Description of Ficus carica L. Italian Cultivars II: Genetic and Chemical Analysis
Abstract
:1. Introduction
2. Results and Discussion
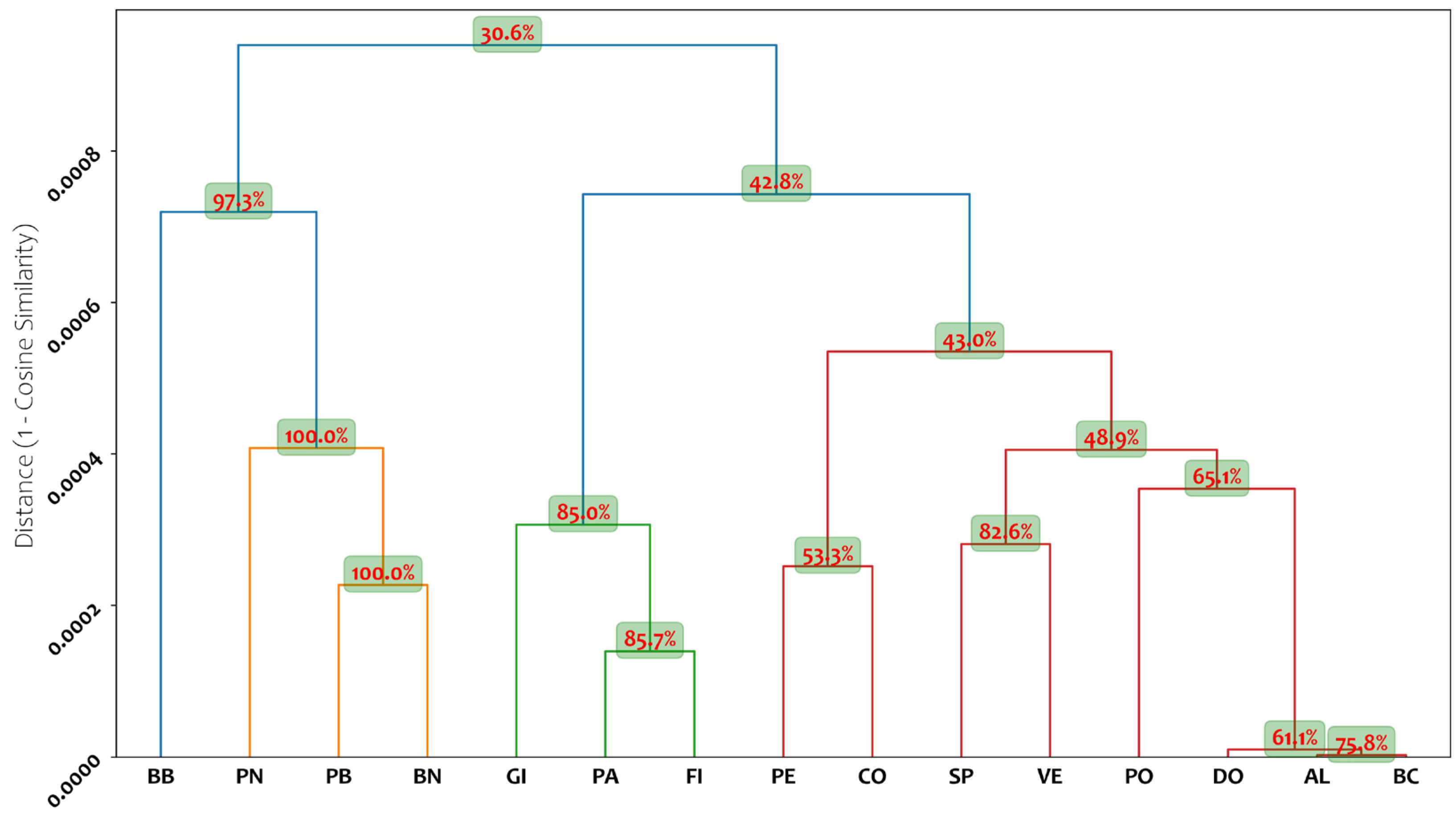
2.1. Genetic Analysis
2.2. Chemical Analysis
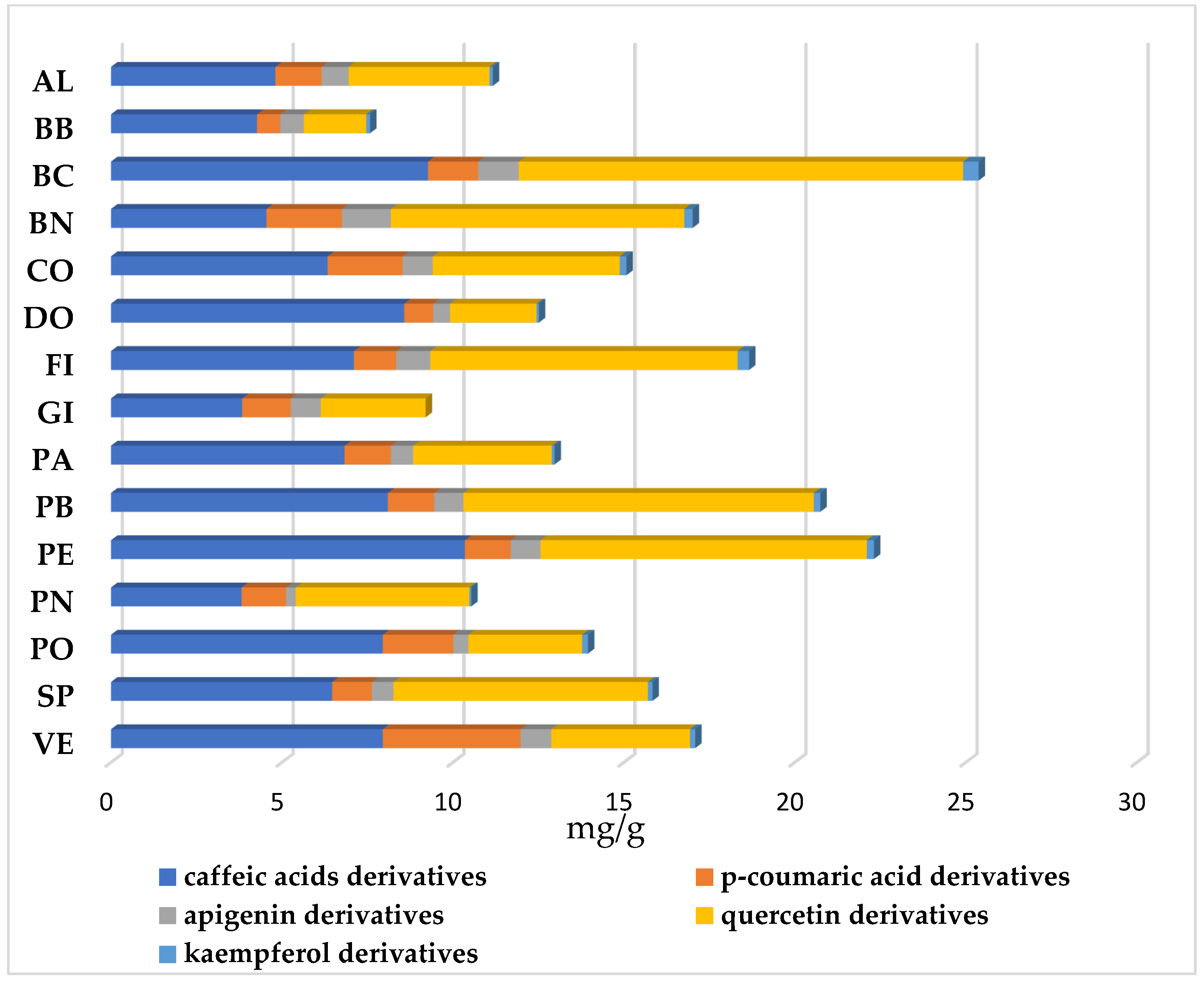
2.2.1. Identification of Phenolic Compounds
2.2.2. Total Polyphenol and Flavonoid Contents
2.2.3. Antioxidant Capacity (EC50) and ORAC Test
2.2.4. Analysis of Sugar Contents
2.3. PCA
3. Materials and Methods
3.1. Plant Material
3.2. Genetic Analysis
3.3. Chemical Analysis
3.3.1. Phenolics Extraction
3.3.2. HPLC-DAD-TOF-MS of Phenolics
3.3.3. Total Phenolic and Flavonoid Contents
3.3.4. Antioxidant Activity (DPPH Test)
3.3.5. Analysis of Sugar Content
3.4. Statistical Analysis
3.4.1. Molecular Analysis
3.4.2. Chemical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Falistocco, E. The World of Figs: An Overview. In Economically Important Trees: Origin, Evolution, Genetic Diversity and Ecology; Springer: Berlin/Heidelberg, Germany, 2024; pp. 261–298. [Google Scholar]
- Zohary, D.; Hopf, M.; Weiss, E. Domestication of Plants in the Old World: The Origin and Spread of Domesticated Plants n SouthwesAsia, Europe, and the Mediterranean Basin; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Goldschmidt, E.E. The Evolution of Fruit Tree Productivity: A Review. Econ. Bot. 2013, 67, 51–62. [Google Scholar] [CrossRef]
- Falistocco, E. The Millenary History of the Fig Tree (Ficus carica L.). Adv. Agric. Hortic. Entomol. 2020, 5, 130. [Google Scholar] [CrossRef]
- Mazzeo, A.; Magarelli, A.; Ferrara, G. The Fig (Ficus carica L.): Varietal Evolution from Asia to Puglia Region, Southeastern Italy. CABI Agric. Biosci. 2024, 5, 1–13. [Google Scholar]
- Nosir, W. Cultivars and Agriculture Practice of Fig (Ficus carica). In Fig (Ficus carica): Production, Processing and Properties; Ramadan, M.F., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 215–246. [Google Scholar]
- Hajam, T.A.; Saleem, H. Phytochemistry, Biological Activities, Industrial and Traditional Uses of Fig (Ficus carica): A Review. Chem. Biol. Interact. 2022, 368, 110237. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, H.; Waheed, R.; Nawaz, M. Phytochemical Composition, Antioxidant Potential, and Medicinal Significance of Ficus. Mod. Fruit. Ind. 2020, 1, 20. [Google Scholar]
- Khadivi, A.; Mirheidari, F. Phenotypic Variability of Fig (Ficus carica L.). In Fig (Ficus carica): Production, Processing, and Properties; Springer: Cham, Switzerland, 2023; pp. 129–174. [Google Scholar]
- Mafrica, R.; De Bruno, A.; Piscopo, A.; Poiana, M.; Bruno, M.; Caruso, T. Cultivar and Accessions of Fig (Ficus carica L.) for Breba Production Selected within the Autochthonous Germplasm of Calabria (South Italy). Acta Hortic. 2021, 1310, 29–34. [Google Scholar] [CrossRef]
- Nuzzo, V.; Gatto, A.; Montanaro, G. Morphological Characterization of Some Local Varieties of Fig (Ficus carica L.) Cultivated in Southern Italy. Sustainability 2022, 14, 15970. [Google Scholar] [CrossRef]
- Ayar, A.; Şahin, B.; Mutlu, D.; Özen, M.; Belge, A.; Karacaoğlan, Ç. Fig (Ficus carica var. domestica L.) Genetic resources Conservation and characterization. Uluslararası Doğu Anadolu Fen Mühendislik Ve Tasarım Dergisi 2023, 5, 1–19. [Google Scholar] [CrossRef]
- Uğur, R.; Gündeşli, M.A.; Özatar, H.O.; Özen, M.; Aras, S. Determination of Fruit Characteristics of Some Fig Genotypes (Ficus carica L.) Obtained by Selection Breeding in the Eastern Mediterranean Region. Int. J. Agric. Environ. Food Sci. 2023, 7, 508–516. [Google Scholar] [CrossRef]
- Gaaliche, B.; Saddoud, O.; Mars, M. Morphological and Pomological Diversity of Fig (Ficus carica L.) Cultivars in Northwest of Tunisia. Int. Sch. Res. Not. 2012, 2012, 326461. [Google Scholar] [CrossRef]
- Essid, A.; Aljane, F.; Neily, M.H.; Ferchichi, A.; Hormaza, J.I. Assessment of Genetic Diversity of Thirty Tunisian Fig (Ficus carica L.) Accessions Using Pomological Traits and SSR Markers. Mol. Biol. Rep. 2021, 48, 335–346. [Google Scholar] [CrossRef]
- Perez-Jiménez, M.; López, B.; Dorado, G.; Pujadas-Salvá, A.; Guzmán, G.; Hernandez, P. Analysis of Genetic Diversity of Southern Spain Fig Tree (Ficus carica L.) and Reference Materials as a Tool for Breeding and Conservation. Hereditas 2012, 149, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Gómez, D.; Legua, P.; Martínez-Nicolás, J.J.; Melgarejo, P. Breba Fruits Characterization from Four Varieties (Ficus carica L.) with Important Commercial Interest in Spain. Foods 2021, 10, 3138. [Google Scholar] [CrossRef]
- Giraldo, E.; López-Corrales, M.; Hormaza, J.I. Selection of the most discriminating morphological qualitative variables for characterization of fig germplasm. J. Am. Soc. Hort. Sci. 2010, 135, 240–249. [Google Scholar] [CrossRef]
- Ciarmiello, L.F.; Piccirillo, P.; Carillo, P.; De Luca, A.; Woodrow, P. Determination of the Genetic Relatedness of Fig (Ficus carica L.) Accessions Using RAPD Fingerprint and Their Agro-Morphological Characterization. S. Afr. J. Bot. 2015, 97, 40–47. [Google Scholar] [CrossRef]
- De Masi, L.; Castaldo, D.; Galano, G.; Minasi, P.; Laratta, B. Genotyping of Fig (Ficus carica L) via RAPD Markers. J. Sci. Food Agric. 2005, 85, 2235–2242. [Google Scholar] [CrossRef]
- Salhi Hannachi, A.; Chatti, K.; Marrakchi, M.; Trifi, M.; Mars, M. Specific Genetic Markers for Tunisian Fig Germplasm: Evidence of Morphological Traits, Random Amplified Polymorphic DNA and Inter Simple Sequence Repeats Markers [RAPD; ISSR; Ficus carica L.]. J. Genet. Breed. Italy 2003, 57, 125–136. [Google Scholar]
- Qurbanova, Q.; Babayeva, S.; Abbasov, M. Analysis of the Genetic Diversity of Azerbaijani Fig Accessions (Ficus carica L.) Using Pomological Traits and Inter Simple Sequence Repeat (ISSR) Markers. Genet. Resour. Crop Evol. 2025, 72, 1985–1998. [Google Scholar] [CrossRef]
- Uçer, V.A.; Aglar, E.; Mortazavi, P.; Qureshi, S.A.; Ali, A.; Tatar, M.; Altaf, M.T.; Bedir, M.; Ercişli, S.; Nadeem, M.A.; et al. Exploring Genetic Diversity of Turkish Fig (Ficus carica L.) Germplasm Using Inter-Primer Binding Site (iPBS) Retrotransposon Markers. Genet. Resour. Crop Evol. 2024, 1–12. [Google Scholar] [CrossRef]
- Sclavounos, A.; Roussos, P.; Milla, S.; Kostas, P.; Samaras, Y.; Pozzi, C.; Molla, J.; Chitikineni, A.; Varshney, R.K.; Voloudakis, A. Genetic Diversity of Fig (Ficus carica L.) Germplasm from the Mediterranean Basin as Revealed by SSR Markers. Genet. Resour. Crop Evol. 2023, 70, 1395–1406. [Google Scholar] [CrossRef]
- Ganopoulos, I.; Xanthopoulou, A.; Molassiotis, A.; Karagiannis, E.; Moysiadis, T.; Katsaris, P.; Aravanopoulos, F.; Tsaftaris, A.; Kalivas, A.; Madesis, P. Mediterranean Basin Ficus carica L.: From Genetic Diversity and Structure to Authentication of a Protected Designation of Origin Cultivar Using Microsatellite Markers. Trees 2015, 29, 1959–1971. [Google Scholar] [CrossRef]
- Akin, M.; Poljuha, D.; Eyduran, S.P.; Ercisli, S.; Radunic, M. SSR Based Molecular Characterization of Local Fig (Ficus carica L.) Germplasm in Northeastern Turkey. Erwerbs-Obstbau 2021, 63, 387–392. [Google Scholar] [CrossRef]
- Belttar, H.; Yahia, A.; Nemli, S.; Ates, D.; Erdogmus, S.; Ertan, B.; Himour, S.; Hepaksoy, S.; Tanyolac, M.B. Determination of the Population Structure of Fig Genotypes from Algeria and Turkey Using Inter Primer Binding Site-Retrotransposon and Simple Sequence Repeat Markers. Agric. Sci. 2017, 8, 1337. [Google Scholar] [CrossRef]
- Baraket, G.; Chatti, K.; Saddoud, O.; Abdelkarim, A.B.; Mars, M.; Trifi, M.; Hannachi, A.S. Comparative Assessment of SSR and AFLP Markers for Evaluation of Genetic Diversity and Conservation of Fig, Ficus carica L., Genetic Resources in Tunisia. Plant Mol. Biol. Rep. 2011, 29, 171–184. [Google Scholar] [CrossRef]
- Haffar, S.; Baraket, G.; Usai, G.; Aounallah, A.; Ben Mustapha, S.; Ben Abdelkrim, A.; Salhi Hannachi, A. Conserved DNA-Derived Polymorphism as a Useful Molecular Marker to Explore Genetic Diversity and Relationships of Wild and Cultivated Tunisian Figs (Ficus carica L.). Trees 2022, 36, 723–735. [Google Scholar] [CrossRef]
- Boudchicha, R.; Hormaza, J.; Benbouza, H. Diversity Analysis and Genetic Relationships among Local Algerian Fig Cultivars (Ficus carica L.) Using SSR Markers. S. Afr. J. Bot. 2018, 116, 207–221. [Google Scholar] [CrossRef]
- Rodolfi, M.; Ganino, T.; Chiancone, B.; Petruccelli, R. Identification and Characterization of Italian Common Figs (Ficus carica) Using Nuclear Microsatellite Markers. Genet. Resour. Crop Evol. 2018, 65, 1337–1348. [Google Scholar] [CrossRef]
- Ferrara, G.; Mazzeo, A.; Pacucci, C.; Matarrese, A.M.S.; Tarantino, A.; Crisosto, C.; Incerti, O.; Marcotuli, I.; Nigro, D.; Blanco, A.; et al. Characterization of Edible Fig Germplasm from Puglia, Southeastern Italy: Is the Distinction of Three Fig Types (Smyrna, San Pedro and Common) Still Valid? Sci. Hortic. 2016, 205, 52–58. [Google Scholar] [CrossRef]
- Giraldo, E.; López-Corrales, M.; Viruel, M.; Hormaza, J. Development of Microsatellite Markers in Fig (Ficus carica L.). In Proceedings of the XI Eucarpia Symposium on Fruit Breeding and Genetics, Angers, France, 1–5 September 2003; Volume 663, pp. 635–638. [Google Scholar]
- Ramawat, K.G. An Introduction to Biodiversity and Chemotaxonomy. Biodivers. Chemotaxon. 2019, 24, 1–14. [Google Scholar]
- Peters, K.; Blatt-Janmaat, K.L.; Tkach, N.; van Dam, N.M.; Neumann, S. Untargeted Metabolomics for Integrative Taxonomy: Metabolomics, DNA Marker-Based Sequencing, and Phenotype Bioimaging. Plants 2023, 12, 881. [Google Scholar] [CrossRef]
- Pagare, S.; Bhatia, M.; Tripathi, N.; Pagare, S.; Bansal, Y. Secondary Metabolites of Plants and Their Role: Overview. Curr. Trends Biotechnol. Pharm. 2015, 9, 293–304. [Google Scholar]
- Misra, A.; Srivastava, S. Chemotaxonomy: An Approach for Conservation and Exploration of Industrially Potential Medicinal Plants. J. Pharmacogn. Nat. Prod. 2016, 2, e108. [Google Scholar] [CrossRef]
- Mannheimer, C.A. An Overview of Chemotaxonomy and Its Role in Creating a Phylogenetic Classification System. Natl. Bot. Res. Inst. Minist. Agric. Water Rural Dev. Windhoek 1999, 87–90. [Google Scholar]
- Ankanna, S.; Suhrulatha, D.; Savithramma, N. Chemotaxonomical Studies of Some Important Monocotyledons. Bot. Res. Int. 2012, 5, 90–96. [Google Scholar]
- Oladipo, O.T.; Akinpelu, B.A.; Folorunso, A.E.; Godwin, A.; Omotoso, S.E.; Dosunmu, O.A.; Joseph, W.A. Chemotaxonomic Study of Six Nigerian Ficus Species (Moraceae). Not. Sci. Biol. 2017, 9, 250–255. [Google Scholar] [CrossRef]
- 41 Giordano, C.; Arcidiaco, L.; Rodolfi, M.; Ganino, T.; Beghè, D.; Petruccelli, R. Description of Ficus carica L. Italian Cultivars—I: Machine Learning Based Analysis of Leaf Morphological Traits. Plants 2025, 14, 333. [Google Scholar] [CrossRef] [PubMed]
- Khadari, B.; Hochu, I.; Santoni, S.; Kjellberg, F. Identification and Characterization of Microsatellite Loci in the Common Fig (Ficus carica L.) and Representative Species of the Genus Ficus. Mol. Ecol. Notes 2001, 1, 191–193. [Google Scholar] [CrossRef]
- Ergül, A.; Büyük, B.P.; Hazrati, N.; Yılmaz, F.; Kazan, K.; Arslan, N.; Özmen, C.Y.; Aydın, S.S.; Bakır, M.; Tan, N.; et al. Genetic Characterisation and Population Structure Analysis of Anatolian Figs (Ficus carica L.) by SSR Markers. Folia Hortic. 2021, 33, 49–78. [Google Scholar] [CrossRef]
- Saddoud, O.; Chatti, K.; Salhi-Hannachi, A.; Mars, M.; Rhouma, A.; Marrakchi, M.; Trifi, M. Genetic Diversity of Tunisian Figs (Ficus carica L.) as Revealed by Nuclear Microsatellites. Hereditas 2007, 144, 149–157. [Google Scholar] [CrossRef]
- Petruccelli, R.; Beghè, D.; Ganino, T.; Bartolini, G.; Ciaccheri, L.; Bernardi, R.; Durante, M. Evaluation of Intra-Cultivar Variability in’Olea europaea’ L. Cv. Leccino Using Morphological, Biochemical and Molecular Markers. Aust. J. Crop Sci. 2020, 14, 588–596. [Google Scholar] [CrossRef]
- Ammar, S.; Contreras, M.D.M.; Belguith-Hadrich, O.; Bouaziz, M.; Segura-Carretero, A. New Insights into the Qualitative Phenolic Profile of Ficus carica L. Fruits and Leaves from Tunisia Using Ultra-High-Performance Liquid Chromatography Coupled to Quadrupole-Time-of-Flight Mass Spectrometry and Their Antioxidant Activity. RSC Adv. 2015, 5, 20035–20050. [Google Scholar] [CrossRef]
- Shiraishi, C.S.H.; Zbiss, Y.; Roriz, C.L.; Dias, M.I.; Prieto, M.A.; Calhelha, R.C.; Alves, M.J.; Heleno, S.A.; V., d.C.M.; Carocho, M.; et al. Fig Leaves (Ficus carica L.): Source of Bioactive Ingredients for Industrial Valorization. Processes 2023, 11, 1179. [Google Scholar] [CrossRef]
- Oliveira, A.P.; Valentão, P.; Pereira, J.A.; Silva, B.M.; Tavares, F.; Andrade, P.B. Ficus carica L.: Metabolic and Biological Screening. Food Chem. Toxicol. 2009, 47, 2841–2846. [Google Scholar] [CrossRef]
- Akhtar, P.; Yaakob, Z.; Ahmed, Y.; Shahinuzzaman, M.; del Mar Contreras, M. Potential of Leaves of Eighteen Cultivars of Ficus carica as Antioxidants and Profiling of Phenolic Compounds as an Active Molecules: Antioxidant Active Compounds Isolation from the Leaves of Ficus carica (Cv. Violette Solise. Iran. J. Pharm. Sci. 2019, 15, 41–60. [Google Scholar]
- Takahashi, T.; Okiura, A.; Kohno, M. Phenylpropanoid Composition in Fig (Ficus carica L.) Leaves. J. Nat. Med. 2017, 71, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Okiura, A.; Saito, K.; Kohno, M. Identification of Phenylpropanoids in Fig (Ficus carica L.) Leaves. J. Agric. Food Chem. 2014, 62, 10076–10083. [Google Scholar] [CrossRef] [PubMed]
- Bruni, R.; Sacchetti, G. Factors Affecting Polyphenol Biosynthesis in Wild and Field Grown St. John’s Wort (Hypericum perforatum L. Hypericaceae/Guttiferae). Molecules 2009, 14, 682–725. [Google Scholar] [CrossRef]
- Imen, M.; Ahmadabadi, A.; Tavousi, S.; Sedaghat, A. The Curious Cases of Burn by Fig Tree Leaves. Indian J. Dermatol. 2019, 64, 71. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, Y.; Huo, B.; Li, B.; Jin, Y.; Hu, X. Extracts and Components of Ficus carica Leaves Suppress Survival, Cell Cycle, and Migration of Triple-Negative Breast Cancer MDA-MB-231 Cells. OncoTargets Ther. 2018, 11, 4377–4386. [Google Scholar] [CrossRef]
- Teruel-Andreu, C.; Andreu-Coll, L.; López-Lluch, D.; Sendra, E.; Hernández, F.; Cano-Lamadrid, M. Ficus carica Fruits, by-Products and Based Products as Potential Sources of Bioactive Compounds: A Review. Agronomy 2021, 11, 1834. [Google Scholar] [CrossRef]
- Del Río, J.A.; Díaz, L.; García-Bernal, D.; Blanquer, M.; Ortuno, A.; Correal, E.; Moraleda, J.M. Furanocoumarins: Biomolecules of Therapeutic Interest. Stud. Nat. Prod. Chem. 2014, 43, 145–195. [Google Scholar]
- Ben Achour Harrabi, N.; Taamalli, W.; Jiljli, H.; Dlima, I.; Yangui, I.; Hachicha, D.; Attia, R.; Mejri, M. Nutritional Profile, Phytochemical Characterization, and Biological Activities of Tunisian Cultivar Ficus carica Zidi Leaves. Euro-Mediterr. J. Environ. Integr. 2024, 9, 1703–1721. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Lohachoompol, V.; Mulholland, M.; Srzednicki, G.; Craske, J. Determination of Anthocyanins in Various Cultivars of Highbush and Rabbiteye Blueberries. Food Chem. 2008, 111, 249–254. [Google Scholar] [CrossRef]
- Brambilla, A.; Lo Scalzo, R.; Bertolo, G.; Torreggiani, D. Steam-Blanched Highbush Blueberry (Vaccinium corymbosum L.) Juice: Phenolic Profile and Antioxidant Capacity in Relation to Cultivar Selection. J. Agric. Food Chem. 2008, 56, 2643–2648. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Chen, S.; Wang, Q.; Jin, B.; Wang, L. Functions, Accumulation, and Biosynthesis of Important Secondary Metabolites in the Fig Tree (Ficus carica). Front. Plant Sci. 2024, 15, 1397874. [Google Scholar] [CrossRef] [PubMed]
- Vemmos, S.N.; Petri, E.; Stournaras, V. Seasonal Changes in Photosynthetic Activity and Carbohydrate Content in Leaves and Fruit of Three Fig Cultivars (Ficus carica L.). Sci. Hortic. 2013, 160, 198–207. [Google Scholar] [CrossRef]
- Cvetković, B.; Bajić, A.; Belović, M.; Pezo, L.; Dragojlović, D.; Šimurina, O.; Djordjević, M.; Korntheuer, K.; Philipp, C.; Eder, R. Assessing Antioxidant Properties, Phenolic Compound Profiles, Organic Acids, and Sugars in Conventional Apple Cultivars (Malus domestica): A Chemometric Approach. Foods 2024, 13, 2291. [Google Scholar] [CrossRef]
- Beccaro, G.L.; Donno, D.; Lione, G.G.; De Biaggi, M.; Gamba, G.; Rapalino, S.; Riondato, I.; Gonthier, P.; Mellano, M.G. Castanea spp. agrobiodiversity conservation: Genotype influence on chemical and sensorial traits of cultivars grown on the same clonal rootstock. Foods 2020, 9, 1062. [Google Scholar] [CrossRef]
- Calani, L.; Bresciani, L.; Rodolfi, M.; Del Rio, D.; Petruccelli, R.; Faraloni, C.; Ganino, T. Characterization of the (Poly) Phenolic Fraction of Fig Peel: Comparison among Twelve Cultivars Harvested in Tuscany. Plants 2022, 11, 3073. [Google Scholar] [CrossRef]
- Torti, S.D.; Dearing, M.D.; Kursar, T.A. Extraction of Phenolic Compounds from Fresh Leaves: A Comparison of Methods. J. Chem. Ecol. 1995, 21, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Petruccelli, R.; Ieri, F.; Ciaccheri, L.; Bonetti, A. Polyphenolic Profiling and Chemometric Analysis of Leaves from Italian Ficus carica L. Varieties. Polyphenol Compounds in Common Fig. Eur. J. Hortic. Sci. 2018, 83, 94–103. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Cao, G.; Prior, R.L. [5] Measurement of Oxygen Radical Absorbance Capacity in Biological Samples. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 50–62. [Google Scholar]
- Beghè, D.; Cirlini, M.; Beneventi, E.; Dall’Asta, C.; Marchioni, I.; Petruccelli, R. Exploring Italian Autochthonous Punica granatum L. Accessions: Pomological, Physicochemical, and Aromatic Investigations. Plants 2024, 13, 2558. [Google Scholar] [CrossRef]
- Weiss, M.L. “DNA Fingerprinting: An Introduction”, by LT Kirby (Book Review). Hum. Biol. 1993, 65, 155. [Google Scholar]
- Brookfield, J. A Simple New Method for Estimating Null Allele Frequency from Heterozygote Deficiency. Mol. Ecol. 1996, 5, 453–455. [Google Scholar] [CrossRef]
- Botstein, D.; White, R.L.; Skolnick, M.; Davis, R.W. Construction of a Genetic Linkage Map in Man Using Restriction Fragment Length Polymorphisms. Am. J. Hum. Genet. 1980, 32, 314. [Google Scholar]
- Marshall, T.; Slate, J.; Kruuk, L.; Pemberton, J. Statistical Confidence for Likelihood-Based Paternity Inference in Natural Populations. Mol. Ecol. 1998, 7, 639–655. [Google Scholar] [CrossRef]
- Kalinowski, S.T.; Taper, M.L.; Marshall, T.C. Revising How the Computer Program CERVUS Accommodates Genotyping Error Increases Success in Paternity Assignment. Mol. Ecol. 2007, 16, 1099–1106. [Google Scholar] [CrossRef]
- Anaconda Software Distribution. Anaconda Doc. 2020. Available online: https://docs.anaconda.com/ (accessed on 15 December 2024).
- Walt, S.; Van der Millman, J. The Pandas development Pandas-Dev/Pandas: Pandas. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28 June–3 July 2010. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental Algorithms for Scientific Computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Seabold, S.; Perktold, J. Statsmodels: Econometric and Statistical Modeling with Python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28 June–3 July 2010. [Google Scholar]
- Vallat, R. Pingouin: Statistics in Python. J. Open Source Softw. 2018, 3, 1026. [Google Scholar] [CrossRef]
- Waskom, M. Seaborn: Statistical Data Visualization. J. Open Source Softw. 2021, 6, 3021. [Google Scholar] [CrossRef]
- Hunter, J.D. Matplotlib: A 2D Graphics Environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Lumivero LLC: XLSTAT Statistical and Data Analysis Solution. 2024. Available online: https://www.xlstat.com/en (accessed on 15 December 2024).
- Caruso, T.; Marra, F.; Costa, F.; Campisi, G.; Macaluso, L.; Marchese, A. Genetic Diversity and Clonal Variation within the Main Sicilian Olive Cultivars Based on Morphological Traits and Microsatellite Markers. Sci. Hortic. 2014, 180, 130–138. [Google Scholar] [CrossRef]


| Locus | Locus | Locus | Locus | Locus | Locus | Locus | |
|---|---|---|---|---|---|---|---|
| MFC2 | MFC3 | MFC4 | LMFC12 | LMC24 | LMFC30 | LMFC31 | |
| a | 159 | 124 | 202 | 353 | 270 | 236 | 229 |
| b | 161 | 126 | 222 | 372 | 276 | 246 | 231 |
| c | 167 | 128 | 226 | 375 | 278 | 252 | 243 |
| d | 169 | 130 | 381 | 280 | 258 | 245 | |
| e | 171 | 132 | 401 | 260 | |||
| f | 173 | 136 | 262 | ||||
| g | 179 | 138 | 266 | ||||
| h | 144 | ||||||
| NA | 7 | 8 | 3 | 5 | 4 | 7 | 4 |
| HE | 0.729 | 0.864 | 0.687 | 0.639 | 0.563 | 0.855 | 0.634 |
| HO | 0.800 | 0.733 | 0.800 | 0.600 | 0.400 | 0.733 | 0.667 |
| r | −0.070 | 0.053 | −0.104 | 0.018 | 0.188 | 0.063 | −0.042 |
| PIC | 0.661 | 0.815 | 0.590 | 0.542 | 0.475 | 0.803 | 0.537 |
| CV | 5-OCaffeoylquinic Acid | Caffeoylmalic ACID | Caffeic Acid Derivatives | p-Coumaroyl Derivative | p-Coumaroylquinic Acid | p-Coumaroyl Malic Acid | Psolaren | Bergapten (5-methoxypsolaren) | Psolaric Acid Isobar |
| mg/g DW | mg/g DW | mg/g DW | mg/g DW | mg/g DW | mg/g DW | mg/g DW | mg/g DW | mg/g DW | |
| PA | 0.550 ± 0.04 | 5.963 ± 0.39 | 0.284 ± 0.02 | 0.426 ± 0.03 | 0.555 ± 0.04 | 0.367 ± 0.03 | 0.786 ± 0.06 | 0.283 ± 0.02 | 0.272 ± 0.02 |
| AL | 0.566 ± 0.03 | 3.847 ± 0.25 | 0.391 ± 0.03 | 0.324 ± 0.02 | 1.063 ± 0.08 | 0.672 ± 0.05 | 0.667 ± 0.05 | 0.383 ± 0.04 | 0.318 ± 0.02 |
| VE | 0.563 ± 0.04 | 6.712 ± 0.48 | 0.675 ± 0.04 | nd | 2.646 ± 0.17 | 1.385 ± 0.09 | 0.729 ± 0.05 | 0.445 ± 0.03 | 0.319 ± 0.03 |
| PO | 0.745 ± 0.05 | 9.221 ± 0.66 | 0.543 ± 0.05 | nd | 0.462 ± 0.03 | 0.364 ± 0.03 | 1.488 ± 0.11 | 0.614 ± 0.08 | 0.381 ± 0.03 |
| CO | 0.479 ± 0.03 | 5.314 ± 0.36 | 0.552 ± 0.04 | nd | 1.222 ± 0.08 | 0.972 ± 0.07 | 0.760 ± 0.07 | 0.451 ± 0.03 | 0.357 ± 0.04 |
| PN | 0.561 ± 0.04 | 2.784 ± 0.22 | 0.465 ± 0.03 | 0.397 ± 0.03 | 0.513 ± 0.04 | 0.394 ± 0.04 | 0.888 ± 0.06 | 0.420 ± 0.03 | 0.407 ± 0.03 |
| BB | 0.764 ± 0.05 | 3.124 ± 0.23 | 0.385 ± 0.04 | nd | 0.217 ± 0.02 | 0.466 ± 0.05 | 0.673 ± 0.05 | 0.581 ± 0.05 | 0.278 ± 0.02 |
| GI | 0.444 ± 0.03 | 3.391 ± 0.24 | nd | 0.384 ± 0.03 | 0.467 ± 0.04 | 0.569 ± 0.04 | nd | nd | nd |
| BC | 2.059 ± 0.14 | 6.674 ± 0.45 | 0.533 ± 0.04 | 0.764 ± 0.06 | 0.363 ± 0.03 | 0.344 ± 0.03 | 0.948 ± 0.08 | 0.354 ± 0.03 | 0.382 ± 0.04 |
| PE | 1.875 ± 0.13 | 7.837 ± 0.57 | 0.620 ± 0.04 | 0.213 ± 0.02 | 0.282 ± 0.02 | 0.842 ± 0.06 | 0.861 ± 0.06 | 0.283 ± 0.02 | 0.182 ± 0.01 |
| PB | 0.759 ±0.05 | 6.843 ± 0.47 | 0.485 ± 0.04 | 0.314 ± 0.02 | 0.372 ± 0.05 | 0.671 ± 0.05 | 0.683 ± 0.05 | 0.262 ± 0.02 | 0.177 ± 0.02 |
| BN | 0.821 ± 0.06 | 3.257 ± 0.06 | 0.454 ± 0.03 | nd | 1.426 ± 0.09 | 0.784 ± 0.05 | 1.158 ± 0.09 | 0.593 ± 0.04 | 0.416 ± 0.05 |
| DO | 0.558 ± 0.04 | 7.483 ± 0.52 | 0.530 ± 0.04 | nd | 0.461 ± 0.03 | 0.386 ± 0.03 | 0.883 ± 0.06 | 0.529 ± 0.04 | nd |
| FI | 0.884 ± 0.06 | 5.759 ± 0.25 | 0.462 ± 0.30 | 0.474 ± 0.03 | 0.384 ± 0.03 | 0.380 ± 0.02 | 0.696 ± 0.05 | 0.342 ± 0.02 | 0.355 ± 0.04 |
| SP | 0.986 ± 0.07 | 4.888 ± 0.35 | 0.590 ± 0.04 | 0.450 ± 0.04 | 0.364 ± 0.04 | 0.347 ± 0.02 | 0.359 ± 0.03 | nd | nd |
| CV | Isoschaftoside | Schaftoside | Quercetin Derivative (Rt 16.5) | Quercetin Derivative (Rt 17.9) | Rutin (quercetin-3-O-rutinoside) | Isoquercitin (quercetin 3-O-glucoside) | Quercetin 3-O-malonyl-glucoside | Kaempferol 3-O-glucoside | Kaempferol Derivative (Rt 21.2) |
| PA | 0.486 ± 0.03 | 0.161 ± 0.01 | 0.135 ± 0.01 | 0.095 ± 0.01 | 3.568 ± 0.27 | 0.074 ± 0.01 | 0.184 ± 0.02 | 0.080 ± 0.02 | nd |
| AL | 0.555 ± 0.03 | 0.237 ± 0.02 | 0.379 ± 0.03 | 0.184 ± 0.02 | 3.333 ± 0.25 | 0.078 ± 0.02 | 0.146 ± 0.01 | 0.095 ± 0.01 | nd |
| VE | 0.665 ± 0.04 | 0.231 ± 0.01 | nd | nd | 2.364 ± 0.16 | 0.067 ± 0.01 | 0.171 ± 0.01 | 0.153 ± 0.02 | nd |
| PO | 0.284 ± 0.02 | 0.189 ± 0.01 | 0.071 ± 0.01 | 0.068 ± 0.01 | 2.518 ± 0.18 | 0.120 ± 0.03 | 0.549 ± 0.04 | 0.170 ± 0.01 | nd |
| CO | 0.654 ± 0.04 | 0.223 ± 0.01 | nd | nd | 4.887 ± 0.32 | 0.193 ± 0.01 | 0.394 ± 0.03 | 0.195 ± 0.03 | nd |
| PN | 0.137 ± 0.01 | 0.145 ± 0.01 | 0.164 ± 0.01 | 0.084 ± 0.02 | 3.728 ± 0.25 | 0.577 ± 0.04 | 0.526 ± 0.03 | 0.055 ± 0.01 | nd |
| BB | 0.455 ± 0.03 | 0.234 ± 0.02 | 0.063 ± 0.01 | 0.043 ± 0.01 | 1.397 ± 0.10 | 0.179 ± 0.01 | 0.142 ± 0.01 | 0.110 ± 0.02 | nd |
| GI | 0.535 ± 0.04 | 0.343 ± 0.03 | 0.457 ± 0.03 | 0.161 ± 0.02 | 2.117 ± 0.15 | 0.132 ± 0.01 | 0.194 ± 0.02 | nd | nd |
| BC | 0.654 ± 0.05 | 0.528 ± 0.04 | 0.236 ± 0.02 | 0.226 ± 0.02 | 8.238 ± 0.58 | 1.625 ± 0.12 | 2.668 ± 0.18 | 0.394 ± 0.03 | 0.053 ± 0.01 |
| PE | 0.513 ± 0.04 | 0.355 ± 0.02 | 0.210 ± 0.01 | 0.186 ± 0.03 | 7.247 ± 0.50 | 0.652 ± 0.04 | 1.243 ± 0.13 | 0.208 ± 0.02 | nd |
| PB | 0.489 ± 0.03 | 0.368 ± 0.03 | 0.255 ± 0.02 | 0.127 ± 0.01 | 8.020 ± 0.55 | 1.183 ± 0.08 | 0.658 ± 0.05 | 0.193 ± 0.01 | nd |
| BN | 0.996 ± 0.07 | 0.434 ± 0.03 | 0.144 ± 0.01 | 0.054 ± 0.01 | 7.583 ± 0.53 | 0.415 ± 0.03 | 0.385 ± 0.03 | 0.242 ± 0.02 | nd |
| DO | 0.293 ± 0.02 | 0.195 ± 0.01 | 0.054 ± 0.01 | 0.082 ± 0.02 | 1.655 ± 0.12 | 0.153 ± 0.01 | 0.585 ± 0.04 | 0.069 ± 0.02 | nd |
| FI | 0.556 ± 0.04 | 0.442 ± 0.03 | 0.306 ± 0.02 | 0.194 ± 0.03 | 5.784 ± 0.39 | 0.962 ± 0.07 | 1.741 ± 0.12 | 0.313 ± 0.02 | 0.021 ± 0.02 |
| SP | 0.399 ± 0.03 | 0.232 ± 0.02 | 0.098 ± 0.01 | 0.075 ± 0.01 | 5.661 ±0.41 | 0.631 ± 0.05 | 0.973 ± 0.07 | 0.134 ± 0.01 | nd |
| CV | Hy.Der./EC50 | Hy.Der/ORAC | Fl. Der./EC50 | Fl.Der./ORAC |
|---|---|---|---|---|
| PA | 0.1293 | 0.1479 | 0.1731 | 0.1940 |
| AL | 0.3912 | 0.3918 | 0.0431 | 0.0434 |
| VE | 0.4323 | 0.4023 | 0.9556 | 0.9423 |
| BC | 0.9643 | 0.9637 | 0.8322 | 0.8334 |
| BB | 0.3007 | 0.2865 | 0.0005 | 0.0015 |
| BN | 0.8338 | 0.7709 | 0.3046 | 0.3796 |
| CO | 0.0332 | 0.0291 | 0.9912 | 0.9932 |
| DO | 0.8355 | 0.8330 | 0.4330 | 0.4296 |
| FI | 0.2927 | 0.2947 | 0.7537 | 0.7519 |
| GI | 0.4453 | 0.4453 | 0.9494 | 0.9494 |
| PB | 0.6460 | 0.6823 | 0.0845 | 0.1071 |
| PN | 0.9868 | 0.9873 | 0.7500 | 0.7519 |
| PE | 0.5819 | 0.5594 | 0.1824 | 0.1805 |
| PO | 0.9935 | 0.9954 | 0.8818 | 0.8736 |
| SP | 0.5715 | 0.5665 | 0.3001 | 0.3048 |
| CV | Fructose | Glucose | Sucrose | Total Sugars |
|---|---|---|---|---|
| AL | 14.19 ± 0.38 h | 6.36 ± 0.14 i | 3.16 ± 0.08 a | 23.72 ± 0.43 i |
| BB | 27.77 ± 0.22 c | 11.90 ± 0.16 e | 1.21 ± 0.03 de | 40.88 ± 0.39 e |
| BC | 13.82 ± 0.21 hi | 5.22 ± 0.12 j | 1.55 ± 0.07 c | 20.60 ± 0.16 k |
| BN | 14.23 ± 0.26 h | 6.49 ± 0.17 hi | 1.18 ± 0.05 de | 21.91 ± 0.25 j |
| CO | 27.81 ± 0.27 c | 13.92 ± 0.12 c | 2.05 ± 0.06 b | 43.74 ± 0.22 d |
| DO | 24.26 ± 0.30 d | 12.90 ± 0.17 d | 2.96 ± 0.04 ab | 43.15 ± 0.14 e |
| FI | 13.31 ± 0.24 i | 6.24 ± 0.18 i | 2.76 ± 0.09 b | 22.31 ± 0.28 j |
| GI | 18.78 ± 0.25 f | 6.81 ± 0.16 h | 2.30 ± 0.05 c | 27.89 ± 0.40 h |
| PA | 28.92 ± 0.25 b | 15.51 ± 0.15 b | 1.50 ± 0.04 c | 45.70 ± 0.18 c |
| PB | 19.66 ± 0.35 e | 10.11 ± 0.1 f1 | 1.13 ± 0.08 e | 30.90 ± 0.36 g |
| PE | 18.69 ± 0.30 f | 6.92 ± 0.14 h | 2.77 ± 0.08 b | 28.38 ± 0.34 h |
| PN | 30.27 ± 0.26 a | 15.56 ± 0.15 b | 1.43 ± 0.06 cd | 47.26 ± 0.44 b |
| PO | 16.69 ± 0.28 g | 9.53 ± 0.17 g | 1.51 ± 0.05 c | 27.73 ± 0.17 h |
| SP | 24.91 ± 0.30 d | 10.24 ± 0.13 f | 1.11 ± 0.03 e | 36.27 ± 0.36 f |
| VE | 30.21 ± 0.28 a | 16.86 ± 0.10 a | 2.12 ± 0.09 b | 49.24 ± 0.25 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petruccelli, R.; Giordano, C.; Beghè, D.; Arcidiaco, L.; Bonetti, A.; Ieri, F.; Rodolfi, M.; Ganino, T. Description of Ficus carica L. Italian Cultivars II: Genetic and Chemical Analysis. Plants 2025, 14, 1238. https://doi.org/10.3390/plants14081238
Petruccelli R, Giordano C, Beghè D, Arcidiaco L, Bonetti A, Ieri F, Rodolfi M, Ganino T. Description of Ficus carica L. Italian Cultivars II: Genetic and Chemical Analysis. Plants. 2025; 14(8):1238. https://doi.org/10.3390/plants14081238
Chicago/Turabian StylePetruccelli, Raffaella, Cristiana Giordano, Deborah Beghè, Lorenzo Arcidiaco, Alessandra Bonetti, Francesca Ieri, Margherita Rodolfi, and Tommaso Ganino. 2025. "Description of Ficus carica L. Italian Cultivars II: Genetic and Chemical Analysis" Plants 14, no. 8: 1238. https://doi.org/10.3390/plants14081238
APA StylePetruccelli, R., Giordano, C., Beghè, D., Arcidiaco, L., Bonetti, A., Ieri, F., Rodolfi, M., & Ganino, T. (2025). Description of Ficus carica L. Italian Cultivars II: Genetic and Chemical Analysis. Plants, 14(8), 1238. https://doi.org/10.3390/plants14081238










