Analysis of Variations in the Flavonoid Profiles of Cuscuta campestris and Cuscuta epithymum in Bulgaria as a Potential Chemotaxonomical Marker
Abstract
1. Introduction
2. Results
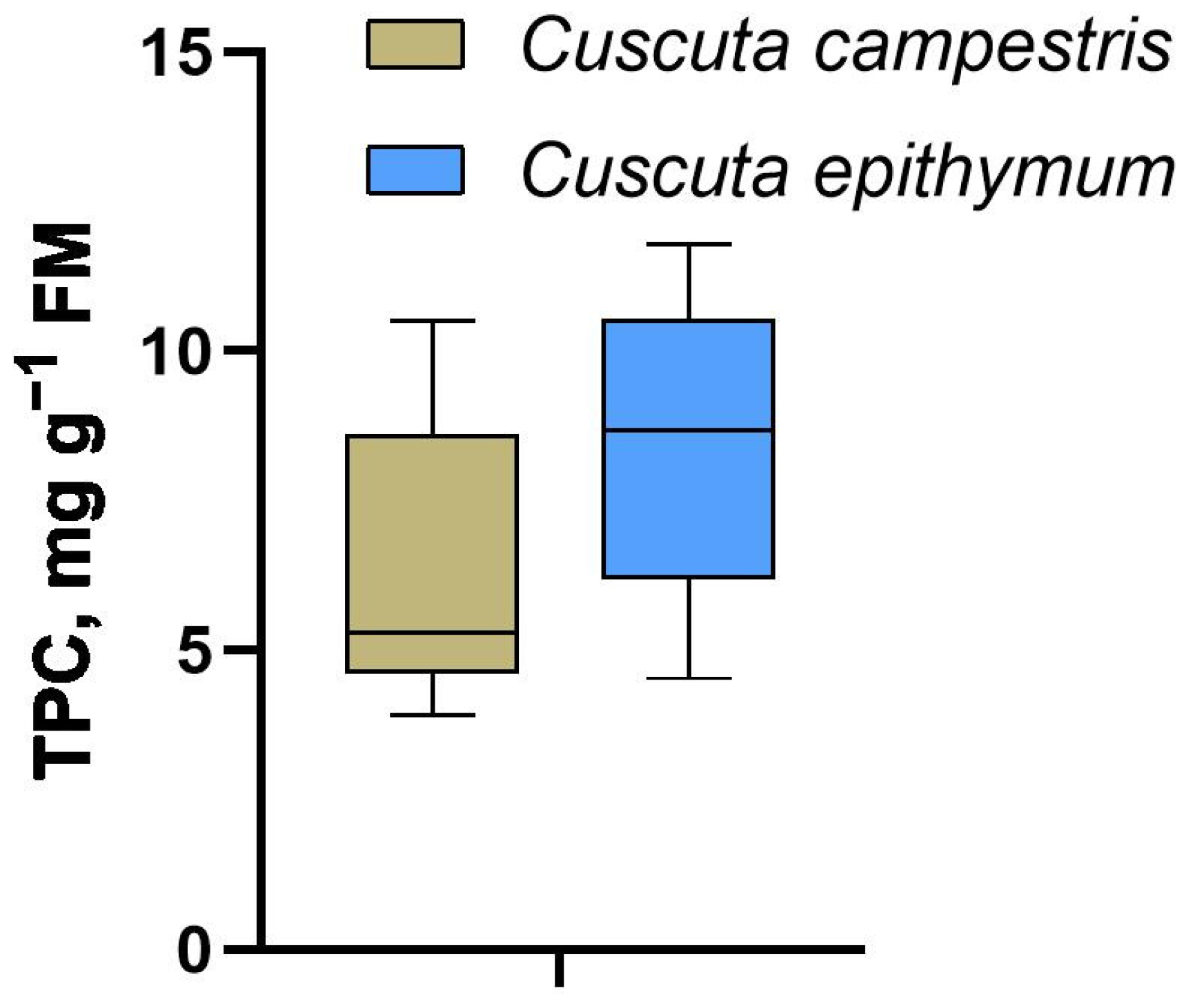
2.1. Total Polyphenolics
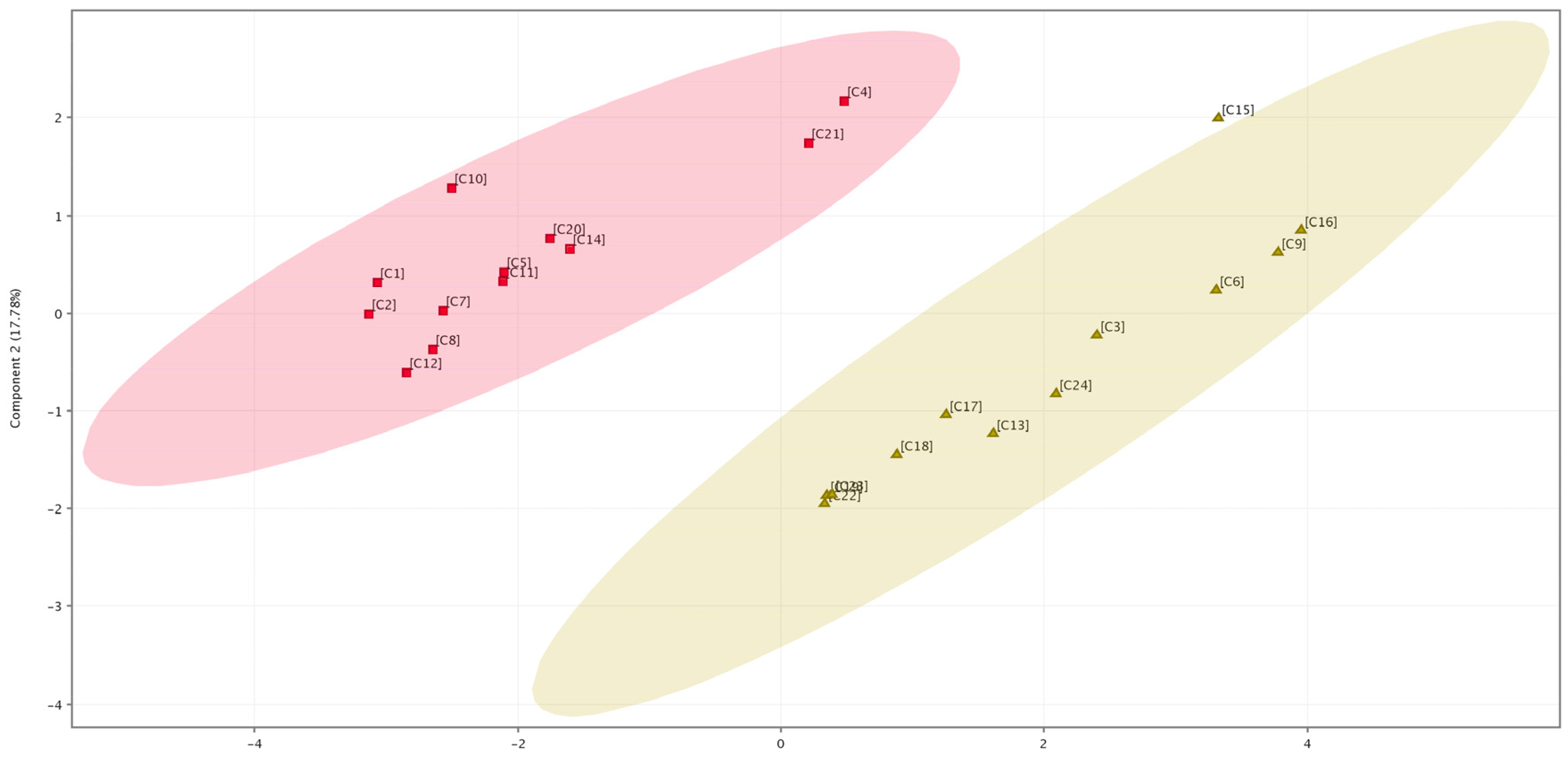
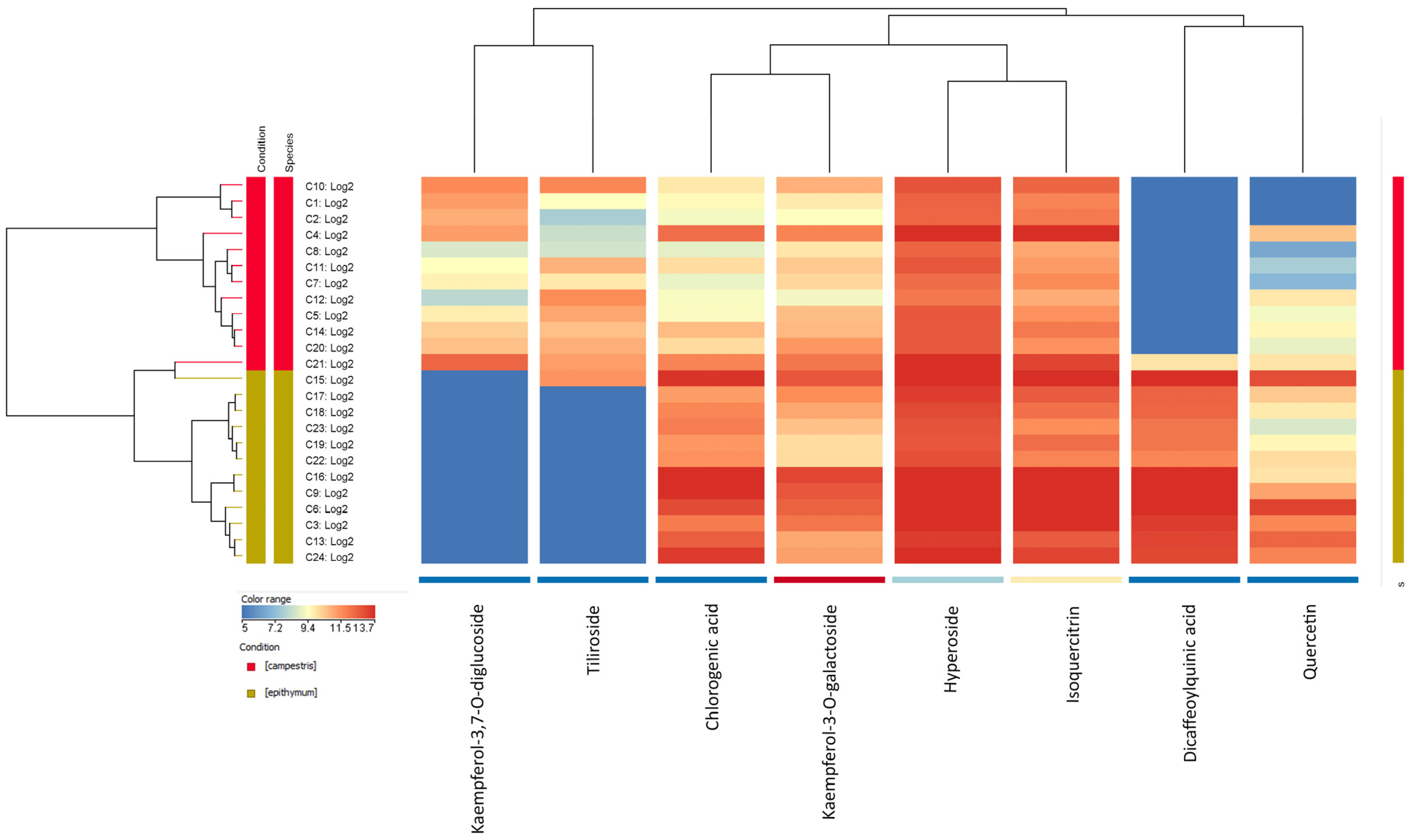
2.2. HPLC Analysis of Flavonoids
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Extraction and Polyphenol Determination
4.3. HPLC-MS Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Löffler, C.; Czygan, F.-C.; Proksch, P. Phenolic constituents as taxonomic markers in the genus Cuscuta (Cuscutaceae). Biochem. Syst. Ecol. 1997, 25, 297–303. [Google Scholar] [CrossRef]
- Watson, D.M.; McLellan, R.C.; Fontúrbel, F.E. Functional roles of parasitic plants in a warming world. Annu. Rev. Ecol. Evol. Syst. 2022, 53, 25–45. [Google Scholar] [CrossRef]
- Heide-Jørgensen, H. Parasitic Flowering Plants; Brill: Buckinghamshire, UK, 2008. [Google Scholar]
- Assyov, B.; Petrova, A. Conspectus of the Vascular Plants in Bulgaria; Bulgarian Biodiversity Foundation: Sofia, Bulgaria, 2012; pp. 1–489. [Google Scholar]
- Stoyanov, K.; Raycheva, T.; Cheschmedzhiev, I. Key to the Native and Foreign Vascular Plants in Bulgaria; Agricultural University Plovdiv Academic Press: Plovdiv, Bulgaria, 2021. [Google Scholar]
- Parker, C. Parasitic weeds: A world challenge. Weed Sci. 2012, 60, 269–276. [Google Scholar] [CrossRef]
- Marinov-Serafimov, P.; Golubinova, I.; Kertikova, D.; Kertikov, T. Opportunities of use of the Segador (organic fertilizer with contact herbicidal effect) for control of dodder (Cuscuta epithymum L.) in alfalfa (Medicago sativa L.). J. Mt. Agric. Balk. 2018, 21, 84–94. [Google Scholar]
- Ahmad, A.; Tandon, S.; Xuan, T.D.; Nooreen, Z. A Review on Phytoconstituents and Biological activities of Cuscuta species. Biomed. Pharmacother. 2017, 92, 772–795. [Google Scholar] [CrossRef]
- Nabavi, S.M.; Šamec, D.; Tomczyk, M.; Milella, L.; Russo, D.; Habtemariam, S.; Suntar, I.; Rastrelli, L.; Daglia, M.; Xiao, J. Flavonoid biosynthetic pathways in plants: Versatile targets for metabolic engineering. Biotechnol. Adv. 2020, 38, 107316. [Google Scholar] [CrossRef]
- He, X.; Yang, W.; Ye, M.; Wang, Q.; Guo, D. Differentiation of Cuscuta chinensis and Cuscuta australis by HPLC-DAD-MS analysis and HPLC-UV quantitation. Planta Medica 2011, 77, 1950–1957. [Google Scholar] [CrossRef]
- Wink, M.; Witte, L. Quinolizidine alkaloids in Genista acanthoclada and its holoparasite, Cuscuta palaestina. J. Chem. Ecol. 1993, 19, 441–448. [Google Scholar] [CrossRef]
- Tanruean, K.; Poolprasert, P.; Kumla, J.; Suwannarach, N.; Lumyong, S. Bioactive compounds content and their biological properties of acetone extract of Cuscuta reflexa Roxb. grown on various host plants. Nat. Prod. Res. 2019, 33, 544–547. [Google Scholar] [CrossRef]
- Ramezan, D.; Farrokhzad, Y.; Zargar, M.; Stybayev, G.; Kipshakbayeva, G.; Baitelenova, A. An Insight into Cuscuta campestris as a Medicinal Plant: Phytochemical Variation of Cuscuta campestris with Various Host Plants. Agriculture 2023, 13, 770. [Google Scholar] [CrossRef]
- Flores-Sánchez, I.J.; Garza-Ortiz, A. Is there a secondary/specialized metabolism in the genus Cuscuta and which is the role of the host plant? Phytochem. Rev. 2019, 18, 1299–1335. [Google Scholar] [CrossRef]
- Stojanov, N.; Kitanov, B. Wild Useful Plants in Bulgaria; Bulgarian Academy of Sciences: Sofia, Bulgaria, 1960. [Google Scholar]
- Leporatti, M.L.; Ivancheva, S. Preliminary comparative analysis of medicinal plants used in the traditional medicine of Bulgaria and Italy. J. Ethnopharmacol. 2003, 87, 123–142. [Google Scholar] [CrossRef] [PubMed]
- Biesaga, M. Influence of extraction methods on stability of flavonoids. J. Chromatogr. A 2011, 1218, 2505–2512. [Google Scholar] [CrossRef] [PubMed]
- Li ZhuanHong, L.Z.; Guo Han, G.H.; Xu WenBin, X.W.; Ge Juan, G.J.; Li Xin, L.X.; Alimu, M.; He DaJun, H.D. Rapid identification of flavonoid constituents directly from PTP1B inhibitive extract of raspberry (Rubus idaeus L.) leaves by HPLC-ESI-QTOF-MS-MS. J. Chromatogr. Sci. 2016, 54, 805–810. [Google Scholar]
- Rusanov, K.; Garo, E.; Rusanova, M.; Fertig, O.; Hamburger, M.; Atanassov, I.; Butterweck, V. Recovery of polyphenols from rose oil distillation wastewater using adsorption resins—A pilot study. Planta Medica 2014, 80, 1657–1664. [Google Scholar] [CrossRef]
- Tallini, L.R.; Pedrazza, G.P.; Bordignon, S.A.d.L.; Costa, A.C.; Steppe, M.; Fuentefria, A.; Zuanazzi, J.A. Analysis of flavonoids in Rubus erythrocladus and Morus nigra leaves extracts by liquid chromatography and capillary electrophoresis. Rev. Bras. Farmacogn. 2015, 25, 219–227. [Google Scholar] [CrossRef]
- Jang, G.H.; Kim, H.W.; Lee, M.K.; Jeong, S.Y.; Bak, A.R.; Lee, D.J.; Kim, J.B. Characterization and quantification of flavonoid glycosides in the Prunus genus by UPLC-DAD-QTOF/MS. Saudi J. Biol. Sci. 2018, 25, 1622–1631. [Google Scholar] [CrossRef]
- López-Angulo, G.; Montes-Avila, J.; Díaz-Camacho, S.P.; Vega-Aviña, R.; López-Valenzuela, J.Á.; Delgado-Vargas, F. Comparison of terpene and phenolic profiles of three wild species of Echeveria (Crassulaceae). J. Appl. Bot. Food Qual. 2018, 91, 145–154. [Google Scholar]
- Dai, L.; Zhu, Q.; Turak, A.; Yusuf, A.; Abudurexiti, A. Flavonoids from the Whole Plant of Cuscuta chinensis and Their Activities. Chem. Nat. Compd. 2023, 59, 537–539. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Meng, Y.; Zhai, C.; Wang, M.; Avula, B.; Yuk, J.; Smith, K.M.; Isaac, G.; Khan, I.A. The Chemical Characterization of Eleutherococcus senticosus and Ci-wu-jia Tea using UHPLC-UV-QTOF/MS. Int. J. Mol. Sci. 2019, 20, 475. [Google Scholar] [CrossRef]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical scheme for LC-MS n identification of chlorogenic acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef] [PubMed]
- Palaiogiannis, D.; Chatzimitakos, T.; Athanasiadis, V.; Bozinou, E.; Makris, D.P.; Lalas, S.I. Successive solvent extraction of polyphenols and flavonoids from Cistus creticus L. leaves. Oxygen 2023, 3, 274–286. [Google Scholar] [CrossRef]
- Xu, M.; Ran, L.; Chen, N.; Fan, X.; Ren, D.; Yi, L. Polarity-dependent extraction of flavonoids from citrus peel waste using a tailor-made deep eutectic solvent. Food Chem. 2019, 297, 124970. [Google Scholar] [CrossRef] [PubMed]
- Chaves, N.; Escudero, J. Variation of flavonoid synthesis induced by ecological factors. In Principles and Practices in Plant Ecology; CRC Press: Boca Raton, FL, USA, 1999; pp. 267–285. [Google Scholar]
- Jaakola, L.; Hohtola, A. Effect of latitude on flavonoid biosynthesis in plants. Plant Cell Environ. 2010, 33, 1239–1247. [Google Scholar] [CrossRef]
- Barath, K.; Csiky, J. Host range and host choice of Cuscuta species in Hungary. Acta Bot. Croat. 2012, 71, 215–227. [Google Scholar] [CrossRef]
- Koch, A.M.; Binder, C.; Sanders, I.R. Does the generalist parasitic plant Cuscuta campestris selectively forage in heterogeneous plant communities? New Phytol. 2004, 162, 147–155. [Google Scholar] [CrossRef]
- García, M.A.; Costea, M.; Kuzmina, M.; Stefanović, S. Phylogeny, character evolution, and biogeography of Cuscuta (dodders; Convolvulaceae) inferred from coding plastid and nuclear sequences. Am. J. Bot. 2014, 101, 670–690. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Zhang, Y.; Xiong, H.; Xu, X.; Xue, X.; Liu, M.; Xu, S.; Liu, H.; Gao, Y.; Zhang, H.; Li, X. Compounds identification in semen cuscutae by ultra-high-performance liquid chromatography (UPLCs) coupled to electrospray ionization mass spectrometry. Molecules 2018, 23, 1199. [Google Scholar] [CrossRef]

| Cuscuta campestris | Cuscuta epithymum | ||||||
|---|---|---|---|---|---|---|---|
| Sample № | Locality | GPS Coordinates, N, E | Main Parasitized Species | Sample № | Locality | GPS Coordinates, N, E | Main Parasitized Species |
| C1 | St. Nicholas, Chernomorets | 42.44795, 27.64192 | Xanthium italicum Moretti. | C3 | Oreshak | 42.88511, 24.76739 | Not determined |
| C2 | Asenovgrad | 42.01476, 24.87629 | Polygonum aviculare L. | C6 | Potochnitsa | 41.61129, 25.68116 | Genista rumelica Velen. |
| C4 | Lozenets | 42.21749, 27.78829 | Peucedanum obtusifolium Sm. | C9 | Dragoman | 42.94693, 22.93126 | Teucrium chamaedrys L. |
| C5 | Potochnitsa | 41.61120, 25.68446 | Paliurus spina-christi Mill. | C13 | Novo Leski | 41.52947, 23.77320 | Teucrium chamaedrys |
| C7 | Karlanovo | 41.54416, 23.41674 | Polygonum aviculare | C15 | Smochevo | 42.13189, 23.10084 | Chondrilla juncea |
| C8 | Mesta | 41.79720, 23.66108 | Cichorium intybus L. | C16 | Seslavtsi | 42.77921, 23.52794 | Astragalus onobrychis L. |
| C10 | Disevitsa | 43.42416, 24.50320 | Chondrilla juncaea L. | C17 | Smochevo | 42.13825, 23.07065 | Sanguisorba officinalis L. |
| C11 | Dobrinishte | 41.82174, 23.56970 | Plantago lanceolata L. | C18 | Smochevo | 42.14144, 23.06889 | Nigella damascena L. |
| C12 | Novo Leski | 41.53070, 23.77537 | Daucus carota subsp. Sativus L. | C19 | Smochevo | 42.14144, 23.06889 | Pinus sylvestris L. |
| C14 | Smochevo | 42.13189, 23.10084 | Chondrilla juncaea | C22 | Mesta | 41.75817, 23.67263 | Potentilla argentea L. |
| C20 | Bebresh | 43.00671, 23.82172 | Xanthium italicum | C23 | Mesta | 41.75817, 23.67263 | Astragalus onobrychis |
| C21 | Nova Lovcha | 41.42828, 23.73052 | Artemisia campestris L. | C24 | Dragoman | 42.94693, 22.93126 | Artemisia alba Asso. |
| Solvent | Cuscuta campestris | Cuscuta epithymum |
|---|---|---|
| 40% aq. MetOH | 6.30 ± 1.4 a | 4.40 ± 0.1 b |
| 40% aq. EtOH | 7.85 ± 0.3 a | 4.19 ± 0.6 b |
| 70% aq. MetOH | 5.06 ± 0.1 b | 6.81 ± 1.0 a |
| 70% aq. EtOH | 4.00 ± 0.2 b | 5.87 ± 0.7 ab |
| 100% MetOH | 7.79 ± 1.0 a | 8.37 ± 0.8 a |
| 100% EtOH | 3.05 ± 0.3 b | 9.45 ± 1.5 a |
| Cuscuta campestris | Cuscuta epithymum | ||
|---|---|---|---|
| Sample № | TPC, mg g−1 FW | Sample № | TPC, mg g−1 FW |
| C1 | 3.91 ± 0.4 a | C3 | 9.64 ± 0.5 b |
| C2 | 4.93 ± 0.7 a | C6 | 11.79 ± 0.5 b |
| C4 | 9.83 ± 0.7 b | C9 | 10.89 ± 0.8 b |
| C5 | 6.32 ± 0.6 ab | C13 | 10.24 ± 0.7 b |
| C7 | 4.58 ± 0.3 a | C15 | 9.33 ± 0.5 b |
| C8 | 4.07 ± 0.2 a | C16 | 10.61 ± 1.0 b |
| C10 | 9.35 ± 1.0 b | C17 | 7.33 ± 0.5 ab |
| C11 | 5.10 ± 0.3 a | C18 | 7.12 ± 0.6 ab |
| C12 | 4.78 ± 0.4 a | C19 | 5.88 ± 0.2 a |
| C14 | 6.00 ± 0.4 a | C22 | 5.89 ± 0.3 a |
| C20 | 5.50 ± 0.7 a | C23 | 4.53 ± 0.6 a |
| C21 | 10.51 ± 0.2 b | C24 | 8.04 ± 0.5 b |
| Peak | tR (min) | Annotated Compounds | Trivial Name | UV λ Max (nm) | [M-H]− m/z | MS2 |
|---|---|---|---|---|---|---|
| 1 | 18.5 | Chlorogenic acid | 218, 300, 325 | 353.0858 | 191.0562, 161.0242 | |
| 2 | 21.56 | Kaempferol-3,7-O-diglucoside | 265, 345 | 609.1439 | 447.0890, 285.0392, 489.1018, 327.0496 | |
| 3 | 26.572 | Quercetin-3-O-galactoside | Hyperoside | 255, 355 | 463.0853 | 300.0268, 271.0240, 178.9980 |
| 4 | 26.94 | Quercetin-3-O-glucoside | Isoquercitrin | 255, 355 | 463.0852 | 300.0268, 271.02839, 178.9980 |
| 5 | 28.16 | Dicaffeoylquinic acid | 300sh, 327 | 515.116 | 353.0868, 191.0556, 135.0449 | |
| 6 | 28.303 | Kaempferol-3-O-galactoside | 265, 343 | 447.0902 | 284.0318, 255.0291, 227.0343, 327.0499, 151.0032 | |
| 7 | 29.079 | Kaempferol 3-O-glucoside | Astragalin | 265, 350 | 447.0902 | 284.0318, 255.0290, 227.0343, 327.0500, 151.0032 |
| 8 | 29.146 | Isorhamnetin-7-glucoside | 255, 353 | 477.1005 | 314.0422, 315.0480, 271.0237, 243.0291, 285.0397 | |
| 9 | 29.58 | Isorhamnetin-3-O-glucoside | 255, 345 | 477.1006 | 314.0422, 315.0476, 271.0240, 243.0292, 285.0395 | |
| 10 | 34.644 | Quercetin | 255, 370 | 301.0336 | 151.0034, 178.9980, 273.0397 | |
| 11 | 37.268 | Kaempferol 3-O-β-(6′′-O-trans-p-coumaroyl)-glucopyranoside | Tiliroside | 268, 315 | 593.1281 | 284.0311, 447.0902 |
| 12 | 38.872 | Kaempferol | 265, 366 | 285.0389 | 151.0033, 229.0501, 257.0448, 185.0603 | |
| 13 | 39.657 | Isorhamnetin | 256, 368 | 315.0492 | 300.0267, 151.0032, 164.0112, 271.0241, 107.0135 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chakarova, B.; Zagorchev, L.; Pachedjieva, K.; Tosheva, A.; Zagorcheva, T.; Rusanov, K.; Teofanova, D. Analysis of Variations in the Flavonoid Profiles of Cuscuta campestris and Cuscuta epithymum in Bulgaria as a Potential Chemotaxonomical Marker. Plants 2025, 14, 1220. https://doi.org/10.3390/plants14081220
Chakarova B, Zagorchev L, Pachedjieva K, Tosheva A, Zagorcheva T, Rusanov K, Teofanova D. Analysis of Variations in the Flavonoid Profiles of Cuscuta campestris and Cuscuta epithymum in Bulgaria as a Potential Chemotaxonomical Marker. Plants. 2025; 14(8):1220. https://doi.org/10.3390/plants14081220
Chicago/Turabian StyleChakarova, Bilyana, Lyuben Zagorchev, Kalina Pachedjieva, Anita Tosheva, Tzvetelina Zagorcheva, Krasimir Rusanov, and Denitsa Teofanova. 2025. "Analysis of Variations in the Flavonoid Profiles of Cuscuta campestris and Cuscuta epithymum in Bulgaria as a Potential Chemotaxonomical Marker" Plants 14, no. 8: 1220. https://doi.org/10.3390/plants14081220
APA StyleChakarova, B., Zagorchev, L., Pachedjieva, K., Tosheva, A., Zagorcheva, T., Rusanov, K., & Teofanova, D. (2025). Analysis of Variations in the Flavonoid Profiles of Cuscuta campestris and Cuscuta epithymum in Bulgaria as a Potential Chemotaxonomical Marker. Plants, 14(8), 1220. https://doi.org/10.3390/plants14081220







