Effects of Simulated Nitrogen and Phosphorus Deposition on Dioecious Populus cathayana Growth and Defense Traits
Abstract
:1. Introduction
2. Results
2.1. Biomass Accumulation and Leaf Areas
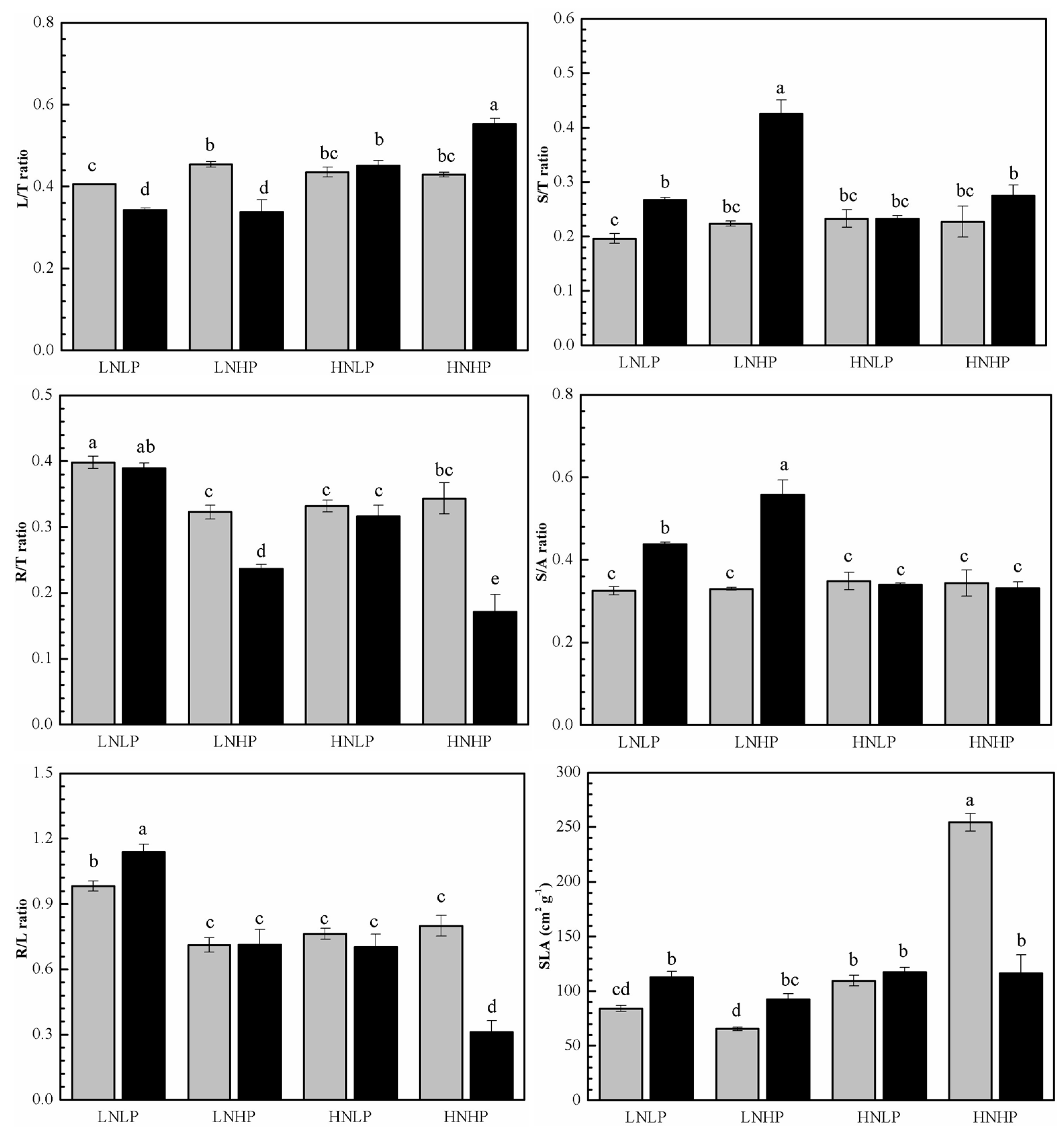
2.2. Biomass Distribution
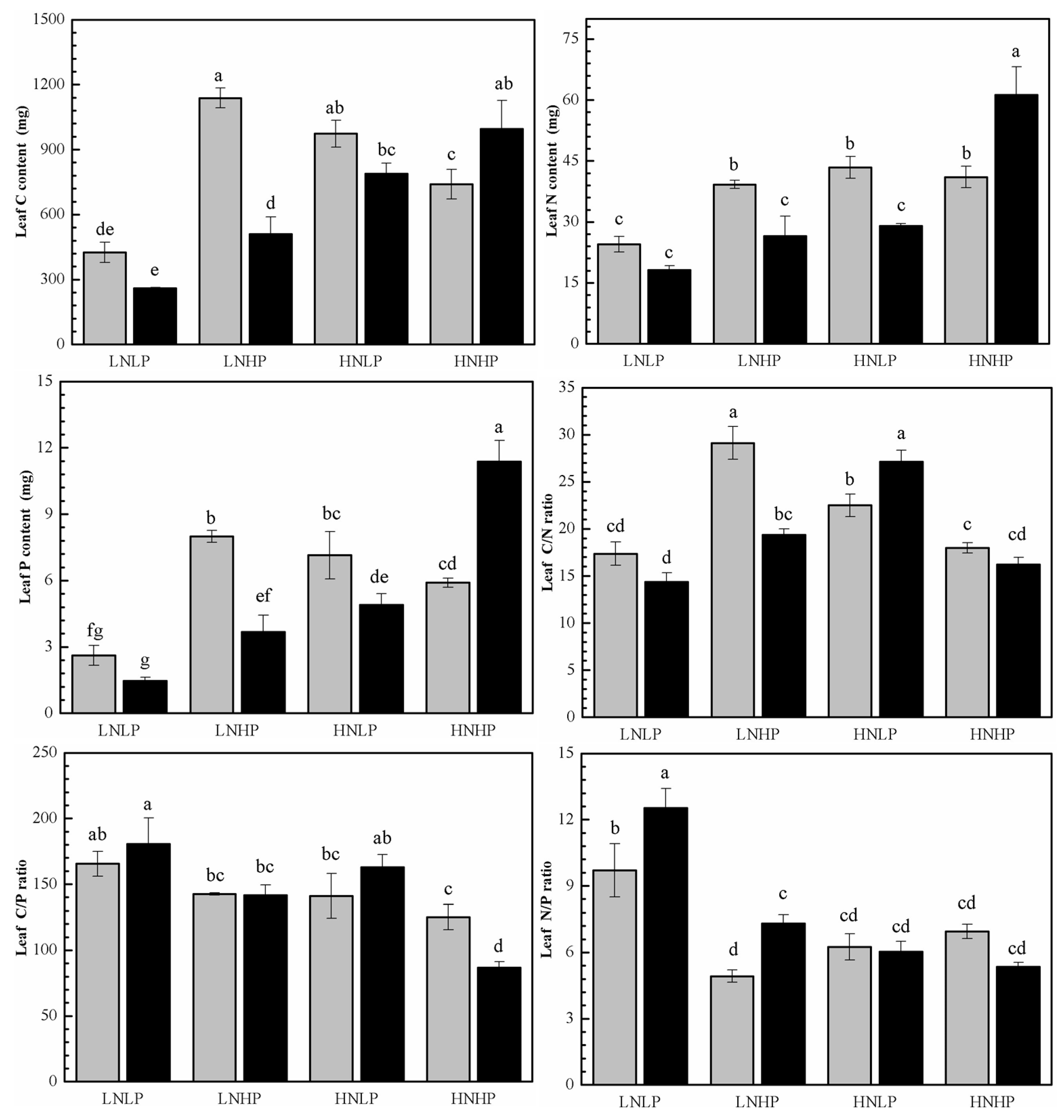
2.3. C, N, and P Content and the Ratios
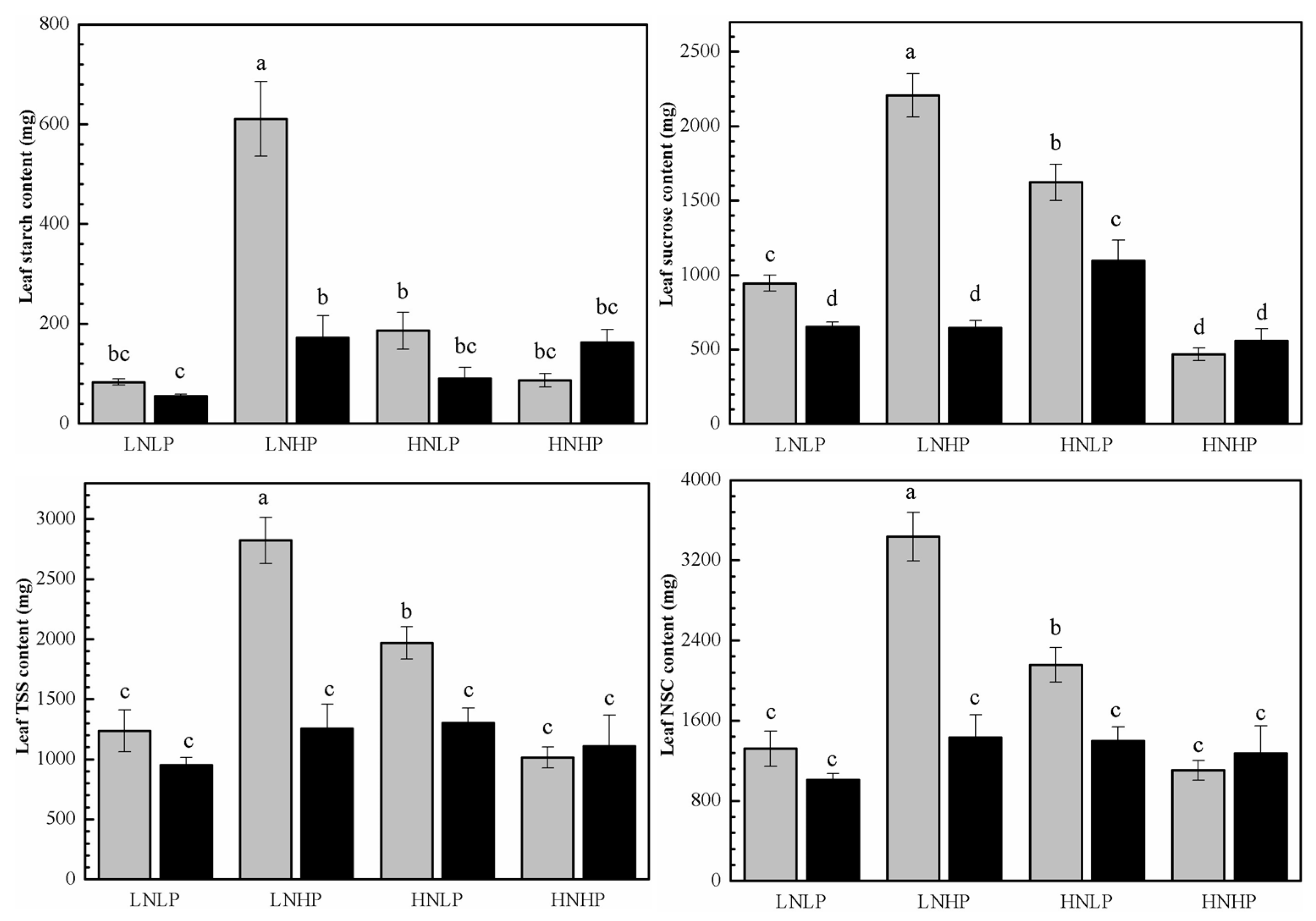
2.4. Carbohydrate Content
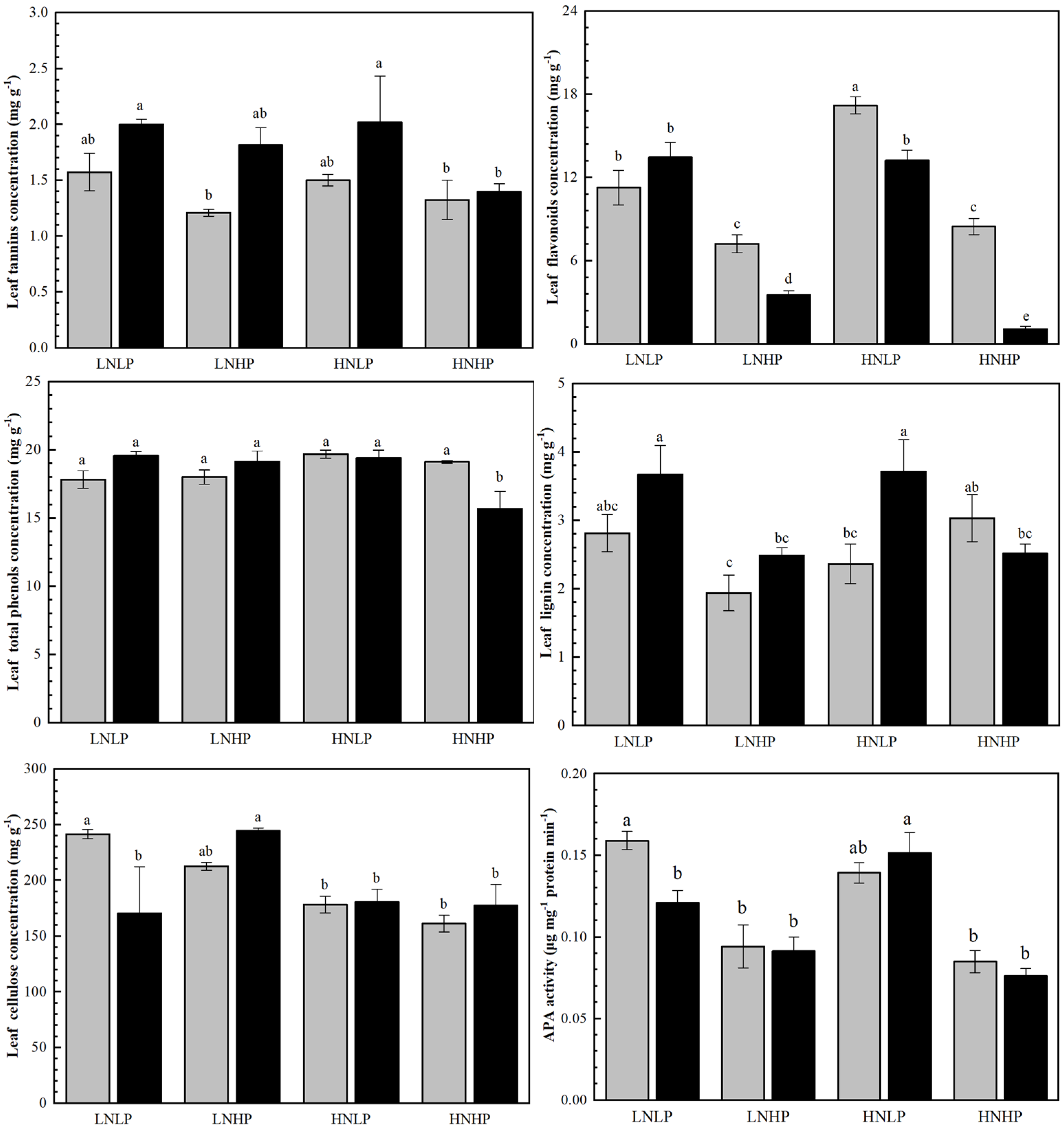
2.5. Content of Defensive Substances and APA Activity in Leaves
2.6. Pearson Correlation Coefficients Between Leaf Defensive Parameters and Nutrient and Carbohydrate Contents
3. Discussion
3.1. The Effects on Biomass Accumulation
3.2. The Effects on Biomass Distribution
3.3. The Effects on Leaf Defensive Traits
4. Materials and Methods
4.1. The Plant Material and Hydroponic Experimental Design
4.2. Growth Measurements
4.3. Foliar C, N, and P Content Measurements
4.4. Foliar Total Soluble Sugar (TSS), Sucrose, Starch, and Non-Structural Carbohydrate Content Measurements
4.5. Foliar Defensive Substance Concentration Measurements: Tannins, Flavonoids, Total Phenols, Lignin, and Cellulose
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CC | Leaf carbon content |
| PC | Leaf phosphorus content |
| NC | Leaf nitrogen content |
| C/N | CC/NC ratio |
| C/P | CC/PC ratio |
| N/P | NC/PC ratio |
| TC | Leaf tannin concentration |
| FC | Leaf flavonoid concentration |
| LC | Leaf lignin concentration |
| CeC | Leaf cellulose concentration |
| TPC | Leaf total phenol concentration |
| LDW | Leaf dry weight |
| StC | Leaf starch content |
| SSC | Leaf total soluble sugar content |
| SuC | Leaf sucrose content |
| NSCC | Leaf non-structural carbohydrate content |
References
- Hultine, K.R.; Grady, K.C.; Wood, T.E.; Shuster, S.M.; Stella, J.C.; Whitham, T.G. Climate change perils for dioecious plant species. Nat. Plants 2016, 2, 16109. [Google Scholar] [CrossRef]
- Guo, Q.; Zhu, Y.; Korpelainen, H.; Niinemets, Ü.; Li, C. How does plant sex alter microbiota assembly in dioecious plants? Trends Microbiol. 2023, 31, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Liu, L.; Liu, J.; Korpelainen, H.; Li, C. Plant sex affects plant-microbiome assemblies of dioecious Populus cathayana trees under different soil nitrogen conditions. Microbiome 2022, 10, 191. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Huang, Z.; Li, Z.; Korpelainen, H.; Li, C. Sex-specific strategies of nutrient resorption associated with leaf economics in Populus euphratica. J. Ecol. 2022, 110, 2062–2073. [Google Scholar] [CrossRef]
- Petry, W.K.; Soule, J.D.; Iler, A.M.; Chicas-Mosier, A.; Inouye, D.W.; Miller, T.E.X.; Mooney, K.A. Sex-specific responses to climate change in plants alter population sex ratio and performance. Science 2016, 353, 69–71. [Google Scholar] [CrossRef]
- Dong, T.; Zhang, R.; Liu, J.; Fowler, J.; Miller, T.; Xu, X. Warming alters sex-specific responses in leaf defense against insect herbivory in Populus cathayana. Environ. Exp. Bot. 2021, 189, 104557. [Google Scholar] [CrossRef]
- Xia, Z.; He, Y.; Yu, L.; Li, Z.; Korpelainen, H.; Li, C. Revealing interactions between root phenolic metabolomes and rhizosphere bacterial communities in Populus euphratica plantations. Biol. Fertil. Soils 2021, 57, 421–434. [Google Scholar] [CrossRef]
- Xu, S.; Cai, Q.; Sun, S.; Liu, Y.; Guan, B.; Wu, L.; Ge, G. The trait co-variation regulates the response of bryophytes to nitrogen deposition: A meta-analysis. Environ. Pollut. 2023, 339, 122739. [Google Scholar] [CrossRef]
- Yu, N.; Dijkstra, F.; Liang, X.; Zhang, X.; Yang, G.; Jiang, L.; Han, X.; Lü, X. Stronger response of plant N:P to nitrogen enrichment when considering roots. Glob. Change Biol. 2025, 31, e70091. [Google Scholar] [CrossRef]
- Peñuelas, J.; Sardans, J. The global nitrogen-phosphorus imbalance. Science 2022, 375, 266–267. [Google Scholar] [CrossRef]
- Wang, G.; Koziol, L.; Foster, B.L.; Bever, J.D. Microbial mediators of plant community response to long-term N and P fertilization: Evidence of a role of plant responsiveness to mycorrhizal fungi. Glob. Change Biol. 2022, 28, 2721–2735. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, J.; Sun, N.; Xu, M.; Zhang, J.; Cai, Z.; Wang, S. Phosphorus addition enhances gross microbial N cycling in phosphorus-poor soils: A 15N study from two long-term fertilization experiments. Biol. Fertil. Soils 2018, 54, 783–789. [Google Scholar] [CrossRef]
- Geng, F.Z.; Li, K.H.; Liu, X.J.; Gong, Y.M.; Yue, P.; Li, Y.G.; Han, W.X. Long-term effects of N deposition on N2O emission in an alpine grassland of Central Asia. Catena 2019, 182, 104100. [Google Scholar] [CrossRef]
- Xiao, J.; Dong, S.; Shen, H.; Li, S.; Zhi, Y.; Mu, Z.; Ding, C. Phosphorus addition promotes nitrogen retention in alpine grassland plants while increasing N deposition. Catena 2022, 210, 105887. [Google Scholar] [CrossRef]
- Peng, Y.; Peng, Z.; Zeng, X.; Houx, J.H. Effects of nitrogen-phosphorus imbalance on plant biomass production: A global perspective. Plant Soil 2019, 436, 245–252. [Google Scholar] [CrossRef]
- Deng, M.; Liu, L.; Sun, Z.; Piao, S.; Ma, Y.; Chen, Y.; Wang, J.; Qiao, C.; Wang, X.; Li, P. Increased phosphate uptake but not resorption alleviates phosphorus deficiency induced by nitrogen deposition in temperate Larix principis-rupprechtii plantations. New Phytol. 2016, 212, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Fernie, A. Plant secondary metabolism in a fluctuating world: Climate change perspectives. Trends Plant Sci. 2023, 29, 560–571. [Google Scholar] [CrossRef]
- Yang, C.; Xia, L.; Fu, M.; Chen, Y.; Kong, X.; Zhang, S. DNA methylation-mediated phenylpropane and starch metabolism causes male poplars to be more tolerant to nitrogen deficiency than females. Plant Physiol. Biochem. 2023, 195, 144–154. [Google Scholar] [CrossRef]
- Xia, Z.; He, Y.; Zhou, B.; Korpelainen, H.; Li, C. Sex-related responses in rhizosphere processes of dioecious Populus cathayana exposed to drought and low phosphorus stress. Environ. Exp. Bot. 2020, 175, 104049. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, D.; Korpelainen, H.; Li, C. Metabolic and physiological analyses reveal that Populus cathayana males adopt an energy-saving strategy to cope with phosphorus deficiency. Tree Physiol. 2019, 39, 1630–1645. [Google Scholar] [CrossRef]
- Obeso, J.R. The costs of reproduction in plants. New Phytol. 2002, 155, 321–348. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Dong, T.; Duan, B. Sex-specific carbon and nitrogen partitioning under N deposition in Populus cathayana. Trees-Struct Funct. 2014, 28, 793–806. [Google Scholar] [CrossRef]
- Chen, J.; Dong, T.; Duan, B.; Korpelainen, H.; Niinemets, Ü.; Li, C. Sexual competition and N supply interactively affect the dimorphism and competiveness of opposite sexes in Populus cathayana. Plant Cell Environ. 2015, 38, 1285–1298. [Google Scholar] [CrossRef]
- Randriamanana, T.R.; Nybakken, L.; Lavola, A.; Aphalo, P.J.; Nissinen, K.; Julkunen-Tiitto, R. Sex-related differences in growth and carbon allocation to defence in Populus tremula as explained by current plant defence theories. Tree Physiol. 2014, 34, 71–87. [Google Scholar] [CrossRef]
- Gao, S.; Song, H. Sex-related response of Salicaceae to drought stress. Chin. J. Appl. Environ. Biol. 2021, 27, 495–502. [Google Scholar] [CrossRef]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Han, W.; Tang, A.; Shen, J.; Cui, Z.; Vitousek, P.; Erisman, J.W.; Goulding, K.W.T.; Christie, P.; et al. Enhanced nitrogen deposition over China. Nature 2013, 494, 459. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, X.; Liu, L.; Cheng, M.; Xu, J. Spatial and seasonal patterns of atmospheric nitrogen deposition in North China. Atmos. Ocean. Sci. Lett. 2019, 13, 188–194. [Google Scholar] [CrossRef]
- Wen, Z.; Wang, R.; Li, Q.; Liu, J.; Ma, X.; Xu, W.; Tang, A.; Collett, J.; Li, H.; Liu, X. Spatiotemporal variations of nitrogen and phosphorus deposition across China. Sci. Total Environ. 2022, 830, 154740. [Google Scholar] [CrossRef]
- Zhu, J.X.; Wang, Q.F.; He, N.P.; Smith, M.D.; Elser, J.J.; Du, J.Q.; Yuan, G.F.; Yu, G.R.; Yu, Q. Imbalanced atmospheric nitrogen and phosphorus depositions in China: Implications for nutrient limitation. J. Geophys. Res. Biogeosci. 2016, 121, 1605–1616. [Google Scholar] [CrossRef]
- Li, L.; Li, X.; Liu, B.; Lei, J.; Yue, Z.; Li, C. Imbalanced stoichiometric patterns in foliar nutrient resorption response to N and P addition in grazing alpine grassland. Acta Oecol. 2020, 102, 103505. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, W.; Wang, J.; Lambers, H.; Yin, H. Extraradical hyphae alleviate nitrogen deposition-induced phosphorus deficiency in ectomycorrhiza-dominated forests. New Phytol. 2023, 239, 1651–1664. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, G.; Guo, Q.; Korpelainen, H.; Li, C. Fast-growing Larix kaempferi suffers under nutrient imbalance caused by phosphorus fertilization in larch plantation soil. For. Ecol. Manag. 2018, 417, 49–62. [Google Scholar] [CrossRef]
- Fageria, N.K.; Melo, L.C.; Carvalho, M.C.S. Influence of nitrogen on growth, yield, and yield components and nitrogen uptake and use efficiency in dry bean genotypes. Commun. Soil Sci. Plant Anal. 2015, 46, 2395–2410. [Google Scholar] [CrossRef]
- Wu, F.Z.; Bao, W.K.; Wu, N. Growth, accumulation and partitioning of biomass, C, N and P of Sophora davidii seedlings in response to N supply in dry valley of upper Minjiang River. Acta Ecol. Sin. 2008, 28, 3817–3824. [Google Scholar]
- Magill, A.H.; Aber, J.D.; Berntson, G.M.; McDowell, W.H.; Nadelhoffer, K.J.; Melillo, J.M.; Steudler, P. Long-term nitrogen additions and nitrogen saturation in two temperate forests. Ecosystems 2000, 3, 238–253. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef]
- Muller, I.; Schmid, B.; Weiner, J. The effect of nutrient availability on biomass allocation patterns in 27 species of herbaceous plants. Perspect. Plant Ecol. Evol. Syst. 2000, 3, 115–127. [Google Scholar] [CrossRef]
- Wittyngham, S.; Carey, J.; Johnson, D. Resource availability and plant age drive defense against herbivory in salt marshes. Oikos 2023, 2023, e09672. [Google Scholar] [CrossRef]
- Bandau, F.; Albrectsen, B.; Robinson, K.; Gundale, M. European aspen with high compared to low constitutive tannin defenses grow taller in response to anthropogenic nitrogen enrichment. For. Ecol. Manag. 2021, 487, 118985. [Google Scholar] [CrossRef]
- López-Goldar, X.; Zas, R.; Sampedro, L. Resource availability drives microevolutionary patterns of plant defences. Func. Ecol. 2020, 34, 1640–1652. [Google Scholar] [CrossRef]
- Hidaka, A.; Kitayama, K. Allocation of foliar phosphorus fractions and leaf traits of tropical tree species in response to decreased soil phosphorus availability on Mount Kinabalu, Borneo. J. Ecol. 2011, 99, 849–857. [Google Scholar] [CrossRef]
- Herms, D.A.; Mattson, W.J. The dilemma of plants: To grow or defend. Q. Rev. Biol. 1992, 67, 283–335. [Google Scholar] [CrossRef]
- Cornelissen, T.; Stiling, P. Sex-biased herbivory: A meta-analysis of the effects of gender on plant-herbivore interactions. Oikos 2005, 111, 488–500. [Google Scholar] [CrossRef]
- Salgado-Luarte, C.; González Teuber, M.; Madriaza, K.; Gianoli, E. Trade-off between plant resistance and tolerance to herbivory: Mechanical defenses outweigh chemical defenses. Ecology 2022, 104, e3860. [Google Scholar] [CrossRef]
- Li, J.; Dong, T.; Guo, Q.; Zhao, H. Populus deltoides females are more selective in nitrogen assimilation than males under different nitrogen forms supply. Trees-Struct Funct. 2015, 29, 143–159. [Google Scholar] [CrossRef]
- Wu, C.; Shu, C.; Zhang, Z.; Zhang, Y.; Liu, Y. Phosphorus deposition accelerates wood decomposition and temperature sensitivity in a subtropical forest. Ecol. Indic. 2021, 128, 107819. [Google Scholar] [CrossRef]
- Crous, K.; O’Sullivan, O.; Zaragoza-Castells, J.; Bloomfield, K.; Alves Negrini, A.C.; Meir, P.; Turnbull, M.; Griffin, K.; Atkin, O. Nitrogen and phosphorus availabilities interact to modulate leaf trait scaling relationships across six plant functional types in a controlled-environment study. New Phytol. 2017, 215, 992–1008. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis. Part 2; Dinauer, R.C., Ed.; American Society of Agronomy, Inc.: Madison, WI, USA; Soil Science Society of America, Inc.: Madison, WI, USA, 1982; pp. 539–579. [Google Scholar]
- Mitchell, A.K. Acclimation of Pacific yew (Taxus brevifolia) foliage to sun and shade. Tree Physiol. 1998, 18, 749–757. [Google Scholar] [CrossRef]
- Hötscher, M.; Hay, M. Genotypic differences in physiological integration, morphological plasticity and utilization of phosphorus induced by variation in phosphate supply in Trifolium repens. J. Ecol. 1997, 85, 341–350. [Google Scholar] [CrossRef]
- Livingston, N.J.; Guy, R.D.; Sun, Z.J.; Ethier, G.J. The effects of nitrogen stress on the stable carbon isotope composition, productivity and water use efficiency of white spruce (Picea glauca (Moench) Voss) seedlings. Plant Cell Environ. 1999, 22, 281–289. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef]
- Murata, T.; Akazawa, T.; Fukuchi, S. Enzymic Mechanism of Starch Breakdown in Germinating Rice Seeds’ I. An Analytical Study. Plant Physiol. 1968, 43, 1899–1905. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Y.; Duan, B.; Korpelainen, H.; Li, C. Sex-related adaptive responses of Populus cathayana to photoperiod transitions. Plant Cell Environ. 2009, 32, 1401–1411. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.; Vercambre, G.; Gomez, L.; Pagès, L. The early spring N uptake of young peach trees (Prunus persica) is affected by past and current fertilizations and levels of C and N stores. Tree Physiol. 2013, 34, 61–72. [Google Scholar] [CrossRef]
- Kenton, P.; Mur, L.A.J.; Draper, J. A requirement for calcium and protein phosphatase in the jasmonate-induced increase in tobacco leaf acid phosphatase specific activity. J. Exp. Bot. 1999, 50, 1331–1341. [Google Scholar] [CrossRef]
- Heil, M.; Delsinne, T.; Hilpert, A.; Schürkens, S.; Andary, C.; Linsenmair, K.E.; Sousa, M.; McKey, D. Reduced chemical defence in plants? A critical re-evaluation of a widely accepted hypothesis. Oikos 2002, 99, 457–468. [Google Scholar] [CrossRef]
- Khorasani Esmaeili, A.; Mat Taha, R.; Mohajer, S.; Banisalam, B. Antioxidant activity and total phenolic and flavonoid content of various solvent extracts from in vivo and in vitro grown Trifolium pratense L. (red clover). BioMed Res. Int. 2015, 2015, 643285. [Google Scholar] [CrossRef]
- Scalbert, A. Quantitative methods for the estimation of tannins in plant tissues. In Plant Polyphenols. Basic Life Sciences; Hemingway, R.W., Laks, P.E., Eds.; Springer: Boston, MA, USA, 1992; Volume 59, pp. 259–280. [Google Scholar]
- Gessner, M. Lignin and Cellulose. In Methods to Study Litter Decomposition: A Practical Guide, 2nd ed.; Graça, M.A.S., Bärlocher, F., Gessner, M.O., Eds.; Springer: Cham, Switzerland, 2020; pp. 179–185. [Google Scholar]


| Parameter | P > FS | P > FN | P > FP | P > FS × N | P > FS × P | P > FN × P | P > FS × N × P |
|---|---|---|---|---|---|---|---|
| Root dry mass (g) | *** | ** | ns | * | ** | *** | ** |
| Stem dry mass (g) | ns | ** | *** | ns | * | *** | * |
| Leaf dry mass (g) | ** | ns | *** | *** | ns | *** | *** |
| Above-ground dry mass (g) | * | ns | *** | ** | ns | *** | *** |
| Total dry mass (g) | *** | ns | *** | ** | ns | *** | *** |
| Total leaf areas (cm2) | ** | *** | ** | ns | ns | ns | ns |
| L/T ratio | ns | *** | ** | *** | ns | ns | ** |
| S/T ratio | *** | ** | *** | *** | ** | ** | ns |
| S/A ratio | *** | *** | ns | *** | ns | * | * |
| R/T ratio | *** | ** | *** | * | *** | * | ns |
| R/L ratio | ** | *** | *** | *** | *** | * | ns |
| SLA (cm2 g−1) | ** | *** | *** | *** | *** | *** | *** |
| Leaf C content (mg) | ** | *** | *** | *** | ns | *** | *** |
| Leaf N content (mg) | ns | *** | *** | * | ** | ns | ** |
| Leaf P content (mg) | ns | *** | *** | *** | * | ns | *** |
| Leaf C/N ratio | ** | ns | ns | *** | ** | *** | ns |
| Leaf C/P ratio | ns | ** | *** | ns | * | ns | ns |
| Leaf N/P ratio | ns | *** | *** | ** | ns | *** | ns |
| Leaf starch content (mg) | *** | *** | *** | *** | * | *** | *** |
| Leaf sucrose content (mg) | *** | ** | ns | *** | * | *** | *** |
| Leaf TSS content (mg) | *** | * | ns | ** | * | *** | *** |
| Leaf NSC content (mg) | *** | ** | ** | ** | ** | *** | *** |
| Leaf tannin concentration (mg g−1) | ** | ns | * | ns | ns | ns | ns |
| Leaf flavonoid concentration (mg g−1) | *** | ns | *** | *** | *** | ** | ns |
| Leaf total phenol concentration (mg g−1) | ns | ns | * | ** | ns | * | ns |
| Leaf lignin concentration (mg g−1) | * | ns | ** | ns | * | ns | ns |
| Leaf cellulose concentration (mg g−1) | ns | ** | ns | ns | * | ns | ns |
| CC | PC | NC | C/N | C/P | N/P | TC | FC | LC | CeC | TPC | LDW | StC | SSC | SuC | NSCC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CC | 0.932 *** | 0.774 ** | 0.867 *** | −0.346 | −0.871 *** | −0.296 | −0.028 | −0.614 * | −0.332 | 0.136 | 0.870 *** | 0.653 * | 0.344 | 0.154 | 0.525 | |
| PC | 0.912 *** | 0.858 *** | 0.706 * | −0.642 * | −0.925 *** | −0.306 | −0.058 | −0.365 | −0.434 | 0.170 | 0.761 ** | 0.524 | 0.218 | −0.026 | 0.376 | |
| NC | 0.880 *** | 0.983 *** | 0.360 | −0.629 * | −0.703 * | −0.097 | 0.113 | −0.276 | −0.686 * | 0.526 | 0.415 | 0.172 | 0.026 | −0.276 | 0.087 | |
| C/N | 0.345 | −0.330 | −0.136 | −0.046 | −0.757 ** | −0.350 | −0.165 | −0.677 * | 0.021 | −0.222 | 0.951 *** | 0.826 ** | 0.469 | 0.407 | 0.691 * | |
| C/P | −0.662 * | −0.853 ** | −0.826 ** | 0.230 | 0.660 ** | 0.208 | 0.214 | −0.325 | 0.583 * | −0.137 | −0.115 | 0.042 | 0.302 | 0.561 | 0.254 | |
| N/P | −0.823 ** | −0.724 ** | −0.633 * | −0.508 | 0.709 * | 0.326 | 0.163 | 0.328 | 0.429 | 0.022 | −0.733 ** | −0.531 | −0.148 | 0.065 | −0.324 | |
| TC | −0.468 | −0.590 * | −0.568 | 0.136 | 0.569 | 0.367 | 0.358 | −0.082 | 0.268 | −0.024 | −0.344 | −0.444 | 0.136 | 0.113 | −0.066 | |
| FC | −0.469 | −0.673 * | −0.672 * | 0.285 | 0.809 ** | 0.567 | 0.611 * | 0.018 | −0.138 | 0.416 | −0.224 | −0.415 | 0.395 | 0.422 | 0.149 | |
| LC | −0.352 | −0.452 | −0.460 | 0.133 | 0.402 | 0.320 | 0.551 | 0.769 ** | −0.130 | 0.025 | −0.686 * | −0.546 | −0.537 | −0.487 | −0.635 * | |
| CeC | −0.139 | −0.180 | −0.210 | 0.179 | 0.169 | −0.092 | 0.099 | −0.330 | −0.558 | 0.622 * | 0.111 | 0.276 | 0.271 | 0.558 | 0.320 | |
| TPC | −0.485 | −0.693 * | −0.663 * | 0.279 | 0.682 * | 0.424 | 0.509 | 0.608 * | 0.511 | −0.046 | −0.207 | −0.240 | 0.114 | −0.116 | −0.004 | |
| LDW | 0.946 *** | 0.935 *** | 0.944 *** | 0.096 | −0.754 ** | −0.732 ** | −0.452 | −0.584 * | −0.446 | −0.140 | −0.529 | 0.878 *** | 0.507 | 0.407 | 0.741 ** | |
| StC | −0.072 | 0.002 | 0.001 | −0.140 | −0.262 | −0.178 | −0.131 | −0.549 | −0.654 * | 0.637 * | −0.210 | 0.103 | 0.373 | 0.319 | 0.683 * | |
| SSC | −0.859 *** | −0.960 *** | −0.955 *** | 0.080 | 0.846 ** | 0.715 ** | 0.459 | 0.713 ** | 0.474 | 0.072 | 0.735 ** | −0.909 *** | −0.070 | 0.803 ** | 0.932 *** | |
| SuC | −0.676 * | −0.878 *** | −0.888 *** | 0.303 | 0.910 *** | 0.619 * | 0.502 | 0.887 *** | 0.626 * | −0.042 | 0.724 ** | −0.805 ** | −0.329 | 0.902 *** | 0.756 ** | |
| NSCC | −0.869 *** | −0.958 *** | −0.954 *** | 0.058 | 0.806 ** | 0.688 * | 0.438 | 0.629 * | 0.374 | 0.169 | 0.703 * | −0.893 *** | 0.082 | 0.998 *** | 0.851 *** |
| Combination | LNLP | LNHP | HNLP | HNHP |
|---|---|---|---|---|
| KNO3 | 0.4 | 0.4 | 5 | 5 |
| KH2(PO4) | 0.002 | 1 | 0.002 | 1 |
| CaCl2 | 0.07 | 0.07 | 0.07 | 0.07 |
| MgSO4·7H2O | 0.45 | 0.45 | 0.45 | 0.45 |
| H3BO3 | 0.0042 | 0.0042 | 0.0042 | 0.0042 |
| MnSO4 | 0.0012 | 0.0012 | 0.0012 | 0.0012 |
| ZnSO4·7H2O | 0.0008 | 0.0008 | 0.0008 | 0.0008 |
| CuSO4·5H2O | 0.00003 | 0.00003 | 0.00003 | 0.00003 |
| Na2MoO4 | 0.00004 | 0.00004 | 0.00004 | 0.00004 |
| CoCl2 | 0.00001 | 0.00001 | 0.00001 | 0.00001 |
| FeSO4·7H2O | 0.008 | 0.008 | 0.008 | 0.008 |
| Na2EDTA | 0.008 | 0.008 | 0.008 | 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Liao, Y.; Wei, W.; Xu, X.; He, J.; Zhao, T. Effects of Simulated Nitrogen and Phosphorus Deposition on Dioecious Populus cathayana Growth and Defense Traits. Plants 2025, 14, 1261. https://doi.org/10.3390/plants14081261
Li J, Liao Y, Wei W, Xu X, He J, Zhao T. Effects of Simulated Nitrogen and Phosphorus Deposition on Dioecious Populus cathayana Growth and Defense Traits. Plants. 2025; 14(8):1261. https://doi.org/10.3390/plants14081261
Chicago/Turabian StyleLi, Junyu, Yongmei Liao, Wanrong Wei, Xiaoqin Xu, Jundong He, and Tingting Zhao. 2025. "Effects of Simulated Nitrogen and Phosphorus Deposition on Dioecious Populus cathayana Growth and Defense Traits" Plants 14, no. 8: 1261. https://doi.org/10.3390/plants14081261
APA StyleLi, J., Liao, Y., Wei, W., Xu, X., He, J., & Zhao, T. (2025). Effects of Simulated Nitrogen and Phosphorus Deposition on Dioecious Populus cathayana Growth and Defense Traits. Plants, 14(8), 1261. https://doi.org/10.3390/plants14081261







