Exploring the Role of TaERF4a in Enhancing Drought Tolerance and Regulating Dehydrin WZY1-2 Gene Expression in Wheat
Abstract
:1. Introduction
2. Results
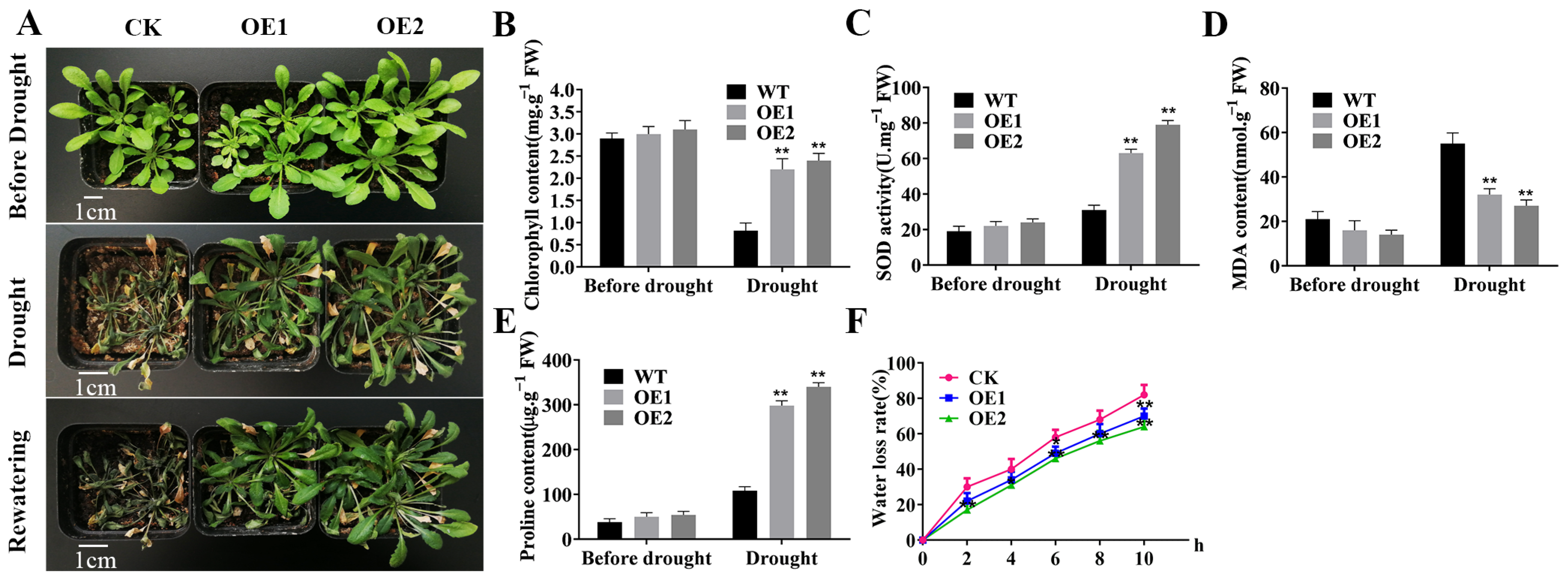
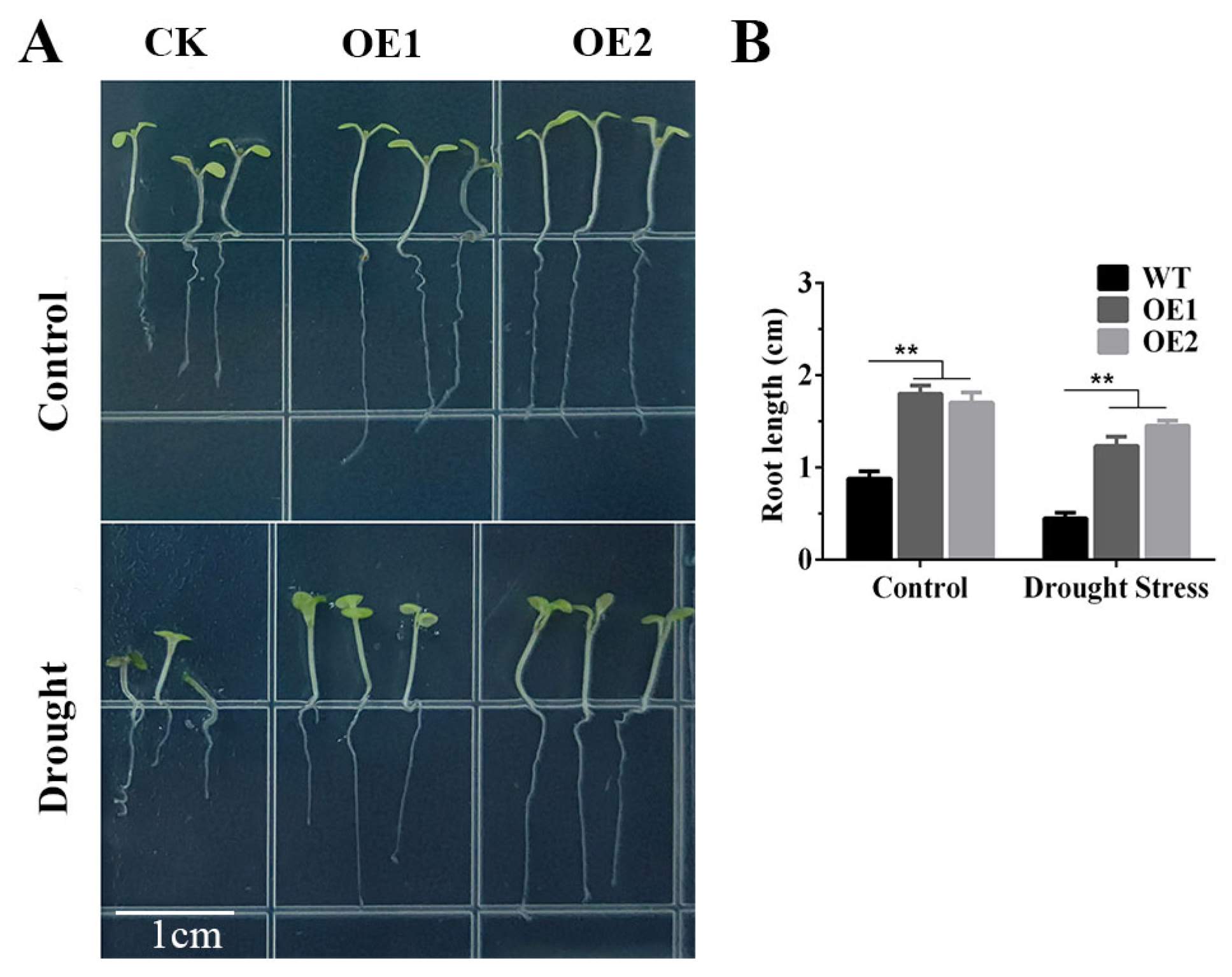
2.1. Overexpression of TaERF4a Enhances Drought Tolerance in Transgenic Arabidopsis thaliana
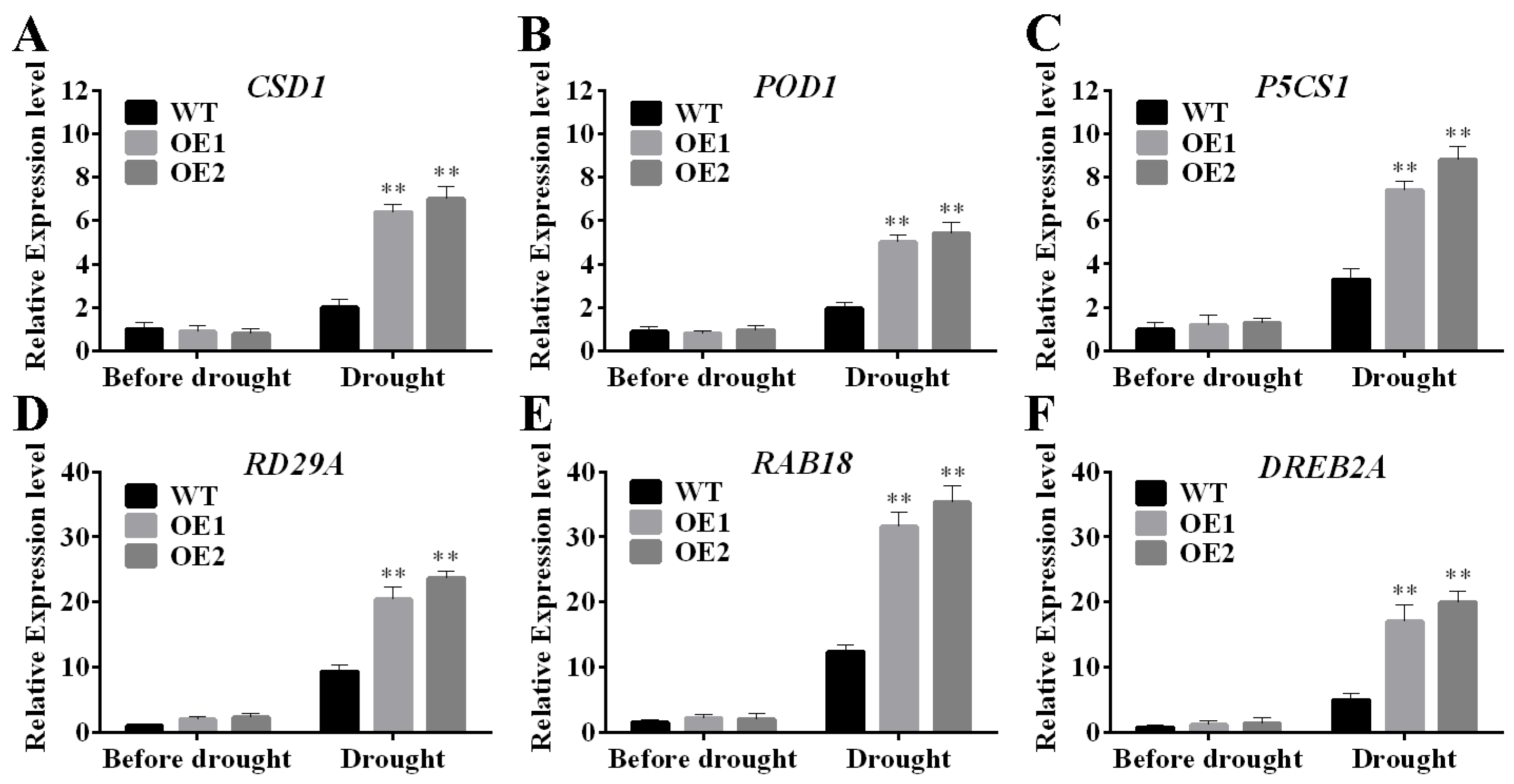
2.2. Measurement of Physiological Indices in TaERF4a-Overexpressing Plants Under Drought Stress
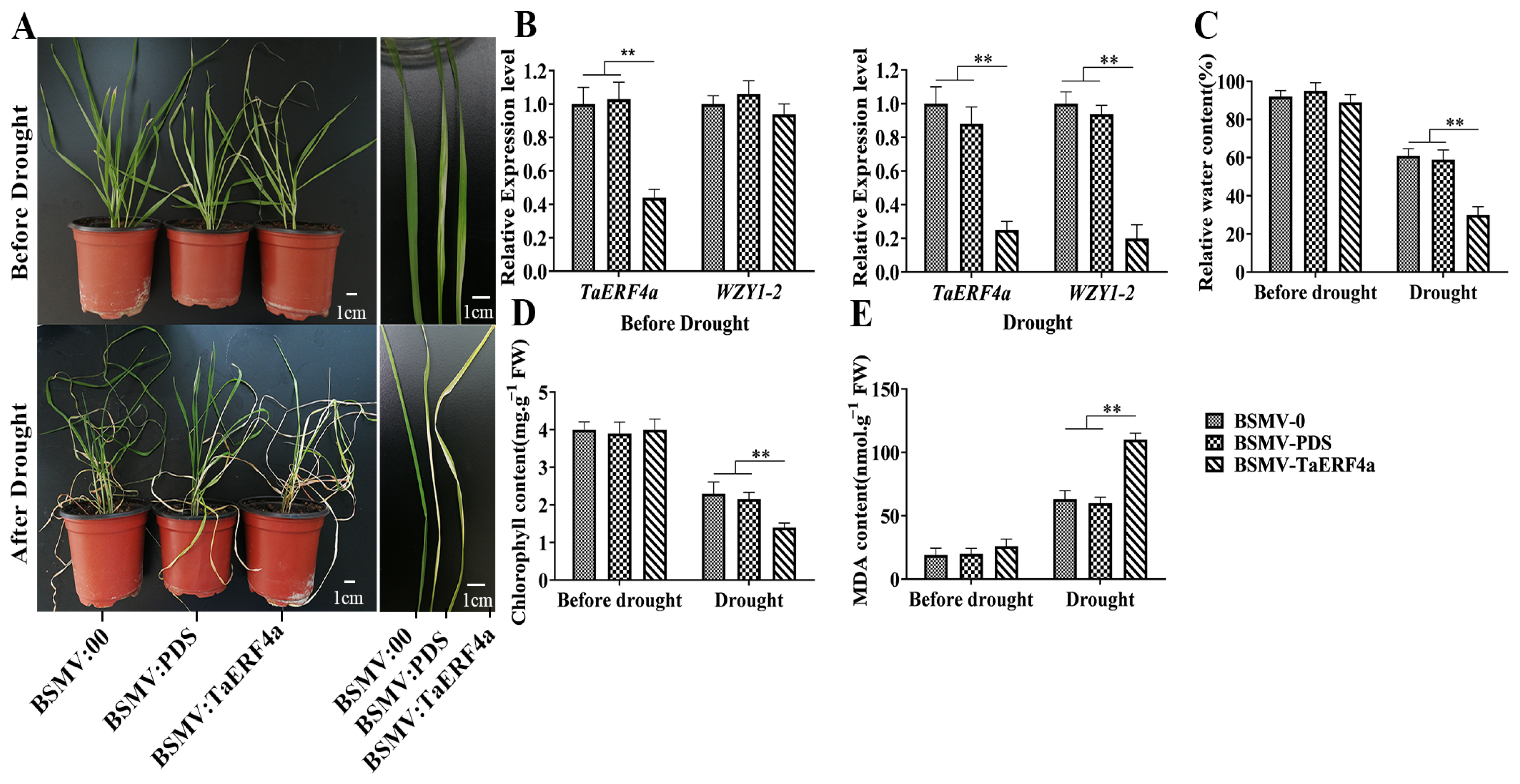
2.3. Silencing of the TaERF4a Gene Affects the Phenotype and Physiological Indices of Wheat Under Drought Stress Treatment
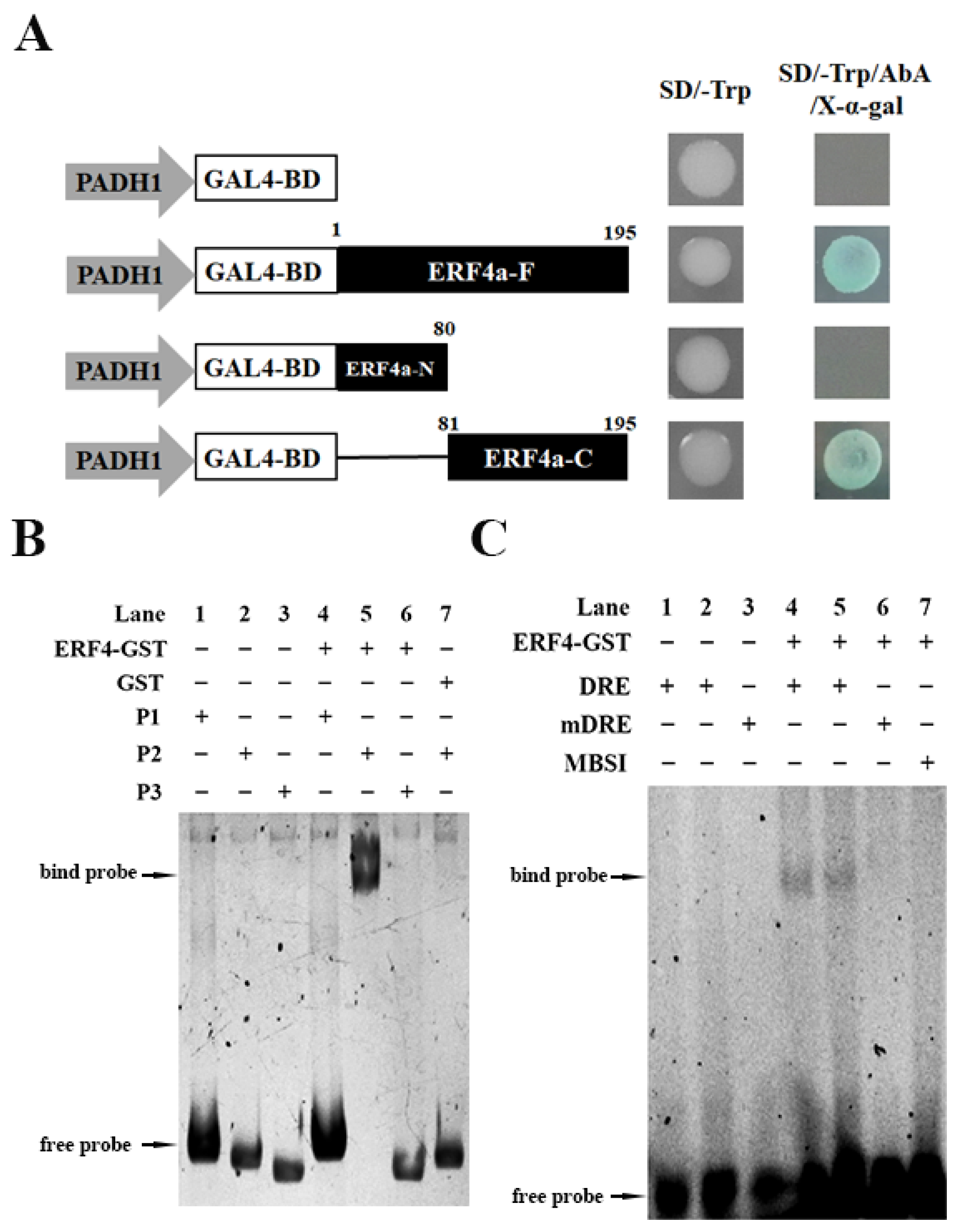
2.4. Transactivation Analysis of TaERF4a Protein
2.5. EMSA Analysis of TaERF4a Binding to the WZY1-2 Promoter
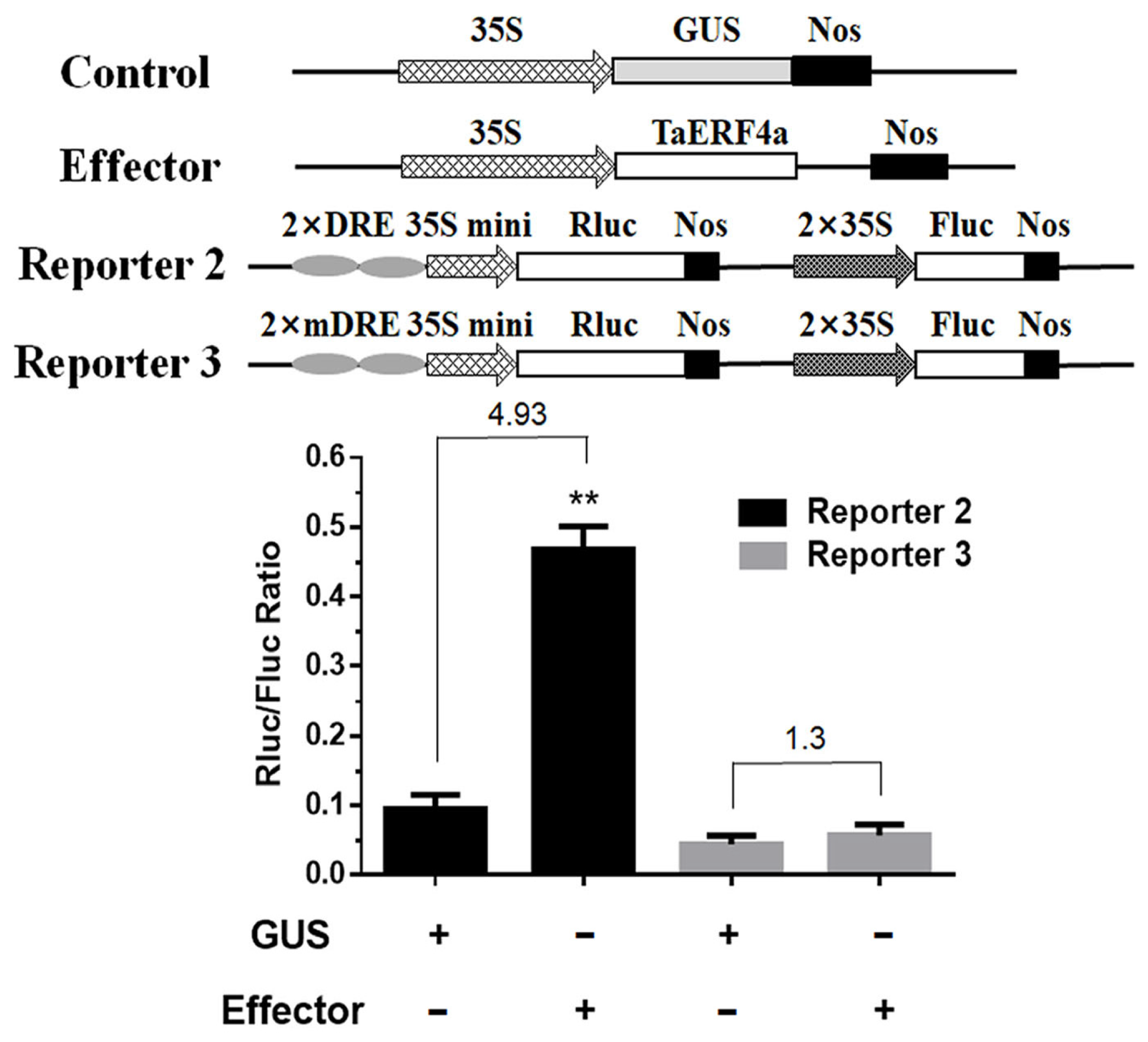
2.6. Dual-Luciferase Assay of TaERF4a in Plants
3. Discussion
4. Materials and Methods
4.1. Plant Growth Conditions
4.2. Acquisition of TaERF4a-Overexpressing Transgenic Arabidopsis thaliana
4.3. VIGS-Mediated TaERF4a Gene Silencing in Wheat
4.4. Quantitative Real-Time PCR
4.5. Performance of TaERF4a-Overexpressing Arabidopsis thaliana Plants Under Drought Conditions
4.6. Measurements of Malondialdehyde (MDA), Water Loss Rate, and Chlorophyll Content Physiological Indices
4.7. Transactivation Analysis of TaERF4a
4.8. Electrophoretic Mobility Shift Assay
4.9. Dual-Luciferase Transient Expression Assay
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Rorat, T.; Szabala, B.M.; Grygorowicz, W.J.; Wojtowicz, B.; Yin, Z.; Rey, P. Expression of SK3-type dehydrin in transporting organs is associated with cold acclimation in Solanum species. Planta 2006, 224, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wang, X.; Zhang, L. Structural and Functional Dynamics of Dehydrins: A Plant Protector Protein under Abiotic Stress. Int. J. Mol. Sci. 2018, 19, 3420. [Google Scholar] [CrossRef]
- Nylander, M.; Svensson, J.; Palva, E.T.; Welin, B.V. Stress-induced accumulation and tissue-specific localization of dehydrins in Arabidopsis thaliana. Plant Mol. Biol. 2001, 45, 263–279. [Google Scholar] [CrossRef]
- Jiang, A.; Liu, X.; Zhu, Z.; Chen, M. Genome-wide identification of the AP2/ERF gene family from Limonium bicolor and functional characterization of LbAP2/ERF32 under salt stress. Plant Physiol. Biochem. 2024, 215, 109035. [Google Scholar] [CrossRef] [PubMed]
- Licausi, F.; Ohme-Takagi, M.; Perata, P. APETALA2/Ethylene Responsive Factor (AP2/ERF) transcription factors: Mediators of stress responses and developmental programs. New Phytol. 2013, 199, 639–649. [Google Scholar] [CrossRef]
- Lakhwani, D.; Pandey, A.; Dhar, Y.V.; Bag, S.K.; Trivedi, P.K.; Asif, M.H. Genome-wide analysis of the AP2/ERF family in Musa species reveals divergence and neofunctionalisation during evolution. Sci. Rep. 2016, 6, 18878. [Google Scholar] [CrossRef]
- Xie, Z.; Nolan, T.M.; Jiang, H.; Yin, Y. AP2/ERF Transcription Factor Regulatory Networks in Hormone and Abiotic Stress Responses in Arabidopsis. Front. Plant Sci. 2019, 10, 228. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Luo, T.; Zhao, H.; Su, Y.; Ji, W.; Li, H. Identification of wheat DREB genes and functional characterization of TaDREB3 in response to abiotic stresses. Gene 2020, 740, 144514. [Google Scholar] [CrossRef]
- Dubouzet, J.G.; Sakuma, Y.; Ito, Y.; Kasuga, M.; Dubouzet, E.G.; Miura, S.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. OsDREB genes in rice, Oryza sativa L., encode transcription activators that function in drought-, high-salt- and cold-responsive gene expression. Plant J. 2003, 33, 751–763. [Google Scholar] [CrossRef]
- Huang, B.; Liu, J.Y. Cloning and functional analysis of the novel gene GhDBP3 encoding a DRE-binding transcription factor from Gossypium hirsutum. Biochim. Biophys. Acta 2006, 1759, 263–269. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Q.Y.; Cheng, X.G.; Xu, Z.S.; Li, L.C.; Ye, X.G.; Xia, L.Q.; Ma, Y.Z. GmDREB2, a soybean DRE-binding transcription factor, conferred drought and high-salt tolerance in transgenic plants. Biochem. Biophys. Res. Commun. 2007, 353, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.C.; Liao, P.M.; Kuo, W.W.; Lin, T.P. The Arabidopsis ETHYLENE RESPONSE FACTOR1 regulates abiotic stress-responsive gene expression by binding to different cis-acting elements in response to different stress signals. Plant Physiol. 2013, 162, 1566–1582. [Google Scholar] [CrossRef]
- Hong, Y.; Xiao, Y.; Song, N.; Zhu, S.; Zhao, R.; Li, K.; Geng, M.; Yu, X.; Wang, H.; Xia, W.; et al. Identification and characterization of MeERF genes and their targets in pathogen response by cassava (Manihot esculenta). Crop J. 2021, 9, 1145–1153. [Google Scholar] [CrossRef]
- McGrath, K.C.; Dombrecht, B.; Manners, J.M.; Schenk, P.M.; Edgar, C.I.; Maclean, D.J.; Scheible, W.R.; Udvardi, M.K.; Kazan, K. Repressor- and activator-type ethylene response factors functioning in jasmonate signaling and disease resistance identified via a genome-wide screen of Arabidopsis transcription factor gene expression. Plant Physiol. 2005, 139, 949–959. [Google Scholar] [CrossRef]
- Liu, H.; Yang, Y.; Liu, D.; Wang, X.; Zhang, L. Transcription factor TabHLH49 positively regulates dehydrin WZY2 gene expression and enhances drought stress tolerance in wheat. Bmc Plant Biol. 2020, 20, 259. [Google Scholar] [CrossRef]
- Vazquez-Hernandez, M.; Romero, I.; Escribano, M.I.; Merodio, C.; Sanchez-Ballesta, M.T. Deciphering the Role of CBF/DREB Transcription Factors and Dehydrins in Maintaining the Quality of Table Grapes cv. Autumn Royal Treated with High CO2 Levels and Stored at 0 °C. Front. Plant Sci. 2017, 8, 1591. [Google Scholar] [CrossRef] [PubMed]
- Lv, A.; Wen, W.; Fan, N.; Su, L.; Zhou, P.; An, Y. Dehydrin MsDHN1 improves aluminum tolerance of alfalfa (Medicago sativa L.) by affecting oxalate exudation from root tips. Plant J. 2021, 108, 441–458. [Google Scholar] [CrossRef]
- Zong, W.; Yang, J.; Fu, J.; Xiong, L. Synergistic regulation of drought-responsive genes by transcription factor OsbZIP23 and histone modification in rice. J. Integr. Plant Biol. 2020, 62, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zheng, L.; Wang, Y.; Zhou, Y.; Gao, F. AmDHN4, a winter accumulated SKn-type dehydrin from Ammopiptanthus mongolicus, and regulated by AmWRKY45, enhances the tolerance of Arabidopsis to low temperature and osmotic stress. Int. J. Biol. Macromol. 2024, 266, 131020. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, H.; Wang, A.; Zhang, L. An ERF-type transcription factor is involved in the regulation of the dehydrin wzy1-2 gene in wheat. Plant Signal. Behav. 2020, 15, 1778920. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C.; Qin, L.; Liu, W.; Wang, Y. ThERF1 regulates its target genes via binding to a novel cis-acting element in response to salt stress. J. Integr. Plant Biol. 2015, 57, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Y.; Wu, M.; Li, L.; Li, C.; Han, Z.; Yuan, J.; Chen, C.; Song, W.; Wang, C. Genome-Wide Identification of AP2/ERF Transcription Factors in Cauliflower and Expression Profiling of the ERF Family under Salt and Drought Stresses. Front. Plant Sci. 2017, 8, 946. [Google Scholar] [CrossRef]
- Weining, Z.; Dapeng, Z.; Xuanxuan, L.; Linsheng, Z.; Zhengyang, Y.; Hui, L.; Hongmei, Z. Characterisation of an SKn-type Dehydrin Promoter from Wheat and Its Responsiveness to Various Abiotic and Biotic Stresses. Plant Mol. Biol. Rep. 2014, 32, 664–678. [Google Scholar]
- Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AP2/ERF family transcription factors in plant abiotic stress responses. Biochim. Biophys. Acta 2012, 1819, 86–96. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, A.; Tewari, K.; Garg, N.K.; Changan, S.S.; Tyagi, A. Isolation and characterization of drought and ABA responsive promoter of a transcription factor encoding gene from rice. Physiol. Mol. Biol. Plants 2022, 28, 1813–1831. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Ma, Z.; Hu, L.; Huang, K.; Zhang, M.; Zhang, S.; Jiang, W.; Wu, T.; Du, X. Involvement of rice transcription factor OsERF19 in response to ABA and salt stress responses. Plant Physiol. Biochem. 2021, 167, 22–30. [Google Scholar] [CrossRef]
- Xu, Z.S.; Xia, L.Q.; Chen, M.; Cheng, X.G.; Zhang, R.Y.; Li, L.C.; Zhao, Y.X.; Lu, Y.; Ni, Z.Y.; Liu, L.; et al. Isolation and molecular characterization of the Triticum aestivum L. ethylene-responsive factor 1 (TaERF1) that increases multiple stress tolerance. Plant Mol. Biol. 2007, 65, 719–732. [Google Scholar] [CrossRef]
- Karlova, R.; Boer, D.; Hayes, S.; Testerink, C. Root plasticity under abiotic stress. Plant Physiol. 2021, 187, 1057–1070. [Google Scholar] [CrossRef]
- Lv, K.; Li, J.; Zhao, K.; Chen, S.; Nie, J.; Zhang, W.; Liu, G.; Wei, H. Overexpression of an AP2/ERF family gene, BpERF13, in birch enhances cold tolerance through upregulating CBF genes and mitigating reactive oxygen species. Plant Sci. 2020, 292, 110375. [Google Scholar] [CrossRef]
- Li, M.; Liu, J.; Hao, J.; Feng, K.; Duan, A.; Yang, Q.; Xu, Z.; Xiong, A. Genomic identification of AP2/ERF transcription factors and functional characterization of two cold resistance-related AP2/ERF genes in celery (Apium graveolens L.). Planta 2019, 250, 1265–1280. [Google Scholar] [CrossRef]
- Meraj, T.A.; Fu, J.; Raza, M.A.; Zhu, C.; Shen, Q.; Xu, D.; Wang, Q. Transcriptional Factors Regulate Plant Stress Responses through Mediating Secondary Metabolism. Genes 2020, 11, 346. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Li, S.; Chen, W.; Zhang, J.; Zhang, L.; Sun, W.; Wang, Z. Plant Dehydrins: Expression, Regulatory Networks, and Protective Roles in Plants Challenged by Abiotic Stress. Int. J. Mol. Sci. 2021, 22, 12619. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, Z.; Liu, H.; Zhang, Y.; Bai, Z.; Zhang, L. Effect of K-/S- segments on subcellular localization and dimerization of wheat dehydrin WZY1-2. Plant Signal. Behav. 2020, 15, 1827583. [Google Scholar] [CrossRef]
- Hernandez-Sanchez, I.E.; Maruri-Lopez, I.; Graether, S.P.; Jimenez-Bremont, J.F. In vivo evidence for homo- and heterodimeric interactions of Arabidopsis thaliana dehydrins AtCOR47, AtERD10, and AtRAB18. Sci. Rep. 2017, 7, 17036. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Yu, Z.; Shi, X.; Qi, Y.; Zhang, D.; Zhang, L. Genetic transformation of wheat with dehydrin wzy1-2 gene and the expression level at different growth stages under drought stress. Agric. Res. Arid. Areas 2019, 37, 1–7. [Google Scholar]
- Yu, Z.; Wang, X.; Mu, X.; Zhang, L. RNAi mediated silencing of dehydrin gene WZY2 confers osmotic stress intolerance in transgenic wheat. Funct. Plant Biol. 2019, 46, 877–884. [Google Scholar] [CrossRef]
- Chen, J.; Gong, Y.; Gao, Y.; Zhou, Y.; Chen, M.; Xu, Z.; Guo, C.; Ma, Y. TaNAC48 positively regulates drought tolerance and ABA responses in wheat (Triticum aestivum L.). Crop J. 2021, 9, 785–793. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Li, X.; Li, Q.; Li, W.; Wang, A.; Liu, H. Exploring the Role of TaERF4a in Enhancing Drought Tolerance and Regulating Dehydrin WZY1-2 Gene Expression in Wheat. Plants 2025, 14, 1214. https://doi.org/10.3390/plants14081214
Yang Y, Li X, Li Q, Li W, Wang A, Liu H. Exploring the Role of TaERF4a in Enhancing Drought Tolerance and Regulating Dehydrin WZY1-2 Gene Expression in Wheat. Plants. 2025; 14(8):1214. https://doi.org/10.3390/plants14081214
Chicago/Turabian StyleYang, Ying, Xinfei Li, Qinying Li, Wenqiang Li, Aina Wang, and Hao Liu. 2025. "Exploring the Role of TaERF4a in Enhancing Drought Tolerance and Regulating Dehydrin WZY1-2 Gene Expression in Wheat" Plants 14, no. 8: 1214. https://doi.org/10.3390/plants14081214
APA StyleYang, Y., Li, X., Li, Q., Li, W., Wang, A., & Liu, H. (2025). Exploring the Role of TaERF4a in Enhancing Drought Tolerance and Regulating Dehydrin WZY1-2 Gene Expression in Wheat. Plants, 14(8), 1214. https://doi.org/10.3390/plants14081214






