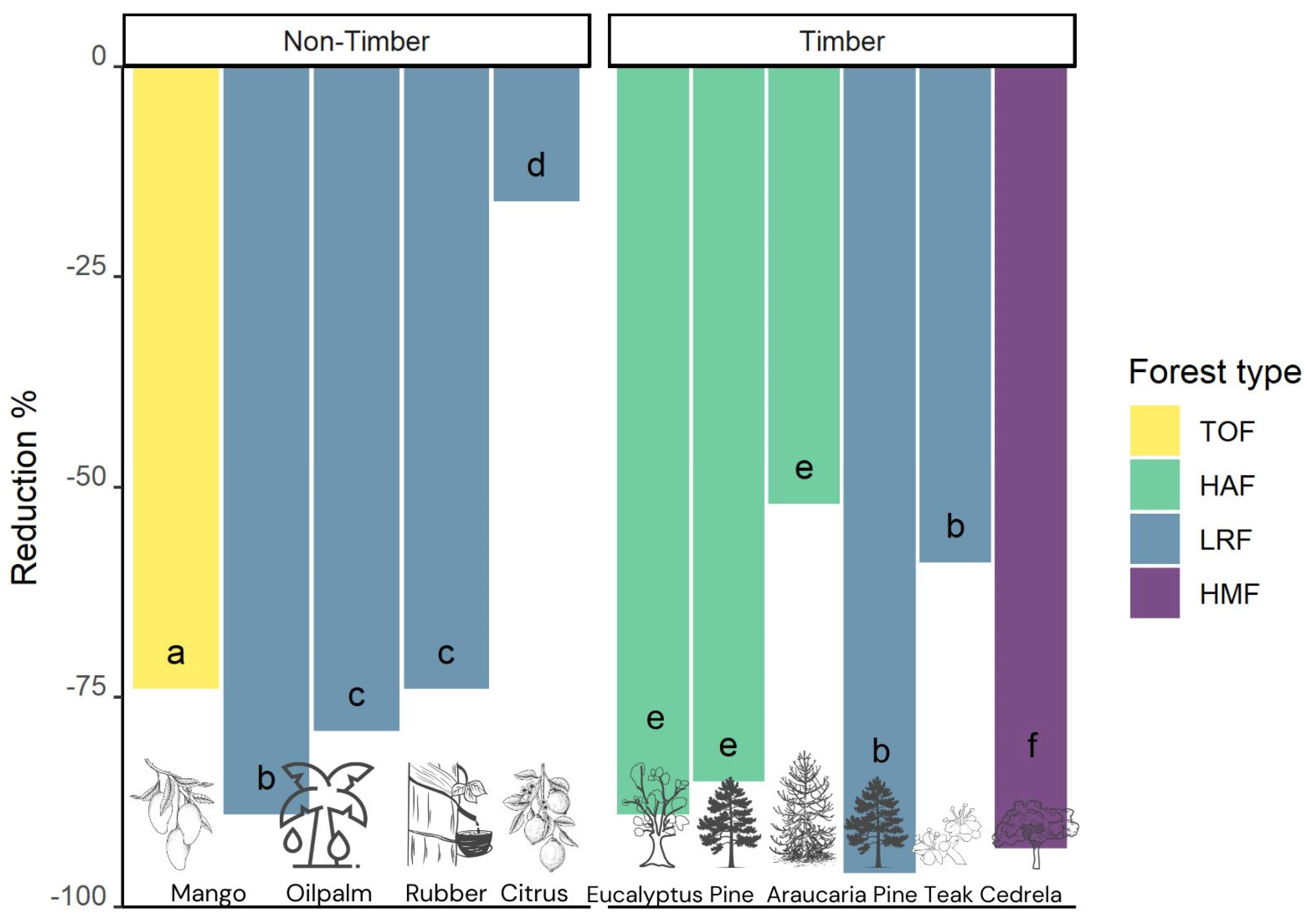
3.5. Tree Plantations
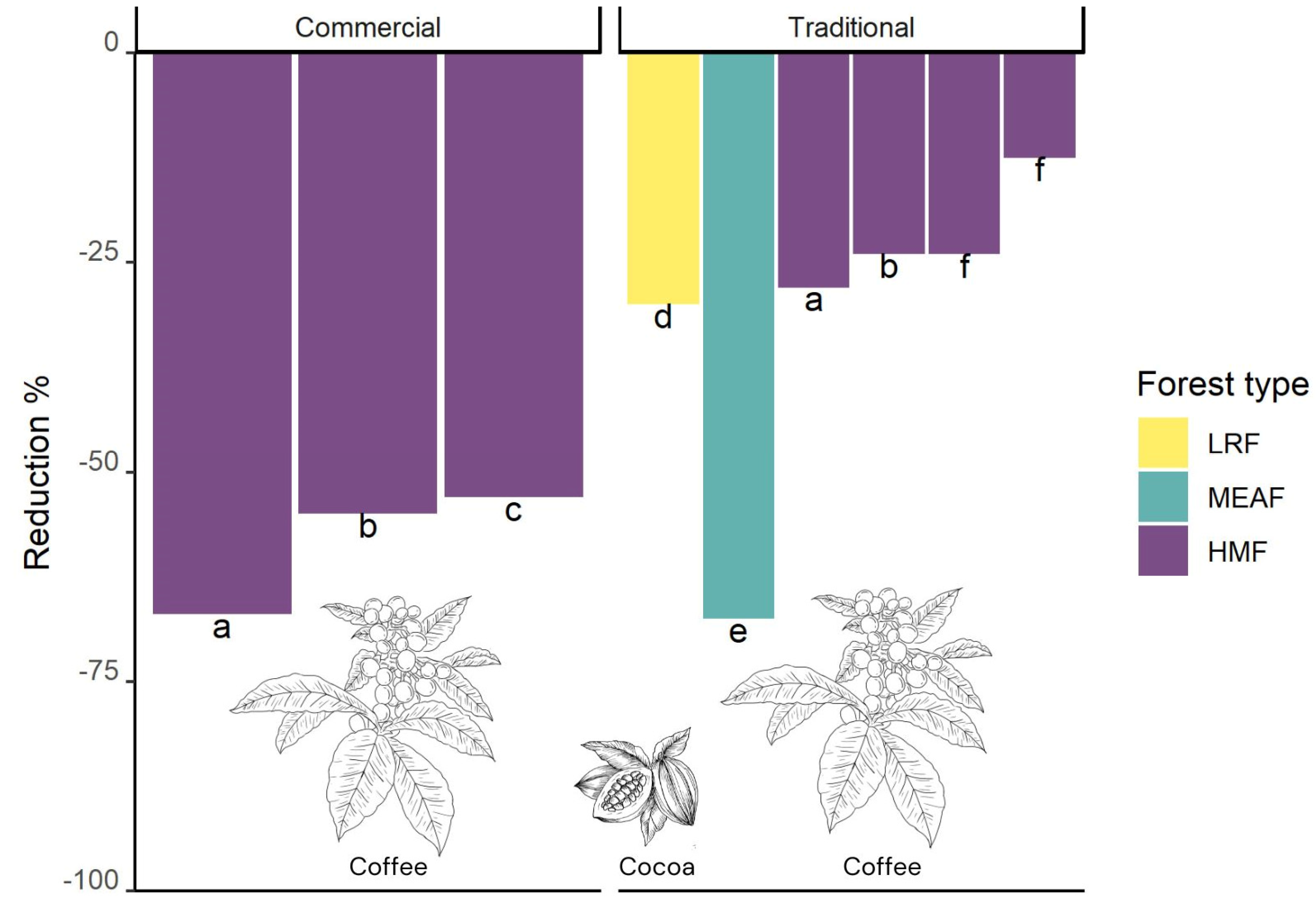
3.6. Pastures with Remnant Trees
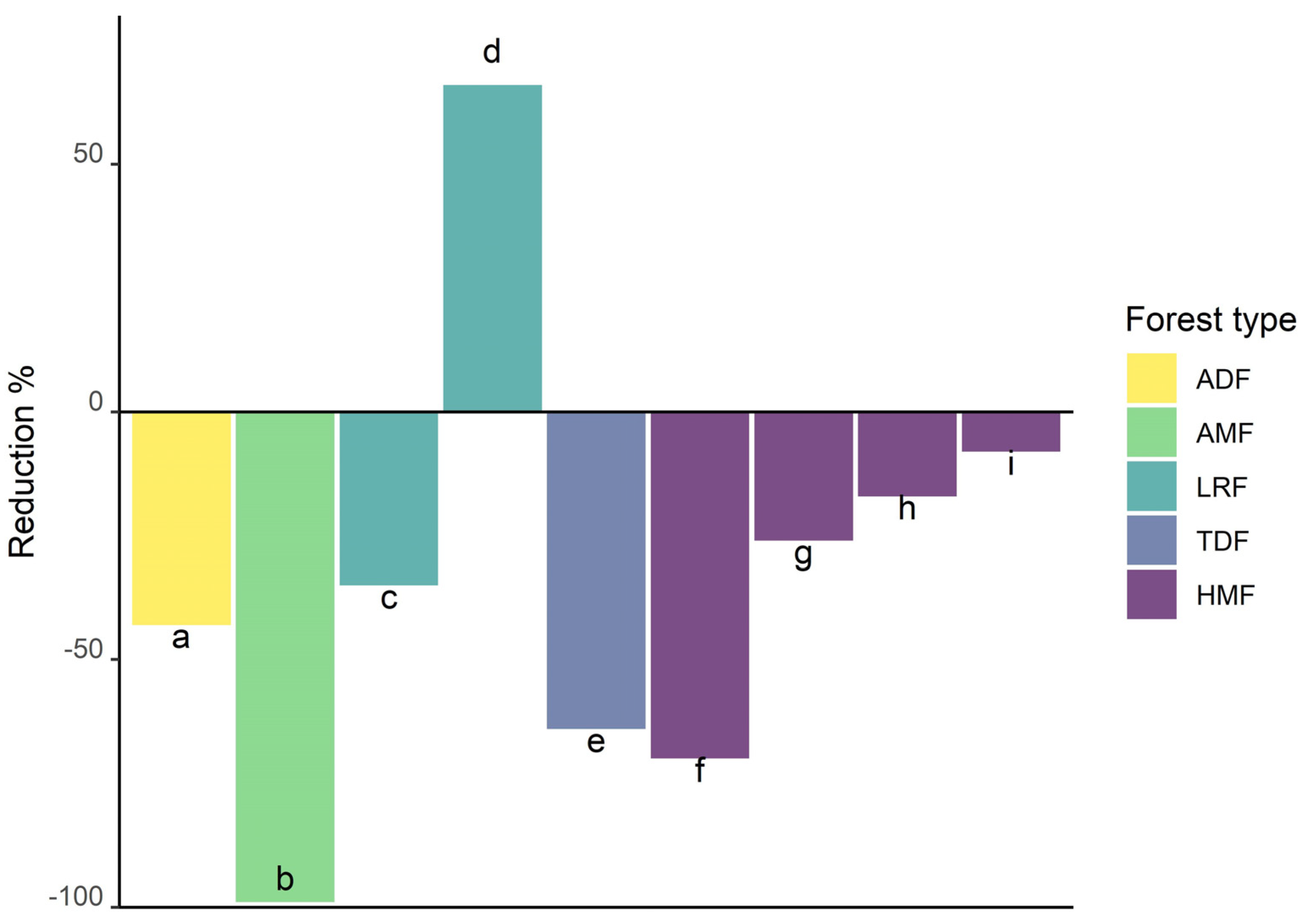
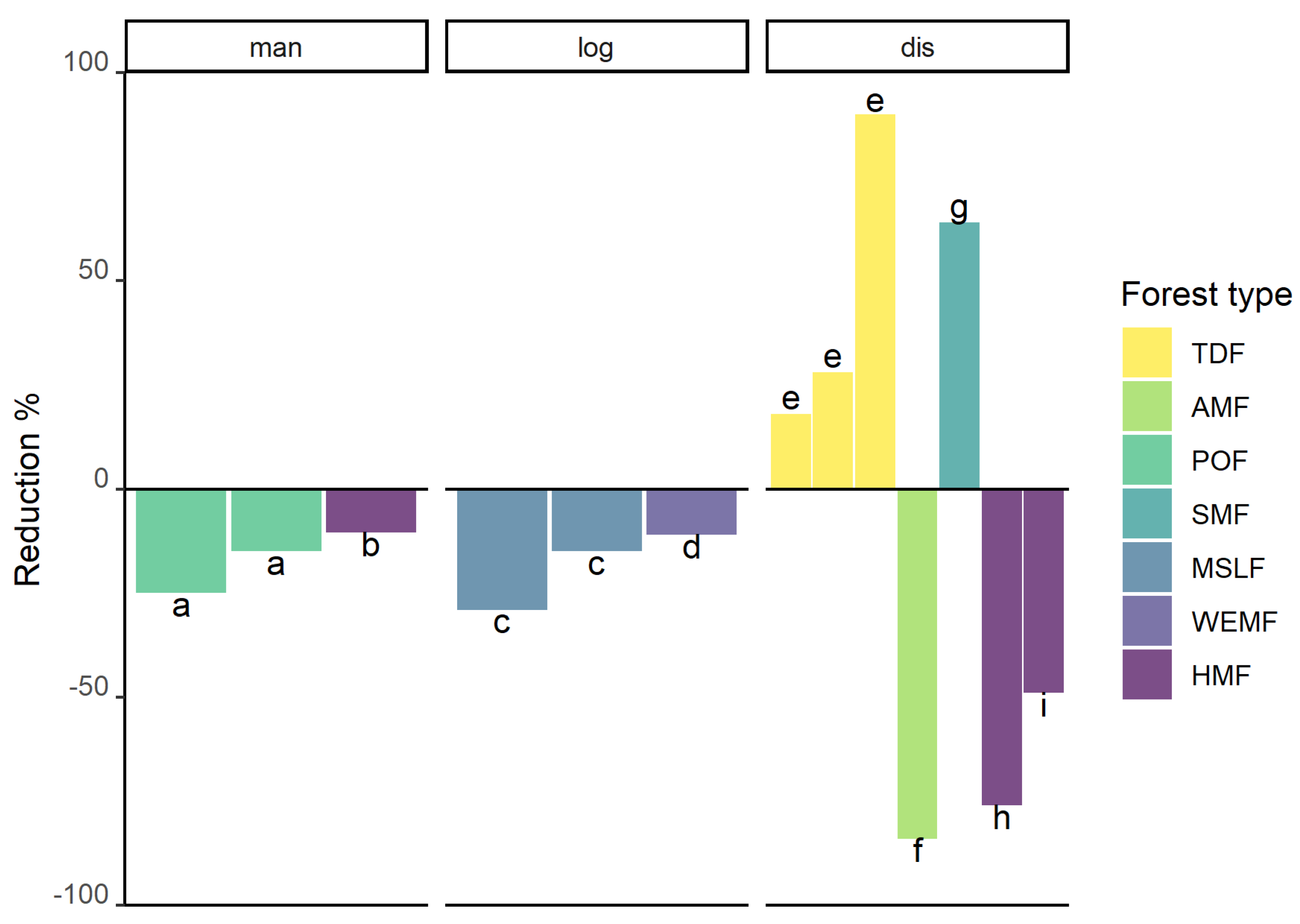
3.7. Fragmented, Disturbed, Degraded and Managed Forests

3.8. Epiphytes in Urban Settings
| Total # Species | Accidental # | Accidental % | Exotics | Study Site | Source |
|---|---|---|---|---|---|
| 10 | 1 | 10 | - | Piratininga, Brazil | [256] |
| 8 | 2 | 25 | 1 | Port Harcourt, Nigeria | [260] |
| 16 | 4 | 25 | - | Bogor, Indonesia | [282] |
| 49 | 18 | 36.7 | 8 | Mar de Espanha, Brazil | [283] |
| 43 | 16 | 37.2 | - | Juiz de Fora, Brazil | [252] |
| 110 | 46 | 41.8 | 30 | Juiz de Fora, Brazil | [270] |
| 47 | 21 | 44.7 | - | Juiz de Fora, Brazil | [258] |
| 15 | 9 | 60 | - | Buenos Aires, Argentina | [284] |
| 72 | 48 | 66.7 | - | Douala, Cameroon | [276] |
| 34 | 23 | 67.6 | 12 | Santo Domingo, R Dominicana | [285] |
| 71 | 60 | 84.5 | 32 | Quito, Ibarra, Riobamba, Mendoza; South America | [275] |
4. Conclusions
5. Future Directions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newbold, T.; Hudson, L.N.; Hill, S.L.L.; Contu, S.; Lysenko, I.; Senior, R.A.; Börger, L.; Bennett, D.J.; Choimes, A.; Collen, B.; et al. Global effects of land use on local terrestrial biodiversity. Nature 2015, 550, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Díaz, S.; Settele, J.; Brondízio, E.S.; Ngo, H.T.; Agard, J.; Arneth, A.; Balvanera, P.; Brauman, K.A.; Butchart, S.H.M.; Chan, K.M.A.; et al. Pervasive human-driven decline of life on Earth points to the need for transformative change. Science 2019, 366, eaax3100. [Google Scholar] [CrossRef]
- Laurance, W.F. Reflections on the tropical deforestation crisis. Biol. Conserv. 1999, 91, 109–117. [Google Scholar] [CrossRef]
- Gibson, L.; Lee, T.M.; Koh, L.P.; Brook, B.W.; Gardner, T.A.; Barlow, J.; Peres, C.A.; Bradshaw, C.J.A.; Laurance, W.F.; Lovejoy, T.E.; et al. Primary forests are irreplaceable for sustaining tropical biodiversity. Nature 2011, 478, 378–381. [Google Scholar] [CrossRef]
- Taubert, F.; Fischer, R.; Groeneveld, J.; Lehmann, S.; Müller, M.S.; Rödig, E.; Wiegand, T.; Huth, A. Global patterns of tropical forest fragmentation. Nature 2018, 554, 519–522. [Google Scholar] [CrossRef]
- Achard, F.; Beuchle, R.; Mayaux, P.; Stibig, H.J.; Bodart, C.; Brink, A.; Carboni, S.; Desclee, B.; Donnay, F.; Eva, H.D.; et al. Determination of tropical deforestation rates and related carbon losses from 1990 to 2010. Glob. Chang. Biol. 2014, 20, 2540–2554. [Google Scholar] [CrossRef]
- Keenan, R.J.; Reams, G.A.; Achard, F.; de Freitas, J.V.; Grainger, A.; Lindquist, E. Dynamics of global forest area: Results from the FAO Global Forest Resources Assessment 2015. For. Ecol. Manag. 2015, 352, 9–20. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. State of the World’s Forests 2014; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014; p. 133. [Google Scholar]
- Oberleitner, F.; Egger, C.; Oberdorfer, S.; Dullinger, S.; Wanek, W.; Hietz, P. Recovery of aboveground biomass, species richness and composition in tropical secondary forests in SW Costa Rica. For. Ecol. Manag. 2021, 479, 118580. [Google Scholar] [CrossRef]
- Chazdon, R.L.; Peres, C.A.; Dent, D.; Sheil, D.; Lugo, A.E.; Lamb, D.; Stork, N.E.; Miller, S.E. The potential for species conservation in tropical secondary forests. Conserv. Biol. 2009, 23, 1406–1417. [Google Scholar] [CrossRef]
- Dent, D.H.; Wright, S.J. The future of tropical species in secondary forests: A quantitative review. Biol. Conserv. 2009, 142, 2833–2843. [Google Scholar] [CrossRef]
- Norden, N.; Chazdon, R.L.; Chao, A.; Jiang, Y.H.; Vilchez-Alvarado, B. Resilience of tropical rain forests: Tree community reassembly in secondary forests. Ecol. Lett. 2009, 12, 385–394. [Google Scholar] [CrossRef]
- Dent, D.H.; DeWalt, S.J.; Denslow, J.S. Secondary forests of central Panama increase in similarity to old-growth forest over time in shade tolerance but not species composition. J. Veg. Sci. 2013, 24, 530–542. [Google Scholar] [CrossRef]
- DeWalt, S.J.; Schnitzer, S.A.; Denslow, J.S. Density and diversity of lianas along a chronosequence in a central Panamanian lowland forest. J. Trop. Ecol. 2000, 16, 1–19. [Google Scholar] [CrossRef]
- Schnitzer, S.A.; Bongers, F.; Burnham, R.J.; Putz, F.E. Ecology of Lianas; John Wiley & Sons Ltd.: Chichester, UK, 2015; p. 504. [Google Scholar]
- Schnitzer, S.A. Testing ecological theory with lianas. New Phytol. 2018, 220, 366–380. [Google Scholar] [CrossRef] [PubMed]
- Schnitzer, S.A.; DeFilippis, D.M.; Visser, M.; Estrada-Villegas, S.; Rivera-Camaña, R.; Bernal, B.; Peréz, S.; Valdéz, A.; Valdéz, S.; Aguilar, A.; et al. Local canopy disturbance as an explanation for long-term increases in liana abundance. Ecol. Lett. 2021, 24, 2635–2647. [Google Scholar] [CrossRef] [PubMed]
- Turner, I.M.; Tan, H.T.W.; Wee, Y.C.; Ibrahim, A.B.; Chew, P.T.; Corlett, R.T. A study of plant species extinction in Singapore: Lessons for the conservation of tropical biodiversity. Conserv. Biol. 1994, 8, 705–712. [Google Scholar] [CrossRef]
- Zotz, G. Plants on Plants—The Biology of Vascular Epiphytes; Springer: Cham, Switzerland, 2016; p. 282. [Google Scholar]
- Gentry, A.H.; Dodson, C.H. Diversity and biogeography of neotropical vascular epiphytes. Ann. Missouri Bot. Gard. 1987, 74, 205–233. [Google Scholar] [CrossRef]
- Krömer, T.; Kessler, M.; Gradstein, S.R.; Acebey, A. Diversity patterns of vascular epiphytes along an elevational gradient in the Andes. J. Biogeogr. 2005, 32, 1799–1809. [Google Scholar] [CrossRef]
- Taylor, A.; Zotz, G.; Weigelt, P.; Cai, L.; Karger, D.N.; König, C.; Kreft, H. Vascular epiphytes contribute disproportionately to global centres of plant diversity. Glob. Ecol. Biogeogr. 2022, 31, 62–74. [Google Scholar] [CrossRef]
- Zotz, G.; Weigelt, P.; Kessler, M.; Kreft, H.; Taylor, A. EpiList 1.0: A global checklist of vascular epiphytes. Ecology 2021, 102, e03326. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.L.; Tanner, E.V.J.; Lughadha, E.N.; Kapos, V. Floristics and biogeography of a rain forest in the Venezuelan Andes. J. Biogeogr. 1994, 21, 421–440. [Google Scholar] [CrossRef]
- Nadkarni, N.M.; Sumera, M.M. Old-growth forest canopy structure and its relationship to throughfall interception. For. Sci. 2004, 50, 290–298. [Google Scholar] [CrossRef]
- Gotsch, S.G.; Nadkarni, N.M.; Amici, A.A. The functional roles of epiphytes and arboreal soils in tropical montane cloud forests. J. Trop. Ecol. 2016, 32, 455–468. [Google Scholar] [CrossRef]
- Mendieta-Leiva, G.; Ramos, F.N.; Elias, J.P.C.; Zotz, G.; Acuña-Tarazona, M.; Alvim, F.S.; Barbosa, D.E.F.; Basílio, G.A.; Batke, S.P.; Benavides, A.M.; et al. EpIG-DB: A database of vascular epiphyte assemblages in the Neotropics. J. Veg. Sci. 2020, 31, 518–528. [Google Scholar] [CrossRef]
- Aguilar-Cruz, Y.; García-Franco, J.G.; Zotz, G. Litter-trapping tank bromeliads in five different forests: Carbon and nutrient pools and fluxes. Biotropica 2021, 54, 170–180. [Google Scholar] [CrossRef]
- Stuntz, S.; Ziegler, C.; Simon, U.; Zotz, G. Diversity and structure of the arthropod fauna within three canopy epiphyte species in central Panama. J. Trop. Ecol. 2002, 18, 161–176. [Google Scholar] [CrossRef]
- Cestari, C. Epiphyte plants use by birds in Brazil. Oecol. Bras. 2009, 13, 689–712. [Google Scholar] [CrossRef]
- Méndez-Castro, F.E.; Bader, M.Y.; Mendieta-Leiva, G.; Rao, D. Islands in the trees: A biogeographic exploration of epiphyte-dwelling spiders. J. Biogeogr. 2018, 45, 2262–2271. [Google Scholar] [CrossRef]
- Aguilar-Rodríguez, P.A.; Krömer, T.; Tschapka, M.; García-Franco, J.G.; Escobedo-Sarti, J.; MacSwiney, G.M.C. Bat pollination in Bromeliaceae. Plant Ecol. Divers. 2019, 12, 1–19. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Altieri, A.H.; Angelini, C.; Bishop, M.J.; Gribben, P.E.; Lear, G.; He, Q.; Schiel, D.R.; Silliman, B.R.; South, P.M.; et al. Secondary foundation species enhance biodiversity. Nat. Ecol. Evol. 2018, 2, 634–639. [Google Scholar] [CrossRef]
- Zotz, G.; Bader, M.Y. Epiphytic plants in a changing world- global change effects on vascular and non-vascular epiphytes. In Progress in Botany; Lüttge, U., Beyschlag, W., Büdel, B., Francis, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 70, pp. 147–170. [Google Scholar]
- Rozendaal, D.M.A.; Bongers, F.; Aide, T.M.; Alvarez-Dávila, E.; Ascarrunz, N.; Balvanera, P.; Becknell, J.M.; Bentos, T.V.; Brancalion, P.H.S.; Cabral, G.A.L.; et al. Biodiversity recovery of Neotropical secondary forests. Sci. Adv. 2019, 5, eaau3114. [Google Scholar] [CrossRef]
- Sodhi, N.S.; Koh, L.P.; Peh, K.S.-H.; Tan, H.T.W.; Chazdon, R.L.; Corlett, R.T.; Lee, T.M.; Colwell, R.K.; Brook, B.W.; Sekercioglu, C.H.; et al. Correlates of extinction proneness in tropical angiosperms. Divers. Distrib. 2008, 14, 1–10. [Google Scholar] [CrossRef]
- Carmona-Higuita, M.J.; Mendieta-Leiva, G.; Gómez-Díaz, J.A.; Villalobos, F.; Nunes Ramos, F.; Costa Elias, J.P.; Jiménez-López, D.A.; Zuluaga, A.; Holst, B.; Kessler, M.; et al. Conservation status of vascular epiphytes in the neotropics. Biodivers. Conserv. 2024, 33, 51–71. [Google Scholar] [CrossRef]
- Barthlott, W.; Schmit-Neuerburg, V.; Nieder, J.; Engwald, S. Diversity and abundance of vascular epiphytes: A comparison of secondary vegetation and primary montane rain forest in the Venezuelan Andes. Plant Ecol. 2001, 152, 145–156. [Google Scholar] [CrossRef]
- Krömer, T.; Gradstein, S.R. Species richness of vascular epiphytes in two primary forests and fallows in the Bolivian Andes. Selbyana 2003, 24, 190–195. [Google Scholar]
- Köster, N.; Friedrich, K.; Nieder, J.; Barthlott, W. Conservation of epiphyte diversity in an Andean landscape transformed by human land use. Conserv. Biol. 2009, 23, 911–919. [Google Scholar] [CrossRef]
- Hietz, P. Diversity and conservation of epiphytes in a changing environment. Pure Appl. Chem. 1999, 70, 1–11. [Google Scholar]
- Hietz-Seifert, U.; Hietz, P.; Guevara, S. Epiphyte vegetation and diversity on remnant trees after forest clearance in southern Veracruz, Mexico. Biol. Conserv. 1996, 75, 103–111. [Google Scholar] [CrossRef]
- Werner, F.A.; Gradstein, S.R. Diversity of dry forest epiphytes along a gradient of human disturbance in the tropical Andes. J. Veg. Sci. 2009, 20, 59–68. [Google Scholar] [CrossRef]
- Larrea, M.L.; Werner, F.A. Response of vascular epiphyte diversity to different land-use intensities in a neotropical montane wet forest. For. Ecol. Manag. 2010, 260, 1950–1955. [Google Scholar] [CrossRef]
- Einzmann, H.J.R.; Zotz, G. How diverse are epiphyte assemblages in plantations and secondary forests in tropical lowlands? Trop. Conserv. Sci. 2016, 9, 629–647. [Google Scholar] [CrossRef]
- Werner, F.A. Reduced growth and survival of vascular epiphytes on isolated remnant trees in a recent tropical montane forest clear-cut. Basic Appl. Ecol. 2011, 12, 172–181. [Google Scholar] [CrossRef]
- Benzing, D.H. Vulnerabilities of tropical forests to climate change: The significance of resident epiphytes. Clim. Change 1998, 39, 519–540. [Google Scholar] [CrossRef]
- Zotz, G. “Hemiepiphyte”: A confusing term and its history. Ann. Bot. 2013, 111, 1015–1020. [Google Scholar] [CrossRef]
- Zotz, G.; Almeda, F.; Bautista-Bello, A.P.; Eskov, A.; Giraldo-Cañas, D.; Hammel, B.; Harrison, R.; Köster, N.; Krömer, T.; Lowry II, P.P.; et al. Hemiepiphytes revisited. Perspect. Plant Ecol. Evol. Syst. 2021, 51, 125620. [Google Scholar] [CrossRef]
- Hoeber, V.; Zotz, G. Accidental epiphytes: Ecological insights and evolutionary implications. Ecol. Monogr. 2022, 92, e1527. [Google Scholar] [CrossRef]
- Muenchow, J.; Dieker, P.; Kluge, J.; Kessler, M.; von Wehrden, H. A review of ecological gradient research in the Tropics: Identifying research gaps, future directions, and conservation priorities. Biodivers. Conserv. 2018, 27, 273–285. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2024. Available online: https://www.R-project.org/ (accessed on 15 October 2024).
- Rinker, T. qdap: Bridging the Gap Between Qualitative Data and Quantitative Analysis. R Package Version 2.4.6. 2023. Available online: https://CRAN.R-project.org/package=qdap (accessed on 15 October 2024).
- Wickham, H. stringr: Simple, Consistent Wrappers for Common String Operations. R Package Version 1.5.1. 2023. Available online: https://CRAN.R-project.org/package=stringr (accessed on 15 October 2024).
- Mori, K. striprtf: Extract Text from RTF File. R Package Version 0.6.0. 2023. Available online: https://CRAN.R-project.org/package=striprtf (accessed on 15 October 2024).
- Feinerer, I.; Hornik, K. tm: Text Mining Package. R Package Version 0.7-14. 2024. Available online: https://CRAN.R-project.org/package=tm (accessed on 15 October 2024).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Barrett, T.; Dowle, M.; Srinivasan, A.; Gorecki, J.; Chirico, M.; Hocking, T.; Schwendinger, B. data.table: Extension of “data.frame”. R Package Version 1.16.2. 2024. Available online: https://CRAN.R-project.org/package=data.table (accessed on 15 October 2024).
- Hill, M.O.; Gauch, H.G. Detrended correspondence analysis: An improved ordination technique. Vegetatio 1980, 42, 47–58. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. vegan: Community Ecology Package. R Package Version 2.6-8. 2024. Available online: https://CRAN.R-project.org/package=vegan (accessed on 15 October 2024).
- Charrad, M.; Ghazzali, N.; Boiteau, V.; Niknafs, A. NbClust: An R Package for Determining the Relevant Number of Clusters in a Data Set. J. Stat. Softw. 2014, 61, 1–36. [Google Scholar] [CrossRef]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning; Springer: New York, NY, USA, 2013; p. 426. [Google Scholar]
- Wickham, H. g gplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer: New York, NY, USA, 2016; p. 213. [Google Scholar] [CrossRef]
- Kassambara, A. ggpubr: “ggplot2” Based Publication Ready Plots. R Package Version 0.6.0. 2023. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 15 October 2024).
- Slowikowski, K. ggrepel: Automatically Position Non-Overlapping Text Labels with ‘ggplot2’. 2024. Available online: https://github.com/slowkow/ggrepel (accessed on 15 October 2024).
- Gradstein, S.R. Epiphytes of tropical montane forests-impact of deforestation and climate change. In The Tropical Mountain Forest, Patterns and Processes in Biodiversity Hotspot; Gradstein, S.R., Homeier, J., Gansert, D., Eds.; Göttingen Centre for Biodiversity and Ecology: Göttingen, Germany, 2008; pp. 51–65. [Google Scholar]
- Nadkarni, N.M. Complex consequences of disturbance on canopy plant communities of world forests: A review and synthesis. New Phytol. 2023, 240, 1366–1380. [Google Scholar] [CrossRef] [PubMed]
- Madison, M. Distribution of epiphytes in a rubber plantation in Sarawak. Selbyana 1979, 5, 207–213. [Google Scholar]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef]
- Melo, F.P.L.; Arroyo-Rodríguez, V.; Fahrig, L.; Martínez-Ramos, M.; Tabarelli, M. On the hope for biodiversity-friendly tropical landscapes. Trends Ecol. Evol. 2013, 28, 462–468. [Google Scholar] [CrossRef]
- Foley, J.A.; DeFries, R.; Asner, G.P.; Barford, C.; Bonan, G.; Carpenter, S.R.; Chapin, F.S.; Coe, M.T.; Daily, G.C.; Gibbs, H.K.; et al. Global consequences of land use. Science 2005, 309, 570–574. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Franklin, J.F.; Fischer, J. General management principles and a checklist of strategies to guide forest biodiversity conservation. Biol. Conserv. 2006, 131, 433–445. [Google Scholar] [CrossRef]
- Wright, S.J. Tropical forests in a changing environment. Trends Ecol. Evol. 2005, 20, 553–560. [Google Scholar] [CrossRef]
- Corlett, R.T. Plant diversity in a changing world: Status, trends, and conservation needs. Plant Divers. 2016, 38, 10–16. [Google Scholar] [CrossRef]
- Christenhusz, M.J.M.; Govaerts, R. Plant extinction in the Anthropocene. Bot. J. Linn. Soc. 2024, 207, 183–196. [Google Scholar] [CrossRef]
- Jim, C.Y. Ecology and conservation of strangler figs in urban wall habitats. Urban Ecosyst. 2014, 17, 405–426. [Google Scholar] [CrossRef]
- Ruas, R.d.B.; de Godoy, S.M.; Feliciano, D.C.; Ruas, C.d.F.; Bered, F. A bromeliad living in the city: A case of a native species resilient to urbanization in South Brazil. Bot. J. Linn. Soc. 2024, 205, 161–176. [Google Scholar] [CrossRef]
- Zotz, G.; Cascante-Marín, A. Life on the wire—Plant growth on power lines in the Americas. Diversity 2024, 16, 573. [Google Scholar] [CrossRef]
- Janzen, D.H. Latent Extinction—The Living Dead. In Encyclopedia of Biodiversity, 2nd ed.; Levin, S.A., Ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 590–598. [Google Scholar]
- Krömer, T.; Viccon-Esquivel, J.; Gómez-Díaz, J.A. Efectos antrópicos sobre la diversidad de epífitas vasculares y orquídeas en el centro de Veracruz. In Las Orquídeas de Veracruz; Viccon-Esquivel, J., Castañeda Zárate, M., Castro Cortés, R., Cetzal Ix, W., Eds.; Editorial de La Universidad Veracruzana: Xalapa, Mexico, 2021; pp. 235–252. [Google Scholar]
- Brown, S.; Lugo, A.E. Tropical secondary forests. J. Trop. Ecol. 1990, 6, 1–32. [Google Scholar] [CrossRef]
- Chazdon, R.L. Tropical forest recovery: Legacies of human impact and natural disturbances. Perspect. Plant Ecol. Evol. Syst. 2003, 6, 51–71. [Google Scholar] [CrossRef]
- Poorter, L.; Craven, D.; Jakovac, C.C.; Van Der Sande, M.T.; Amissah, L.; Bongers, F.; Chazdon, R.L.; Farrior, C.E.; Kambach, S.; Meave, J.A.; et al. Multidimensional tropical forest recovery. Science 2021, 374, 1370–1376. [Google Scholar] [CrossRef]
- Guariguata, M.R.; Ostertag, R. Neotropical secondary forest succession: Changes in structural and functional characteristics. For. Ecol. Manag. 2001, 148, 185–206. [Google Scholar] [CrossRef]
- Chazdon, R.L.; Letcher, S.G.; van Breugel, M.; Martínez-Ramos, M.; Bongers, F.; Finegan, B. Rates of change in tree communities of secondary Neotropical forests following major disturbances. Philosphical Trans. R. Soc. Lond. B Biol. Sci. 2007, 362, 273–289. [Google Scholar] [CrossRef]
- Woods, C.L.; DeWalt, S.J. The conservation value of secondary forests for vascular epiphytes in Central Panama. Biotropica 2013, 45, 119–127. [Google Scholar] [CrossRef]
- DeWalt, S.J.; Maliakal, S.K.; Denslow, J.S. Changes in vegetation structure and composition along a tropical forest chronosequence: Implications for wildlife. For. Ecol. Manag. 2003, 182, 139–151. [Google Scholar] [CrossRef]
- Carvajal-Hernández, C.I.; Krömer, T.; López-Acosta, J.C.; Gómez-Díaz, J.A.; Kessler, M. Conservation value of disturbed and secondary forests for ferns and lycophytes along an elevational gradient in Mexico. Appl. Veg. Sci. 2017, 20, 662–672. [Google Scholar] [CrossRef]
- Van Vuuren, D.P.; Sala, O.E.; Pereira, H.M. The future of vascular plant diversity under four global scenarios. Ecol. Soc. 2006, 11, 25. [Google Scholar] [CrossRef]
- Benavides, A.-M.; Wolf, J.H.D.; Duivenvoorden, J.F. Recovery and succession of epiphytes in upper Amazonian fallows. J. Trop. Ecol. 2006, 22, 705–717. [Google Scholar] [CrossRef]
- Pérez-Peña, A.; Krömer, T. ¿Qué pueden aportar los acahuales y las plantaciones de cítricos a la conservación de las epífitas vasculares en los Tuxtlas, Veracruz? In Avances y Perspectivas en la Investigación de los Bosques Tropicales y Sus Alrededores: La Región de Los Tuxtlas; Reynoso, V.H., Coates, R.I., Vázquez-Cruz, M.d.L., Eds.; Instituto de Biología, Universidad Nacional Autónoma de México: Ciudad de México, Mexico, 2017; pp. 569–580. [Google Scholar]
- Ceballos, S.J. Vascular epiphyte communities in secondary and mature forests of a subtropical montane area. Acta Oecol. 2020, 105, 103571. [Google Scholar] [CrossRef]
- Cuevas Reyes, P.; Vega Gutiérrez, J.I. Cambios en la estructura, composición y fenología de plantas epífitas bajo diferentes estadios de sucesión vegetal en un bosque tropical seco. Biol. Rev. Cienc. Biol. Agropecu. Univ. Michoacana San Nicolás Hidalgo 2012, 14, 37–44. [Google Scholar]
- Reid, J.L.; Chaves-Fallas, J.M.; Holl, K.D.; Zahawi, R.A. Tropical forest restoration enriches vascular epiphyte recovery. Appl. Veg. Sci. 2016, 19, 508–517. [Google Scholar] [CrossRef]
- Bacles, C.F.; Lowe, A.J.; Ennos, R.A. Effective seed sispersal across a fragmented landscape. Science 2006, 311, 628. [Google Scholar] [CrossRef]
- Thomson, F.J.; Moles, A.T.; Auld, T.D.; Kingsford, R.T. Seed dispersal distance is more strongly correlated with plant height than with seed mass. J. Ecol. 2011, 99, 1299–1307. [Google Scholar] [CrossRef]
- Einzmann, H.J.R.; Zotz, G. Dispersal and establishment of vascular epiphytes in human-modified landscapes. AoB Plants 2017, 9, plx052. [Google Scholar] [CrossRef]
- Cascante-Marín, A.; von Meijenfeldt, N.; de Leeuw, H.M.H.; Wolf, J.H.D.; Oostermeijer, J.G.B.; den Nijs, J.C.M. Dispersal limitation in epiphytic bromeliad communities in a Costa Rican fragmented montane landscape. J. Trop. Ecol. 2009, 25, 63–73. [Google Scholar] [CrossRef]
- Werth, S.; Wagner, H.H.; Gugerli, F.; Holderegger, R.; Csencsics, D.; Kalwij, J.M.; Scheidegger, C. Quantifying dispersal and establishment limitation in a population of an epiphytic lichen. Ecology 2006, 87, 2037–2046. [Google Scholar] [CrossRef] [PubMed]
- Burns, K.C.; Zotz, G. A hierarchical framework for investigating epiphyte assemblages: Networks, meta-communities, and scale. Ecology 2010, 91, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Krömer, T.; García-Franco, J.G.; Toledo-Aceves, T. Epífitas vasculares como bioindicadores de la calidad forestal: Impacto antrópico sobre su diversidad y composición. In Bioindicadores: Guardianes de Nuestro Futuro Ambiental; González-Zuarth, C.A., Vallarino, A., Pérez-Jimenez, J.C., Low-Pfeng, A.M., Eds.; Instituto Nacional de Ecología y Cambio Climático (INECC)—El Colegio de la Frontera Sur (ECOSUR): Ciudad de México, México, 2014; pp. 605–623. [Google Scholar]
- Costa Elias, J.P.; Borges e Silva, B.A.; Gonçalves de Carvalho, R.; Sampaio, M.B.; Mendieta-Leiva, G.; Nunes Ramos, F. Tree structure instead of microclimatic zones determines differences in vascular epiphyte assemblages between forest and pasture. For. Ecol. Manag. 2024, 552, 121567. [Google Scholar] [CrossRef]
- Zotz, G.; Vollrath, B. The epiphyte vegetation of the palm Socratea exorrhiza—Correlations with tree size, tree age and bryophyte cover. J. Trop. Ecol. 2003, 19, 81–90. [Google Scholar] [CrossRef]
- Nadkarni, N.M. Colonization of stripped branch surfaces by epiphytes in a Lower Montane Cloud Forest, Monteverde, Costa Rica. Biotropica 2000, 32, 358–363. [Google Scholar] [CrossRef]
- Hietz, P.; Ausserer, J.; Schindler, G. Growth, maturation and survival of epiphytic bromeliads in a Mexican humid montane forest. J. Trop. Ecol. 2002, 18, 177–191. [Google Scholar] [CrossRef]
- Schmidt, G.; Zotz, G. Inherently slow growth in two Caribbean epiphytic species: A demographic approach. J. Veg. Sci. 2002, 13, 527–534. [Google Scholar] [CrossRef]
- Werner, F.A.; Köster, N.; Kessler, M.; Gradstein, S.R. Is the resilience of epiphyte assemblages to human disturbance a function of local climate. Ecotropica 2011, 17, 15–20. [Google Scholar] [CrossRef]
- Guzmán-Jacob, V.; Zotz, G.; Craven, D.; Taylor, A.; Krömer, T.; Monge-González, M.L.; Kreft, H.; Pfeifer, M. Effects of forest-use intensity on vascular epiphyte diversity along an elevational gradient. Divers. Distrib. 2020, 26, 4–15. [Google Scholar] [CrossRef]
- Graham, E.A.; Andrade, J.L. Drought tolerance associated with vertical stratification of two co-occurring epiphytic bromeliads in a tropical dry forest. Am. J. Bot. 2004, 91, 699–706. [Google Scholar] [CrossRef]
- Carvajal-Hernández, C.I.; Krömer, T.; Vázquez-Torres, M. Riqueza y composición florística de pteridobiontes en bosque mesófilo de montaña y ambientes asociados en el centro de Veracruz, México. Rev. Mex. Biodivers. 2014, 85, 491–501. [Google Scholar] [CrossRef]
- McCormick, M.K.; Jacquemyn, H. What constrains the distribution of orchid populations? New Phytol. 2014, 202, 392–400. [Google Scholar] [CrossRef]
- Hietz, P.; Briones, O. Correlation between water relations and within-canopy distribution of epiphytic ferns in a Mexican cloud forest. Oecologia 1998, 114, 305–316. [Google Scholar] [CrossRef]
- Zotz, G.; Andrade, J.L. La ecología y fisiología de las epífitas y hemiepífitas. In Ecología y Conservación de Bosques Neotropicales; Guariguata, M.R., Kattan, G.H., Eds.; Libro Universitario Regional del Instituto Tecnológico de Costa Rica, San José: San José, Costa Rica, 2002; pp. 271–296. [Google Scholar]
- Zotz, G.; Hietz, P.; Einzmann, H.J.R. Functional ecology of vascular epiphytes. Annu. Plant Rev. Online 2021, 4, 869–906. [Google Scholar] [CrossRef]
- Dunn, R.R. Bromeliad communities in isolated trees and three successional stages of an Andean cloud forest in Ecuador. Selbyana 2000, 21, 137–143. [Google Scholar]
- Cascante-Marín, A.; Wolf, J.H.D.; Oostermeijer, J.G.B.; den Nijs, J.C.M.; Sanahuja, O.; Durán-Apuy, A. Epiphytic bromeliad communities in secondary and mature forest in a tropical premontane area. Basic Appl. Ecol. 2006, 7, 520–532. [Google Scholar] [CrossRef]
- Alanís-Méndez, J.L.; Muñoz-Arteaga, F.O.; López-Ortega, M.; Cuervo-López, L.; Raya-Cruz, B.E. Aportes al conocimiento de las epífitas (Bromeliaceae, Cactaceae y Orchidaceae) en dos tipos de vegetación del Municipio de Pánuco, Veracruz, México. Rev. Cient. UDO Agríc. 2007, 7, 160–174. [Google Scholar]
- Flores-Argüelles, A.; Espejo-Serna, A.; López-Ferrari, A.R.; Krömer, T. Diversity and vertical distribution of epiphytic angiosperms, in natural and disturbed forest on the Northern Coast of Jalisco, Mexico. Front. For. Glob. Change 2022, 5, 828851. [Google Scholar] [CrossRef]
- Griffiths, H.; Smith, J.A.C. Photosynthetic pathways in the Bromeliaceae of Trinidad: Relations between life-forms, habitat preference and the occurrence of CAM. Oecologia 1983, 60, 176–184. [Google Scholar] [CrossRef]
- Winkler, M.; Huelber, K.; Hietz, P. Effect of canopy position on germination and seedling survival of epiphytic bromeliads in a Mexican humid montane forest. Ann. Bot. 2005, 95, 1039–1047. [Google Scholar] [CrossRef]
- Winkler, M.; Hülber, K.; Hietz, P. Population dynamics of epiphytic bromeliads: Life strategies and the role of host branches. Basic Appl. Ecol. 2007, 8, 183–196. [Google Scholar] [CrossRef]
- Köster, N.; Kreft, H.; Nieder, J.; Barthlott, W.; Jetz, W. Range size and climatic niche correlate with the vulnerability of epiphytes to human land use in the tropics. J. Biogeogr. 2013, 40, 963–976. [Google Scholar] [CrossRef]
- Flores-Palacios, A.; García-Franco, J.G. Effect of isolation on the structure and nutrient content of oak epiphyte communities. Plant Ecol. 2004, 173, 259–269. [Google Scholar] [CrossRef]
- Hietz, P.; Buchberger, G.; Winkler, M. Effect of forest disturbance on abundance and distribution of epiphytic bromeliads and orchids. Ecotropica 2006, 12, 103–112. [Google Scholar]
- Curtis, P.G.; Slay, C.M.; Harris, N.L.; Tyukavina, A.; Hansen, M.C. Classifying drivers of global forest loss. Science 2018, 361, 1108–1111. [Google Scholar] [CrossRef] [PubMed]
- Pendrill, F.; Persson, U.M.; Godar, J.; Kastner, T. Deforestation displaced: Trade in forest-risk commodities and the prospects for a global forest transition. Environ. Res. Lett. 2019, 14, 055003. [Google Scholar] [CrossRef]
- Stephens, W.; Hamilton, A.P.; Carr, M.K.V. Plantation crops. In Agriculture in the Tropics, 3rd ed.; Webster, C.C., Williams, C.N., Eds.; Blackwell: Oxford, UK, 1998; pp. 200–221. [Google Scholar]
- Hartemink, A.E. Plantation agriculture in the tropics: Environmental issues. Outlook Agric. 2005, 34, 11–21. [Google Scholar] [CrossRef]
- Fagan, M.E.; Kim, D.-H.; Settle, W.; Ferry, L.; Drew, J.; Carlson, H.; Slaughter, J.; Schaferbien, J.; Tyukavina, A.; Harris, N.L.; et al. The expansion of tree plantations across tropical biomes. Nat. Sustain. 2022, 5, 681–688. [Google Scholar] [CrossRef]
- Evans, J. Plantation Forestry in the Tropics, 2nd ed.; Cambridge University Press: Cambridge, UK, 1992; p. 403. [Google Scholar]
- Onyekwelu, J.C.; Stimm, B.; Evans, J. Review Plantation Forestry. In Silviculture in the Tropics (Tropical Forestry); Günter, S., Weber, M., Stimm, B., Mosandl, R., Eds.; Springer: Berlin, Germany, 2011; Volume 8, pp. 399–454. [Google Scholar]
- Boelter, C.R.; Zartman, C.E.; Fonseca, C.R. Exotic tree monocultures play a limited role in the conservation of Atlantic Forest epiphytes. Biodivers. Conserv. 2011, 20, 1255–1272. [Google Scholar] [CrossRef]
- Hietz, P.; Hietz-Seifert, U. Composition and ecology of vascular epiphyte communities along an altitudinal gradient in central Veracruz, Mexico. J. Veg. Sci. 1995, 6, 487–498. [Google Scholar] [CrossRef]
- Wolf, J.H.D. The response of epiphytes to anthropogenic disturbance of pine-oak forests in the highlands of Chiapas, Mexico. For. Ecol. Manag. 2005, 212, 376–393. [Google Scholar] [CrossRef]
- Zotz, G. Vascular epiphytes in the temperate zones—A review. Plant Ecol. 2005, 176, 173–183. [Google Scholar] [CrossRef]
- Callaway, R.M.; Reinhart, K.O.; Moore, G.W.; Moore, D.J.; Pennings, S.C. Epiphyte host preferences and host traits: Mechanisms for species-specific interactions. Oecologia 2002, 132, 221–230. [Google Scholar] [CrossRef]
- Böhnert, T.; Wenzel, A.; Altenhövel, C.; Beeretz, L.; Tjitrosoedirdjo, S.S.; Meijide, A.; Rembold, K.; Kreft, H. Effects of land-use change on vascular epiphyte diversity in Sumatra (Indonesia). Biol. Conserv. 2016, 202, 20–29. [Google Scholar] [CrossRef]
- Carrasco, L.R.; Larrosa, C.; Milner-Gulland, E.J.; Edwards, D.P. A double-edged sword for tropical forests. Science 2014, 346, 38–40. [Google Scholar] [CrossRef]
- Furumo, P.R.; Aide, T.M. Characterizing commercial oil palm expansion in Latin America: Land use change and trade. Environ. Res. Lett. 2017, 12, 024008. [Google Scholar] [CrossRef]
- Jayathilake, H.M.; Jamaludin, J.; De Alban, J.D.T.; Webb, E.L.; Carrasco, L.R. The conversion of rubber to oil palm and other landcover types in Southeast Asia. Appl. Geogr. 2023, 150, 102838. [Google Scholar] [CrossRef]
- Piggott, A.G. The fern flora of oil palm plantations in West Malaysia. Fern Gaz. 1980, 12, 93–102. [Google Scholar]
- Gill, L.S.; Onyibe, H.I. Phytosociological studies of epiphytic flora of oil palm (Elaeis guineensis Jacq.) in Benin City, Nigeria. Feddes Repert. 1986, 97, 691–695. [Google Scholar] [CrossRef]
- Prescott, G.W.; Edwards, D.P.; Foster, W.A. Retaining biodiversity in intensive farmland: Epiphyte removal in oil palm plantations does not affect yield. Ecol. Evol. 2015, 5, 1944–1954. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.D.; Gillbanks, R.A. Oil Palm Cultivation and Management; The Incorporated Society of Planters: Kuala Lumpur, Malaysia, 2003; p. 672. [Google Scholar]
- Nunes-Freitas, A.F.; de Asis Ribeiro, D.C.; Meireles, A.S.; Azevedo, D.B.; Almeida, G.H.; Oliveira, W.F.; Rocha-Pessôa, T.C. Invasive exotic oil palm trees modify the structure of vascular epiphytes community on an Atlantic Forest Island. In Invasive Species. Ecology, Impacts, and Potential Uses; Londe, V., Ed.; Nova Science Publishers: New York, NY, USA, 2020; pp. 157–188. [Google Scholar]
- Wagner, W.H. Ferns on Pacific island coconut trees. Am. Fern J. 1945, 35, 74–76. [Google Scholar] [CrossRef]
- Porembski, S.; Biedinger, N. Epiphytic ferns for sale: Influence of commercial plant collection on the frequency of Platycerium stemaria (Polypodiaceae) in coconut plantations on the southeastern Ivory Coast. Plant Biol. 2001, 3, 72–76. [Google Scholar] [CrossRef]
- Schimper, A.F.W. Die Epiphytische Vegetation Amerikas; Fischer: Frankfurt am Main, Germany, 1888; p. 130. [Google Scholar]
- Johansson, D. Ecology of Vascular Epiphytes in West African Rain Forest. Ph.D. Thesis, Uppsala University, Uppsala, Sweden, 1974; p. 138. [Google Scholar]
- Nir, M.A. The survivor: Orchids on a Puerto Rican coffee finca. Am. Orchid. Soc. Bull. 1988, 57, 989–995. [Google Scholar]
- Einzmann, H.J.R.; Döcke, L.; Zotz, G. Epiphytes in human settlements in rural Panama. Plant Ecol. Divers. 2016, 9, 277–287. [Google Scholar] [CrossRef]
- Cook, M.T. Epiphytic orchids a serious pest on citrus trees. J. Agric. Univ. Puerto Rico 1926, 10, 5–9. [Google Scholar] [CrossRef]
- García-González, A.; Riverón-Giró, F.B. Organización espacial y estructura de una población de Lonopsis utricularioides (Orchidaceae) en un área suburbana de Pinar del Río, Cuba. Lankesteriana 2013, 13, 419–427. [Google Scholar] [CrossRef]
- Mondragón, D.; Santos-Moreno, A.; Damon, A. Epiphyte diversity on coffee bushes: A management question? J. Sustain. Agric. 2009, 33, 703–715. [Google Scholar] [CrossRef]
- Moguel, P.; Toledo, V.M. Biodiversity conservation in traditional coffee systems of Mexico. Conserv. Biol. 1999, 13, 11–21. [Google Scholar] [CrossRef]
- Hernández-Martínez, G.; Manson, R.H.; Contreras Hernández, A. Quantitative classification of coffee agroecosystems spanning a range of production intensities in central Veracruz, Mexico. Agric. Ecosyst. Environ. 2009, 134, 89–98. [Google Scholar] [CrossRef]
- Hietz, P. Conservation of vascular epiphyte diversity in Mexican coffee plantations. Conserv. Biol. 2005, 19, 391–399. [Google Scholar] [CrossRef]
- Espejo-Serna, A.; López-Ferrari, A.R.; Jiménez-Machorro, R.; Sánchez-Saldaña, L. Orchids from coffee-plantations in Mexico: An alternative for the sustainable use of tropical ecosystems. Rev. Biol. Trop. 2005, 53, 73–84. [Google Scholar]
- Hylander, K.; Nemomissa, S. Home garden coffee as a repository of epiphyte biodiversity in Ethiopia. Front. Ecol. Environ. 2008, 6, 524–528. [Google Scholar] [CrossRef]
- Hundera, K.; Aerts, R.; De Beenhouwer, M.; Van Overtveld, K.; Helsen, K.; Muys, B.; Honnay, O. Both forest fragmentation and coffee cultivation negatively affect epiphytic orchid diversity in Ethiopian moist evergreen Afromontane forests. Biol. Conserv. 2013, 159, 285–291. [Google Scholar] [CrossRef]
- Moorhead, L.C.; Philpott, S.M.; Bichier, P. Epiphyte biodiversity in the coffee agricultural matrix: Canopy stratification and distance from forest fragments. Conserv. Biol. 2010, 24, 737–746. [Google Scholar] [CrossRef]
- Richards, J.H.; Luna Torrez, I.M.; Waller, D.M. Tree longevity drives conservation value of shade coffee farms for vascular epiphytes. Agric. Ecosyst. Environ. 2020, 301, 107025. [Google Scholar] [CrossRef]
- Osie, M.; Shibru, S.; Dalle, G.; Nemomissa, S. Habitat fragmentation effects on vascular epiphytes diversity in Kafa biosphere reserve and nearby coffee agroecosystem, southwestern Ethiopia. Trop. Ecol. 2022, 63, 561–571. [Google Scholar] [CrossRef]
- Williams-Linera, G.; Sosa, V.; Platas, T. The fate of epiphytic orchids after fragmentation of a Mexican cloud forest. Selbyana 1995, 16, 36–40. [Google Scholar]
- Solís-Montero, L.; Flores-Palacios, A.; Cruz-Angón, A. Shade-coffee plantations as refuges for tropical wild orchids in central Veracruz, Mexico. Conserv. Biol. 2005, 19, 908–916. [Google Scholar] [CrossRef]
- García-Franco, J.G.; Toledo, T. Vascular epiphytes: Bromeliads and orchids. In Agroecosistemas Cafetaleros de Veracruz: Biodiversidad, Manejo y Conservación; Manson, R.H., Hernández-Ortiz, V., Gallina, S., Mehltreter, K., Eds.; Instituto de Ecología A.C. (INECOL) e Instituto Nacional de Ecología (INE-SEMARNAT): Xalapa, México, 2008; pp. 69–82. [Google Scholar]
- Toledo-Aceves, T.; García-Franco, J.G.; Hernández-Rojas, A.; MacMillan, K. Recolonization of vascular epiphytes in a shaded coffee agroecosystem. Appl. Veg. Sci. 2012, 15, 99–107. [Google Scholar] [CrossRef]
- Toledo-Aceves, T.; Mehltreter, K.; Garcia-Franco, J.G.; Hernandez-Rojas, A.; Sosa, V.J. Benefits and costs of epiphyte management in shade coffee plantations. Agric. Ecosyst. Environ. 2013, 181, 149–156. [Google Scholar] [CrossRef]
- Solís-Montero, L.; Quintana-Palacios, V.; Damon, A. Impact of moss and epiphyte removal on coffee production and implications for epiphyte conservation in shade coffee plantations in southeast Mexico. Agroecol. Sustain. Food Syst. 2019, 43, 1124–1144. [Google Scholar] [CrossRef]
- Cruz-Angón, A.; Greenberg, R. Are epiphytes important for birds in coffee plantations? An experimental assessment. J. Appl. Ecol. 2005, 42, 150–159. [Google Scholar] [CrossRef]
- Cruz-Angón, A.; Baena, M.L.; Greenberg, R. The contribution of epiphytes to the abundance and species richness of canopy insects in a Mexican coffee plantation. J. Trop. Ecol. 2009, 25, 453–463. [Google Scholar] [CrossRef]
- De Beenhouwer, M.; Aerts, R.; Hundera, K.; Van Overtveld, K.; Honnay, O. Management intensification in Ethiopian coffee forests is associated with crown habitat contraction and loss of specialized epiphytic orchid species. Basic Appl. Ecol. 2015, 16, 592–600. [Google Scholar] [CrossRef]
- Morales-Linares, J.; Garcia-Franco, J.G.; Flores-Palacios, A.; Krömer, T.; Toledo-Aceves, T. The role of shaded cocoa plantations in the maintenance of epiphytic orchids and their interactions with phorophytes. J. Plant Ecol. 2020, 13, 27–35. [Google Scholar] [CrossRef]
- Andersson, M.S.; Gradstein, S.R. Impact of management intensity on non-vascular epiphyte diversity in cacao plantations in western Ecuador. Biodivers. Conserv. 2005, 14, 1101–1120. [Google Scholar] [CrossRef]
- Haro-Carrión, X.; Lozada, T.; Navarrete, H.; de Koning, G.H.J. Conservation of vascular epiphyte diversity in shade cacao plantations in the Chocó region of Ecuador. Biotropica 2009, 41, 520–529. [Google Scholar] [CrossRef]
- Morales-Linares, J.; García-Franco, J.G.; Flores-Palacios, A.; Valenzuela-González, J.E.; Mata-Rosas, M.; Díaz-Castelazo, C. Vascular epiphytes and host trees of ant-gardens in an anthropic landscape in southeastern Mexico. Sci. Nat. 2016, 103, 96. [Google Scholar] [CrossRef]
- Qi, D.-H.; Guo, H.-J.; Sheng, C.-Y. Assessment of plant species diversity of ancient tea garden communities in Yunnan, Southwest of China. Agrofor. Syst. 2013, 87, 465–474. [Google Scholar] [CrossRef]
- Qi, D.-H.; Guo, H.-J.; Cui, J.-Y.; Sheng, C.-I. Plant biodiversity assessment of the ancient tea garden ecosystem in Jingmai of Lancang, Yunnan. Biodivers. Sci. 2005, 13, 221–231. [Google Scholar] [CrossRef]
- Wang, Q.; Guan, W.-B.; Gi Wong, M.H.; Ranjitkar, S.; Sun, W.-N.; Pan, Y.; El-Kassaby, Y.A.; Shen, L.-X. Tree size predicts vascular epiphytic richness of traditional cultivated tea plantations in Southwestern China. Glob. Ecol. Conserv. 2017, 10, 147–153. [Google Scholar] [CrossRef]
- Wu, S.-M.; Li, T.-Q.; Yang, W.-K.; Liu, Q.; Gao, J.-Y. Ancient tea gardens play important role on in situ conservation of epiphytic orchids in southwest Yunnan, China. Glob. Ecol. Conserv. 2024, 49, e02778. [Google Scholar] [CrossRef]
- Chowdhury, R.; Chowdhury, M. Diversity of vascular epiphytes on preferred shade trees in tea gardens of sub-Himalayan tracts in West Bengal, India. J. Threat. Taxa 2024, 16, 25720–25729. [Google Scholar] [CrossRef]
- Guevara, S.; Meave, J.; Moreno-Casasola, P.; Laborde, J. Floristic composition and structure of vegetation under isolated trees in neotropical pastures. J. Veg. Sci. 1992, 3, 655–664. [Google Scholar] [CrossRef]
- Flores-Palacios, A.; García-Franco, J.G. The relationship between tree size and epiphyte species richness: Testing four different hypotheses. J. Biogeogr. 2006, 33, 323–330. [Google Scholar] [CrossRef]
- Parra-Sanchez, E.; Banks-Leite, C. Value of human-modified forests for the conservation of canopy epiphytes. Biotropica 2022, 54, 958–968. [Google Scholar] [CrossRef]
- Werner, F.A.; Homeier, J.; Gradstein, S.R. Diversity of vascular epiphytes on isolated remnant trees in the montane forest belt of southern Ecuador. Ecotropica 2005, 11, 21–40. [Google Scholar]
- Werner, F.A.; Gradstein, S.R. Seedling establishment of vascular epiphytes on isolated and enclosed forest trees in an Andean landscape, Ecuador. Biodivers. Conserv. 2008, 17, 3195–3207. [Google Scholar] [CrossRef]
- Trejo-Cruz, I.A.; Martínez-Camilo, R.; Martínez-Meléndez, N.; Jiménez-López, D.A. Diversidad de epífitas vasculares en árboles remanentes del género Ficus (Moraceae) en sistemas silvopastoriles del sureste de México. Acta Bot. Mex. 2021, 128, e1827. [Google Scholar] [CrossRef]
- Poltz, K.; Zotz, G. Vascular epiphytes on isolated pasture trees along a rainfall gradient in the lowlands of Panama. Biotropica 2011, 43, 165–172. [Google Scholar] [CrossRef]
- Nadkarni, N.M.; Haber, W.A. Canopy seed banks as time capsules of biodiversity in pasture-remnant tree crowns. Conserv. Biol. 2009, 23, 1117–1126. [Google Scholar] [CrossRef]
- Werner, F.A.; Gradstein, S.R. Spatial distribution and abundance of epiphytes along a gradient of human disturbance in an Interandean dry valley, Ecuador. Selbyana 2010, 30, 208–215. [Google Scholar]
- Flores-Palacios, A.; García-Franco, J.G. Habitat isolation changes the beta diversity of the vascular epiphyte community in lower montane forest, Veracruz, Mexico. Biodivers. Conserv. 2008, 17, 191–207. [Google Scholar] [CrossRef]
- Amici, A.A.; Nadkarni, N.M.; Williams, C.B.; Gotsch, S.G. Differences in epiphyte biomass and community composition along landscape and within-crown spatial scales. Biotropica 2020, 52, 46–58. [Google Scholar] [CrossRef]
- Einzmann, H.J.R.; Zotz, G. “No signs of saturation”: Long-term dynamics of vascular epiphyte communities in a human-modified landscape. Biodivers. Conserv. 2017, 26, 1393–1410. [Google Scholar] [CrossRef]
- Elias, J.P.C.; Mortara, S.R.; Nunes-Freitas, A.F.; van den Berg, E.; Ramos, F.N. Host tree traits in pasture areas affect forest and pasture specialist epiphyte species differently. Am. J. Bot. 2021, 108, 598–606. [Google Scholar] [CrossRef]
- Trapnell, D.W.; Hamrick, J.L. Partitioning nuclear and chloroplast variation at multiple spatial scales in the neotropical epiphytic orchid, Laelia rubescens. Mol. Ecol. 2004, 13, 2655–2666. [Google Scholar] [CrossRef]
- Svahnström, V.J.; Lughadha, E.N.; Forest, F.; Leão, T.C. A global study of the geographic range size of epiphytes. bioRxiv 2023. [Google Scholar] [CrossRef]
- Winkler, M.; Koch, M.; Hietz, P. High gene flow in epiphytic ferns despite habitat loss and fragmentation. Conserv. Genet. 2011, 12, 1411–1420. [Google Scholar] [CrossRef]
- Laurance, W.F. Have we overstated the tropical biodiversity crisis? Trends Ecol. Evol. 2006, 22, 65–70. [Google Scholar] [CrossRef]
- Murcia, C. Edge effects in fragmented forests: Implications for conservation. Trends Ecol. Evol. 1995, 10, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Fahrig, L. Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef]
- Silva, V.L.; Mehltreter, K.; Schmitt, J.L. Ferns as potential ecological indicators of edge effects in two types of Mexican forests. Ecol. Indic. 2018, 93, 669–676. [Google Scholar] [CrossRef]
- Haddad, N.M.; Brudvig, L.A.; Clobert, J.; Davies, K.F.; Gonzalez, A.; Holt, R.D.; Lovejoy, T.E.; Sexton, J.O.; Austin, M.P.; Collins, C.D.; et al. Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci. Adv. 2015, 1, e1500052. [Google Scholar] [CrossRef]
- Laurance, W.F. Edge effects in tropical forest fragments: Applications of a model for the design of nature reserves. Biol. Conserv. 1991, 57, 205–219. [Google Scholar] [CrossRef]
- Willmer, J.N.G.; Puettker, T.; Prevedello, J.A. Global impacts of edge effects on species richness. Biol. Conserv. 2022, 272, 109654. [Google Scholar] [CrossRef]
- Bataghin, F.A.; Rodrigues Pires, J.S.; de Barros, F. Epifitismo vascular em sítios de borda e interior em Floresta Estacional Semidecidual no Sudeste do Brasil. Hoehnea 2012, 39, 235–245. [Google Scholar] [CrossRef]
- Bianchi, J.S.; Kersten, R.d.A. Edge effect on vascular epiphytes in a subtropical Atlantic Forest. Acta Bot. Bras. 2014, 28, 120–126. [Google Scholar] [CrossRef]
- Parra-Sanchez, E.; Banks-Leite, C. The magnitude and extent of edge effects on vascular epiphytes across the Brazilian Atlantic Forest. Sci. Rep. 2020, 10, 18847. [Google Scholar] [CrossRef]
- Lippert, A.P.U.; Silva, V.L.; Mallmann, I.T.; Müller, A.; Droste, A.; Schmitt, J.L. Edge effect on vascular epiphytes in a subtropical Atlantic Forest fragment. J. Environ. Anal. Prog. 2022, 7, 135–149. [Google Scholar] [CrossRef]
- Ghazoul, J.; Burivalova, Z.; Garcia-Ulloa, J.; King, L.A. Conceptualizing forest degradation. Trends Ecol. Evol. 2015, 30, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Putz, F.E. Critical need for new definitions of “forest” and “forest degradation” in global climate change agreements. Conserv. Lett. 2009, 2, 226–232. [Google Scholar] [CrossRef]
- Vásquez-Grandón, A.; Donoso, P.J.; Gerding, V. Forest degradation: When is a forest degraded? Forest 2018, 9, 726. [Google Scholar] [CrossRef]
- Stanturf, J.A.; Palik, B.J.; Williams, M.I.; Dumroese, R.K.; Madsen, P. Forest restoration paradigms. J. Sustain. For. 2014, 33, S161–S194. [Google Scholar] [CrossRef]
- Vancutsem, C.; Achard, F.; Pekel, J.-F.; Vieilledent, G.; Carboni, S.; Simonetti, D.; Gallego, J.; Aragão, L.E.O.C.; Nasi, R. Long-term (1990–2019) monitoring of forest cover changes in the humid tropics. Sci. Adv. 2021, 7, eabe1603. [Google Scholar] [CrossRef]
- Hosonuma, N.; Herold, M.; De Sy, V.; De Fries, R.S.; Brockhaus, M.; Verchot, L.; Angelsen, A.; Romijn, E. An assessment of deforestation and forest degradation drivers in developing countries. Environ. Res. Lett. 2012, 7, 44009. [Google Scholar] [CrossRef]
- Ticktin, T. The ecological implications of harvesting non-timber forest products. J. Appl. Ecol. 2004, 41, 11–21. [Google Scholar] [CrossRef]
- Torres-Rojo, J.M.; Moreno-Sánchez, R.; Mendoza-Briseño, M.A. Sustainable forest management in Mexico. Curr. For. Rep. 2016, 2, 93–105. [Google Scholar] [CrossRef]
- Martínez-Meléndez, N.; Ramírez-Marcial, N.; García-Franco, J.G.; Cach-Pérez, M.J.; Martínez-Zurimendi, P. Importance of Quercus spp. for diversity and biomass of vascular epiphytes in a managed pine-oak forest in Southern Mexico. For. Ecosyst. 2022, 9, 100034. [Google Scholar] [CrossRef]
- Rutten, G.; Ensslin, A.; Hemp, A.; Fischer, M. Forest structure and composition of previously selectively logged and non-logged montane forests at Mt. Kilimanjaro. For. Ecol. Manag. 2015, 337, 61–66. [Google Scholar] [CrossRef]
- Shima, K.; Yamada, T.; Okuda, T.; Fletcher, C.; Kassim, A.R. Dynamics of tree species diversity in unlogged and selectively logged Malaysian forests. Sci. Rep. 2018, 8, 1024. [Google Scholar] [CrossRef]
- Flores-Palacios, A.; Valencia-Díaz, S. Local illegal trade reveals unknown diversity and involves a high species richness of wild vascular epiphytes. Biol. Conserv. 2007, 136, 372–387. [Google Scholar] [CrossRef]
- Cruz-Garcia, G.; Lagunez-Rivera, L.; Chavez-Angeles, M.G.; Solano-Gomez, R. The wild orchid trade in a Mexican local market: Diversity and economics. Econ. Bot. 2015, 69, 291–305. [Google Scholar] [CrossRef]
- Mondragón, D.; del Carmen Méndez-García, E.M.; Morillo, I.R. Prioritizing the conservation of epiphytic bromeliads using ethnobotanical information from a traditional Mexican market. Econ. Bot. 2016, 70, 29–36. [Google Scholar] [CrossRef]
- Krömer, T.; Acebey, A.; Toledo-Aceves, T. Aprovechamiento de plantas epífitas: Implicaciones para su conservación y manejo sustentable. In De la Recolección a los Agroecosistemas: Soberanía Alimentaria y Conservación de la Biodiversidad; Silva-Rivera, E., Martínez-Valdéz, V., Lascuráin, M., Rodríguez-Luna, E., Eds.; Editorial de la Universidad Veracruzana: Xalapa, Veracruz, 2018; pp. 175–196. [Google Scholar]
- Jiménez-López, D.A.; Pérez-García, E.A.; Martínez-Meléndez, N.; Solano, R. Orquídeas silvestres comercializadas en un mercado tradicional de Chiapas, México. Bot. Sci. 2019, 97, 691–700. [Google Scholar] [CrossRef]
- Elliott, D.D.; Ticktin, T. Epiphytic plants as NTFPs from the forest canopies: Priorities for management and conservation. In Treetops at Risk; Springer: Berlin, Germany, 2013; pp. 435–444. [Google Scholar]
- Chinsamy, M.; Finnie, J.F.; Van Staden, J. The ethnobotany of South African medicinal orchids. S. Afr. J. Bot. 2011, 77, 2–9. [Google Scholar] [CrossRef]
- Hossain, M.M. Therapeutic orchids: Traditional uses and recent advances—An overview. Fitoterapia 2011, 82, 102–140. [Google Scholar] [CrossRef]
- Subedi, A.; Kunwar, B.; Choi, Y.; Dai, Y.; van Andel, T.; Chaudhary, R.P.; de Boer, H.J.; Gravendee, B. Collection and trade of wild-harvested orchids in Nepal. J. Ethnobiol. Ethnomed. 2013, 9, 64. [Google Scholar] [CrossRef]
- Liu, H.; Luo, Y.-B.; Heinen, J.; Bhat, M.; Liu, Z.-J. Eat your orchid and have it too: A potentially new conservation formula for Chinese epiphytic medicinal orchids. Biodivers. Conserv. 2014, 23, 1215–1228. [Google Scholar] [CrossRef]
- Hinsley, A.; de Boer, H.J.; Fay, M.F.; Gale, S.W.; Gardiner, L.M.; Gunasekara, R.S.; Kumar, P.; Masters, S.; Metusala, D.; Roberts, D.L.; et al. A review of the trade in orchids and its implications for conservation. Bot. J. Linn. Soc. 2018, 186, 435–455. [Google Scholar] [CrossRef]
- Mondragón, D. Population viability analysis for Guarianthe aurantiaca, an ornamental epiphytic orchid harvested in Southeast Mexico. Plant Species Biol. 2009, 24, 35–41. [Google Scholar] [CrossRef]
- Mondragón, D.; Ticktin, T. Demographic effects of harvesting epiphytic bromeliads and an alternative approach to collection. Conserv. Biol. 2011, 25, 797–807. [Google Scholar] [CrossRef]
- Emeterio-Lara, A.; García-Franco, J.G.; Hernández-Apolinar, M.; Toledo-Hernández, V.H.; Valencia-Díaz, S.; Flores-Palacios, A. Does extraction of orchids affect their population structure? Evidence from populations of Laelia autumnalis (Orchidaceae). For. Ecol. Manag. 2021, 480, 118667. [Google Scholar] [CrossRef]
- Orozco-Ibarrola, O.; Solano, R.; Valverde, T. Sustainable harvesting and conservation of Laelia furfuracea, a rare epiphytic orchid from Oaxaca, Mexico. Biotropica 2021, 53, 142–151. [Google Scholar] [CrossRef]
- Ticktin, T.; Charitonidou, M.; Douglas, J.; Halley, J.M.; Hernández-Apolinar, M.; Liu, H.; Mondragón, D.; Pérez-García, E.A.; Tremblay, R.L.; Phelps, J. Wild orchids: A framework for identifying and improving sustainable harvest. Biol. Conserv. 2023, 277, 109816. [Google Scholar] [CrossRef]
- Toledo-Aceves, T.; García-Franco, J.G.; López-Barrera, F. Bromeliad rain: An opportunity for cloud forest management. For. Ecol. Manag. 2014, 329, 129–136. [Google Scholar] [CrossRef]
- Francisco-Ventura, E.; Menchaca-García, R.A.; Toledo-Aceves, T.; Krömer, T. Potencial de aprovechamiento de epífitas vasculares caídas en un bosque mesófilo de montaña de Los Tuxtlas, Veracruz, México. Rev. Mex. Biodivers. 2018, 89, 1263–1279. [Google Scholar] [CrossRef]
- Jiménez-Bautista, L.; Damon, A.; Ochoa-Gaona, S.; Tapia, R.C. Impact of silvicultural methods on vascular epiphytes (ferns, bromeliads and orchids) in a temperate forest in Oaxaca, Mexico. For. Ecol. Manag. 2014, 329, 10–20. [Google Scholar] [CrossRef]
- Seshadri, K.S.; Ganesan, R.; Devy, S.M. Persistent effects of historical selective logging on a vascular epiphyte assemblage in the forest canopy of the Western Ghats, India. Front. For. Glob. Change 2021, 4, 727422. [Google Scholar] [CrossRef]
- Padmawathe, R.; Qureshi, Q.; Rawat, G.S. Effects of selective logging on vascular epiphyte diversity in a moist lowland forest of Eastern Himalaya, India. Biol. Conserv. 2004, 119, 81–92. [Google Scholar] [CrossRef]
- Aguirre, A.; Guevara, R.; García, M.; López, J.C. Fate of epiphytes on phorophytes with different architectural characteristics along the perturbation gradient of Sabal mexicana forests in Veracruz, Mexico. J. Veg. Sci. 2010, 21, 6–15. [Google Scholar] [CrossRef]
- Obermüller, F.A.; Silveira, M.; Salimon, C.I.; Daly, D.C. Epiphytic (including hemiepiphytes) diversity in three timber species in the southwestern Amazon, Brazil. Biodivers. Conserv. 2012, 21, 565–575. [Google Scholar] [CrossRef]
- Duarte, M.M.; Gandolfi, S. Diversifying growth forms in tropical forest restoration: Enrichment with vascular epiphytes. For. Ecol. Manag. 2017, 401, 89–98. [Google Scholar] [CrossRef]
- Fernandez-Barrancos, E.P.; Reid, J.L.; Aronson, J. Tank bromeliad transplants as an enrichment strategy in southern Costa Rica. Restor. Ecol. 2017, 25, 569–576. [Google Scholar] [CrossRef]
- Izuddin, M.; Yam, T.W.; Webb, E.L. Specific niche requirements drive long-term survival and growth of translocated epiphytic orchids in an urbanised tropical landscape. Urban Ecosyst. 2018, 21, 531–540. [Google Scholar] [CrossRef]
- Benavides, A.-M.; Calderón-Caro, J.; Canal, D. Surviving in a new host: Eight years of monitoring translocated aroids, bromeliads, and orchids in the Andean forests in Colombia. For. Glob. Change 2023, 6, 83466. [Google Scholar] [CrossRef]
- Orozco Ávila, J.; Valencia Marín, A.; Betancur Pérez, J.F. Estimation of the transfer of vascular epiphytes, as a conservation strategy in the municipality of Aguazul, Casanare, Colombia. Rev. Investig. Agrar. Ambiental 2017, 8, 27–37. [Google Scholar] [CrossRef]
- Piana, M.R.; Aronson, M.F.J.; Pickett, S.T.A.; Handel, S.N. Plants in the city: Understanding recruitment dynamics in urban landscapes. Front. Ecol. Environ. 2019, 17, 455–463. [Google Scholar] [CrossRef]
- Bryan, C.L. Ecology of Vascular Epiphytes in Urban Forests with Special Reference to the Shrub Epiphyte Griselinia lucida. Master’s Thesis, The University of Waikato, Hamilton, New Zealand, 2011. [Google Scholar]
- Chang, C.-R.; Chen, M.-C.; Su, M.-H. Natural versus human drivers of plant diversity in urban parks and the anthropogenic species-area hypotheses. Landsc. Urban Plan. 2021, 208, 104023. [Google Scholar] [CrossRef]
- Furtado Gomes, S.; Menini Neto, L. Diversity of vascular epiphytes in urban environment: A case study in a biodiversity hotspot, the Brazilian Atlantic forest. CES Rev. 2015, 29, 82–101. [Google Scholar]
- Baltazar-Bernal, O.; Zavala-Ruiz, J.; Hernández-García, A. Orchid diversity (Orchidaceae) in two urban sites in the state of Veracruz, Mexico. Agrociencia 2024, 58, 571–583. [Google Scholar] [CrossRef]
- Asharo, R.K.; Novitasari, A.; Azizah, S.D.N.; Saraswati, R.A.; Setyaningsih, F.; Apriliani, P.; Priambodo, R.; Pasaribu, P.O.; Rizkawati, V.; Usman. Araceae floristic and potential study in Bogor Botanical Gardens, West Java, Indonesia. J. Biol. Res. Appl. 2022, 4, 8–18. [Google Scholar] [CrossRef]
- Yam, T.W.; Tay, F.; Ang, P.; Soh, W. Conservation and reintroduction of native orchids of Singapore—The next phase. Eur. J. Environ. Sci. 2011, 1, 143–147. [Google Scholar] [CrossRef]
- Fabricante, J.R.; de Andrade, L.A.; Marques, F.J. Componente epifítico vascular ocorrente em árvores urbanas. CERNE 2006, 12, 399–405. [Google Scholar]
- Adhikari, Y.P.; Fischer, A.; Fischer, H.S. Micro-site conditions of epiphytic orchids in a human impact gradient in Kathmandu valley, Nepal. J. Mt. Sci. 2012, 9, 331–342. [Google Scholar] [CrossRef]
- Santana, L.D.; Gomes Furtado, S.; Nardy, C.; Silveira Leite, F.; Neto Menini, L. Diversity, vertical structure and floristic relationships of vascular epiphytes in an urban remnant of the Brazilian Atlantic Forest. Hoehnea 2017, 44, 123–138. [Google Scholar] [CrossRef]
- Riefner, R.E., Jr.; Smith, A.R. New and noteworthy epiphytic ferns from the urban forests of Coastal Southern California, U.S.A. Phytologia 2019, 101, 81–112. [Google Scholar]
- Alex, A.; Chima, U.D.; Uzoamaka, D.U. Diversity and phorophyte preference of vascular epiphytic flora on avenues within the University of Port Harcourt, Nigeria. J. For. Environ. Sci. 2021, 37, 217–225. [Google Scholar] [CrossRef]
- Kimpouni, V.; Lenga-Sacadura, M.Y.; Kalath, R.S.; Kiangana-Ngoyi, L. Diversité floristique des épiphytes et hémiparasites vasculaires de l’écosystème forestier urbain de Brazzaville, Congo. J. Appl. Biosci. 2017, 117, 11704–11719. [Google Scholar] [CrossRef]
- Brandes, D. Some Observations on the Urban Flora in Albania. 2023. Available online: http://www.ruderal-vegetation.de/epub/Urban_flora_in_Albania_2.pdf (accessed on 15 October 2024).
- Spennemann, D.H.R. Growth of ornamental palms, Phoenix and Washingtonia, as epiphytes on suburban street trees in Albury, NSW, Australia. Cunninghamia 2019, 19, 113–119. Available online: https://www.botanicgardens.org.au/sites/default/files/2023-06/BGD0562_CunninghamiaSPENNEMANN-Epiphyt-palms.pdf (accessed on 8 April 2025).
- Rogers, H.C.; Clarkson, B.D. Epiphyte-host relationships of remnant and recombinant urban ecosystems in Hamilton, New Zealand: The importance of Dicksonia squarrosa (G.Forst.) Sw., whekī. N. Z. J. Bot. 2023, 63, 150–159. [Google Scholar] [CrossRef]
- Hall, C.F.; Gomes Klein, V.L.; de Barros, F. Orchidaceae no município de Caldas Novas, Goiás, Brasil. Rodriguésia 2013, 64, 685–704. [Google Scholar] [CrossRef]
- Aoki-Gonçalves, F.; Pena, J.C.; Toledo-Aceves, T.; MacGregor-Fors, I. Urban epiphytes: Bromeliad diversity in a green cover gradient across a Neotropical streetscape. Urban For. Urban Green. 2023, 83, 127901. [Google Scholar] [CrossRef]
- Quail, M.R.; Ramos, F.N.; Dallimore, T.; Ashton, P.; Clayton-Brown, J.; Provan, J.; Batke, S.P. Surrounded by concrete: Genetic isolation of Tillandsia recurvata L. in an urban landscape in southeastern Brazil. Bot. J. Linn. Soc. 2023, 203, 390–400. [Google Scholar] [CrossRef]
- Morajkar, S.; Sajeev, S.; Hegde, S. Ferns: A thriving group of urban dwellers. Bionature 2015, 35, 13–21. [Google Scholar]
- Yañez, A.; Marquez, G.J.; Berrueta, P.C.; García, R.A. An urban fern refugium: Municipal Ecological Reserve of Avellaneda (Eco Área) (Buenos Aires, Argentina). Blumea 2021, 66, 227–235. [Google Scholar] [CrossRef]
- Alvim, F.S.; Gomes Furtado, S.; Menini Neto, L. Diversity of vascular epiphytes in urban green areas of Juiz de Fora, Minas Gerais, Brazil. Floresta Ambiente 2020, 27, e20190116. [Google Scholar] [CrossRef]
- Mondragón, D.; Mora-Flores, M.P. First steps to study the demography of vascular epiphytes in cities. Braz. J. Bot. 2024, 84, e270998. [Google Scholar] [CrossRef]
- Landi, M.; Angiolini, C. Population structure of Osmunda regalis in relation to environment and vegetation: An example in the Mediterranean area. Folia Geobot. 2011, 46, 49–68. [Google Scholar] [CrossRef]
- Martins, P.L.S.S.; Furtado, S.G.; Menini Neto, L. Could epiphytes be xenophobic? Evaluating the use of native versus exotic phorophytes by the vascular epiphytic community in an urban environment. Community Ecol. 2020, 21, 91–101. [Google Scholar] [CrossRef]
- Izuddin, M.; Webb, E.L. The influence of tree architecture, forest remnants, and dispersal syndrome on roadside epiphyte diversity in a highly urbanized tropical environment. Biodivers. Conserv. 2015, 24, 2063–2077. [Google Scholar] [CrossRef]
- Cerón Martínez, C.E.; Reyes Tello, C.I. Epifitas de Phoenix canariensis Chabaud (Arecaceae) en cinco localidades Sudamericanas. Cinchonia 2021, 16, 197–216. [Google Scholar]
- Ngotta, B.J.B.; Bell, D.M.; Mvogo, O.P.B.; Nguimfack, D.J.; Doumbe, M.L.C.; Wafo, T.Y.D.; Betti, J.L.; Priso, R.J. Diversity and composition of the epiphytic flora in an urban agglomeration: The case of city of Douala, Cameroon. J. Ecol. Nat. Environ. 2023, 15, 9–17. [Google Scholar] [CrossRef]
- Moreno-Barreto, E.; Medina-Sánchez, A.M. Caracterización florística y funcional de las epífitas vasculares asociadas a palmas del género Phoenix L. en Bogotá, Colombia. Biota Colomb. 2024, 25, 1–14. [Google Scholar] [CrossRef]
- Ritter, C.M.; Rocha Santos, F.; Pezenti Crespão, L.M.; Ardengui, T.C.; Galeazzi Caxambu, M. Levantamento de epífitas presentes na arborização urbana no Município de Farol—Paraná. Rev. Soc. Bras. Arboriz. Urbana 2015, 9, 18–28. [Google Scholar] [CrossRef]
- Devens, K.U.; Geraldini, A.P.B.; Amadeo, R.M.; Caxambu, M.G.; Magnoni, P.H.J. Levantamento de epífitas na arborização urbana do município de Luiziana-Paraná. Soc. Bras. Arboriz. Urbana 2016, 10, 1–11. [Google Scholar] [CrossRef]
- Wu, J.; Brock, J. The invasion of non-native epiphyte Platycerium bifurcatum in Auckland’s urban forest canopy. N. Z. J. Ecol. 2023, 47, 3542. [Google Scholar] [CrossRef]
- Neo, L.; Chong, K.Y.; Lindsay, S.; Middleton, D.J.; Tan, P.Y.; Er, K.B.H. A botanical oasis rather than a biological desert: Rediscoveries, new species and new records in a tropical city. Plants People Planet 2024, 6, 697–709. [Google Scholar] [CrossRef]
- Praptosuwiryo, T.N.; Sumanto, S.; Cahyaningsih, R. Diversity and host preferences of ferns and lycopods epiphytes on palm trees. Biodiversitas J. Biol. Divers. 2019, 20, 3731–3740. [Google Scholar] [CrossRef]
- dos Santos Kaeser, S.; Chiavegatto, B.; Ulguim Bordoni, P.S.; Furtado Gomes, S.; Menini Neto, L. Composição florística e ecologia de epífitas vasculares na praça central do município de Mar de Espanha, Minas Gerais, Brasil. Rev. Bras. Arboriz. Urbana 2020, 15, 26–38. [Google Scholar]
- Romero Zapiola, A.; Devoto, M. Factores que afectan la riqueza de especies epífitas vasculares del arbolado de alineación en la Ciudad Autónoma de Buenos Aires. Ecol. Austral 2024, 34, 579–592. [Google Scholar] [CrossRef]
- Jiménez-Orozco, C.; Lebrón-Liriano, B.V.; Fernández-Gutiérrez, R.; Urbáez, R.; Guerrero, Á. Caracterización de la flora epífita vascular del Parque Iberoamérica, Santo Domingo, República Dominicana. Cienc. Ambiente Clima 2019, 2, 23–33. [Google Scholar] [CrossRef]
- Hietz, P.; Wagner, K.; Nunes Ramos, F.; Sarmento Cabral, J.; Agudelo, C.; Benavides, A.M.; Cach-Pérez, M.J.; Cardelús, C.L.; Chilpa Galván, N.; Nascimento da Costa, L.E.; et al. Putting vascular epiphytes on the traits map. J. Ecol. 2022, 110, 340–358. [Google Scholar] [CrossRef]
- Martinson, G.O.; Werner, F.A.; Scherber, C.; Conrad, R.; Corre, M.D.; Flessa, H.; Wolf, K.; Klose, M.; Gradstein, S.R.; Veldkamp, E. Methane emissions from tank bromeliads in neotropical forests. Nat. Geosci. 2010, 3, 766–769. [Google Scholar] [CrossRef]
- Rapp, J.M.; Silman, M.R. Epiphyte response to drought and experimental warming in an Andean cloud forest. F1000Research 2014, 3, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Lugo, A.E.; Scatena, F.N. Epiphytes and climate change research in the Caribbean: A proposal. Selbyana 1992, 13, 123–130. [Google Scholar]
- Foster, P. The potential negative impacts of global climate change on tropical montane cloud forests. Earth-Sci. Rev. 2001, 55, 73–106. [Google Scholar] [CrossRef]
- Barlow, J.; Gardner, T.A.; Araujo, I.S.; Ávila-Pires, T.C.; Bonaldo, A.B.; Costa, J.E.; Esposito, M.C.; Ferreira, L.V.; Hawes, J.; Hernandez, M.I.M.; et al. Quantifying the biodiversity value of tropical primary, secondary, and plantation forests. Proc. Natl. Acad. Sci. USA 2007, 104, 18555–18560. [Google Scholar] [CrossRef]
- Crouzeilles, R.; Curran, M.; Ferreira, M.S.; Lindenmayer, D.B.; Grelle, C.E.; Rey Benayas, J.M. A global meta-analysis on the ecological drivers of forest restoration success. Nat. Commun. 2016, 7, 11666. [Google Scholar] [CrossRef]
- Matos, F.A.R.; Magnago, L.F.S.; Chan Miranda, C.A.; de Menezes, L.F.T.; Gastauer, M.; Safar, N.V.H.; Schaefer, C.E.G.R.; da Silva, M.P.; Simonelli, M.; Edwards, F.A.; et al. Secondary forest fragments offer important carbon and biodiversity cobenefits. Glob. Change Biol. 2020, 26, 509–522. [Google Scholar] [CrossRef]
- Foo, Y.Z.; O’Dea, R.E.; Koricheva, J.; Nakagawa, S.; Lagisz, M. A practical guide to question formation, systematic searching and study screening for literature reviews in ecology and evolution. Methods Ecol. Evol. 2021, 12, 1705–1720. [Google Scholar] [CrossRef]
- Fox, J. Why Do Ecologists Publish so Many More Meta-Analyses Than Evolutionary Biologists? 2021. Available online: https://dynamicecology.wordpress.com/2021/07/08/why-do-ecologists-publish-so-many-more-meta-analyses-than-evolutionary-biologists/ (accessed on 15 October 2024).
- Laube, S.; Zotz, G. Long-term changes of the vascular epiphyte assemblage on the palm Socratea exorrhiza in a lowland forest in Panama. J. Veg Sci. 2006, 17, 307–314. [Google Scholar] [CrossRef]
- Einzmann, H.J.R.; Weichgrebe, L.; Zotz, G. Long-term community dynamics in vascular epiphytes on Annona glabra along the shoreline of Barro Colorado Island, Panama. J. Ecol. 2021, 109, 1931–1946. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krömer, T.; Einzmann, H.J.R.; Mendieta-Leiva, G.; Zotz, G. Impact of Land-Use Change on Vascular Epiphytes: A Review. Plants 2025, 14, 1188. https://doi.org/10.3390/plants14081188
Krömer T, Einzmann HJR, Mendieta-Leiva G, Zotz G. Impact of Land-Use Change on Vascular Epiphytes: A Review. Plants. 2025; 14(8):1188. https://doi.org/10.3390/plants14081188
Chicago/Turabian StyleKrömer, Thorsten, Helena J. R. Einzmann, Glenda Mendieta-Leiva, and Gerhard Zotz. 2025. "Impact of Land-Use Change on Vascular Epiphytes: A Review" Plants 14, no. 8: 1188. https://doi.org/10.3390/plants14081188
APA StyleKrömer, T., Einzmann, H. J. R., Mendieta-Leiva, G., & Zotz, G. (2025). Impact of Land-Use Change on Vascular Epiphytes: A Review. Plants, 14(8), 1188. https://doi.org/10.3390/plants14081188








