Integrated Phenotypic and Molecular Evaluation of Powdery Mildew Resistance in Egyptian Barley: Identification of Resistance-Associated Markers
Abstract
1. Introduction
2. Results
2.1. Differential Symptom Progression in Barley Genotypes Challenged with Powdery Mildew at the Seedling Stage
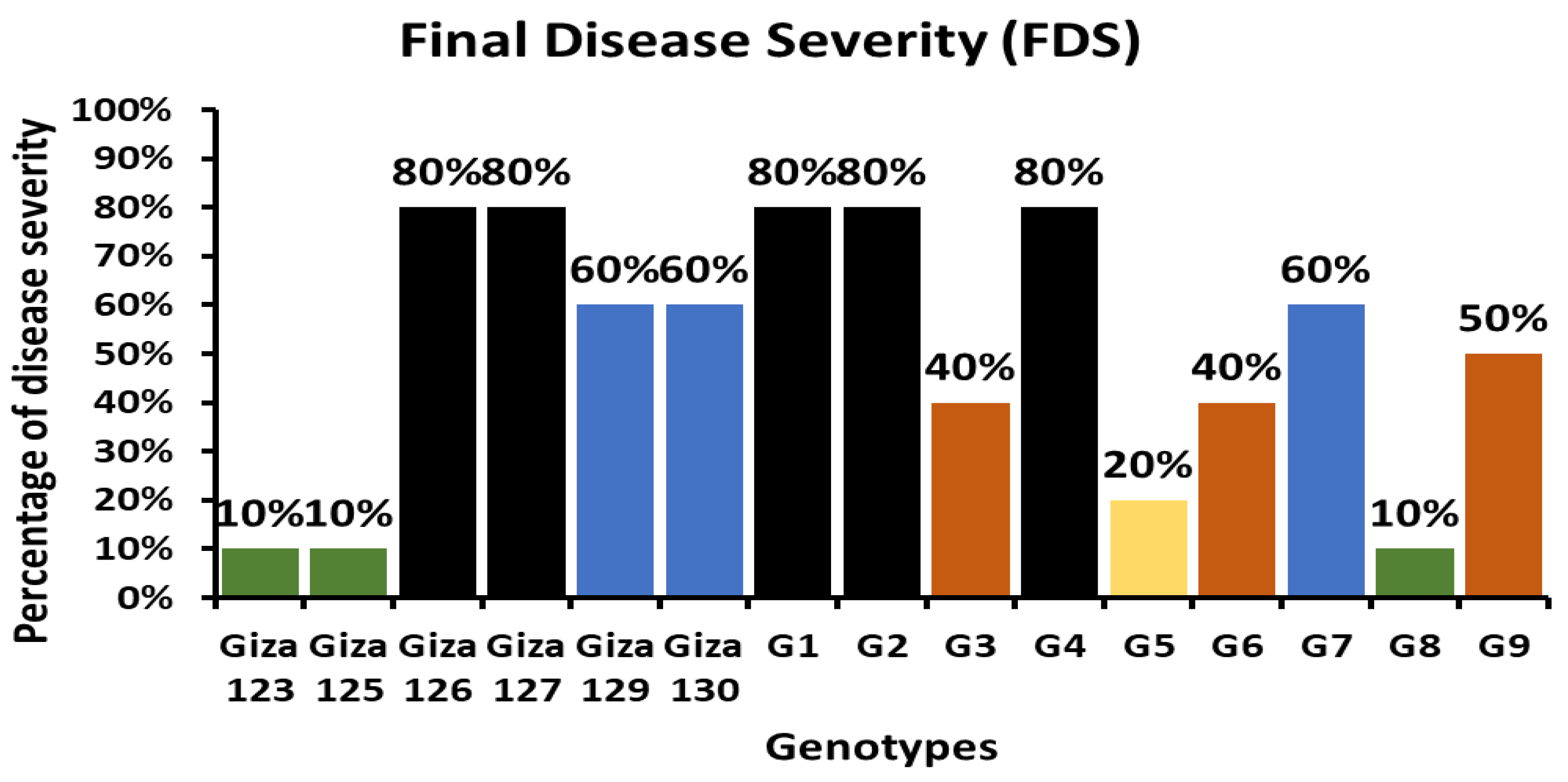
2.2. Field-Based Evaluation of Powdery Mildew Resistance in Egyptian Barley Genotypes
2.3. AUDPC-Based Assessment of Powdery Mildew Resistance
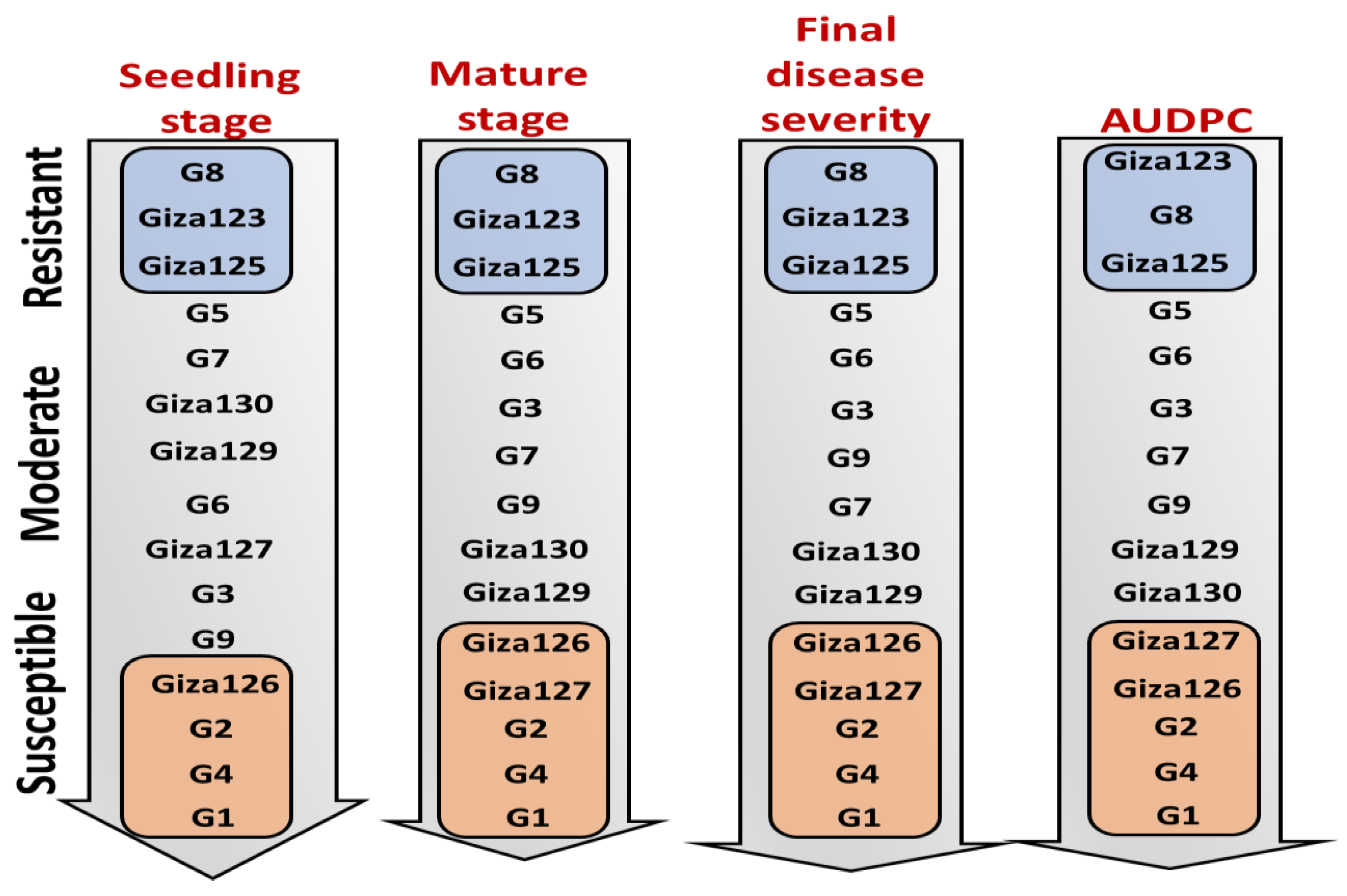
2.4. Resistance–Susceptibility Gradients to Powdery Mildew in Tested Genotypes
2.5. SSR Unveiled Band-Associated Resistance to Powdery Mildew in Barley Genotypes
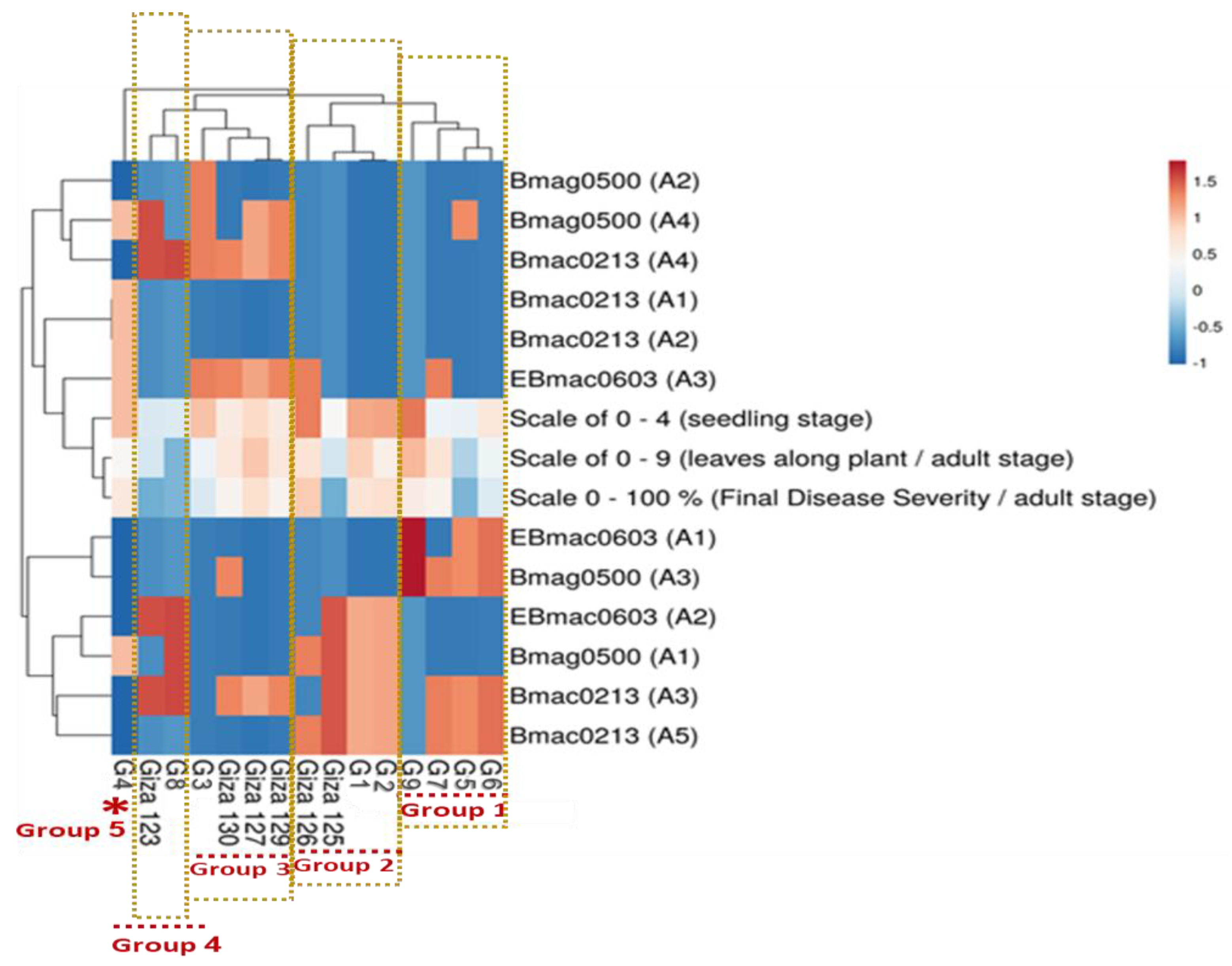
2.6. Integrated Analysis of Egyptian Genotypes
3. Discussion
4. Materials and Methods
4.1. Experimental Sites
4.2. Plant Materials
4.3. Seedling Test
4.4. Seedling-Stage Evaluation of Powdery Mildew Resistance
4.5. Maturity Stage Test
4.6. Evaluation of Maturity Stage Test
4.7. Hierarchical Clustering and Heatmap Visualization
4.8. Genomic DNA Extraction
4.9. SSR-PCR
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohamed, A.H.; Omar, A.A.; Attya, A.M.; Elashtokhy, M.M.A.; Zayed, E.M.; Rizk, R.M. Morphological and Molecular Characterization of Some Egyptian Six-Rowed Barley (Hordeum vulgare L.). Plants 2021, 10, 2527. [Google Scholar] [CrossRef]
- Czembor, J.H.; Czembor, E. Mlo Resistance to Powdery Mildew (Blumeria graminis f. sp. hordei) in Barley Landraces Collected in Yemen. Agronomy 2021, 11, 1582. [Google Scholar] [CrossRef]
- Guo, J.; Zhao, C.; Gupta, S.; Platz, G.; Snyman, L.; Zhou, M. Genome-wide association mapping for seedling and adult resistance to powdery mildew in barley. Theor. Appl. Genet. 2024, 137, 50. [Google Scholar] [CrossRef] [PubMed]
- Jankovics, T.; Komáromi, J.; Fábián, A.; Jäger, K.; Vida, G.; Kiss, L. New insights into the life cycle of the wheat powdery mildew: Direct observation of ascosporic infection in Blumeria graminis f. sp. tritici. Phytopathology 2015, 105, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, A.; Zhang, C.; Xu, F.; Liu, W.; Fan, J.; Ma, Z.; Zhou, Y. Key infection stages defending heat stress in high-temperature-resistant Blumeria graminis f. sp. tritici isolates. Front. Microbiol. 2022, 13, 1045796. [Google Scholar] [CrossRef]
- Liu, M.; Braun, U.; Takamatsu, S.; Hambleton, S.; Shoukouhi, P.; Bisson, K.R.; Hubbard, K. Taxonomic revision of Blumeria based on multi-gene DNA sequences, host preferences and morphology. Mycoscience 2021, 62, 143–165. [Google Scholar] [CrossRef]
- Dreiseitl, A. Powdery Mildew Resistance Genes in European Barley Cultivars Registered in the Czech Republic from 2016 to 2020. Genes 2022, 13, 1274. [Google Scholar] [CrossRef] [PubMed]
- Hautsalo, J.; Novakazi, F.; Jalli, M.; Göransson, M.; Manninen, O.; Isolahti, M.; Reitan, L.; Bergersen, S.; Krusell, L.; Damsgård Robertsen, C.; et al. Pyramiding of scald resistance genes in four spring barley MAGIC populations. Theor. Appl. Genet. 2021, 134, 3829–3843. [Google Scholar] [CrossRef]
- Collard, B.C.; Mackill, D.J. Marker-assisted selection: An approach for precision plant breeding in the twenty-first century. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 557–572. [Google Scholar] [CrossRef]
- Mohammadi, S.A.; Abdollahi Sisi, N.; Sadeghzadeh, B. The influence of breeding history, origin and growth type on population structure of barley as revealed by SSR markers. Sci. Rep. 2020, 10, 19165. [Google Scholar] [CrossRef]
- Hickey, L.T.; Lawson, W.; Platz, G.J.; Dieters, M.; Arief, V.N.; Germán, S.; Fletcher, S.; Park, R.F.; Singh, D.; Pereyra, S.; et al. Mapping Rph20: A gene conferring adult plant resistance to Puccinia hordei in barley. Theor. Appl. Genet. 2011, 123, 55–68. [Google Scholar] [CrossRef]
- Mains, E.B.; Dietz, S.M. Physiologic Forms of Barley Mildew, Erysiphe Graminis Hordei Marchal. Phytopathology 1930, 20, 229–239. [Google Scholar]
- Esmail, S.M.; Jarquín, D.; Börner, A.; Sallam, A. Genome-wide association mapping highlights candidate genes and immune genotypes for net blotch and powdery mildew resistance in barley. Comput. Struct. Biotechnol. J. 2023, 21, 4923–4932. [Google Scholar] [CrossRef] [PubMed]
- Büschges, R.; Hollricher, K.; Panstruga, R.; Simons, G.; Wolter, M.; Frijters, A.; van Daelen, R.; van der Lee, T.; Diergaarde, P.; Groenendijk, J.; et al. The barley Mlo gene: A novel control element of plant pathogen resistance. Cell 1997, 88, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Koebner, R.; Summers, R. The impact of molecular markers on the wheat breeding paradigm. Cell Mol. Biol. Lett. 2002, 7, 695–702. [Google Scholar]
- Kamel, A.M.; Metwally, K.; Sabry, M.; Albalawi, D.A.; Abbas, Z.K.; Darwish, D.B.E.; Al-Qahtani, S.M.; Al-Harbi, N.A.; Alzuaibr, F.M.; Khalil, H.B. The Expression of Triticum aestivum Cysteine-Rich Receptor-like Protein Kinase Genes during Leaf Rust Fungal Infection. Plants 2023, 12, 2932. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.B. Genome-Wide Characterization and Expression Profiling of Phytosulfokine Receptor Genes (PSKRs) in Triticum aestivum with Docking Simulations of Their Interactions with Phytosulfokine (PSK): A Bioinformatics Study. Genes 2024, 15, 1306. [Google Scholar] [CrossRef]
- Arabi, M.I.E.; Jawhar, M.; Al-Shehadah, E. Identification of Barley Lines with Resistance to Powdery Mildew Based on Seedling and Adult Plant Responses. Acta Phytopathol. Entomol. Hung. 2020, 55, 3–10. [Google Scholar] [CrossRef]
- Zadoks, J.; Chang, T.T.; Konzak, C.F. A Decimal Code for the Growth Stages of Cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Arabi, M.I.E.; Jawhar, M. The Use of Cochliobolus sativus Culture Filtrates to Evaluate Barley Resistance to Spot Blotch. Sydowia 2012, 64, 13–18. [Google Scholar]
- Moseman, J.G.; Baenziger, P.S.; Kilpatrick, R.A. Genes Conditioning Resistance of Hordeum spontaneum to Erysiphe graminis f. sp. hordei. Crop Sci. 1981, 21, 229–232. [Google Scholar] [CrossRef]
- Peterson, R.F.; Campbell, A.B.; Hannah, A.E. A diagrammatic Scale for Estimating Rust Intensity on Leaves and Stems of Cereals. Can. J. Res. 1948, 26, 496–500. [Google Scholar] [CrossRef]
- Das, M.; Rajaram, S.; Kronstad, W.E.; Mundt, C.C.; Singh, R.P. Associations and Genetics of Three Components of Slow Rusting in Leaf Rust of Wheat. Euphytica 1993, 68, 99–109. [Google Scholar] [CrossRef]
- Pandey, H.N.; Menon, T.C.M.; Rao, M.V. A Simple Formula for Calculating Area Under Disease Progress Curve. Rachis 1989, 8, 38–39. [Google Scholar]
- Van der Plank, J.E. Plant Disease: Epidemics and Control; Academic Press: New York, NY, USA, 1963; p. 349. [Google Scholar]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef]
- Khoothiam, K.; Prapasawat, W.; Yosboonruang, A.; Rawangkan, A.; Phuangsri, C.; Rupprom, K.; Kraivuttinun, P.; Tanomsridachchai, W.; Suthienkul, O.; Siriphap, A. Prevalence, Antimicrobial Resistance, and Enterotoxin Gene Profiles of Staphylococcus aureus Isolated from Mobile Phones of the Food Vendors in Phayao Province, Thailand. Ann. Clin. Microbiol. Antimicrob. 2023, 22, 68. [Google Scholar] [CrossRef]
- Elakhdar, A.; EL-Sattar, M.A.; Amer, K.; Kumamaru, T. Genetic Diversity and Association Analysis Among Egyptian Barley (Hordeum vulgare L.) Genotypes with Different Adaptations to Saline Conditions Analyzed by SSR Markers. Aust. J. Crop Sci. 2016, 10, 637–645. [Google Scholar] [CrossRef]
- Marzougui, S.; Kharrat, M.; Younes, M.B. Assessment of Genetic Diversity and Population Structure of Tunisian Barley Accessions (Hordeum vulgare L.) Using SSR Markers. Acta Agrobot. 2020, 73, 7343. [Google Scholar] [CrossRef]
- Yumurtaci, S.H. Dissection of Barley Landraces Originated from Twelve Different Countries by Using Simple Sequence Repeats Markers. Tarım. Bilim. Derg. 2015, 21, 420–430. [Google Scholar] [CrossRef]
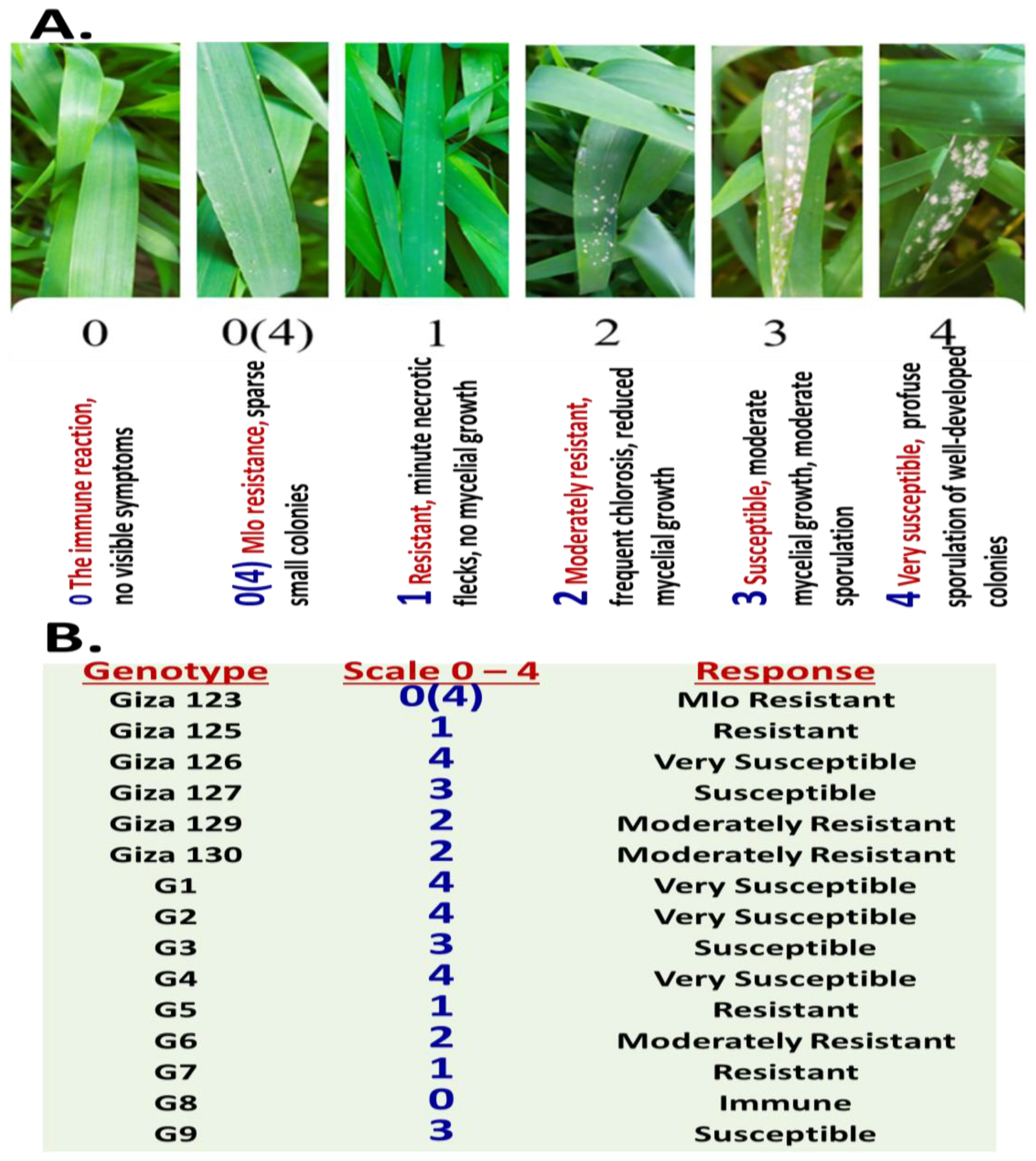

| Genotypes | Scale of IT0–9 (Leaves Along Plant) | ||
|---|---|---|---|
| 1st Read | 2nd Read | 3rd Read | |
| Giza 123 | 0 | 1 | 3 |
| Giza 125 | 1 | 1 | 3 |
| Giza 126 | 5 | 7 | 7 |
| Giza 127 | 5 | 7 | 9 |
| Giza 129 | 5 | 5 | 7 |
| Giza 130 | 5 | 5 | 7 |
| G1 | 7 | 7 | 9 |
| G2 | 7 | 7 | 7 |
| G3 | 3 | 5 | 5 |
| G4 | 7 | 7 | 7 |
| G5 | 3 | 3 | 3 |
| G6 | 3 | 5 | 5 |
| G7 | 3 | 4 | 7 |
| G8 | 1 | 1 | 1 |
| G9 | 3 | 7 | 7 |
| Genotypes | Final Disease Severity (0–100%) | ||
|---|---|---|---|
| 1st Read | 2nd Read | 3rd Read | |
| Giza 123 | 0% | 5% | 10% |
| Giza 125 | 5% | 10% | 10% |
| Giza 126 | 70% | 80% | 80% |
| Giza 127 | 50% | 70% | 80% |
| Giza 129 | 40% | 50% | 60% |
| Giza 130 | 40% | 50% | 60% |
| G1 | 70% | 80% | 80% |
| G2 | 70% | 80% | 80% |
| G3 | 30% | 40% | 40% |
| G4 | 70% | 80% | 80% |
| G5 | 10% | 20% | 20% |
| G6 | 30% | 40% | 40% |
| G7 | 20% | 40% | 60% |
| G8 | 5% | 10% | 10% |
| G9 | 40% | 50% | 50% |
| Genotypes | AUDPC | r-Value |
|---|---|---|
| Giza 123 | 301 | 0.0198 |
| Giza 125 | 385 | 0.0116 |
| Giza 126 | 3290 | 0.0084 |
| Giza 127 | 3010 | 0.0215 |
| Giza 129 | 2240 | 0.0126 |
| Giza 130 | 2240 | 0.0126 |
| G1 | 3360 | 0.0084 |
| G2 | 3360 | 0.0084 |
| G3 | 1680 | 0.0069 |
| G4 | 3360 | 0.0084 |
| G5 | 840 | 0.0126 |
| G6 | 1610 | 0.0069 |
| G7 | 1960 | 0.0278 |
| G8 | 385 | 0.0116 |
| G9 | 2030 | 0.0063 |
| Genotype | Row Type | Growth Habit | Flag Leaf | Pedigree |
|---|---|---|---|---|
| Giza 123 | Six | Semi-prostrate | Horizontal | Giza 117/FAO 86 |
| Giza 125 | Six | Semi-prostrate | Semi-drooping | Giza 117/Bahteem52//Giza118/FAO86 (sister line to Giza 124) |
| Giza 126 | Six | Semi-prostrate | Horizontal | Baladi Bahteem/S D729-Por12762-BC |
| Giza 127 | Two | Intermediate | Semi-erect | W12291/B0gs//Hamal-02 |
| Giza 129 | Six | Semi-prostrate | Semi-drooping | Deir Alla 106/Cel//As46/Aths * 2’’ Comp.cross” |
| Giza 130 | Six | Intermediate | Semi-erect | 229//Bco.Mr./DZ02391/3/Deir Alla 106 CM67B/CENTENO//CAMB/3/ROW906.73/4/GLORIABAR/COME-B/5/ |
| G1 | Six | Intermediate | Semi-erect | El Minya (Egy., GenBank, code No. 11331) |
| G2 | Six | Intermediate | Horizontal | El Minya (Egy., GenBank, code No. 11333) |
| G3 | Six | Intermediate | Semi-erect | El Minya (Egy., GenBank, code No. 11337) |
| G4 | Six | Semi-erect | Semi-erect | El Minya (Egy., GenBank, code No. 11338) |
| G5 | Six | Semi-erect | Erect | El Minya (Egy., GenBank, code No. 11342) |
| G6 | Six | Semi-prostrate | Semi-drooping | Alexandria (Egy., GenBank, code No. 11343) |
| G7 | Six | Intermediate | Horizontal | El Wadi El Gadid, El Dakhla, Mut Agricultural School |
| G8 | Two | Intermediate | Semi-erect | Kafr El-Sheikh—Muhammad Issa El Nataq |
| G9 | Six | Semi-prostrate | Semi-drooping | Kafr El-Sheikh—Wanis Nazih—Al-Rawda |
| Primer | Sequences (5′ to 3′) | References |
|---|---|---|
| EBmac0603-F | ACCGAAACTAAATGAACTACTTCG | Elakhdar et al. [28] |
| EBmac0603-R | TGCAAACTGTGCTATTAAGGG | |
| Bmag0500-F | GGGAACTTGCTAATGAAGAG | Marzougui et al. [29] |
| Bmag0500-R | AATGTAAGGGAGTGTCCATAG | |
| Bmac0213-F | ATGGATGCAAGACCAAAC | Yumurtaci [30] |
| Bmac0213-R | CTATGAGAGGTAGAGCAGCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Nabawy, M.H.M.; Najeeb, K.M.A.; Khalil, H.B.; Soliman, K.A.; El-Seoudy, A.A. Integrated Phenotypic and Molecular Evaluation of Powdery Mildew Resistance in Egyptian Barley: Identification of Resistance-Associated Markers. Plants 2025, 14, 1231. https://doi.org/10.3390/plants14081231
El Nabawy MHM, Najeeb KMA, Khalil HB, Soliman KA, El-Seoudy AA. Integrated Phenotypic and Molecular Evaluation of Powdery Mildew Resistance in Egyptian Barley: Identification of Resistance-Associated Markers. Plants. 2025; 14(8):1231. https://doi.org/10.3390/plants14081231
Chicago/Turabian StyleEl Nabawy, Mariam H. M., Khadegah M. A. Najeeb, Hala B. Khalil, Khaled A. Soliman, and Alia A. El-Seoudy. 2025. "Integrated Phenotypic and Molecular Evaluation of Powdery Mildew Resistance in Egyptian Barley: Identification of Resistance-Associated Markers" Plants 14, no. 8: 1231. https://doi.org/10.3390/plants14081231
APA StyleEl Nabawy, M. H. M., Najeeb, K. M. A., Khalil, H. B., Soliman, K. A., & El-Seoudy, A. A. (2025). Integrated Phenotypic and Molecular Evaluation of Powdery Mildew Resistance in Egyptian Barley: Identification of Resistance-Associated Markers. Plants, 14(8), 1231. https://doi.org/10.3390/plants14081231








