The Geographic Distribution and Natural Variation of the Rice Blast Fungus Avirulence Gene AVR-Pita1 in Southern China
Abstract
:1. Introduction
2. Materials and Methods
2.1. M. oryzae Strains
2.2. Plant Materials and Pathogenicity Assay
2.3. DNA Extraction, PCR Amplification, and Sequencing
2.4. Phylogenetic Analysis
2.5. Analysis for the Distribution of Ptr Alleles in Elite Rice Parental Lines
3. Results
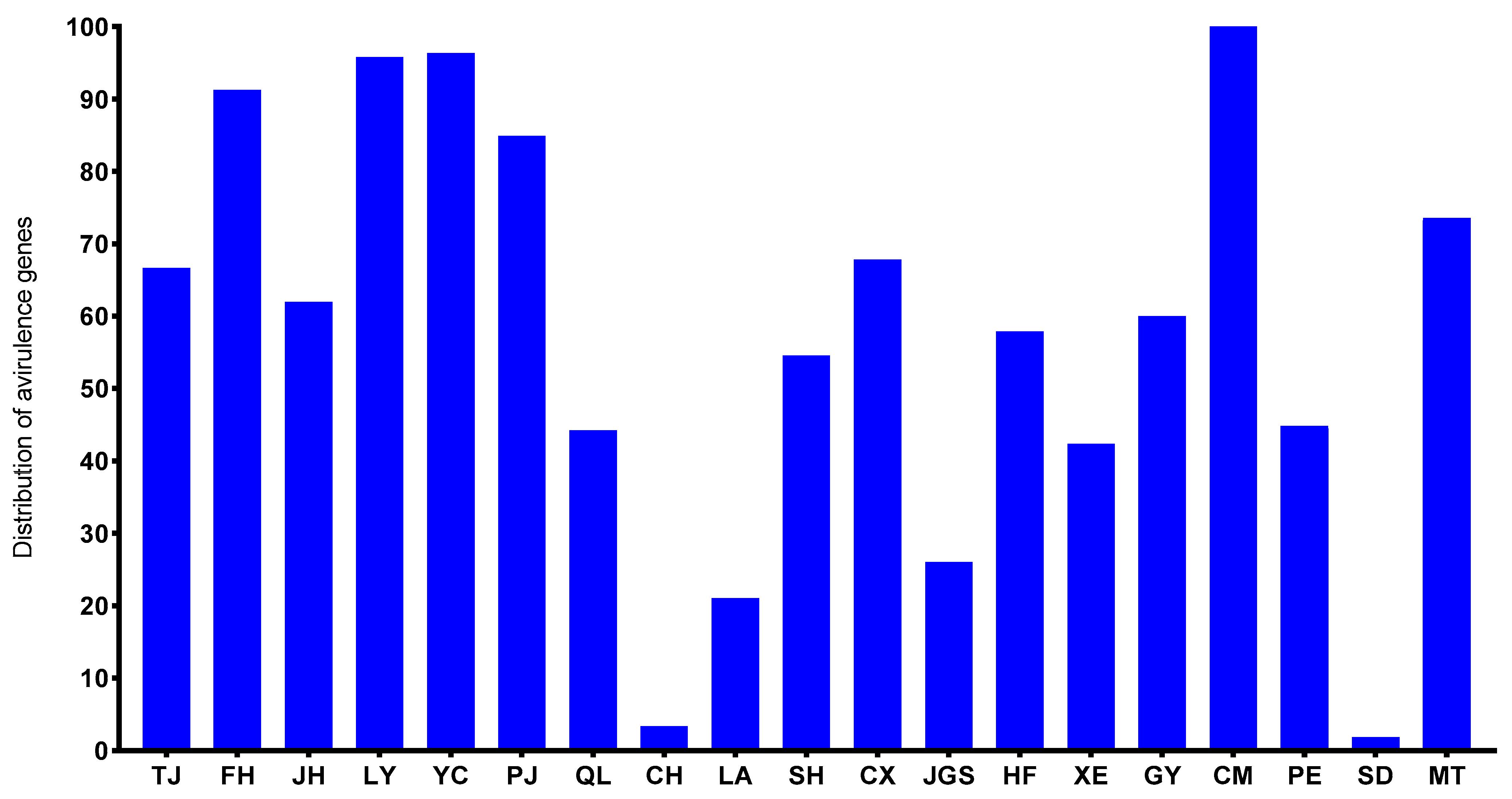
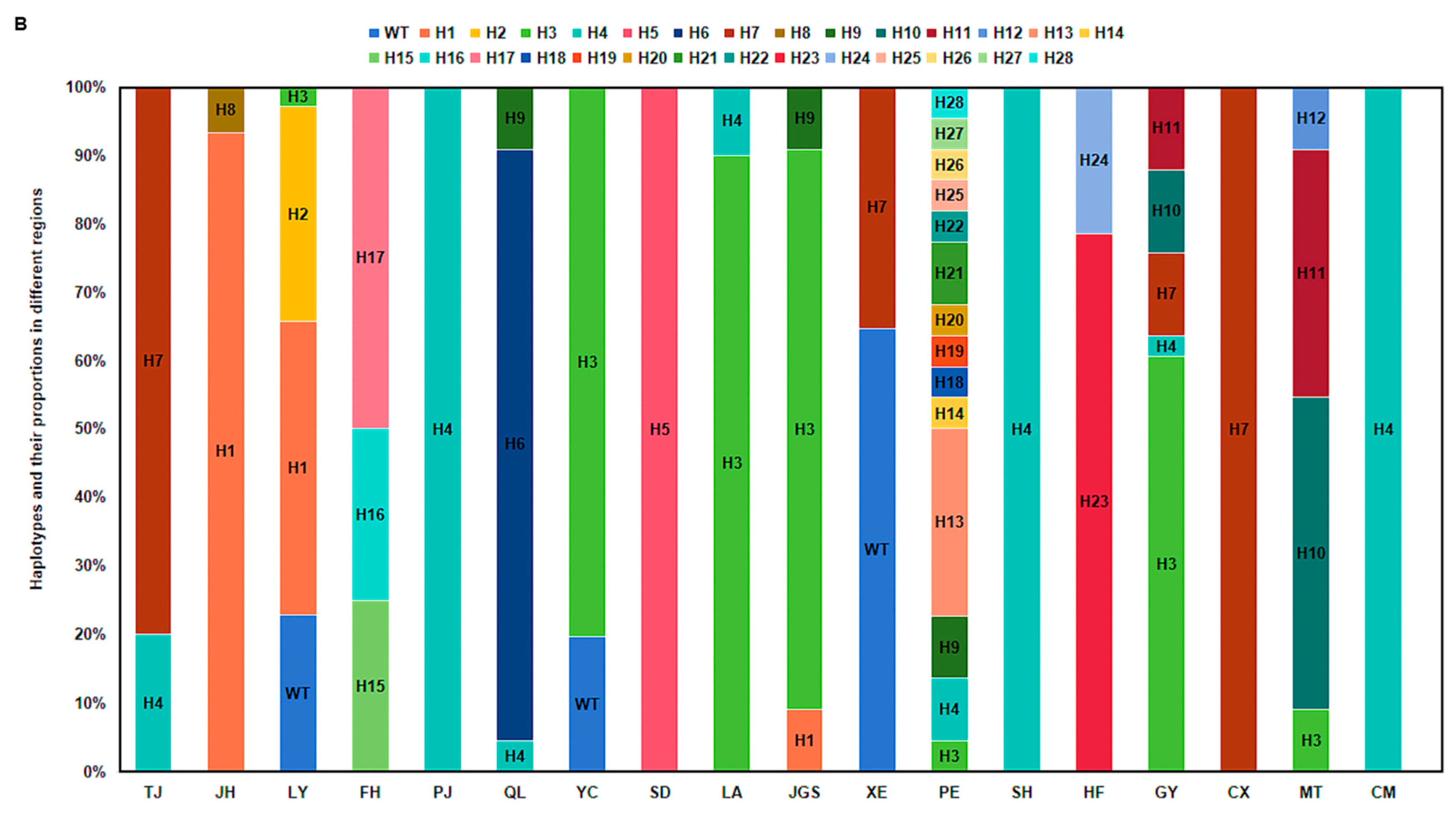
3.1. The Presence/Absence Variation of AVR-Pita1 in M. oryzae Strains from Southern China
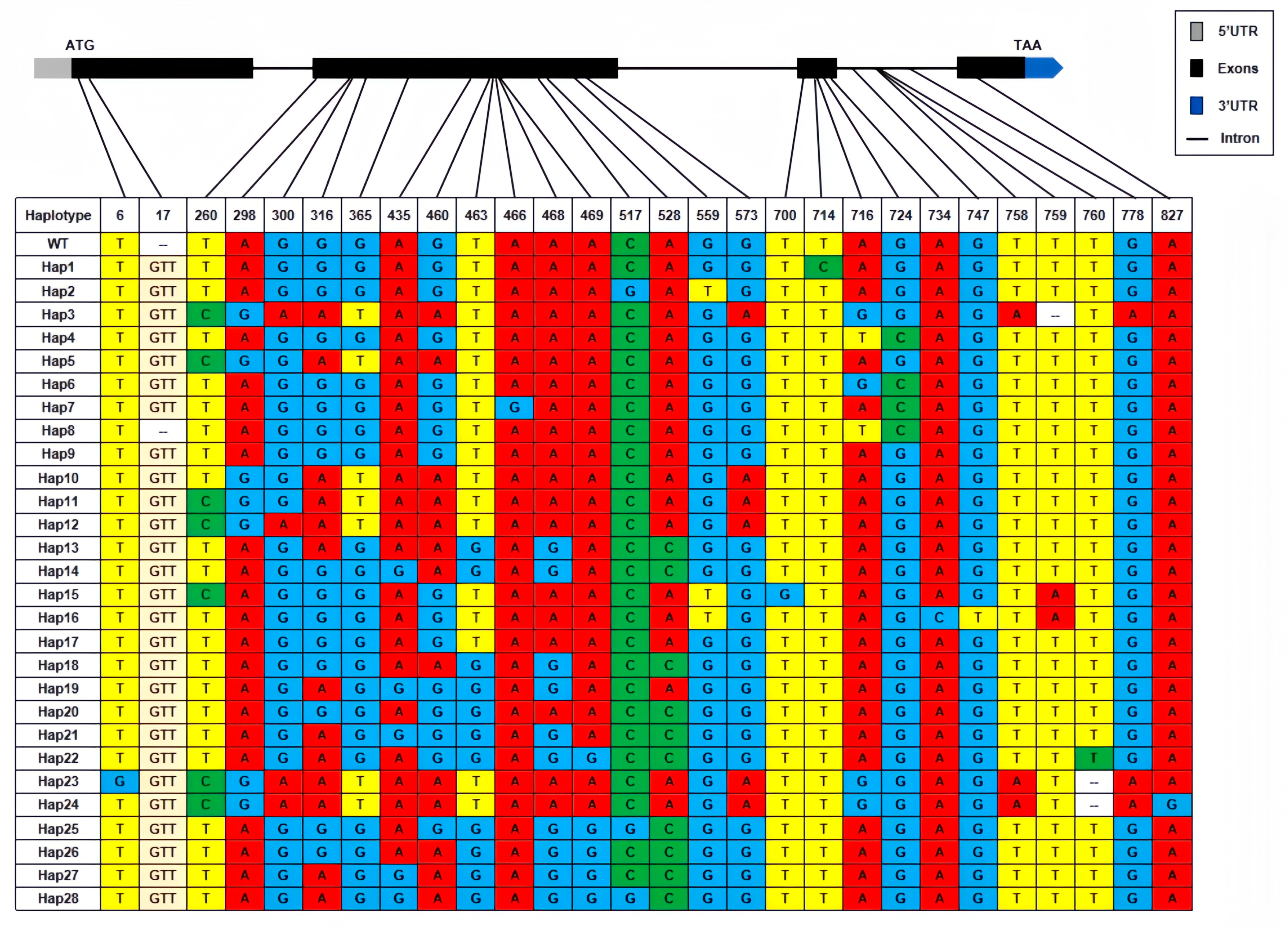
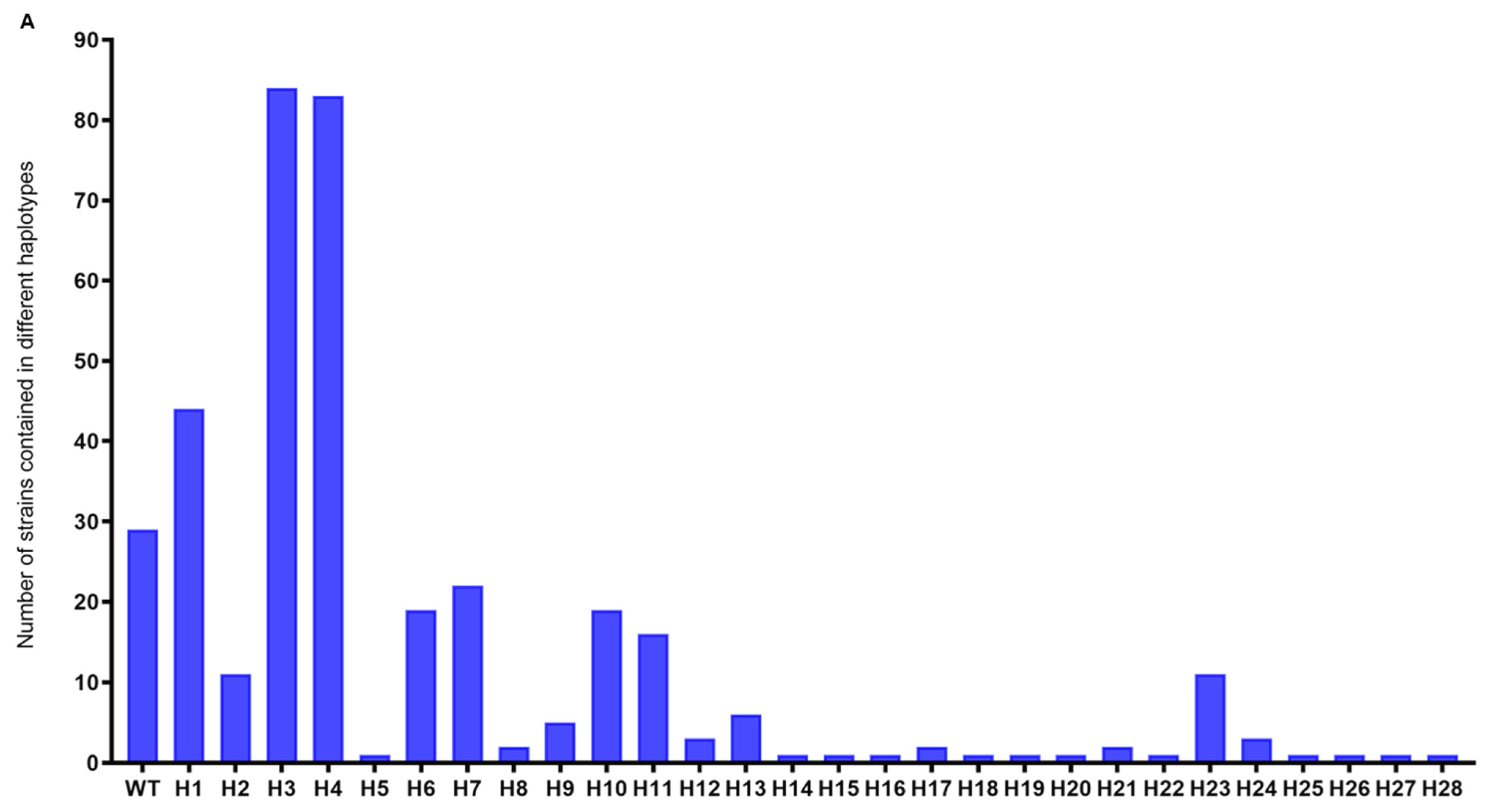
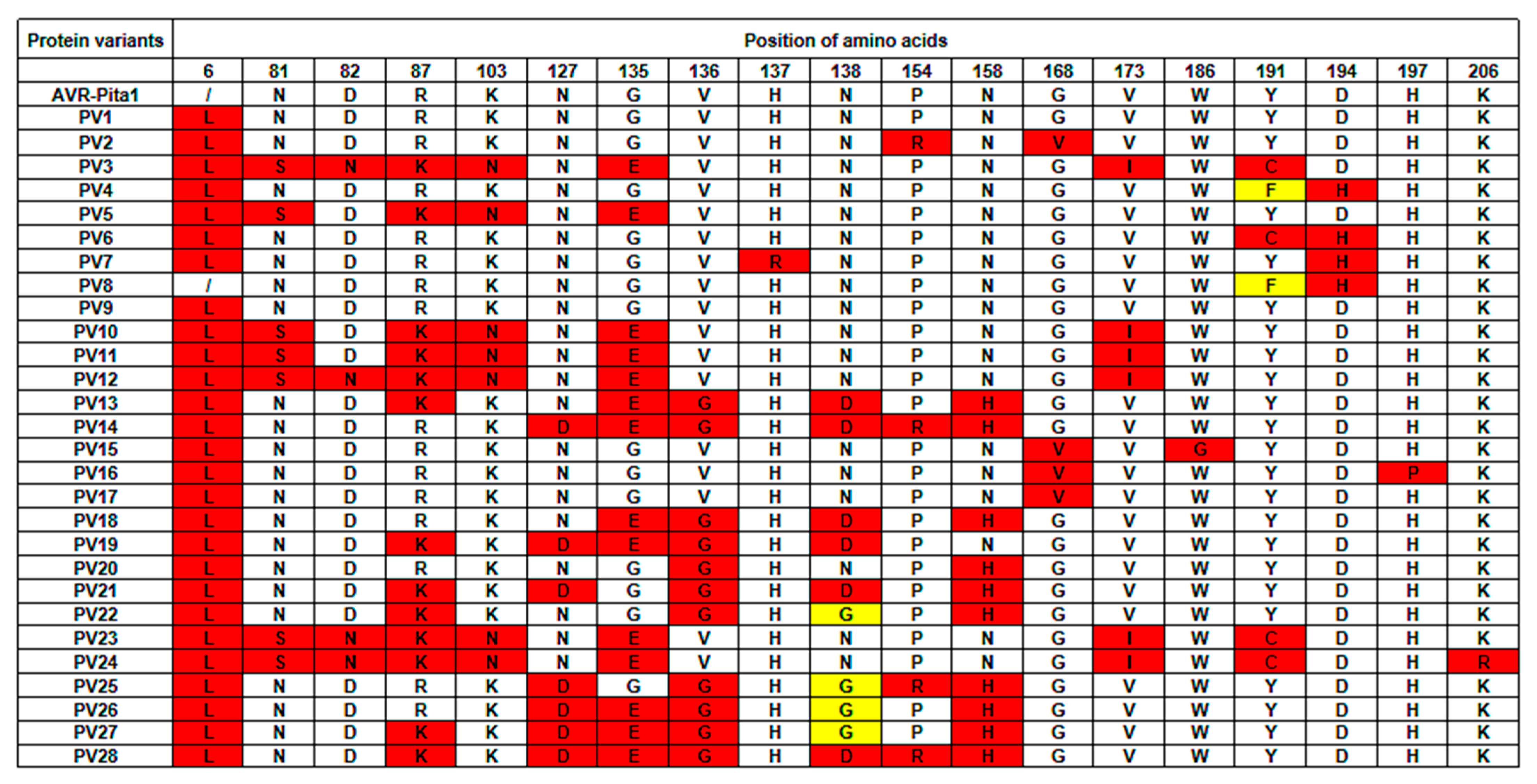
3.2. Nucleotide Diversity and the Phylogenetic Analysis of AVR-Pita1
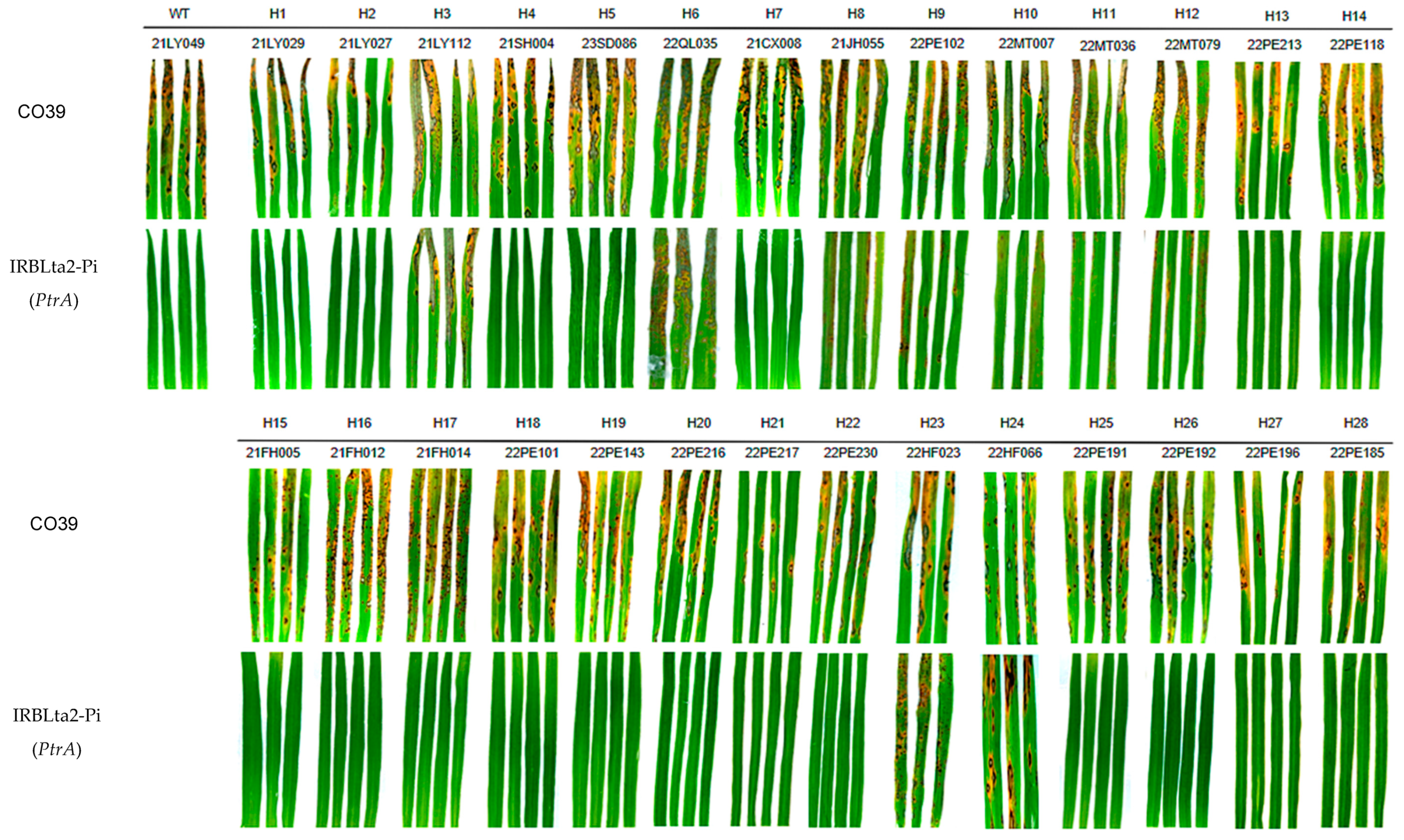
3.3. The Virulence Assay of Different AVR-Pita1 Haplotypes
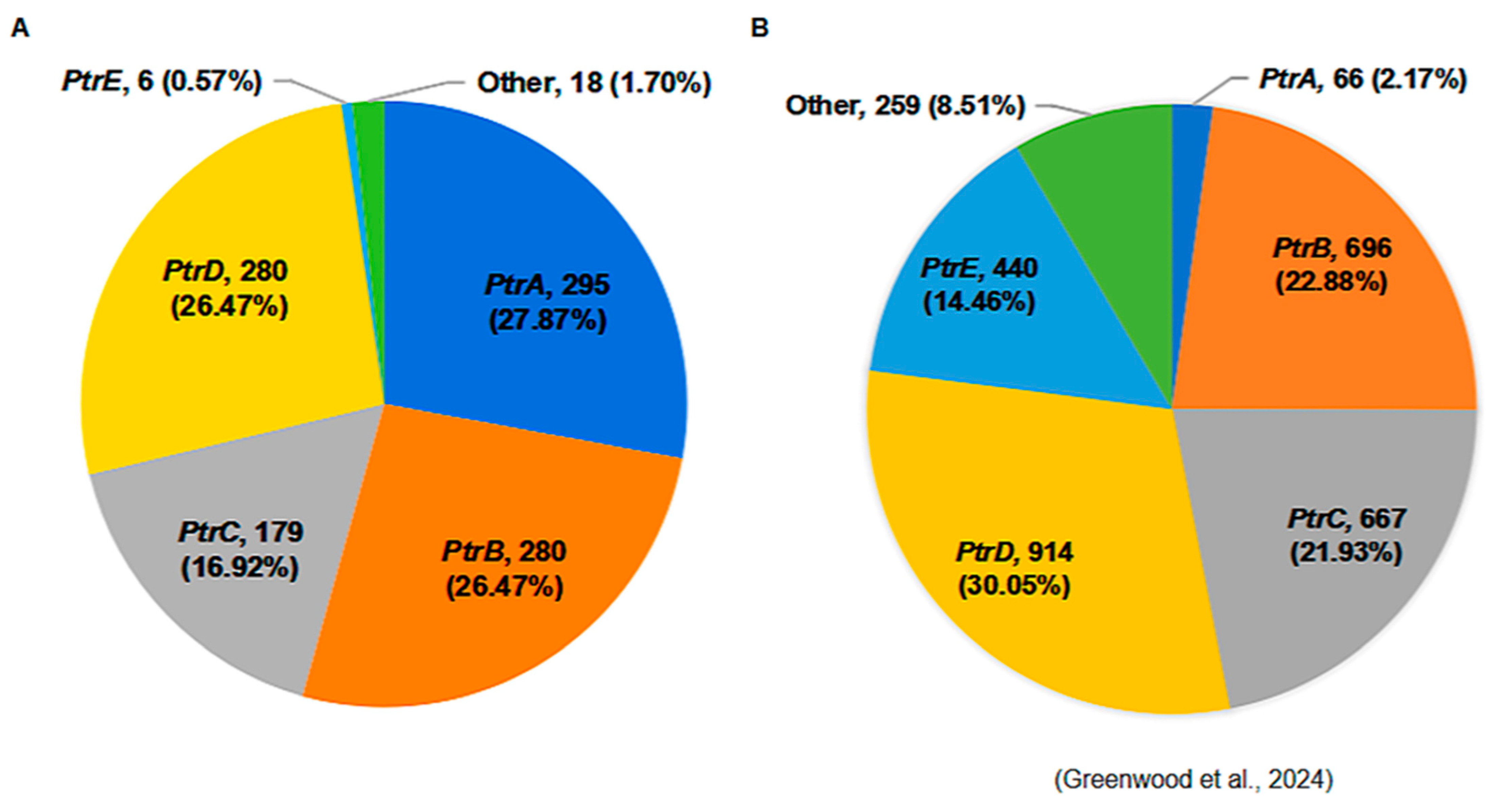
3.4. The Distribution of the Ptr Alleles in Elite Rice Parental Lines
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ou, S.H. A look at worldwide rice blast disease control. Plant Dis. 1980, 64, 439–445. [Google Scholar] [CrossRef]
- Dean, R.; van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Khush, G.S.; Jena, K.K. Current status and future prospects for research on blast resistance in rice (Oryza sativa L.). In Advances in Genetics, Genomics and Control of Rice Blast Disease, 1st ed.; Wang, G.-L., Valent, B., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 1–10. ISBN 978-1-4020-9499-6. [Google Scholar]
- Deng, Y.; Zhai, K.; Xie, Z.; Yang, D.; Zhu, X.; Liu, J.; Wang, X.; Qin, P.; Yang, Y.; Zhang, G.; et al. Epigenetic regulation of antagonistic receptors confers rice blast resistance with yield balance. Science 2017, 355, 962–965. [Google Scholar] [CrossRef]
- Fan, Y.; Ma, L.; Pan, X.; Tian, P.; Wang, W.; Liu, K.; Xiong, Z.; Li, C.; Wang, Z.; Wang, J.; et al. Genome-wide association study identifies rice panicle blast-resistant gene Pb4 encoding a wall-associated kinase. Int. J. Mol. Sci. 2024, 25, 830. [Google Scholar] [CrossRef]
- Liu, X.; Hu, X.; Tu, Z.; Sun, Z.; Qin, P.; Liu, Y.; Chen, X.; Li, Z.; Jiang, N.; Yang, Y. The roles of Magnaporthe oryzae avirulence effectors involved in blast resistance/susceptibility. Front. Plant Sci. 2024, 15, 1478159. [Google Scholar] [CrossRef]
- Wang, B.; Ebbole, D.J.; Wang, Z. The arms race between Magnaporthe oryzae and rice: Diversity and interaction of Avr and R genes. J. Integr. Agric. 2017, 16, 2746–2760. [Google Scholar] [CrossRef]
- Chaipanya, C.; Telebanco-Yanoria, M.J.; Quime, B.; Longya, A.; Korinsak, S.; Korinsak, S.; Toojinda, T.; Vanavichit, A.; Jantasuriyarat, C.; Zhou, B. Dissection of broad-spectrum resistance of the Thai rice variety Jao Hom Nin conferred by two resistance genes against rice blast. Rice 2017, 10, 18. [Google Scholar] [CrossRef]
- Wang, Z.; Jia, Y.; Rutger, J.N.; Xia, Y. Rapid survey for presence of a blast resistance gene Pi-ta in rice cultivars using the dominant DNA markers derived from portions of the Pi-ta gene. Plant Breed. 2007, 126, 36–42. [Google Scholar] [CrossRef]
- Skamnioti, P.; Gurr, S.J. Against the grain: Safeguarding rice from rice blast disease. Trends Biotechnol. 2009, 27, 141–150. [Google Scholar] [CrossRef]
- Flor, H.H. Current status of the gene-for-gene concept. Annu. Rev. Phytopathol. 1971, 9, 275–296. [Google Scholar] [CrossRef]
- Huang, J.; Si, W.; Deng, Q.; Li, P.; Yang, S. Rapid evolution of avirulence genes in rice blast fungus Magnaporthe oryzae. BMC Genet. 2014, 15, 45. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Ray, S.; Thakur, S.; Rathour, R.; Sharma, V.; Sharma, T.R. Co-evolutionary interactions between host resistance and pathogen avirulence genes in rice-Magnaporthe oryzae pathosystem. Fungal Genet Biol. 2018, 115, 9–19. [Google Scholar] [CrossRef]
- Kang, S.; Sweigard, J.A.; Valent, B. The PWL host specificity gene family in the blast fungus Magnaporthe grisea. Mol. Plant-Microbe Interact. 1995, 8, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Sweigard, J.A.; Carroll, A.M.; Kang, S.; Farrall, L.; Chumley, F.G.; Valent, B. Identification, cloning, and characterization of PWL2, a gene for host species specificity in the rice blast fungus. Plant Cell 1995, 7, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Orbach, M.J.; Farrall, L.; Sweigard, J.A.; Chumley, F.G.; Valent, B. A telomeric avirulence gene determines efficacy for the rice blast resistance gene Pi-ta. Plant Cell 2000, 12, 2019–2032. [Google Scholar] [CrossRef]
- Li, W.; Wang, B.; Wu, J.; Lu, G.; Hu, Y.; Zhang, X.; Zhang, Z.; Zhao, Q.; Feng, Q.; Zhang, H.; et al. The Magnaporthe oryzae avirulence gene AvrPiz-t encodes a predicted secreted protein that triggers the immunity in rice mediated by the blast resistance gene Piz-t. Mol. Plant-Microbe Interact. 2009, 22, 411–420. [Google Scholar] [CrossRef]
- Yoshida, K.; Saitoh, H.; Fujisawa, S.; Kanzaki, H.; Matsumura, H.; Yoshida, K.; Tosa, Y.; Chuma, I.; Takano, Y.; Win, J.; et al. Association genetics reveals three novel avirulence genes from the rice blast fungal pathogen Magnaporthe oryzae. Plant Cell 2009, 21, 1573–1591. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, L.; Wu, W.; He, L.; Yang, X.; Pan, Q. Function and evolution of Magnaporthe oryzae avirulence gene AvrPib responding to the rice blast resistance gene Pib. Sci. Rep. 2015, 5, 11642. [Google Scholar] [CrossRef]
- Wu, J.; Kou, Y.; Bao, J.; Li, Y.; Tang, M.; Zhu, X.; Ponaya, A.; Xiao, G.; Li, J.; Li, C.; et al. Comparative genomics identifies the Magnaporthe oryzae avirulence effector AvrPi9 that triggers Pi9-mediated blast resistance in rice. New Phytol. 2015, 206, 1463–1475. [Google Scholar] [CrossRef]
- Böhnert, H.U.; Fudal, I.; Dioh, W.; Tharreau, D.; Notteghem, J.L.; Lebrun, M.H. A putative polyketide synthase/peptide synthetase from Magnaporthe grisea signals pathogen attack to resistant rice. Plant Cell 2004, 16, 2499–2513. [Google Scholar] [CrossRef]
- Ray, S.; Singh, P.K.; Gupta, D.K.; Mahato, A.K.; Sarkar, C.; Rathour, R.; Singh, N.K.; Sharma, T.R. Analysis of Magnaporthe oryzae genome reveals a fungal effector, which is able to induce resistance response in transgenic rice line containing resistance gene, Pi54. Front. Plant Sci. 2016, 7, 1140. [Google Scholar] [CrossRef] [PubMed]
- Ribot, C.; Césari, S.; Abidi, I.; Chalvon, V.; Bournaud, C.; Vallet, J.; Lebrun, M.H.; Morel, J.B.; Kroj, T. The Magnaporthe oryzae effector AVR1-CO39 is translocated into rice cells independently of a fungal-derived machinery. Plant J. 2013, 74, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Vy, T.T.P.; Yoshida, K.; Asano, H.; Mitsuoka, C.; Asuke, S.; Anh, V.L.; Cumagun, C.J.R.; Chuma, I.; Terauchi, R.; et al. Evolution of the wheat blast fungus through functional losses in a host specificity determinant. Science 2017, 357, 80–83. [Google Scholar] [CrossRef]
- Shimizu, M.; Hirabuchi, A.; Sugihara, Y.; Abe, A.; Takeda, T.; Kobayashi, M.; Hiraka, Y.; Kanzaki, E.; Oikawa, K.; Saitoh, H.; et al. A genetically linked pair of NLR immune receptors shows contrasting patterns of evolution. Proc. Natl. Acad. Sci. USA 2022, 119, e2116896119. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, Y.; Abe, Y.; Takagi, H.; Abe, A.; Shimizu, M.; Ito, K.; Kanzaki, E.; Oikawa, K.; Kourelis, J.; Langner, T.; et al. Disentangling the complex gene interaction networks between rice and the blast fungus identifies a new pathogen effector. PLoS Biol. 2023, 21, e3001945. [Google Scholar] [CrossRef]
- Bryan, G.T.; Wu, K.S.; Farrall, L.; Jia, Y.; Hershey, H.P.; McAdams, S.A.; Faulk, K.N.; Donaldson, G.K.; Tarchini, R.; Valent, B. A single amino acid difference distinguishes resistant and susceptible alleles of the rice blast resistance gene Pi-ta. Plant Cell 2000, 12, 2033–2046. [Google Scholar] [CrossRef]
- Khang, C.H.; Park, S.Y.; Lee, Y.H.; Valent, B.; Kang, S. Genome organization and evolution of the AVR-Pita avirulence gene family in the Magnaporthe grisea species complex. Mol. Plant-Microbe Interact. 2008, 21, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, X.; Jia, Y.; Minkenberg, B.; Wheatley, M.; Fan, J.; Jia, M.H.; Famoso, A.; Edwards, J.D.; Wamishe, Y.; et al. The rice blast resistance gene Ptr encodes an atypical protein required for broad-spectrum disease resistance. Nat. Commun. 2018, 9, 2039. [Google Scholar] [CrossRef]
- Meng, X.; Xiao, G.; Telebanco-Yanoria, M.J.; Siazon, P.M.; Padilla, J.; Opulencia, R.; Bigirimana, J.; Habarugira, G.; Wu, J.; Li, M.; et al. The broad-spectrum rice blast resistance (R) gene Pita2 encodes a novel R protein unique from Pita. Rice 2020, 13, 19. [Google Scholar] [CrossRef]
- Kiyosawa, S. Inheritance of resistance of the rice variety Pi No. 4 to blast. Jpn. J. Breed. 1967, 17, 165–172. [Google Scholar] [CrossRef]
- Kiyosawa, S. Genetical approach to the biochemical nature of plant disease resistance. Jarq-Jpn. Agric. Res. Q. 1971, 6, 73–80. [Google Scholar]
- Rybka, K.; Miyamoto, M.; Ando, I.; Saito, A.; Kawasaki, S. High resolution mapping of the indica-derived rice blast resistance genes II. Pi-ta2 and Pi-ta and a consideration of their origin. Mol. Plant-Microbe Interact. 1997, 10, 517–524. [Google Scholar]
- Xiao, G.; Laksanavilat, N.; Cesari, S.; Lambou, K.; Baudin, M.; Jalilian, A.; Telebanco-Yanoria, M.J.; Chalvon, V.; Meusnier, I.; Fournier, E.; et al. The unconventional resistance protein PTR recognizes the Magnaporthe oryzae effector AVR-Pita in an allele-specific manner. Nat. Plants 2024, 10, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Moldenhauer, K.; Lee, F.; Norman, R.; Helms, R.; Wells, B.; Dilday, R.; Rohman, P.; Marchetti, M. Registration of ‘Katy’ rice. Crop Sci. 1990, 30, 747–748. [Google Scholar] [CrossRef]
- Jia, Y. Artificial introgression of a large chromosome fragment around the rice blast resistance gene Pi-ta in backcross progeny and several elite rice cultivars. Heredity 2009, 103, 333–339. [Google Scholar] [CrossRef]
- Wang, X.; Lee, S.; Wang, J.; Ma, J.; Bianco, T.; Jia, Y. Current advances on genetic resistance to rice blast disease. In Rice: Germplasm, Genetics and Improvement, 1st ed.; Wengui, Y., Ed.; Springer: Berlin, Germany, 2014; pp. 195–217. ISBN 978-953-51-1240-2. [Google Scholar]
- Li, J.; Lu, L.; Jia, Y.; Li, C. Effectiveness and durability of the rice Pi-ta gene in Yunnan province of China. Phytopathology 2014, 104, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Zhou, E.; Jia, Y.; Singh, P.; Correll, J.C.; Lee, F.N. Instability of the Magnaporthe oryzae avirulence gene AVR-Pita alters virulence. Fungal Genet. Biol. 2007, 44, 1024–1034. [Google Scholar] [CrossRef]
- Dai, Y.; Jia, Y.; Correll, J.; Wang, X.; Wang, Y. Diversification and evolution of the avirulence gene AVR-Pita1 in field isolates of Magnaporthe oryzae. Fungal Genet. Biol. 2010, 47, 973–980. [Google Scholar] [CrossRef]
- Valent, B.; Chumley, F.G. Molecular genetic analysis of the rice blast fungus, Magnaporthe grisea. Annu. Rev. Phytopathol. 1991, 29, 443–467. [Google Scholar] [CrossRef]
- Takahashi, M.; Ashizawa, T.; Hirayae, K.; Moriwaki, J.; Sone, T.; Sonoda, R.; Noguchi, M.T.; Nagashima, S.; Ishikawa, K.; Arai, M. One of two major paralogs of AVR-Pita1 is functional in Japanese rice blast isolates. Phytopathology 2010, 100, 612–618. [Google Scholar] [CrossRef]
- Kasetsomboon, T.; Kate-Ngam, S.; Sriwongchai, T.; Zhou, B.; Jantasuriyarat, C. Sequence variation of avirulence gene AVR-Pita1 in rice blast fungus, Magnaporthe oryzae. Mycol. Prog. 2013, 12, 617–628. [Google Scholar] [CrossRef]
- Kang, S.; Lebrun, M.H.; Farrall, L.; Valent, B. Gain of virulence caused by insertion of a Pot3 transposon in a Magnaporthe grisea avirulence gene. Mol. Plant-Microbe Interact. 2001, 14, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.J.; Huang, Y.Y.; Lin, X.Y.; Feng, H.; Zhou, S.X.; Xie, Y.; Liu, X.X.; Liu, C.; Zhao, R.M.; Zhao, W.S.; et al. Loss and natural variations of blast fungal avirulence genes breakdown rice resistance genes in the Sichuan Basin of China. Front. Plant Sci. 2022, 13, 788876. [Google Scholar] [CrossRef]
- Yasuda, Y. Climate change and the origin and development of rice cultivation in the Yangtze River basin, China. Ambio 2008, 37, 502–506. [Google Scholar] [CrossRef]
- Ma, X.; Wu, S.; Li, Y.e.; Zhang, X.; Gao, Q.; Wu, Y. Rice re-cultivation in southern China: An option for enhanced climate change resilience in rice production. J. Geogr. Sci. 2013, 23, 67–84. [Google Scholar] [CrossRef]
- Li, R.; Qiu, J.; Shi, H.; Jiang, N.; Lan, B.; Kou, Y. Variation of avirulence genes in the blast fungus from Jiangxi Province, China. Plant Dis. 2024; advance online publication. [Google Scholar] [CrossRef]
- Fei, L.W.; Lu, W.B.; Xu, X.Z.; Yan, F.C.; Zhang, L.W.; Liu, J.T.; Bai, Y.J.; Li, Z.Y.; Zhao, W.S.; Yang, J.; et al. A rapid approach for isolating a single fungal spore from rice blast diseased leaves. J. Integr. Agric. 2019, 18, 1415–1418. [Google Scholar] [CrossRef]
- Harmon, P.F.; Dunkle, L.D.; Latin, R. A rapid PCR-based method for the detection of Magnaporthe oryzae from infected perennial ryegrass. Plant Dis. 2003, 87, 1072–1076. [Google Scholar] [CrossRef] [PubMed]
- Telebanco-Yanoria, M.J.; Koide, Y.; Fukuta, Y.; Imbe, T.; Tsunematsu, H.; Kato, H.; Ebron, L.A.; Nguyen, T.M.N. A set of near-isogenic lines of Indica-type rice variety CO39 as differential varieties for blast resistance. Mol. Breeding 2011, 27, 357–373. [Google Scholar] [CrossRef]
- Standard Evaluation System for Rice (SES). Available online: http://www.knowledgebank.irri.org/images/docs/rice-standard-evaluation-system.pdf (accessed on 4 April 2025).
- Longya, A.; Chaipanya, C.; Franceschetti, M.; Maidment, J.H.R.; Banfield, M.J.; Jantasuriyarat, C. Gene duplication and mutation in the emergence of a novel aggressive allele of the AVR-Pik effector in the rice blast fungus. Mol. Plant-Microbe Interact. 2019, 32, 740–749. [Google Scholar] [CrossRef]
- Greenwood, J.R.; Lacorte-Apostol, V.; Kroj, T.; Padilla, J.; Telebanco-Yanoria, M.J.; Glaus, A.N.; Roulin, A.; Padilla, A.; Zhou, B.; Keller, B.; et al. Genome-wide association analysis uncovers rice blast resistance alleles of Ptr and Pia. Commun. Biol. 2024, 7, 607. [Google Scholar] [CrossRef]
- Damchuay, K.; Longya, A.; Sriwongchai, T.; Songkumarn, P.; Parinthawong, N.; Darwell, K.; Talumphai, S.; Tasanasuwan, P.; Jantasuriyarat, C. High nucleotide sequence variation of avirulent gene, AVR-Pita1, in Thai rice blast fungus population. J. Genet. 2020, 99, 45. [Google Scholar] [CrossRef]
- Wang, L.; Zhong, D.; Chen, X.; Niu, Z.; Cao, Q. Impact of climate change on rice growth and yield in China: Analysis based on climate year type. Geogr. Sustain. 2024, 5, 548–560. [Google Scholar] [CrossRef]
- Ji, Y.; Zhou, Y.; Li, Z.; Feng, K.; Sun, X.; Xu, Y.; Wu, W.; Zou, H. Carbon footprint research and mitigation strategies for rice-cropping systems in China: A review. Front. Sustain. Food Syst. 2024, 8, 1375092. [Google Scholar] [CrossRef]
- Hang, S.; Wang, Q.; Wang, Y.; Xiang, H. Evolution of rice cultivar performance across China: A multi-dimensional study on yield and agronomic characteristics over three decades. Agronomy 2024, 14, 2780. [Google Scholar] [CrossRef]
- Hu, H.W.; Chen, Q.L.; He, J.Z. The end of hunger: Fertilizers, microbes and plant productivity. Microb. Biotechnol. 2022, 15, 1050–1054. [Google Scholar] [CrossRef]
- Sutthiphai, T.; Damchuay, K.; Neupane, R.C.; Longya, A.; Sriwongchai, T.; Songkumarn, P.; Parinthawong, N.; Darwell, K.; Jantasuriyarat, C. Genetic variation of avirulence genes (AVR-Pi9, AVR-Pik, AVR-Pita1) and genetic diversity of rice blast fungus, Pyricularia oryzae, in Thailand. Plant Pathol. 2022, 71, 322–333. [Google Scholar] [CrossRef]
- Trang, H.T.T.; Chau, N.N.B.; Thao, N.P.; Jantasuriyarat, C.; Quoc, N.B. Analyzing sequence variation of the avirulence Avr-Pita1 gene of rice blast isolates, Magnaporthe oryzae in vietnam. Agric. Nat. Resour. 2019, 53, 20–25. [Google Scholar]
- Saleh, D.; Milazzo, J.; Adreit, H.; Fournier, E.; Tharreau, D. South-East Asia is the center of origin, diversity and dispersion of the rice blast fungus, Magnaporthe oryzae. New Phytol. 2014, 201, 1440–1456. [Google Scholar] [CrossRef]
- Ceresini, P.C.; Castroagudín, V.L.; Rodrigues, F.Á.; Rios, J.A.; Eduardo Aucique-Pérez, C.; Moreira, S.I.; Alves, E.; Croll, D.; Maciel, J.L.N. Wheat blast: Past, present, and future. Annu. Rev. Phytopathol. 2018, 56, 427–456. [Google Scholar] [CrossRef]
- Gladieux, P.; Ravel, S.; Rieux, A.; Cros-Arteil, S.; Adreit, H.; Milazzo, J.; Thierry, M.; Fournier, E.; Terauchi, R.; Tharreau, D. Coexistence of multiple endemic and pandemic lineages of the rice blast pathogen. mBio 2018, 9, e01806-17. [Google Scholar] [CrossRef]
- Zhong, Z.; Chen, M.; Lin, L.; Han, Y.; Bao, J.; Tang, W.; Lin, L.; Lin, Y.; Somai, R.; Lu, L.; et al. Population genomic analysis of the rice blast fungus reveals specific events associated with expansion of three main clades. ISME J. 2018, 12, 1867–1878. [Google Scholar] [CrossRef] [PubMed]
- Latorre, S.M.; Reyes-Avila, C.S.; Malmgren, A.; Win, J.; Kamoun, S.; Burbano, H.A. Differential loss of effector genes in three recently expanded pandemic clonal lineages of the rice blast fungus. BMC Biol. 2020, 18, 88. [Google Scholar] [CrossRef] [PubMed]
- Thierry, M.; Charriat, F.; Milazzo, J.; Adreit, H.; Ravel, S.; Cros-Arteil, S.; Borron, S.; Sella, V.; Kroj, T.; Ioos, R.; et al. Maintenance of divergent lineages of the Rice Blast Fungus Pyricularia oryzae through niche separation, loss of sex and post-mating genetic incompatibilities. PLoS Pathog. 2022, 18, e1010687. [Google Scholar] [CrossRef]
- Qi, Z.; Ju, F.; Guo, Y.; Du, Y.; Yu, J.; Zhang, R.; Yu, M.; Cao, H.; Song, T.; Pan, X.; et al. A rapid, equipment-free method for detecting avirulence genes of Pyricularia oryzae using a lateral flow strip-based RPA assay. Plant Dis. 2024, 108, 2283–2290. [Google Scholar] [CrossRef]
- Bao, J.; Wang, Z.; Chen, M.; Chen, S.; Chen, X.; Xie, J.; Tang, W.; Zheng, H.; Wang, Z. Pan-genomics reveals a new variation pattern of secreted proteins in Pyricularia oryzae. J. Fungi 2022, 8, 1238. [Google Scholar] [CrossRef]
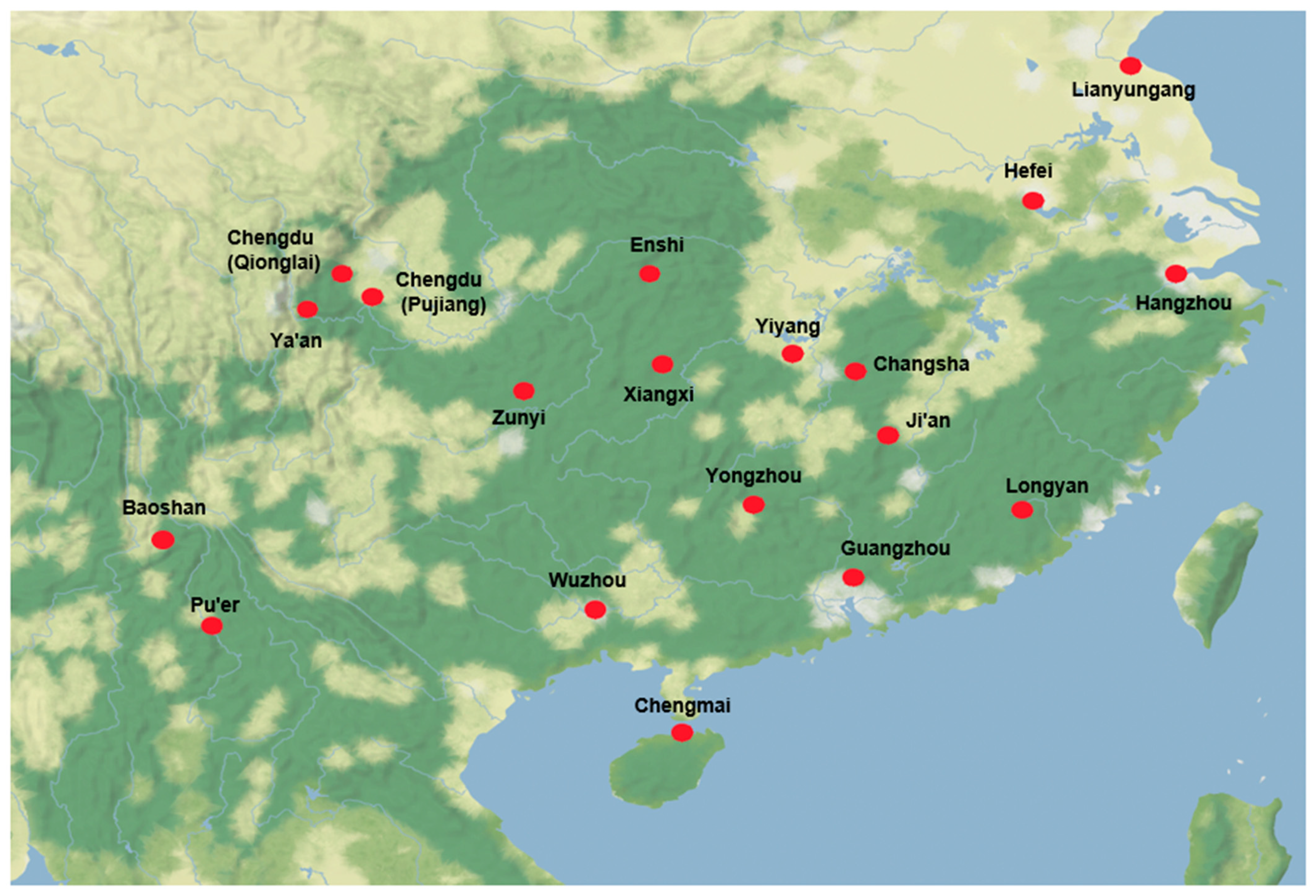

| Province | City/Prefecture | County/District | Abbreviation | Ecological Zone |
|---|---|---|---|---|
| Hunan | Changsha | Liuyang | LY | The middle and lower reaches of the Yangtze River |
| Yiyang | Taojiang | TJ | The middle and lower reaches of the Yangtze River | |
| Xiangxi | Fenghuang | FH | Wuling Mountainous Area | |
| Yongzhou | Jianghua | JH | The middle and lower reaches of the Yangtze River | |
| Sichuan | Chengdu | Pujiang | PJ | The upper reaches of the Yangtze River |
| Qionglai | QL | The upper reaches of the Yangtze River | ||
| Ya’an | Yucheng | YC | The upper reaches of the Yangtze River | |
| Jiangsu | Lianyungang | Ganyu | GY | The middle and lower reaches of the Yangtze River |
| Zhejiang | Hangzhou | Lin’an | LA | The middle and lower reaches of the Yangtze River |
| Anhui | Hefei | / | HF | The middle and lower reaches of the Yangtze River |
| Fujian | Longyan | Shanghang | SH | South China |
| Guangdong | Guangzhou | Conghua | CH | South China |
| Guangxi | Wuzhou | Cenxi | CX | South China |
| Jiangxi | Ji’an | Jinggangshan | JGS | The middle and lower reaches of the Yangtze River |
| Hubei | Enshi | Xuan’en | XE | Wuling Mountainous Area |
| Hainan | / | Chengmai | CM | South China |
| Yunnan | Pu’er | / | PE | The upper reaches of the Yangtze River |
| Baoshan | ShiDian | SD | The upper reaches of the Yangtze River | |
| Guizhou | Zunyi | Meitan | MT | The upper reaches of the Yangtze River |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Liu, X.; Hu, X.; Tu, Z.; Fu, J.; Zhong, L.; Jiang, N.; Yang, Y. The Geographic Distribution and Natural Variation of the Rice Blast Fungus Avirulence Gene AVR-Pita1 in Southern China. Plants 2025, 14, 1210. https://doi.org/10.3390/plants14081210
Chen X, Liu X, Hu X, Tu Z, Fu J, Zhong L, Jiang N, Yang Y. The Geographic Distribution and Natural Variation of the Rice Blast Fungus Avirulence Gene AVR-Pita1 in Southern China. Plants. 2025; 14(8):1210. https://doi.org/10.3390/plants14081210
Chicago/Turabian StyleChen, Xinwei, Xin Liu, Xiaochun Hu, Zhouyi Tu, Jun Fu, Liping Zhong, Nan Jiang, and Yuanzhu Yang. 2025. "The Geographic Distribution and Natural Variation of the Rice Blast Fungus Avirulence Gene AVR-Pita1 in Southern China" Plants 14, no. 8: 1210. https://doi.org/10.3390/plants14081210
APA StyleChen, X., Liu, X., Hu, X., Tu, Z., Fu, J., Zhong, L., Jiang, N., & Yang, Y. (2025). The Geographic Distribution and Natural Variation of the Rice Blast Fungus Avirulence Gene AVR-Pita1 in Southern China. Plants, 14(8), 1210. https://doi.org/10.3390/plants14081210






