Mechanisms of Salt and Drought Stress Responses in Foxtail Millet
Abstract
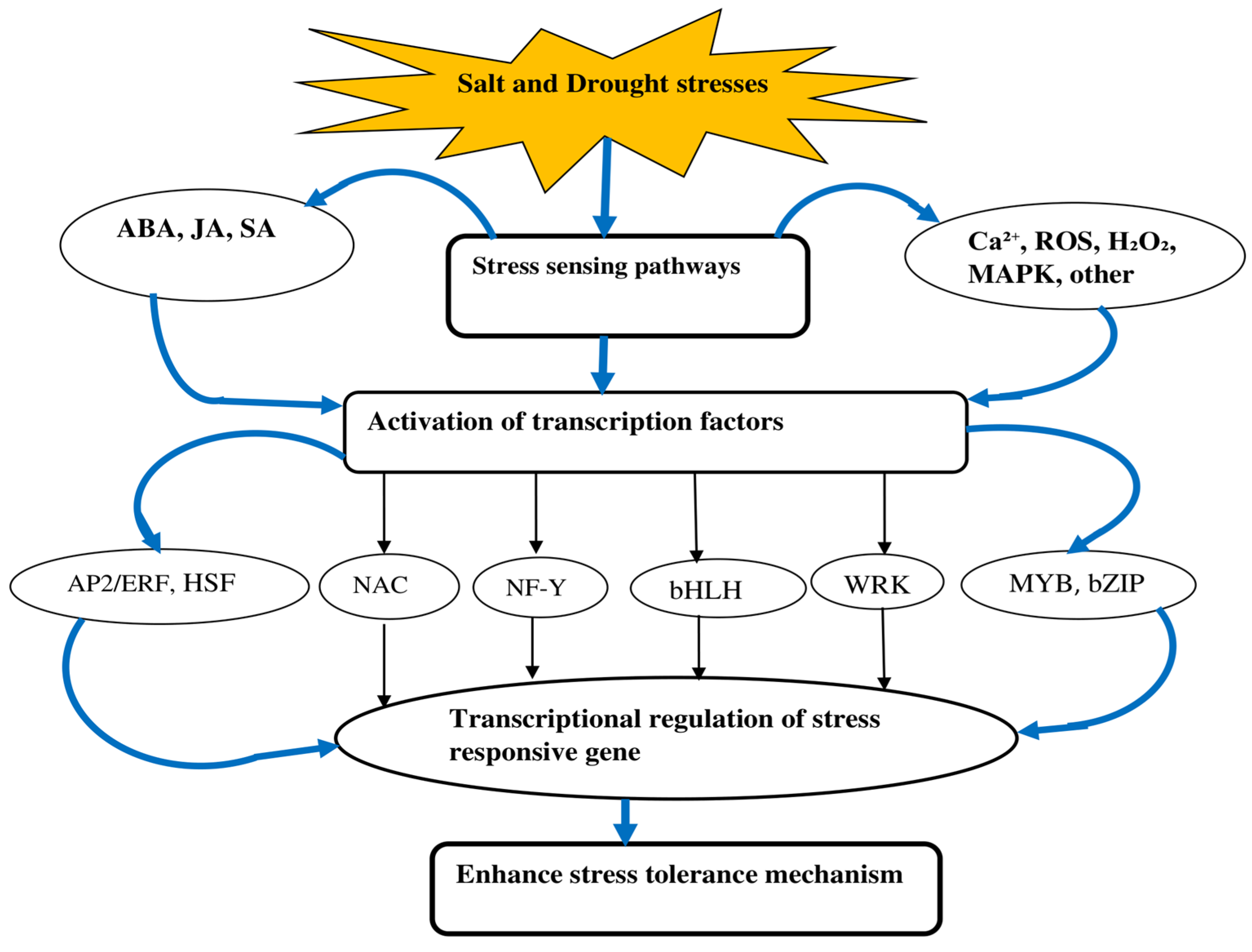
:1. Introduction
2. Response to Salt and Drought Stresses
2.1. Transcription Factor Response
2.2. Osmotic Adjustment
2.3. Oxidative Response
2.4. Water Use Efficiency
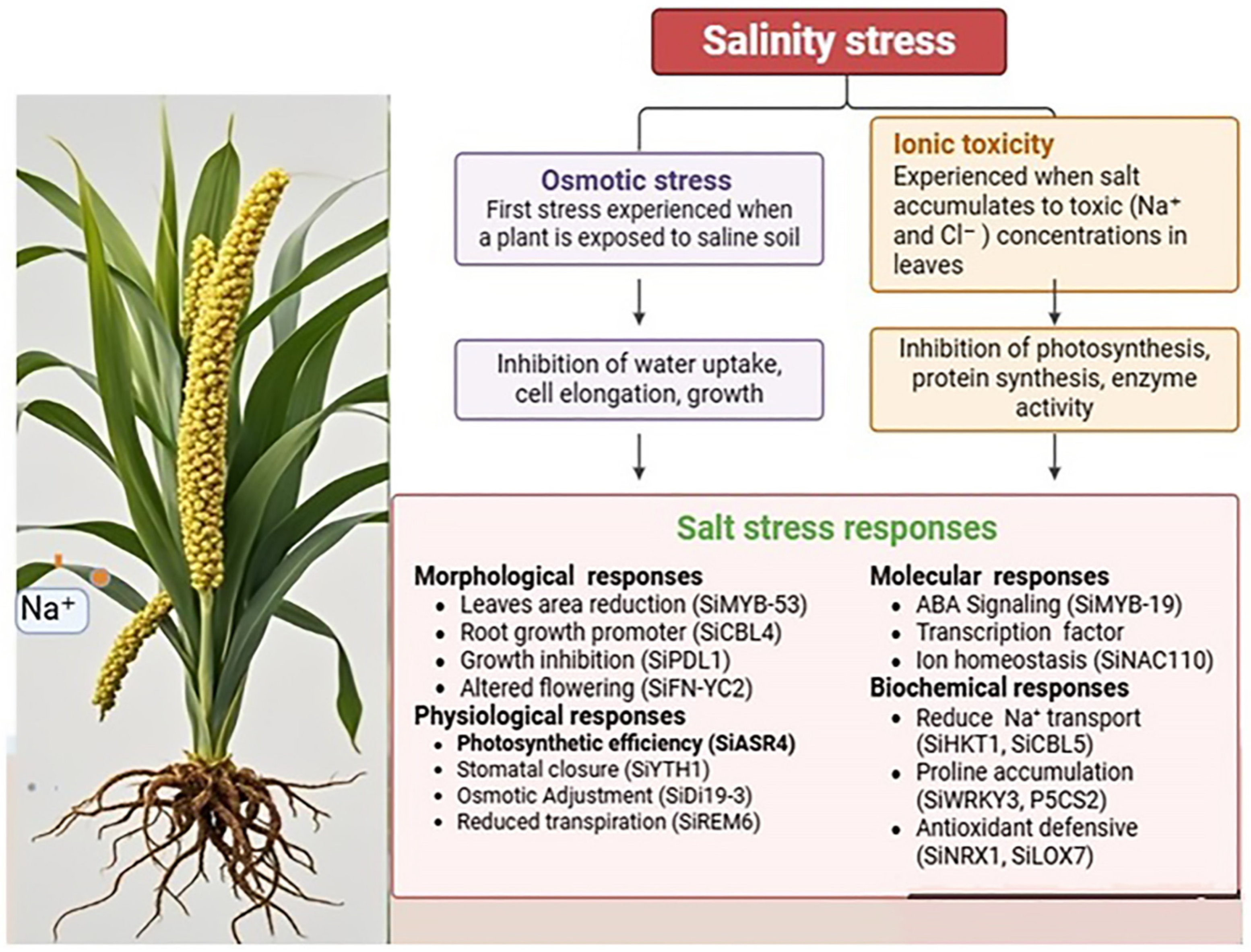
3. Salinity Stress Response
3.1. Regulation of Gene Expression Under Salt Stress
| Name of Protein/TF | Gene Name | Functions | Reference |
|---|---|---|---|
| Abscisic acid stress and ripening induction | SiASR4 | Reduce ROS accumulation. Increase chlorophyll content. | [43] |
| Later embryogenesis abundant | SiLEA | Increases proline and soluble sugar. | [62] |
| Thioredoxin (TRX) | SiNRX1 | Promotes antioxidant enzyme (CAT, SOD, and POD) activity system. | [63] |
| Alternative splicing | SiCYP19-b | Increases the proline content and reduces ROS accumulation. | [90] |
| Nuclear Factor Y | SiNF-YA1 | Increases ROS scavenging and enhances the antioxidant system. | [44] |
| SiNF-YB8 | Enhances antioxidant system and increases ROS scavenging. | [44] | |
| SiFN-YC2 | Regulates early flowering. | [46] | |
| PLATZ | SiPLATZ12 | Negatively regulates salt tolerance by lowering Na+/H+ antiporter activities of SOS1 and NHXs. | [91] |
| Cys2/His2-type | SiDi19-3 | Upregulates NHX, SOS, and CBL genes under salt stress. | [84] |
| High-affinity K+ transporter (HKT) transporter | SiHKT1 | Minimizes Na+ transfer from roots to shoots and Na+ accumulation in shoots. | [81] |
| DREB | SiARDP | Promotes proline accumulation | [42] |
| PTI1 | SiPTI1-5 | Regulates dynamic ROS balance. | [92] |
| REM | SiREM6 | Reduces electrolyte leakage and increases proline accumulation. | [93] |
| LOXs | SiLOX7 | Promote antioxidant enzyme activities. | [89] |
| PLETHORA-LIKE 1 | SiPLT-L1 | Promotes primary root growth. | [94] |
| MYB | SiMYB19 | Regulates ABA synthesis and signal transduction. | [85] |
| SiMYB16 | Increases the flavonoid and lignin contents and activities of fatty acid synthesis enzymes. Regulates lignin and suberin biosynthesis. | [95] | |
| Nonspecific lipid transfer protein | SiLTP | Involved in the ABA-dependent transduction signal pathways. Promotes proline and soluble sugar content. | [96] |
| Δ1-pyrroline-5-carboxylate synthetase | SiP5CS2 | ROS scavenging. | [60] |
| Calcineurin B-like protein | SiCBL5 | Lower Na+ accumulation and stronger Na+ efflux. | [97] |
| Calcineurin B-like protein | SiCBL4 | Enhances root growth. | [98] |
| CIPK | SiCIPK24 | Promotes root elongation. | [98] |
| C-terminal-encoding peptides | SiCEP3 | Promote ABA import. | [99] |
| bZIP | SibZIP67 | Reduces malondialdehyde, enhances antioxidant enzyme activities. | [100] |
| WRKY | SiWRKY3 | Reduces oxidative stress and proline contents. | [101] |
| NAC | SiNAC110 | Regulates proline biosynthesis, ion homeostasis, and ABA signaling pathway-independent osmotic balance | [40] |
3.2. Ionic Balance Maintenance
3.3. Oxidative Responses
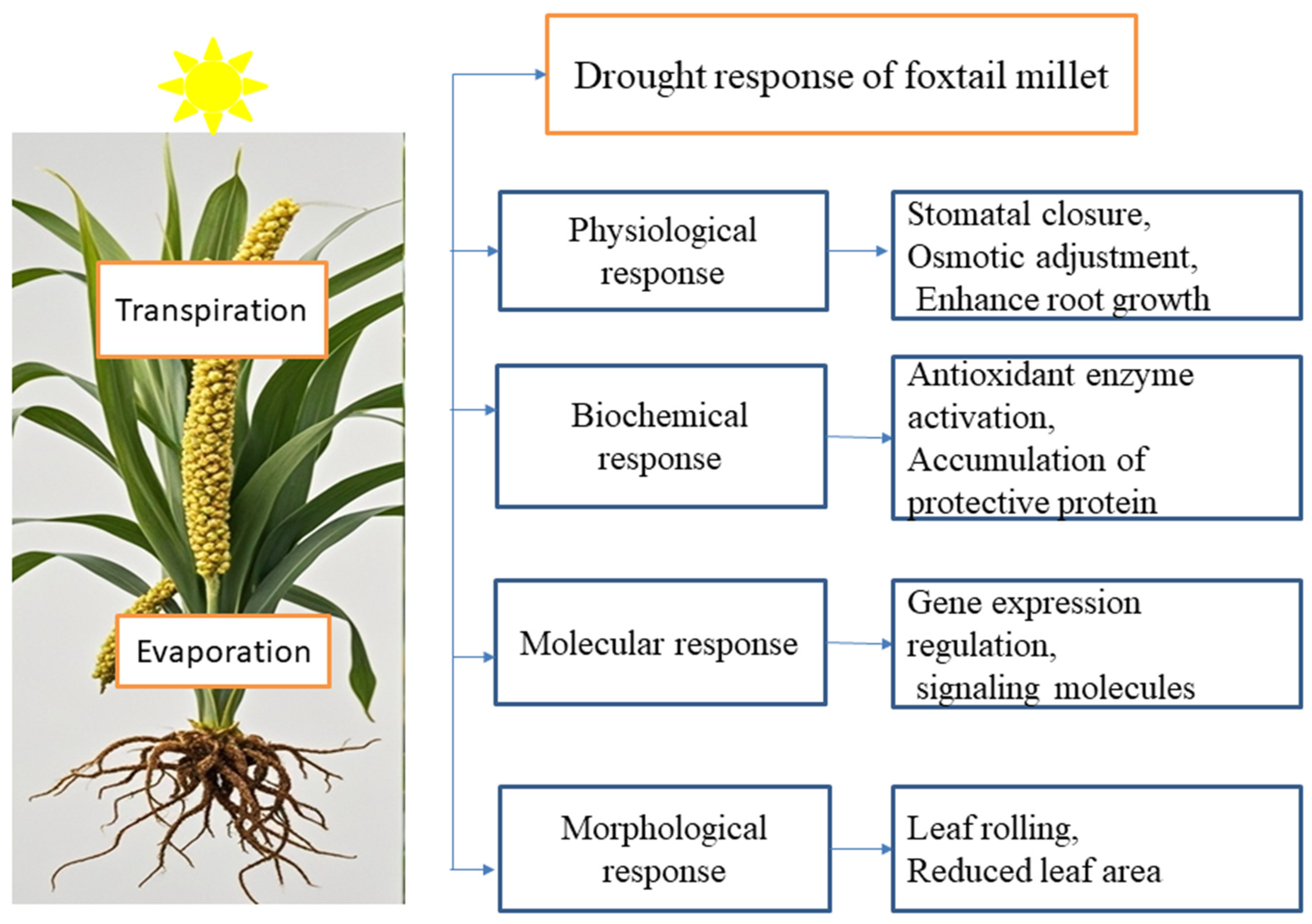
4. Response to Drought Stress
4.1. Morphological, Physiological, and Biochemical Responses
4.2. Regulation of Gene Expression Under Drought Stress
| Name of Protein/TF | Gene Name | Functions | Reference |
|---|---|---|---|
| Abscisic acid stress repining | SiASR1 | Reduces ROS accumulation. Improves antioxidant enzyme activities. | [142] |
| miRNA | SimiR396d | Regulates root growth. | [143] |
| YTH | SiYTH1 | Reduces excessive H2O2 accumulation. Reduces stomatal closure. | [144] |
| Phospholipase D | SiPDLα1 | Reduces ion leakage, chlorosis, and growth inhibition. | [145] |
| MYB | SiMYB56 | Regulates ABA signaling pathways and lignin biosynthesis. | [146] |
| SiMYB53 | Regulates plant growth and development. Provides drought stress signals. | [46] | |
| Lateral organ boundaries domain (LBD) | SiLBD21 | Involved in root development. | [147] |
| Δ1-pyrroline-5-carboxylate synthetase | SiP5CS2 | Promotes proline accumulation. | [60] |
| Autophagy-related gene | SiATG8a | Increases chlorophyll and proline contents. Reduces malondialdehyde content. | [148] |
| NAC | SiNAC110 | Regulates proline biosynthesis, ion homeostasis, and ABA signaling pathway-independent osmotic balance. | [40] |
| 9-cis-epoxycarotenoid dioxygenase (NCED) | SiNCED1 | Modulates ABA biosynthesis and enhances ABA accumulation | [139] |
| Calcium-dependent protein kinases (CDPKs) | SiCDPK24 | Activate drought stress response gene expression and promote drought stress recovery | [132] |
4.3. Oxidative Response to Drought Stress
4.4. Relative Water Content
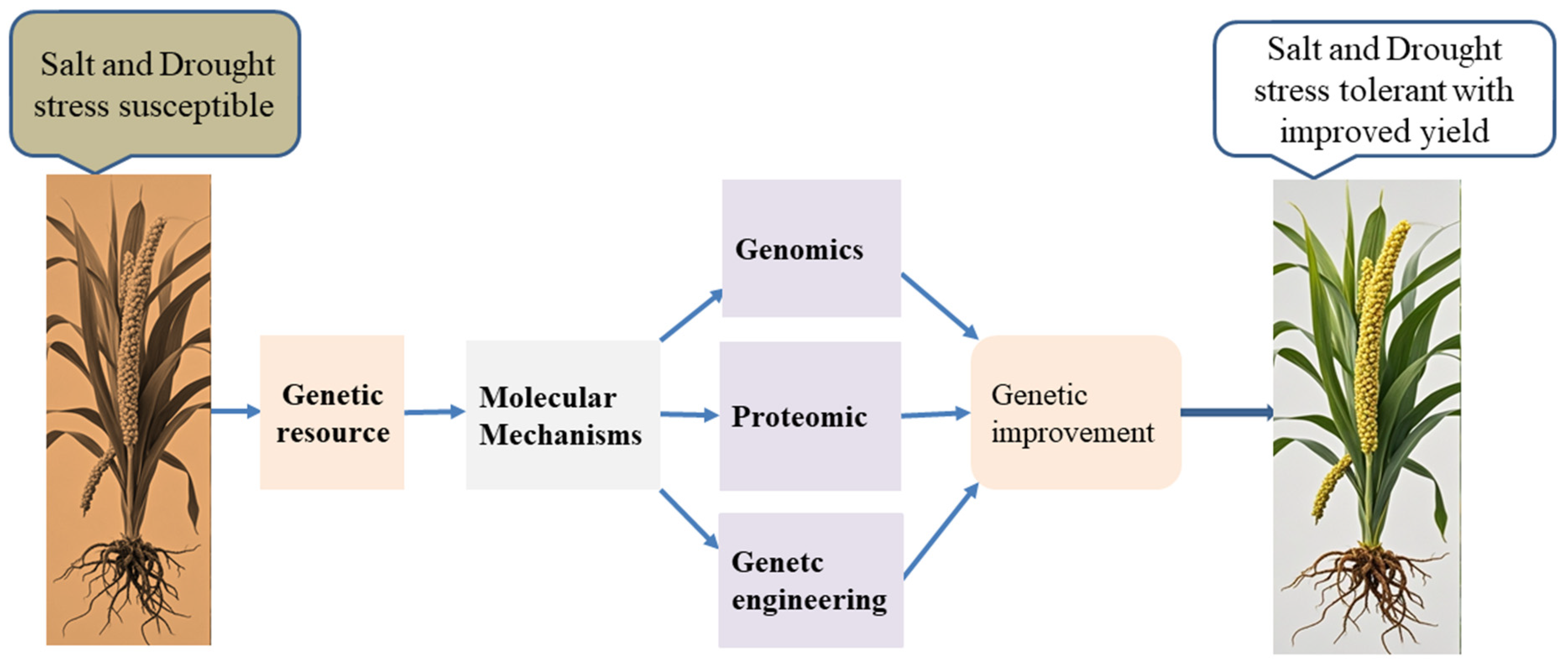
5. Conclusions and Future Prospective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wagner, G. Recalculate the social cost of carbon. Nat. Clim. Change 2021, 11, 293–294. [Google Scholar] [CrossRef]
- Hertel, T.W.; Burke, M.B.; Lobell, D.B. The poverty implications of climate-induced crop yield changes by 2030. Glob. Environ. Change 2010, 20, 577–585. [Google Scholar] [CrossRef]
- Cataldo, E.; Puccioni, S.; Eichmeier, A.; Storchi, P. Prevention of drought damage through zeolite treatments on Vitis vinifera: A promising sustainable solution for soil management. Soil Use Manag. 2024, 40, e13036. [Google Scholar] [CrossRef]
- Angon, P.B.; Tahjib-Ul-Arif, M.; Samin, S.I.; Habiba, U.; Hossain, M.A.; Brestic, M. How do plants respond to combined drought and salinity stress?—A systematic review. Plants 2022, 11, 2884. [Google Scholar] [CrossRef] [PubMed]
- Yi, F.; Huo, M.; Li, J.; Yu, J. Time-series transcriptomics reveals a drought-responsive temporal network and crosstalk between drought stress and the circadian clock in foxtail millet. Plant J. Cell Mol. Biol. 2022, 110, 1213–1228. [Google Scholar] [CrossRef]
- Rana, S.; Pramitha, L.; Muthamilarasan, M. Genomic designing for abiotic stress tolerance in foxtail millet (Setaria italica L.). In Genomic Designing for Abiotic Stress Resistant Cereal Crops; Springer: Cham, Switzerland, 2021; pp. 255–289. [Google Scholar]
- Zhang, W.; Wang, B.; Liu, B.; Chen, Z.; Lu, G.; Ge, Y.; Bai, C. Trait selection for sield improvement in foxtail millet (Setaria italica Beauv.) under Climate Change in the North China Plain. Agronomy 2022, 12, 1500. [Google Scholar] [CrossRef]
- Kole, C.; Muthamilarasan, M.; Henry, R.; Edwards, D.; Sharma, R.; Abberton, M.; Batley, J.; Bentley, A.; Blakeney, M.; Bryant, J.; et al. Application of genomics-assisted breeding for generation of climate resilient crops: Progress and prospects. Front. Plant Sci. 2015, 6, 563. [Google Scholar] [CrossRef]
- Krishnamurthy, L.; Upadhyaya, H.D.; Kashiwagi, J.; Purushothaman, R.; Dwivedi, S.L.; Vadez, V. Variation in drought-tolerance components and their interrelationships in the minicore collection of finger millet germplasm. Crop Sci. 2016, 56, 1914–1926. [Google Scholar] [CrossRef]
- Singh, R.K.; Prasad, M. Foxtail Millet: A climate-resilient crop species with potential to ensure food and agriculture security amidst global climate change. Int. J. Plant Environ. 2020, 6, 165–169. [Google Scholar] [CrossRef]
- Das, I.K.; Rakshit, S. Millets, Their importance, and production constraints. In Biotic Stress Resistance in Millets; Academic Press: Cambridge, MA, USA, 2016; pp. 3–19. [Google Scholar]
- Saxena, R.; Vanga, S.K.; Wang, J.; Orsat, V.; Raghavan, V. Millets for food security in the context of climate change: A review. Sustainability 2018, 10, 2228. [Google Scholar] [CrossRef]
- FAO. The global agro-ecological zoning version 4, crop profile: Pearl and foxtail millets. In GAEZ Data Portal; FAO: Rome, Italy, 2023. [Google Scholar]
- Tripathi, T. Foxtail millet: Rediscovering a superfood for the 21st century. J. Curr. Res. Food Sci. 2024, 5, 110–115. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, W.; Qiao, Y.; Qiao, W.; Yang, H.; Zhong, Y.; Yang, H.; Wang, H.; Li, Y.; Dong, B.; et al. Effects of soil salinity on foxtail millet osmoregulation, grain yield, and soil water utilization under varying water conditions. Agric. Water Manag. 2023, 284, 108354. [Google Scholar] [CrossRef]
- Li, P.; Brutnell, T.P. Setaria viridis and Setaria italica, model genetic systems for the panicoid grasses. J. Exp. Bot. 2011, 62, 3031–3037. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef]
- Charu, L.; Radha, S. Genetic determinants of abiotic stress tolerance in foxtail millet. In The Foxtail Millet Genome; Springer: Cham, Switzerland, 2017; pp. 85–104. [Google Scholar]
- Han, F.; Sun, M.; He, W.; Guo, S.; Feng, J.; Wang, H.; Yang, Q.; Pan, H.; Lou, Y.; Zhuge, Y. Transcriptome analysis reveals molecular mechanisms under salt stress in leaves of foxtail millet (Setaria italica L.). Plants 2022, 11, 1864. [Google Scholar] [CrossRef]
- Pan, J.; Li, Z.; Wang, Q.; Garrell, A.K.; Liu, M.; Guan, Y.; Zhou, W.; Liu, W. Comparative proteomic investigation of drought responses in foxtail millet. BMC Plant Biol. 2018, 18, 315. [Google Scholar] [CrossRef] [PubMed]
- Lata, C.; Jha, S.; Dixit, V.; Sreenivasulu, N.; Prasad, M. Differential antioxidative responses to dehydration-induced oxidative stress in core set of foxtail millet cultivars [Setaria italica (L.)]. Protoplasma 2011, 248, 817–828. [Google Scholar] [CrossRef]
- Nadeem, F.; Ahmad, Z.; Ul Hassan, M.; Wang, R.; Diao, X.; Li, X. Adaptation of foxtail millet (Setaria italica L.) to abiotic stresses: A special perspective of responses to nitrogen and phosphate limitations. Front. Plant Sci. 2020, 11, 187. [Google Scholar] [CrossRef]
- Zhu, C.; Zhao, L.; Zhao, S.; Niu, X.; Li, L.; Gao, H.; Liu, J.; Wang, L.; Zhang, T.; Cheng, R.; et al. Utilizing machine learning and bioinformatics analysis to identify drought-responsive genes affecting yield in foxtail millet. Int. J. Biol. Macromol. 2024, 277, 134288. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, N.; Kang, F.; Wang, J.; Wang, X. Cultivar replacement increases water use efficiency in foxtail millet in Shaanxi Province, China. Plant Physiol. Biochem. 2021, 164, 73–81. [Google Scholar] [CrossRef]
- Nahar, A.; Mannan, M.A.; Mamun, M.A.A.; Ghosh, T.K. Growth and yield performance of foxtail millets under salinity. Bangladesh Agron. J. 2018, 21, 51–59. [Google Scholar] [CrossRef]
- Zhi, H.; Diao, X.; Lu, P.; Li, W.; Akolova, Z. Methodology analysis on screening of salt tolerant genotypes from foxtail millet and other Setaria species. J. Hebei Agric. Res. 2004, 8, 15–18. [Google Scholar]
- Muthamilarasan, M.; Venkata Suresh, B.; Pandey, G.; Kumari, K.; Parida, S.K.; Prasad, M. Development of 5123 intron-length polymorphic markers for large-scale genotyping applications in foxtail millet. DNA Res. 2013, 21, 41–52. [Google Scholar] [CrossRef]
- Nematpour, A.; Eshghizadeh, H.R.; Zahedi, M. Drought-tolerance mechanisms in foxtail millet (Setaria italica) and proso millet (Panicum miliaceum) under different nitrogen supply and sowing dates. Crop Pasture Sci. 2019, 70, 442. [Google Scholar] [CrossRef]
- Loni, F.; Ismaili, A.; Nakhoda, B.; Darzi Ramandi, H.; Shobbar, Z.-S. The genomic regions and candidate genes associated with drought tolerance and yield-related traits in foxtail millet: An integrative meta-analysis approach. Plant Growth Regul. 2023, 101, 169–185. [Google Scholar] [CrossRef]
- Sun, M.; Wang, T.; Fan, L.; Wang, H.; Pan, H.; Cui, X.; Lou, Y.; Zhuge, Y. Foliar applications of spermidine improve foxtail millet seedling characteristics under salt stress. Biol. Plant. 2020, 64, 353–362. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, J.; Chen, T.T.; Zhao, X.H.; Zhang, S.P.; Liu, S.D.; Dong, H.L.; Feng, L.; Yu, S.X. Effect of drought stress on lipid peroxidation and proline contentin cotton roots. J. Anim. Plant Sci. 2014, 24, 1729–1736. [Google Scholar]
- Liu, M.; Li, M.; Liu, K.; Sui, N. Effects of drought stress on seed germination and seedling growth of different maize varieties. J. Agric. Sci. 2015, 7, 231. [Google Scholar] [CrossRef]
- Gollan, P.J.; Tikkanen, M.; Aro, E.M. Photosynthetic light reactions: Integral to chloroplast retrograde signalling. Curr. Opin. Plant Biol. 2015, 27, 180–191. [Google Scholar] [CrossRef]
- Chowdhary, A.A.; Mishra, S.; Mehrotra, S.; Upadhyay, S.K.; Bagal, D.; Srivastava, V. Plant transcription factors: An overview of their role in plant life. In Plant Transcription Factors; Academic Press: Cambridge, MA, USA, 2023; pp. 3–20. [Google Scholar]
- Rabeh, K.; Hnini, M.; Oubohssaine, M.A. comprehensive review of transcription factor-mediated regulation of secondary metabolites in plants under environmental stress. Stress Biol. 2025, 5, 15. [Google Scholar] [CrossRef]
- Prusty, A.; Panchal, A.; Singh, R.K.; Prasad, M. Major transcription factor families at the nexus of regulating abiotic stress response in millets: A comprehensive review. Planta 2024, 259, 118. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Li, M.Z.; Wang, S.M.; Yin, H.J. Revisiting the role of plant transcription factors in the battle against abiotic stress. Int. J. Mol. Sci. 2018, 19, 1634. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Q.; Asim, M.; Zhang, R.; Khan, R.; Farooq, S.; Wu, J. Transcription factors interact with ABA through gene expression and signaling pathways to mitigate drought and salinity stress. Biomolecules 2021, 11, 1159. [Google Scholar] [CrossRef]
- Lata, C.; Mishra, A.K.; Muthamilarasan, M.; Bonthala, V.S.; Khan, Y.; Prasad, M. Genome-wide investigation and expression profiling of AP2/ERF transcription factor superfamily in foxtail millet (Setaria italica L.). PLoS ONE 2014, 9, e113092. [Google Scholar] [CrossRef]
- Xie, L.-N.; Chen, M.; Min, D.-H.; Feng, L.; Xu, Z.-S.; Zhou, Y.-B.; Xu, D.-B.; Li, L.-C.; Ma, Y.-Z.; Zhang, X.-H. The NAC-like transcription factor SiNAC110 in foxtail millet (Setaria italica L.) confers tolerance to drought and high salt stress through an ABA independent signaling pathway. J. Integr. Agric. 2017, 16, 559–571. [Google Scholar] [CrossRef]
- Puranik, S.; Sahu, P.P.; Mandal, S.N.; Parida, S.K.; Prasad, M. Comprehensive genome-wide survey, genomic constitution and expression profiling of the NAC transcription factor family in foxtail millet (Setaria italica L.). PLoS ONE 2013, 8, e64594. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yue, J.; Wu, X.; Xu, C.; Yu, J. An ABA-responsive DRE-binding protein gene from Setaria italica, SiARDP, the target gene of SiAREB, plays a critical role under drought stress. J. Exp. Bot. 2014, 65, 5415–5427. [Google Scholar] [CrossRef]
- Li, J.; Dong, Y.; Li, C.; Pan, Y.; Yu, J. SiASR4, the target gene of SiARDP from Setaria italica, improves abiotic stress adaption in plants. Front. Plant. Sci. 2016, 7, 2053. [Google Scholar] [CrossRef]
- Feng, Z.-J.; He, G.-H.; Zheng, W.-J.; Lu, P.-P.; Chen, M.; Gong, Y.-M.; Ma, Y.-Z.; Xu, Z.-S. Foxtail millet NF-Y families: Genome-wide survey and evolution analyses identified two functional genes important in abiotic stresses. Front. Plant Sci. 2015, 6, 1142. [Google Scholar] [CrossRef]
- Rani, V.; Joshi, D.C.; Joshi, P.; Singh, R.; Yadav, D. “Millet models” for harnessing nuclear factor-Y transcription factors to engineer stress tolerance in plants: Current knowledge and emerging paradigms. Planta 2023, 258, 29. [Google Scholar] [CrossRef]
- Niu, J.; Guan, Y.; Yu, X.; Wang, R.; Qin, L.; Chen, E.; Yang, Y.; Zhang, H.; Wang, H.; Li, F. SiNF-YC2 regulates early maturity and salt tolerance in Setaria italica. Int. J. Mol. Sci. 2023, 24, 7217. [Google Scholar] [CrossRef] [PubMed]
- Verslues, P.E.; Lasky, J.R.; Juenger, T.E.; Liu, T.W.; Kumar, M.N. Genome-wide association mapping combined with reverse genetics identifies new effectors of low water potential-induced proline accumulation in Arabidopsis. Plant Physiol. 2014, 164, 144–159. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Thangavel, P.; Rasheed, F.; Masood, A.; Pirasteh-Anosheh, H.; Khan, N.A. Osmolytes: Efficient oxidative stress-busters in plants. In Global Climate Change and Plant Stress Management; Wiley: Hoboken, NJ, USA, 2023; pp. 399–409. [Google Scholar]
- Dutta, T.; Neelapu, N.R.; Wani, S.H.; Challa, S. Compatible solute engineering of crop plants for improved tolerance toward abiotic stresses. In Biochemical, Physiological and Molecular Avenues for Combating Abiotic Stress Tolerance in Plants; Elsevier: Amsterdam, The Netherlands, 2018; pp. 221–254. [Google Scholar]
- Zivcak, M.; Brestic, M.; Sytar, O. Osmotic adjustment and plant adaptation to drought stress. In Drought Stress Tolerance in Plants, Vol 1: Physiology and Biochemistry; Springer: Cham, Switzerland, 2016; pp. 105–143. [Google Scholar]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Khan, M.A.R. Understanding the roles of osmolytes for acclimatizing plants to changing environment: A review of potential mechanism. Plant Signal. Behav. 2021, 16, 1913306. [Google Scholar] [CrossRef]
- Ejaz, S.; Hussain, S.; Anjum, M.A.; Ahmad, S. Application of osmolytes in improving abiotic stress tolerance in plant. In Plant Tolerance to Environmental Stress; CRC Press: Boca Raton, FL, USA, 2019; pp. 47–58. [Google Scholar]
- Hare, P.; Cress, W. Metabolic implications of stress-induced proline accumulation in plants. Plant growth Regul. 1997, 21, 79–102. [Google Scholar] [CrossRef]
- Ozturk, M.; Turkyilmaz Unal, B.; Garcia-Caparros, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant. 2021, 172, 1321–1335. [Google Scholar] [CrossRef]
- Ghaffari, A. Role of inorganic and organic ions in response to salt and drought stresses. J. Plant Stress Physiol. 2022, 8, 17–25. [Google Scholar] [CrossRef]
- Rajasheker, G.; Jawahar, G.; Jalaja, N.; Kumar, S.A.; Kumari, P.H.; Punita, D.L.; Karumanchi, A.R.; Reddy, P.S.; Rathnagiri, P.; Sreenivasulu, N. Role and regulation of osmolytes and ABA interaction in salt and drought stress tolerance. In Plant Signaling Molecules; Elsevier: Amsterdam, The Netherlands, 2019; pp. 417–436. [Google Scholar]
- Xiong, L.; Zhu, J.K. Molecular and genetic aspects of plant responses to osmotic stress. Plant Cell Environ. 2002, 25, 131–139. [Google Scholar] [CrossRef]
- Lata, C.; Muthamilarasan, M.; Prasad, M. Drought stress responses and signal transduction in plants. In Elucidation of Abiotic Stress Signaling in Plants; Springer: New York, NY, USA, 2015; pp. 195–225. [Google Scholar]
- Mukami, A.; Ngetich, A.; Mweu, C.; Oduor, R.O.; Muthangya, M.; Mbinda, W.M. Differential characterization of physiological and biochemical responses during drought stress in finger millet varieties. Physiol. Mol. Biol. Plants Int. J. Funct. Plant Biol. 2019, 25, 837–846. [Google Scholar] [CrossRef]
- Qin, L.; Chen, E.; Li, F.; Yu, X.; Liu, Z.; Yang, Y.; Wang, R.; Zhang, H.; Wang, H.; Liu, B.; et al. Genome-wide gene expression profiles analysis reveal novel insights into drought stress in foxtail millet (Setaria italica L.). Int. J. Mol. Sci. 2020, 21, 8520. [Google Scholar] [CrossRef]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Cao, X.; Khan, M.A.R. Proline, a multifaceted signalling molecule in plant responses to abiotic stress: Understanding the physiological mechanisms. Plant Biol. 2022, 24, 227–239. [Google Scholar] [CrossRef]
- Wang, M.; Li, P.; Li, C.; Pan, Y.; Jiang, X.; Zhu, D.; Zhao, Q.; Yu, J. SiLEA14, a novel atypical LEA protein, confers abiotic stress resistance in foxtail millet. BMC Plant Biol. 2014, 14, 290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yu, Y.; Song, T.; Zhang, M.; Li, N.; Yu, M.; Zhou, H.; Yang, Y.; Guo, S.; Xu, C.; et al. Genome-wide identification of foxtail millet’s TRX family and a functional analysis of SiNRX1 in response to drought and salt stresses in transgenic Arabidopsis. Front. Plant Sci. 2022, 13, 946037. [Google Scholar] [CrossRef]
- Bhinda, M.S.; Sanadya, S.K.; Kumari, A.; Kant, L.; Debnath, A. Omics for abiotic stress tolerance in foxtail millet. In Omics of Climate Resilient Small Millets; Pudake, R.N., Solanke, A.U., Sevanthi, A.M., Rajendrakumar, P., Eds.; Springer: Singapore, 2022. [Google Scholar]
- Raturi, A.; Shekhar, S.; Jha, R.K.; Chauhan, D.; Pandey, S.; Kumari, S.; Singh, A. Genome-wide comparative analysis of photosynthetic enzymatic genes provides novel insights into foxtail millet and other cereals. Front. Genet. 2024, 15, 1449113. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, T.; Muthamilarasan, M.; Prasad, M. Millets for next generation climate-smart agriculture. Front. Plant Sci. 2017, 8, 1266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, X.; Quan, Z.; Cheng, S.; Xu, X.; Pan, S.; Xie, M.; Zeng, P.; Yue, Z.; Wang, W.; et al. Genome sequence of foxtail millet (Setaria italica) provides insights into grass evolution and biofuel potential. Nat. Biotechnol. 2012, 30, 549–554. [Google Scholar] [CrossRef]
- Paes de Melo, B.; Carpinetti, P.A.; Fraga, O.T.; Rodrigues-Silva, P.L.; Fioresi, V.S.; de Camargos, L.F.; Ferreira, M. Abiotic stresses in plants and their markers: A practice view of plant stress responses and programmed cell death mechanisms. Plants 2022, 11, 1100. [Google Scholar] [CrossRef]
- Qamer, Z.; Chaudhary, M.T.; Du, X.; Hinze, L.; Azhar, M.T. Review of oxidative stress and antioxidative defense mechanisms in Gossypium hirsutum L. in response to extreme abiotic conditions. J. Cotton Res. 2021, 4, 9. [Google Scholar] [CrossRef]
- Wang, T.; Song, H.; Zhang, B.; Lu, Q.; Liu, Z.; Zhang, S.; Guo, R.; Wang, C.; Zhao, Z.; Liu, J.; et al. Genome-wide identification, characterization, and expression analysis of superoxide dismutase (SOD) genes in foxtail millet (Setaria italica L.). 3 Biotech 2018, 8, 486. [Google Scholar] [CrossRef]
- Muhammad, M.; Waheed, A.; Wahab, A.; Majeed, M.; Nazim, M.; Liu, Y.-H.; Li, L.; Li, W.-J. Soil salinity and drought tolerance: An evaluation of plant growth, productivity, microbial diversity, and amelioration strategies. Plant Stress 2024, 11, 100319. [Google Scholar] [CrossRef]
- Yadav, C.B.; Prasad, M. Transposable elements in setaria genomes. In The Foxtail Millet Genome; Springer: Cham, Switzerland, 2017; pp. 23–35. [Google Scholar]
- Tang, S.; Li, L.; Wang, Y.; Chen, Q.; Zhang, W.; Jia, G.; Zhi, H.; Zhao, B.; Diao, X. Genotype-specific physiological and transcriptomic responses to drought stress in Setaria italica (an emerging model for Panicoideae grasses). Sci. Rep. 2017, 7, 10009. [Google Scholar] [CrossRef]
- Li, Y. Drought-resistant mechanism and genetic expression of foxtail millet. In Foxtail Millet Breeding; Agricultural Press: Beijing, China, 1997; pp. 433–434. [Google Scholar]
- Yang, X.; Liu, R.; Jing, M.; Zhang, N.; Liu, C.; Yan, J. Variation of root soluble sugar and starch response to drought stress in foxtail millet. Agronomy 2023, 13, 359. [Google Scholar] [CrossRef]
- Anwar, A.; Zhang, S.; Lilong, H.E.; Jianwei, G.A.O. Understanding the physiological and molecular mechanism of salinity stress tolerance in plantsof salinity stress tolerance in plantsof salinity stress tolerance in plants of salinity stress tolerance in plant. Not. Bot. Horti Agrobot. Cluj-Napoca 2022, 49, 12959. [Google Scholar] [CrossRef]
- Fedoreyeva, L.I.; Lazareva, E.M.; Shelepova, O.V.; Baranova, E.N.; Kononenko, N.V. Salt-induced autophagy and programmed cell death in wheat. Agronomy 2022, 12, 1909. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, M.; Liang, Y.; Zhang, L.; Diao, X. Salt stress responses in foxtail millet: Physiological and molecular regulation. Crop J. 2023, 11, 1011–1021. [Google Scholar] [CrossRef]
- Rathinapriya, P.; Pandian, S.; Rakkammal, K.; Balasangeetha, M.; Alexpandi, R.; Satish, L.; Rameshkumar, R.; Ramesh, M. The protective effects of polyamines on salinity stress tolerance in foxtail millet (Setaria italica L.), an important C4 model crop. Physiol. Mol. Biol. Plants Int. J. Funct. Plant Biol. 2020, 26, 1815–1829. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, C.; Veeranagamallaiah, G.; Nareshkumar, A.; Sudhakarbabu, O.; Sivakumar, M.; Pandurangaiah, M.; Kiranmai, K.; Lokesh, U. Polyamine metabolism influences antioxidant defense mechanism in foxtail millet (Setaria italica L.) cultivars with different salinity tolerance. Plant Cell Rep. 2015, 34, 141–156. [Google Scholar] [CrossRef]
- Pan, J.; Li, Z.; Dai, S.; Ding, H.; Wang, Q.; Li, X.; Ding, G.; Wang, P.; Guan, Y.; Liu, W. Integrative analyses of transcriptomics and metabolomics upon seed germination of foxtail millet in response to salinity. Sci. Rep. 2020, 10, 13660. [Google Scholar] [CrossRef]
- Zhang, B.; Xia, T.; Duan, W.; Zhang, Z.; Li, Y.; Fang, B.; Xia, M.; Wang, M. Effects of organic acids, amino acids and phenolic compounds on antioxidant characteristic of Zhenjiang aromatic vinegar. Molecules 2019, 24, 3799. [Google Scholar] [CrossRef]
- Zhang, X.; Ding, Y.; Yang, M.; Wei, A.; Huo, D. The role of NaHS pretreatment in improving salt stress resistance in foxtail millet seedlings: Physiological and molecular mechanisms. Plant Signal. Behav. 2023, 18, 2276611. [Google Scholar] [CrossRef]
- Xiao, S.; Wan, Y.; Guo, S.; Fan, J.; Lin, Q.; Zheng, C.; Wu, C. Transcription factor SiDi19-3 enhances salt tolerance of foxtail millet and Arabidopsis. Int. J. Mol. Sci. 2023, 24, 2592. [Google Scholar] [CrossRef]
- Xu, C.; Luo, M.; Sun, X.; Yan, J.; Shi, H.; Yan, H.; Yan, R.; Wang, S.; Tang, W.; Zhou, Y.; et al. SiMYB19 from Foxtail Millet (Setaria italica) Confers Transgenic Rice Tolerance to High Salt Stress in the Field. Int. J. Mol. Sci. 2022, 23, 756. [Google Scholar] [CrossRef]
- Muthamilarasan, M.; Bonthala, V.S.; Khandelwal, R.; Jaishankar, J.; Shweta, S.; Nawaz, K.; Prasad, M. Global analysis of WRKY transcription factor superfamily in setaria identifies potential candidates involved in abiotic stress signaling. Front. Plant Sci. 2015, 6, 910. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Zhao, Y.; Gao, H.; Li, Y.; Tang, W.; Ma, L.; Yang, G.; Sun, J. Genomic characterization and expression analysis of TCP transcription factors in Setaria italica and Setaria viridis. Plant signal. Behav. 2022, 17, 2075158. [Google Scholar] [CrossRef] [PubMed]
- Lai, D.; Fan, Y.; Xue, G.; He, A.; Yang, H.; He, C.; Li, Y.; Ruan, J.; Yan, J.; Cheng, J. Genome-wide identifcation and characterization of the SPL gene family and its expression in the various developmental stages and stress conditions in foxtail millet (Setaria italica). BMC Genom. 2022, 23, 389. [Google Scholar]
- Zhang, Q.; Zhao, Y.; Zhang, J.; Li, X.; Ma, F.; Duan, M.; Zhang, B.; Li, H. The responses of the lipoxygenase gene family to salt and drought stress in foxtail millet (Setaria italica). Life 2021, 11, 1169. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Tian, H.; Wu, Y.; Kong, Y.; Wang, X.; Sui, N. Alternative splicing plays a crucial role in the salt tolerance of foxtail millet. J. Agric. Food Chem. 2024, 72, 10814–10827. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, L.; Wan, Y.; Tang, S.; Sui, Y.; Bai, Y.; Wang, Y.; Liu, M.; Fan, J.; Zhang, S.; et al. SiPLATZ12 transcription factor negatively regulates salt tolerance in foxtail millet (Setaria italica) by suppressing the expression of SiNHX, SiCBL and SiSOS genes. Environ. Exp. Bot. 2023, 213, 105417. [Google Scholar] [CrossRef]
- Huangfu, Y.; Pan, J.; Li, Z.; Wang, Q.; Mastouri, F.; Li, Y.; Yang, S.; Liu, M.; Dai, S.; Liu, W. Genome-wide identification of PTI1 family in Setaria italica and salinity-responsive functional analysis of SiPTI1-5. BMC Plant Biol. 2021, 21, 319. [Google Scholar] [CrossRef]
- Yue, J.; Li, C.; Liu, Y.; Yu, J. A Remorin Gene SiREM6, the target gene of SiARDP, from foxtail millet (Setaria italica) promotes high salt tolerance in transgenic Arabidopsis. PLoS ONE 2014, 9, e100772. [Google Scholar] [CrossRef]
- Zhao, Z.; Tang, S.; Li, W.; Yang, X.; Wang, R.; Diao, X.; Tang, W. Overexpression of a BRASSINAZOLE RESISTANT 1 homolog attenuates drought tolerance by suppressing the expression of PLETHORA-LIKE 1 in Setaria italica. Crop J. 2021, 9, 1208–1213. [Google Scholar] [CrossRef]
- Yu, Y.; Guo, D.D.; Min, D.H.; Cao, T.; Ning, L.; Jiang, Q.Y.; Sun, X.J.; Zhang, H.; Tang, W.S.; Gao, S.Q.; et al. Foxtail millet MYB-like transcription factor SiMYB16 confers salt tolerance in transgenic rice by regulating phenylpropane pathway. Plant Physiol. Biochem. PPB 2023, 195, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Li, J.; Jiao, L.; Li, C.; Zhu, D.; Yu, J. A Non-specific Setaria italica lipid transfer protein gene plays a critical role under abiotic Stress. Front. Plant Sci. 2016, 7, 1752. [Google Scholar] [CrossRef]
- Yan, J.; Yang, L.; Liu, Y.; Zhao, Y.; Han, T.; Miao, X.; Zhang, A. Calcineurin B-like protein 5 (SiCBL5) in Setaria italica enhances salt tolerance by regulating Na+ homeostasis. Crop J. 2022, 10, 234–242. [Google Scholar] [CrossRef]
- Zhang, Y.; Linghu, J.; Wang, D.; Liu, X.; Yu, A.; Li, F.; Zhao, J.; Zhao, T. Foxtail millet CBL4 (SiCBL4) interacts with SiCIPK24, modulates plant salt stress tolerance. Plant Mol. Biol. Rep. 2017, 35, 634–646. [Google Scholar] [CrossRef]
- Zhang, L.; Ren, Y.; Xu, Q.; Wan, Y.; Zhang, S.; Yang, G.; Huang, J.; Yan, K.; Zheng, C.; Wu, C. SiCEP3, a C-terminally encoded peptide from Setaria italica, promotes ABA import and signaling. J. Exp. Bot. 2021, 72, 6260–6273. [Google Scholar] [CrossRef]
- Jia, X.; Gao, H.; Zhang, L.; Tang, W.; Wei, G.; Sun, J.; Xiong, W. Expression of foxtail millet bZIP transcription factor SibZIP67 enhances drought tolerance in Arabidopsis. Biomolecules 2024, 14, 958. [Google Scholar] [CrossRef] [PubMed]
- Hichri, I.; Muhovski, Y.; Zizkova, E.; Dobrev, P.I.; Gharbi, E.; Franco-Zorrilla, J.M.; Lopez-Vidriero, I.; Solano, R.; Clippe, A.; Errachid, A.; et al. The solanum lycopersicum WRKY3 transcription factor SlWRKY3 is involved in salt stress tolerance in tomato. Front. Plant Sci. 2017, 8, 1343. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, W.; Yu, W.; Yao, L.; Li, L.; Wei, J.; Li, R. Foxtail millet SiHAK1 excites extreme high-affinity K(+) uptake to maintain K(+) homeostasis under low K(+) or salt stress. Plant Cell Rep. 2018, 37, 1533–1546. [Google Scholar] [CrossRef]
- Zhang, M.; Cao, Y.; Wang, Z.; Wang, Z.Q.; Shi, J.; Liang, X.; Song, W.; Chen, Q.; Lai, J.; Jiang, C. A retrotransposon in an HKT1 family sodium transporter causes variation of leaf Na+ exclusion and salt tolerance in maize. New Phytol. 2018, 217, 1161–1176. [Google Scholar] [CrossRef]
- Hao, S.; Wang, Y.; Yan, Y.; Liu, Y.; Wang, J.; Chen, S. A review on plant responses to salt stress and their mechanisms of salt resistance. Horticulturae 2021, 7, 132. [Google Scholar] [CrossRef]
- Han, F.; Sun, M.; He, W.; Cui, X.; Pan, H.; Wang, H.; Song, F.; Lou, Y.; Zhuge, Y. Ameliorating effects of exogenous Ca2+ on foxtail millet seedlings under salt stress. Funct. Plant Biol. 2019, 46, 407. [Google Scholar] [CrossRef]
- Dzinyela, R.; Alhassan, A.R.; Suglo, P.; Movahedi, A. Advanced study of functional proteins involved in salt stress regulatory pathways in plants. S. Afr. J. Bot. 2023, 159, 425–438. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, Q.H.; Yu, Y.N.; Qiao, Y.M.; Haq, S.U.; Gong, Z.H. The CBL-CIPK pathway in plant response to stress signals. Int. J. Mol. Sci. 2020, 21, 5668. [Google Scholar] [CrossRef]
- Nath, M.; Bhatt, D.; Jain, A.; Saxena, S.C.; Saifi, S.K.; Yadav, S.; Negi, M.; Prasad, R.; Tuteja, N. Salt stress triggers augmented levels of Na+, Ca2+ and ROS and alter stress-responsive gene expression in roots of CBL9 and CIPK23 knockout mutants of Arabidopsis thaliana. Environ. Exp. Bot. 2019, 161, 265–276. [Google Scholar] [CrossRef]
- Cheong, Y.H.; Sung, S.J.; Kim, B.G.; Pandey, G.K.; Cho, J.S.; Kim, K.N.; Luan, S. Constitutive overexpression of the calcium sensor CBL5 confers osmotic or drought stress tolerance in Arabidopsis. Mol. Cells 2010, 29, 159–165. [Google Scholar] [CrossRef]
- Hussain, S.; Hussain, S.; Ali, B.; Ren, X.; Chen, X.; Li, Q.; Saqib, M.; Ahmad, N. Recent progress in understanding salinity tolerance in plants: Story of Na(+)/K(+) balance and beyond. Plant Physiol. Biochem. PPB 2021, 160, 239–256. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Saleem, S.; Alghamdi, K.M.; Mushtaq, N.U.; Tahir, I.; Bahieldin, A.; Henrissat, B.; Alghamdi, M.K.; Rehman, R.U.; Hakeem, K.R. Computational and experimental analysis of foxtail millet under salt stress and selenium supplementation. Environ. Sci. Pollut. Res. Int. 2023, 30, 112695–112709. [Google Scholar] [CrossRef]
- Chapman, J.M.; Muhlemann, J.K.; Gayomba, S.R.; Muday, G.K. RBOH-Dependent ROS Synthesis and ROS scavenging by plant specialized metabolites to modulate plant development and stress responses. Chem. Res. Toxicol. 2019, 32, 370–396. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Z.; Tian, L.; Sun, W.; Wang, X.; Wang, Z.; Wang, Z.; Li, Z.; Liu, W.; Ma, Q.; et al. Transcriptome-metabolome and anatomy conjoint analysis of vital component change of photosynthesis of foxtail millet under different drought conditions. J. Integr. Agric. 2024; in press. [Google Scholar] [CrossRef]
- Chang, X.; Zhang, S.; Cao, C.; Zhou, J.; Wang, X.; Zhang, D.; Xiang, J. Transcriptome analysis and characteristics of drought resistance related genes in four varieties of foxtail millet [Setaria italica]. Heliyon 2024, 10, e38083. [Google Scholar] [CrossRef]
- Cui, X.; Wang, B.; Chen, Z.; Guo, J.; Zhang, T.; Zhang, W.; Shi, L. Comprehensive physiological, transcriptomic, and metabolomic analysis of the key metabolic pathways in millet seedling adaptation to drought stress. Physiol. Plant. 2023, 175, e14122. [Google Scholar] [CrossRef]
- Liu, T.Y.; Ye, N.; Song, T.; Cao, Y.; Gao, B.; Zhang, D.; Zhu, F.; Chen, M.; Zhang, Y.; Xu, W.; et al. Rhizosheath formation and involvement in foxtail millet (Setaria italica) root growth under drought stress. J. Integr. Plant Biol. 2019, 61, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Ajithkumar, I.P.; Panneerselvam, R. Osmolyte accumulation, photosynthetic pigment and growth of Setaria italica (L.) P. Beauv. under drought stress. Asian Pac. J. Reprod. 2013, 2, 220–224. [Google Scholar] [CrossRef]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Gene expression and signal transduction in water-stress response. Plant Physiol. 1997, 115, 327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, T.; Fu, J.; Zhu, Y.; Jia, J.; Zheng, J.; Zhao, Y.; Zhang, Y.; Wang, G. Construction and application of EST library from setaria italica in response to dehydration stress. Genomics 2007, 90, 121–131. [Google Scholar] [CrossRef]
- Heinemann, B.; Künzler, P.; Eubel, H.; Braun, H.-P.; Hildebrandt, T.M. Estimating the number of protein molecules in a plant cell: Protein and amino acid homeostasis during drought. Plant Physiol. 2021, 185, 385–404. [Google Scholar] [CrossRef]
- Sadeghipour, O. Polyamines protect mung bean [Vigna radiata (L.) Wilczek] plants against drought stress. Biol. Futur. 2019, 70, 71–78. [Google Scholar] [CrossRef]
- Graether, S.P.; Boddington, K.F. Disorder and function: A review of the dehydrin protein family. Front. Plant Sci. 2014, 5, 576. [Google Scholar] [CrossRef]
- Ren, W.; Shi, Z.; Zhou, M.; Zhao, B.; Li, H.; Wang, J.; Liu, Y.; Zhao, J. iTRAQ-based quantitative proteomic analysis provides insight into the drought-stress response in maize seedlings. Sci. Rep. Nat. Portf. 2022, 12, 1952. [Google Scholar] [CrossRef] [PubMed]
- Priya, M.; Dhanker, O.P.; Siddique, K.H.M.; HanumanthaRao, B.; Nair, R.M.; Pandey, S.; Singh, S.; Varshney, R.K.; Prasad, P.V.V.; Nayyar, H. Drought and heat stress-related proteins: An update about their functional relevance in imparting stress tolerance in agricultural crops. Theor. Appl. Genet. 2019, 132, 1607–1638. [Google Scholar] [CrossRef]
- Manfre, A.J.; Lanni, L.M.; Marcotte, W.R. The Arabidopsis group 1 LATE EMBRYOGENESIS ABUNDANT Protein ATEM6 is required for normal seed development. Plant physiol. 2006, 140, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Roychoudhury, A. Group II late embryogenesis abundant (LEA) proteins: Structural and functional aspects in plant abiotic stress. Plant Growth Regul. 2015, 79, 1–17. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, X.; Lv, Q.; Zhu, D.; Qiu, T.; Xu, Y.; Bao, F.; He, Y.; Hu, Y. Physcomitrella patens dehydrins (PpDHNA and PpDHNC) confer salinity and drought tolerance to transgenic Arabidopsis plants. Front. Plant Sci. 2017, 8, 1316. [Google Scholar] [CrossRef]
- Guo, Y.; Hao, D.; Wang, X.; Wang, H.; Wu, Z.; Yang, P.; Zhang, B. Comparative transcriptomics reveals key genes contributing to the differences in drought tolerance among three cultivars of foxtail millet (Setaria italica). Plant Growth Regul. 2022, 99, 45–64. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, C.; Kang, X.; Pei, Z.Q.; Bai, X.; Wang, J.; Zheng, S.; Zhang, T.G. Identification and expression analysis of MAPK cascade gene family in foxtail millet (Setaria italica). Plant Signal. Behav. 2023, 18, 2246228. [Google Scholar] [CrossRef]
- Yu, T.-F.; Zhao, W.-Y.; Fu, J.-D.; Liu, Y.-W.; Chen, M.; Zhou, Y.-B.; Ma, Y.-Z.; Xu, Z.-S.; Xi, Y.-J. Genome-wide analysis of CDPK family in foxtail millet and determination of SiCDPK24 functions in drought stress. Front. Plant Sci. 2018, 9, 651. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, Q.; Liu, H.; Tang, S.; Shang, C.; Zhang, W.; Sui, Y.; Zhang, Y.; Zheng, C.; Zhang, H.; et al. The osmotic stress-activated receptor-like kinase DPY1 mediates SnRK2 kinase activation and drought tolerance in Setaria. Plant Cell 2023, 35, 3782–3808. [Google Scholar] [CrossRef]
- Amoah, J.N.; Adu-Gyamfi, M.O. Effect of drought acclimation on sugar metabolism in millet. Protoplasma 2024, 262, 35–49. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, K.; Li, Z.; Li, W.; Zhang, X.; Liu, S.; Meng, R.; Lu, B.; Li, X.; Ren, J.; et al. Transcriptome analysis reveals brassinolide signaling pathway control of foxtail millet seedling starch and sucrose metabolism under freezing stress, with implications for growth and development. Int. J. Mol. Sci. 2023, 24, 11590. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 2007, 58, 221–227. [Google Scholar] [CrossRef]
- Huang, Z.; Zhao, L.; Chen, D.; Liang, M.; Liu, Z.; Shao, H.; Long, X. Salt stress encourages proline accumulation by regulating proline biosynthesis and degradation in Jerusalem artichoke plantlets. PLoS ONE 2013, 8, e62085. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.Q.; Gao, X.L.; Gao, J.F.; Li, J.; Yang, P.; Feng, B.L. Transcriptome profiling using RNA-seq to provide insights into foxtail millet seedling tolerance to short-term water deficit stress induced by PEG-6000. J. Integr. Agric. 2019, 18, 2457–2471. [Google Scholar] [CrossRef]
- Huang, Y.; Jiao, Y.; Yang, S.; Mao, D.; Wang, F.; Chen, L.; Liang, M. SiNCED1, a 9-cis-epoxycarotenoid dioxygenase gene in Setaria italica, is involved in drought tolerance and seed germination in transgenic Arabidopsis. Front. Plant Sci. 2023, 14, 1121809. [Google Scholar] [CrossRef]
- Cao, X.; Hu, L.; Chen, X.; Zhang, R.; Cheng, D.; Li, H.; Xu, Z.; Li, L.; Zhou, Y.; Liu, A.; et al. Genome-wide analysis and identification of the low potassium stress responsive gene SiMYB3 in foxtail millet (Setaria italica L.). BMC Genom. 2019, 20, 136. [Google Scholar] [CrossRef]
- Qi, X.; Xie, S.; Liu, Y.; Yi, F.; Yu, J. Genome-wide annotation of genes and noncoding RNAs of foxtail millet in response to simulated drought stress by deep sequencing. Plant Mol. Biol. 2013, 83, 459–473. [Google Scholar] [CrossRef]
- Feng, Z.J.; Xu, Z.S.; Sun, J.; Li, L.C.; Chen, M.; Yang, G.X.; He, G.Y.; Ma, Y.Z. Investigation of the ASR family in foxtail millet and the role of ASR1 in drought/oxidative stress tolerance. Plant Cell Rep. 2016, 35, 115–128. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, T.; Yi, F.; Yu, J. SimiR396d targets SiGRF1 to regulate drought tolerance and root growth in foxtail millet. Plant Sci. Int. J. Exp. Plant Biol. 2023, 326, 111492. [Google Scholar] [CrossRef]
- Luo, W.; Tang, Y.; Li, S.; Zhang, L.; Liu, Y.; Zhang, R.; Diao, X.; Yu, J. The m(6) A reader SiYTH1 enhances drought tolerance by affecting the messenger RNA stability of genes related to stomatal closure and reactive oxygen species scavenging in Setaria italica. J. Integr. Plant Biol. 2023, 65, 2569–2586. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, J.; Cao, G.; Xie, Y.; Liu, X.; Lu, M.; Wang, G. Overexpression of a PLDα1 gene from Setaria italica enhances the sensitivity of Arabidopsis to abscisic acid and improves its drought tolerance. Plant Cell Rep. 2010, 29, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Tang, W.; Wang, C.; Ge, L.; Sun, J.; Qi, X.; He, Z.; Zhou, Y.; Chen, J.; Xu, Z.; et al. SiMYB56 confers drought stress tolerance in transgenic rice by regulating lignin biosynthesis and ABA signaling pathway. Front. Plant Sci. 2020, 11, 785. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wei, Y.; Wang, Y.; Tan, B.; Chen, S.; Li, H.G. Genome-wide identification of LBD genes in foxtail millet (Setaria italica) and functional characterization of SiLBD21. Int. J. Mol. Sci. 2023, 23, 7110. [Google Scholar] [CrossRef] [PubMed]
- Li, W.W.; Chen, M.; Zhong, L.; Liu, J.M.; Xu, Z.S.; Li, L.C.; Zhou, Y.B.; Guo, C.H.; Ma, Y.Z. Overexpression of the autophagy-related gene SiATG8a from foxtail millet (Setaria italica L.) confers tolerance to both nitrogen starvation and drought stress in Arabidopsis. Biochem. Biophys. Res. Commun. 2015, 468, 800–806. [Google Scholar] [CrossRef]
- Nachimuthu, V.V.; Pandian, B.A.; Robin, S. Role of reactive oxygen species in water-deficit stress response. In Reactive Oxygen Species and Antioxidant Systems in Plants: Role and Regulation Under Abiotic Stress; Springer: Singapore, 2017; pp. 283–295. [Google Scholar]
- Borges, C.V.; Orsi, R.O.; Maraschin, M.; Lima, G.P.P. Oxidative stress in plants and the biochemical response mechanisms. In Plant Stress Mitigators; Elsevier: Amsterdam, The Netherlands, 2023; pp. 455–468. [Google Scholar]
- Avashthi, H.; Pathak, R.K.; Gaur, V.S.; Singh, S.; Gupta, V.K.; Ramteke, P.W.; Kumar, A. Comparative analysis of ROS-scavenging gene families in finger millet, rice, sorghum, and foxtail millet revealed potential targets for antioxidant activity and drought tolerance improvement. Netw. Model. Anal. Health Inform. Bioinform. 2020, 9, 33. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Z.; Wang, X.; Tang, Y.; Li, X.; Ren, C.; Ren, J.; Wang, X.; Jiang, C.; Zhong, C.; et al. Transcriptome-based analysis of key pathways relating to yield formation stage of foxtail millet under different drought stress conditions. Front. Plant Sci. 2023, 13, 910. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Li, Z.; Han, X.; Song, X.; Zhang, Y.; Jiang, J.; Han, Q.; Liu, M.; Qiao, G.; Zhuo, R. Overexpressing the sedum alfredii Cu/Zn superoxide dismutase increased resistance to oxidative stress in transgenic Arabidopsis. Front. Plant Sci. 2017, 8, 1010. [Google Scholar] [CrossRef]
- Han, X.M.; Chen, Q.X.; Yang, Q.; Zeng, Q.Y.; Lan, T.; Liu, Y.J. Genome-wide analysis of superoxide dismutase genes in Larix kaempferi. Gene 2019, 686, 29–36. [Google Scholar] [CrossRef]
- Hsiao, T.C. Plant responses to water stress. Annu. Rev. Plant Physiol. 1973, 24, 519–570. [Google Scholar] [CrossRef]
- Boominathan, P.; Geethanjali, S.; Sivakumar, R.; Vinothini, M. Physiological studies on effect of drought during flowering in foxtail millet (Setaria italica L.). Madras Agric. J. 2018, 105, 373–377. [Google Scholar] [CrossRef]
- Sapaki Suneetha, R.S.; Babu, K.; Thimma Naik, S. PEG-6000 mediated screening of foxtail millet (Setaria italica L.) varieties for drought tolerance. Bull. Environ. Pharmacol. Life Sci. 2022, 11, 116–122. [Google Scholar]
- Divya, K.; Mk, K.; Jeyakumar, P.; Kr, L.; Nirmala, K. Screening for drought and heat tolerance in foxtail millet by physiological and biochemical indices. J. Pharm. Innov. 2019, 8, 665–669. [Google Scholar]
- Ramesh, P.; Mallikarjuna, G.; Sameena, S.; Yugandhar, P.; Pandit, V.; Singh, R.K.; Reddy, P.C.O.; AKILA, C.S. Agro-morphological, Physiological and Biochemical Analysis of Foxtail Millet (Setaria italica L.) Landraces Along with Released Cultivars Under Induced Drought Stress. Available online: https://ssrn.com/abstract=4489736 (accessed on 20 November 2024). [CrossRef]
- Yang, R.; Chen, M.; Sun, J.C.; Yu, Y.; Min, D.H.; Chen, J.; Xu, Z.S.; Zhou, Y.B.; Ma, Y.Z.; Zhang, X.H. Genome-wide analysis of LIM family genes in foxtail millet (Setaria italica L.) and characterization of the role of SiWLIM2b in drought tolerance. Int. J. Mol. Sci. 2019, 20, 1303. [Google Scholar] [CrossRef]
- Chen, Y.E.; Mao, J.J.; Sun, L.Q.; Huang, B.; Ding, C.B.; Gu, Y.; Liao, J.Q.; Hu, C.; Zhang, Z.W.; Yuan, S.; et al. Exogenous melatonin enhances salt stress tolerance in maize seedlings by improving antioxidant and photosynthetic capacity. Physiol. Plant. 2018, 164, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Muchate, N.S.; Nikalje, G.C.; Rajurkar, N.S.; Suprasanna, P.; Nikam, T.D. Plant salt stress: Adaptive responses, tolerance mechanism and bioengineering for salt tolerance. Bot. Rev. 2016, 82, 371–406. [Google Scholar] [CrossRef]
- Patan, S.; Vallepu, S.; Shaik, K.B.; Shaik, N.; Adi Reddy, N.R.Y.; Terry, R.G.; Sergeant, K.; Hausman, J.F. Drought resistance strategies in minor millets: A review. Planta 2024, 260, 29. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terfa, G.N.; Pan, W.; Hu, L.; Hao, J.; Zhao, Q.; Jia, Y.; Nie, X. Mechanisms of Salt and Drought Stress Responses in Foxtail Millet. Plants 2025, 14, 1215. https://doi.org/10.3390/plants14081215
Terfa GN, Pan W, Hu L, Hao J, Zhao Q, Jia Y, Nie X. Mechanisms of Salt and Drought Stress Responses in Foxtail Millet. Plants. 2025; 14(8):1215. https://doi.org/10.3390/plants14081215
Chicago/Turabian StyleTerfa, Gemechu Nedi, Wenqiu Pan, Longjiao Hu, Junwei Hao, Qinlong Zhao, Yanzhe Jia, and Xiaojun Nie. 2025. "Mechanisms of Salt and Drought Stress Responses in Foxtail Millet" Plants 14, no. 8: 1215. https://doi.org/10.3390/plants14081215
APA StyleTerfa, G. N., Pan, W., Hu, L., Hao, J., Zhao, Q., Jia, Y., & Nie, X. (2025). Mechanisms of Salt and Drought Stress Responses in Foxtail Millet. Plants, 14(8), 1215. https://doi.org/10.3390/plants14081215







