Optimization of Fermentation Conditions for Bacillus amyloliquefaciens JL54 and Preparation of Powder Through Spray Drying
Abstract
:1. Introduction
2. Results
2.1. Analysis of Optimization Results of Strain Fermentation Medium Components
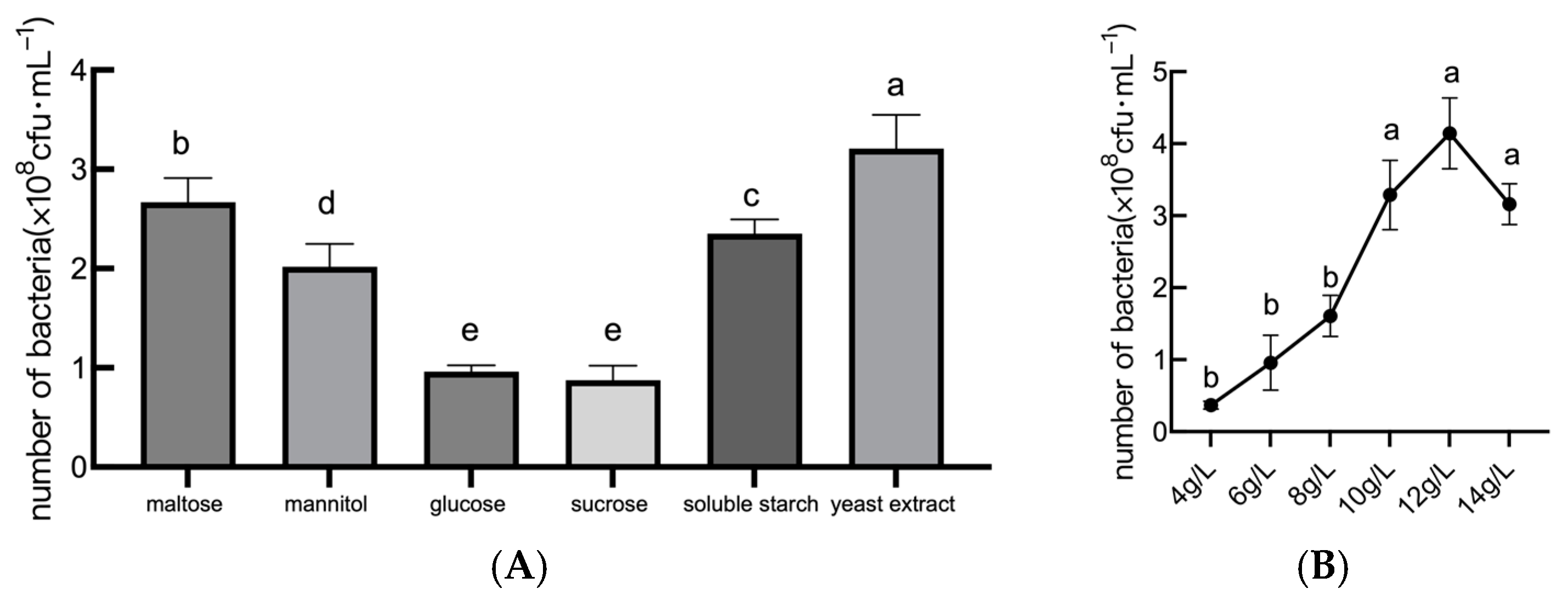
2.1.1. Analysis of Carbon Source Optimization Results
2.1.2. Analysis of Nitrogen Source Optimization Results
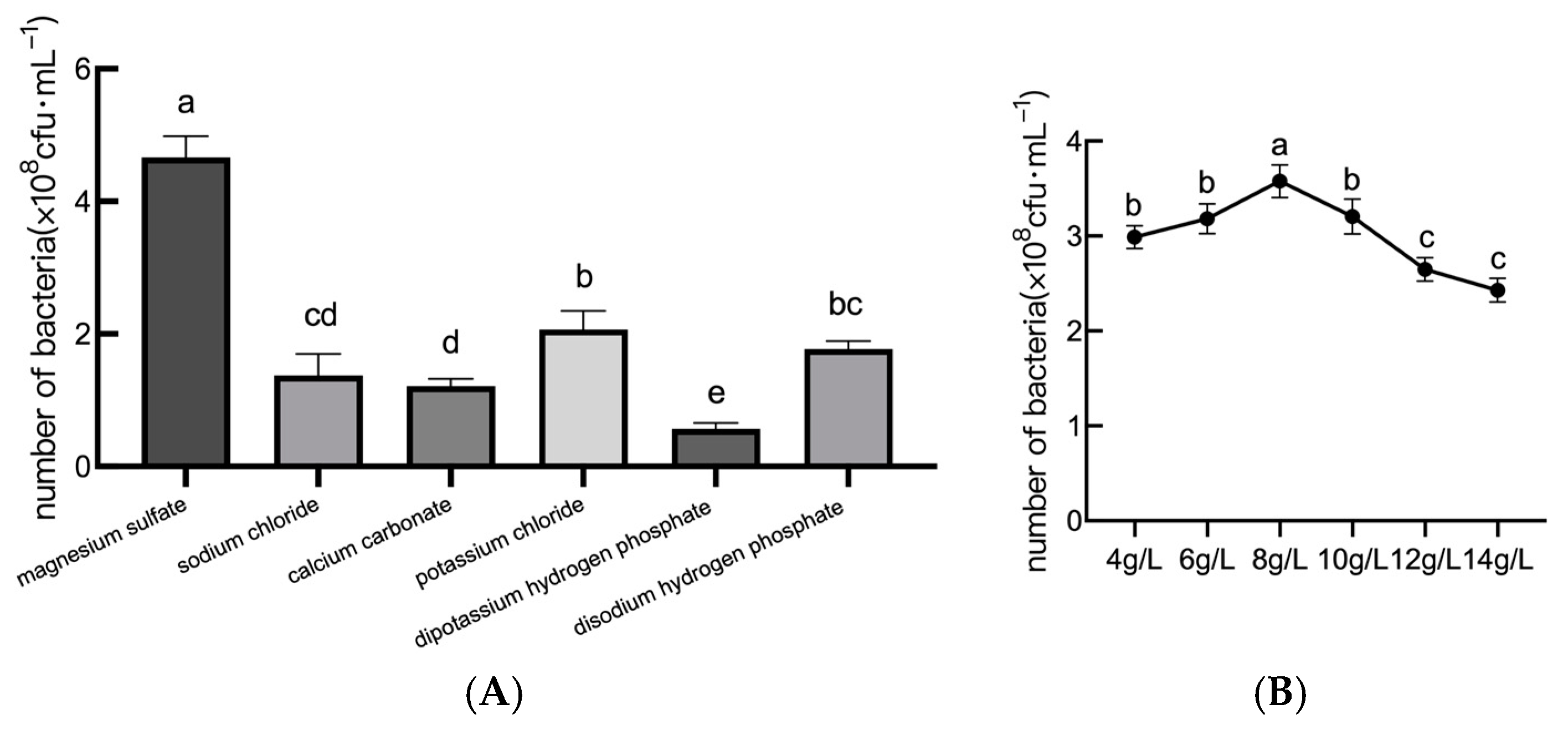
2.1.3. Analysis of Inorganic Salt Optimization Results
2.2. Analysis of Optimization Results of Strain Fermentation Conditions
2.3. Response Surface Optimization of Fermentation Culture of B. amyloliquefaciens JL54
2.3.1. Plackett–Burman Test
2.3.2. Steepest Climb Test
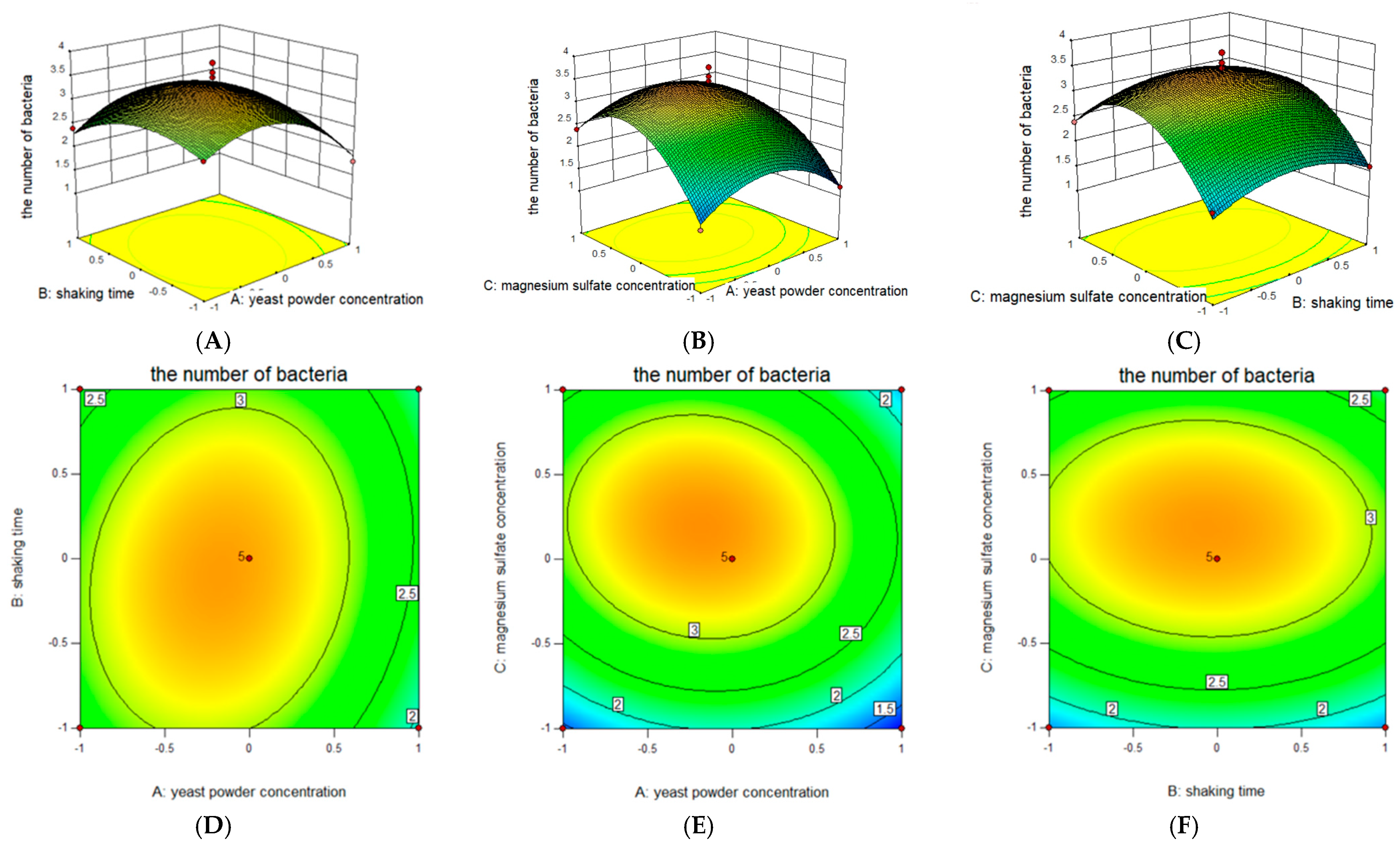
2.3.3. Box–Behnken Design (BBD) Analysis
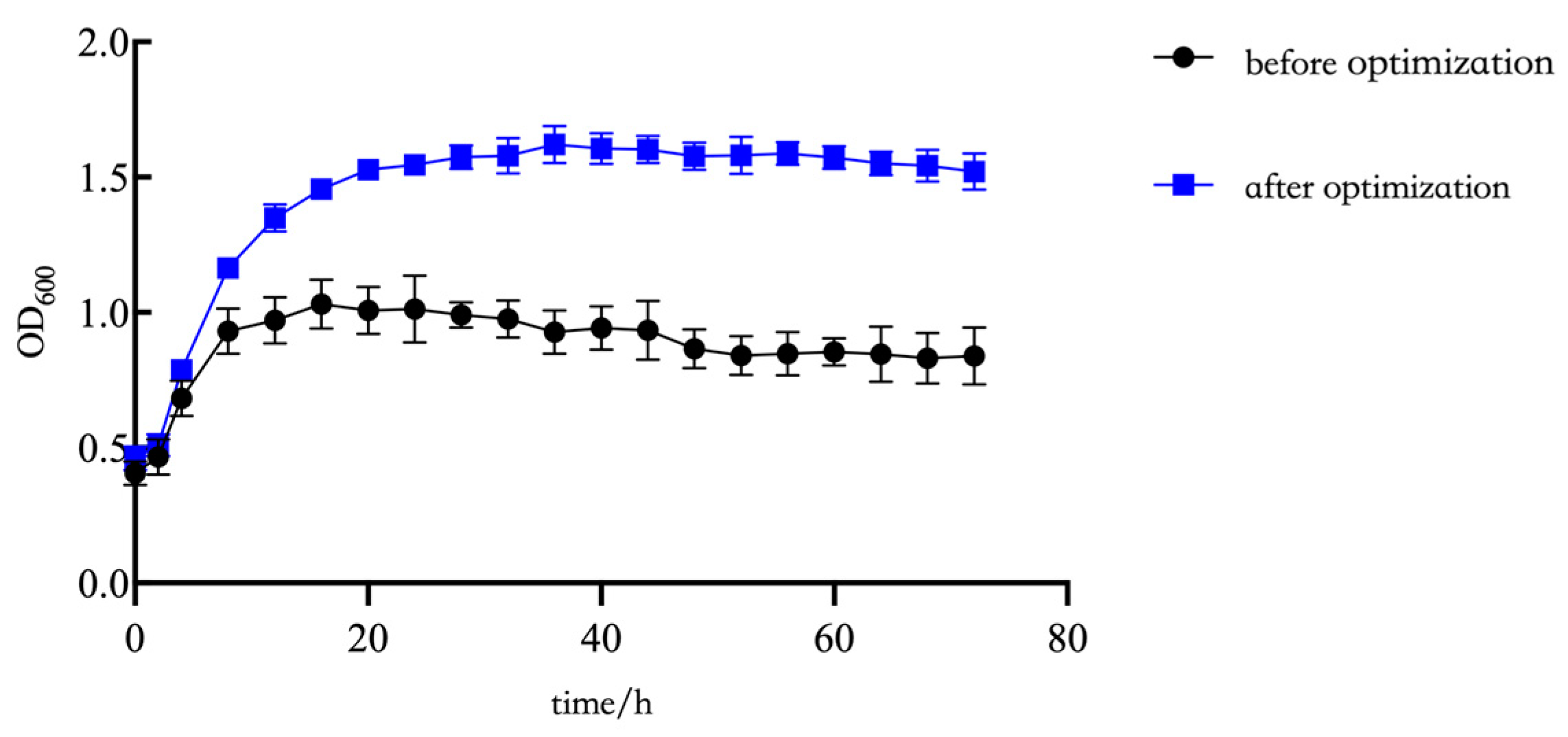
2.4. Comparison of Viable Bacteria Numbers in Fermentation Broth Before and After Fermentation Optimization
2.5. Field Experiments
2.6. Results of Spray Drying Single Factor Test
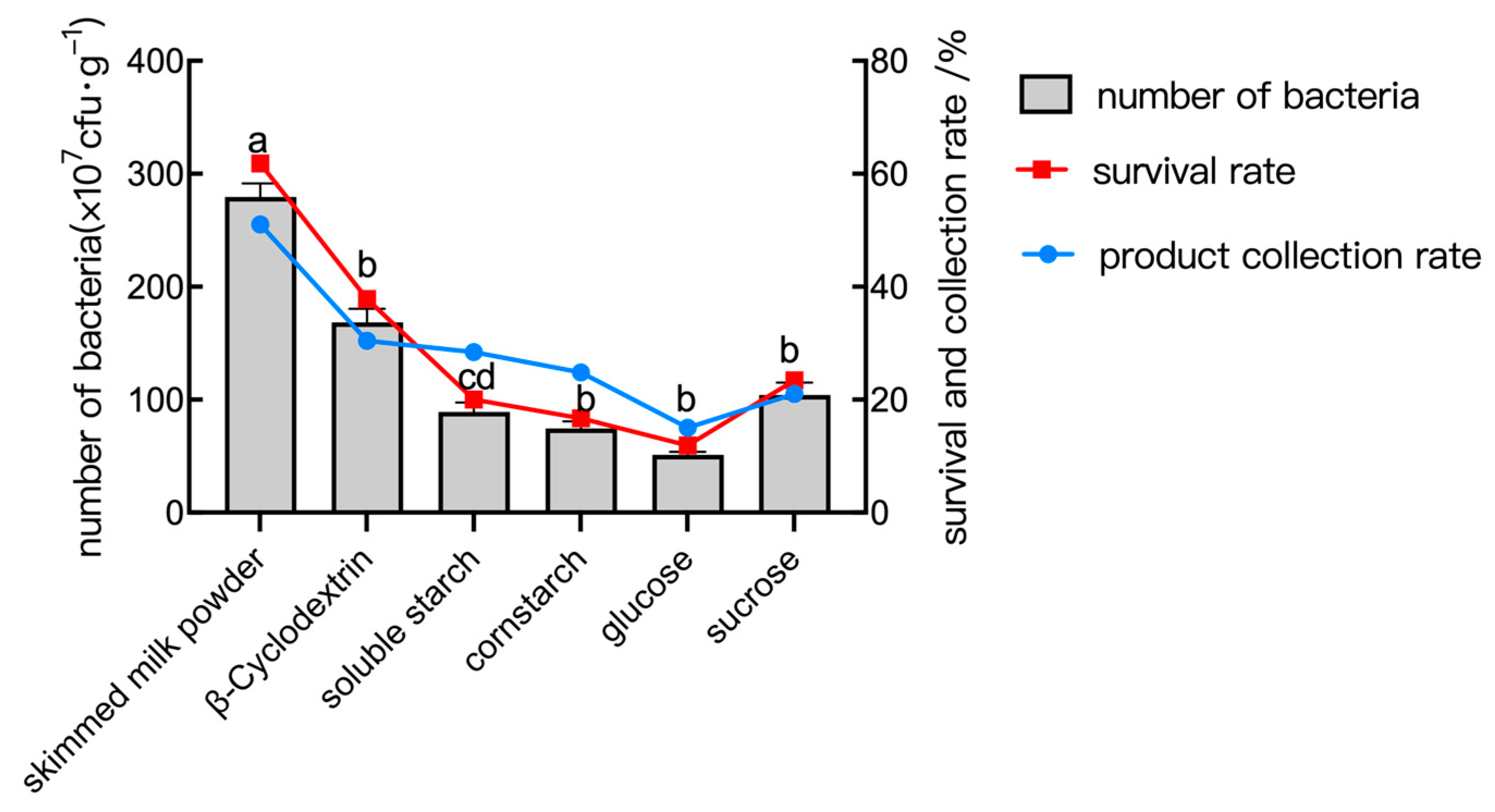
2.6.1. The Effect of Protective Agent Type
2.6.2. The Effect of the Material/Liquid Ratio
2.6.3. The Effect of Inlet Temperature
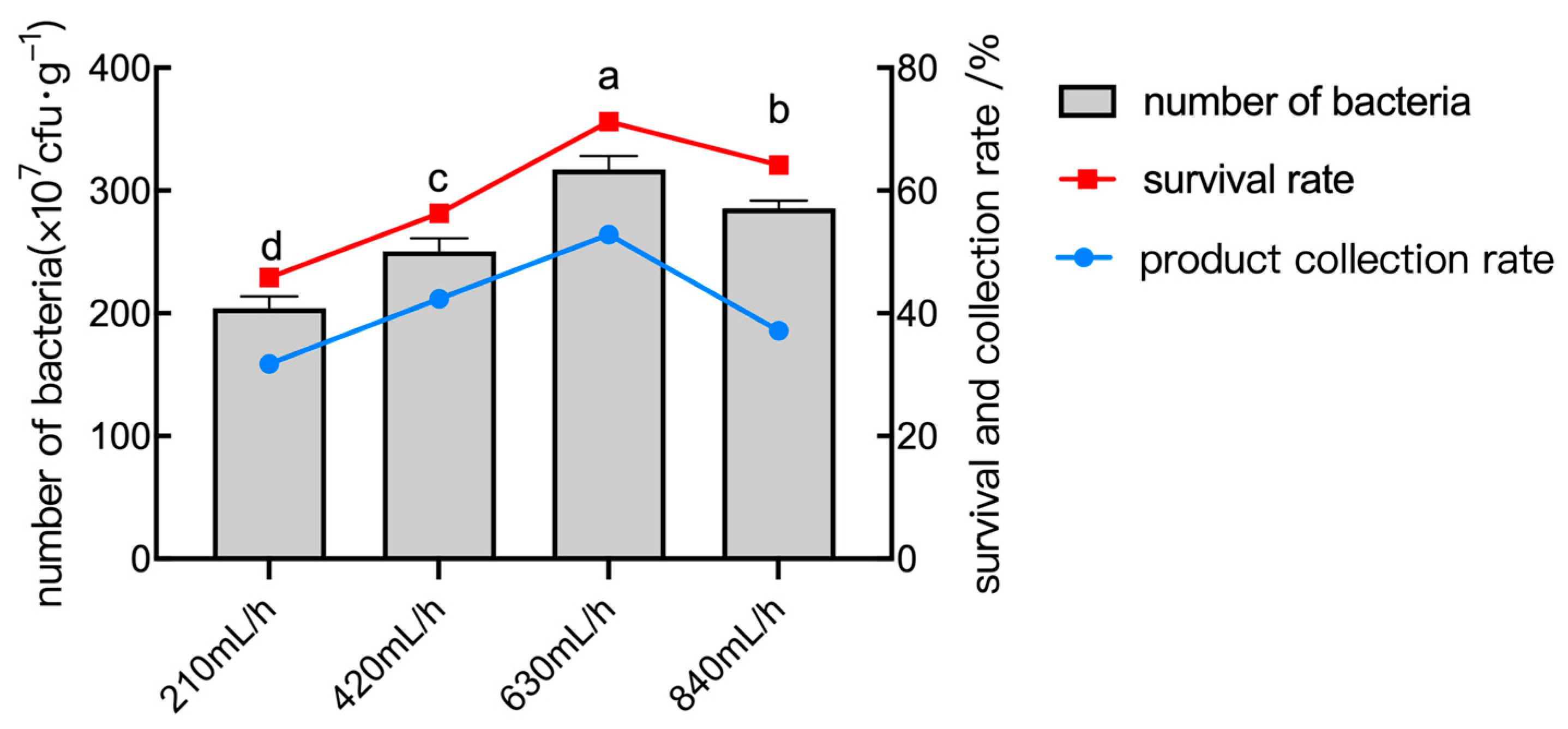
2.6.4. The Effect of Inlet Flow Rate
2.7. Orthogonal Test of Spray Drying Conditions
3. Discussion
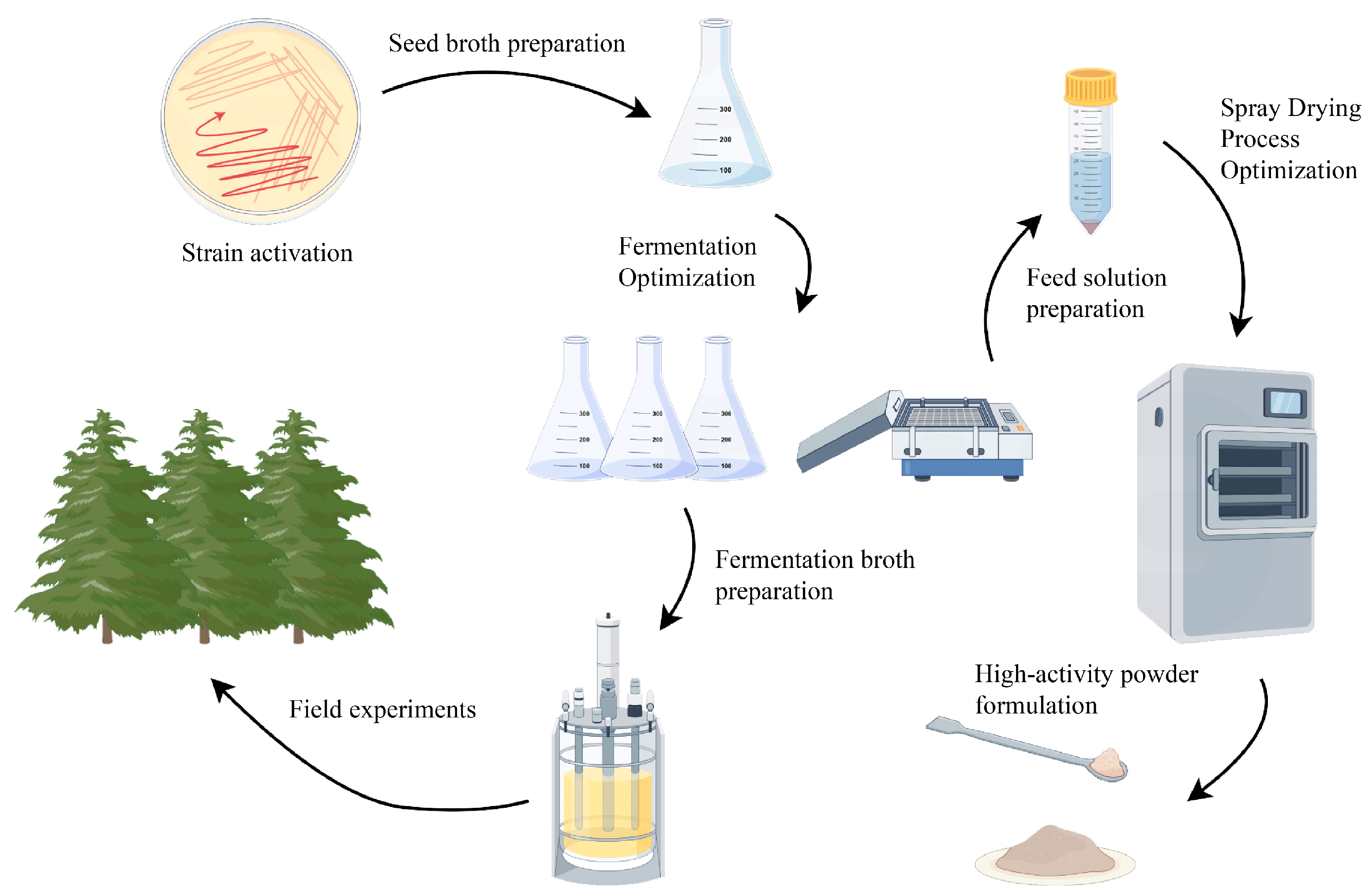
4. Materials and Methods
4.1. Medium and Source of Strains
4.2. Strain Activation, Preparation of Seed Broth, and Obtaining of Fermentation Broth and Bacterial Suspension
4.3. Optimization of Fermentation Medium and Conditions
4.3.1. Optimization of Fermentation Medium
4.3.2. Optimization of Fermentation Conditions
4.4. Response Surface Methodology (RSM) Optimization
4.4.1. Plackett–Burman (PB) Test
4.4.2. Steepest Climb Test
4.4.3. Box–Behnken Design (BBD)
4.5. Determination of the Growth Curve of B. amyloliquefaciens JL54
4.6. Data Analysis and Processing
4.7. Field Experiments
4.8. Single-Factor Test of the Main Parameters of Spray Drying
4.9. Orthogonal Optimization Test
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hattori, Y.; Ando, Y.; Nakashima, C. Taxonomical re-examination of the genus Neofusicoccum in Japan. Mycoscience 2021, 62, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Kazuo, I. A New Lecture on the Illustration of Forest Trees; Terre des Hommes Press, Inc.: Toyonaka City, Japan, 1964. [Google Scholar]
- Zhao, J.Z.; Yu, W.X.; Wang, N.Y. Current status of domestic and international research on larch shoot blight. For. Sci. Technol. 1995, 5, 23–25. [Google Scholar]
- Sawada, K. Fungi of Conifers in Tohoku district (2)-fungi of Conifers other than cedar. J. For. Sci. Technol. 1950, 46, 111–154. [Google Scholar]
- Yamamoto, W.; Kazuo, I. Species of the genera of Glomerella and Guignardia with special reference to their imperfect stages. Sci. Rep. Hyogo Univ. Agric. 1961, 5, 1–12. [Google Scholar]
- Sun, M.L.; Huang, L.; Ye, J.R.; He, J.; Wang, Z. Advances on pathogenic mechanisms and endophytes-employed biological control of fungal diseases on major timber forests in China. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2022, 46, 225–232. [Google Scholar]
- CSFA. State Forestry Administration Announcement (no. 4, 2013) National Forestry Quarantine Pest List. 2013. Available online: https://www.gov.cn/gzdt/2013-01/15/content_2312345.htm (accessed on 1 December 2024).
- SFGA. State Forestry and Grassland Administration Notice (No. 567), ‘List of Priority Invasive Alien Species for Management’. 2023. Available online: http://www.moa.gov.cn/govpublic/KJJYS/202211/t20221109_6415160.htm (accessed on 20 April 2024).
- EPPO. Neofusicoccum laricinum (GUIGLA) Associated EPPO Standards; EPPO: Paris, France, 2022. [Google Scholar]
- Xu, Z.G. General Plant Pathology, 4th ed.; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Liu, M.G.; Zhang, D.; Lu, W.Y.; Wang, J. Pathogen of Pine Shoot Blight: Research Progress. Pathog. Pine Shoot Blight Res. Prog. 2018, 34, 97–101. [Google Scholar]
- Jorge, P.; Andrea, R.R.; Rosa, M.M.; Alejandra, F.M. Microorganisms as biocontrol agents against bacterial citrus diseases. Biol. Control 2021, 158, 104602. [Google Scholar]
- Yavuz, E.; Gunes, H.; Harsa, S.; Bulut, C.; Yenidunya, A.F. Optimization of pulsed field gel electrophoresis (PFGE) conditions for thermophilic bacilli. World J. Microbiol. Biotechnol. 2004, 20, 871–874. [Google Scholar] [CrossRef]
- Ling, P.S.; Pirasannah, E.; AlAdil, B.M.M.; Mohd, Y.N.; Ahmad, K.W.N.I.W.; Mohamad, A.M.S.; Aqlima, A.S.; Nurbaya, O.S.; Sooa, L.; Suriana, S. Antimicrobial peptides from Bacillus spp. and strategies to enhance their yield. Appl. Microbiol. Biotechnol. 2023, 107, 5569–5593. [Google Scholar]
- Dai, L.; Li, L.; Liu, Y.; Shi, Y.; Cai, Z. Whole genome sequencing and genomics analysis of Bacillus amyloliquefaciens BS-3 with biocontrol activity. Microbiol. China 2021, 48, 2073–2088. [Google Scholar] [CrossRef]
- Qin, X.; Xiao, Y.; Xiong, Q.; Kong, W.L.; Borriss, R.; Gao, Z.; Fan, B. Four antimicrobial compounds and ISR induction are involved in biocontrol of crown gall disease by the plant beneficial rhizobacterium Bacillus velezensis FZB42. Plant Dis. 2025. [Google Scholar] [CrossRef] [PubMed]
- Ying, T.T.; Wu, P.J.; Gao, L.L.; Wang, C.C.; Zhang, T.H.; Liu, S.S.; Huang, R.Q. Isolation and characterization of a new strain of Bacillus amyloliquefaciens and its effect on strawberry preservation. LWT 2022, 165, 113712. [Google Scholar] [CrossRef]
- Michael, B.; Danai, E.; Sabrina, C.; Peter, B.; Roger, S.; Monika, E.S.; Sophia, J. Whole Genome Sequencing Reveals Biopesticidal Origin of Bacillus thuringiensis in Foods
. Front. Microbiol. 2022, 12, 775669. [Google Scholar]
- Pan, X.H.; Chen, F.; Guo, X.P.; Wang, Y.L.; Cui, Z.Q.; Huang, T.P.; Hou, Y.M.; Guan, X. Development of a safe and effective Bacillus thuringiensis-based nanobiopesticide for controlling tea pests. J. Agric. Food Chem. 2024, 72, 7807–7817. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.M.; Savocchia, S.; Lindbeck, K.D.; Ash, G.J. Biology and biocontrol of Sclerotinia sclerotiorum (Lib.) de Bary in oilseed Brassicas. Australas. Plant Pathol. 2016, 45, 1–14. [Google Scholar] [CrossRef]
- Femina, C.C.; Senthil, K.P.; Tsopbou, N.P. A review on new aspects of lipopeptide biosurfactant: Types, production, properties and its application in the bioremediation process. J. Hazard. Mater. 2021, 407, 124827. [Google Scholar]
- Wang, R.; Lan, B.F.; Zhou, L.Q.; Yang, H.; Meng, J.Z.; Chen, W.C. Optimization of fermentation conditions of Penicillium sp. antagonistic bacterium Bacillus velezensis wr8 in postharvest citrus by response surface method. China Brew. 2023, 42, 197–202. [Google Scholar]
- Zhang, C.F.; Si, H.L.; Chen, L.; Liang, X.J.; Zhang, J.L. Isolation and identification of nicosulfuron-degrading enzymes from Bacillus velezensis CF57. J. Hebei Agric. Univ. 2020, 43, 43–48. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, J.; Zhao, J.; Hao, Y.S.; Liang, H. Isolation and Identification of Pathogenic Fungi of Strawberry Root Rot and the Inhibition of Antagonistic Bacteria CM3 on These Fungi. Biotechnol. Bull. 2018, 34, 135–141. [Google Scholar] [CrossRef]
- Chai, Q.K.; Zhang, B.; Chang, R.K.; Liu, H.Q.; Tian, X.W.; Wang, Y.H. Preliminary study on the effect of the induced resistance in cucumber with Bacillus amyloliquefaciens LJ02 against Botrytis cinerea. Acta Phytopathol. Sin. 2019, 49, 828–835. [Google Scholar] [CrossRef]
- Hou, B.H.; Xu, B.L.; Xun, T.; Zhang, S.W. Determination of the stability and antibacterial spectrum of antibacterial substances of the Bacillus amyloliquefaciens TS-1203 against Valsa mali. J. Gansu Agric. Univ. 2017, 52, 80–86. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Liu, C.; Song, M.; Xu, Q.; Liu, Y.; Yan, H. Genome analysis of a newly isolated Bacillus velezensis-YW01 for biodegrading acetaldehyde. Biodegradation 2024, 35, 539–549. [Google Scholar] [CrossRef]
- Wang, S.W.; Wang, Q.H.; Zheng, C.X.; Liu, X.; Li, B. Advance in the drug form, preparation and applicationof Bacillus amyloliquefaciens. J. Sci. Teach. Coll. Univ. 2023, 43, 60–65. [Google Scholar]
- Jin, W.J.; Zhang, F.J.; Zhang, X.M.; Yu, J.J.; Zou, L.; Sun, B.S.; Yan, Y.Z.; Xue, J. Bacteriostatic and antiseptic effects of Bacillus amyloliquefaciens B15 wettable powder. Jiangsu Agric. Sci. 2021, 49, 169–172. [Google Scholar] [CrossRef]
- Qian, Y.X.; Kang, J.C.; Luo, Y.K.; Geng, K.; Lei, B.X.; Zhang, B. Development of Wettable Powder of Bacillus amyloliquefaciens X17 for Control of Botrytis cinerea on Kiwifruits. Chin. J. Biol. Control 2016, 32, 342–348. [Google Scholar] [CrossRef]
- Cai, X.C.; Liu, J.D.; Gao, X.; Wang, B.T.; Liu, C.H. Preparation of Endophyte Bacillus amyloliquefaciens CC09 WP. Mod. Agrochem. 2015, 14, 8–12+20. [Google Scholar]
- Huang, M.; Yao, L.; Zhao, L.H. Research on Microencapsulation of Linseed Oil by Spray Drying. Farm Prod. Process. 2015, 7, 42–45. [Google Scholar]
- Meng, X.K.; Yu, J.J.; Yin, X.L.; Nie, Y.F.; Yu, M.N.; Chen, Z.Y.; Liu, Y.F. Study on the Spray Drying Technology of Antagonistic Bacteria Bacillus subtilis T429. Chin. J. Biol. Control 2014, 30, 101–106. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Han, S.J.; Song, L.W.; Li, L.M.; Wang, H.F.; Pan, M.; Tan, J.J. Screening of bacterial endophytes of larch against Neofusicoccum laricinum and validation of their safety. Microbiol. Spectr. 2024, 12, e04112–e04123. [Google Scholar] [CrossRef]
- Pan, M.; Wang, Y.; Tan, J.; Liu, F.; Hu, J. Optimization of Fermentation Conditions for Bacillus pumilus LYMC-3 to Antagonize Sphaeropsis sapinea. Fermentation 2023, 9, 482. [Google Scholar] [CrossRef]
- Wei, H.; Liu, S.H.; Song, Q.F.; Bao, F.; Shang, Q.X.; Liu, Z.P.; Wei, Y.M. Studies on Fermentation Process of Strain BJ-6 of Bacillus amyloliquefaciens. Chin. Agric. Sci. Bull. 2009, 25, 151–155. [Google Scholar]
- Li, Y.; Gao, Z.; Kong, W.; Xiao, Y.; Adjei, M.O.; Fan, B. Biocontrol of Crown Gall Disease of Cherry Trees by Bacillus velezensis. Plants 2025, 14, 475. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.S.; Guo, N.Q. Research on spray drying process of pesticide water dispersible granules. Pesticides 2001, 11, 14–15. [Google Scholar]
- Peng, X.Q.; Wang, R.; Xu, X.L.; Fan, H.M. DVS meitauza starter preparation by spray drying process. China Brew. 2014, 33, 34–37. [Google Scholar]
- Li, P.T.; Hsiao, W.L.; Yu, R.C.; Chou, C.C. Effect of heat shock on the fatty acid and protein profiles of Cronobacter sakazakii BCRC 13988 as well as its growth and survival in the presence of various carbon, nitrogen sources and disinfectants. Food Microbiol. 2013, 36, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Kuang, J.H.; Huang, Y.Y.; Hu, J.S.; Yu, J.J.; Zhou, Q.Y.; Zhao, S.; Liu, D.M. Optimization of fermentation conditions and anti-oxidation of exopolysaccharide produced by Bacillus amyloliquefaciens DMBA-K4. Food Ferment. Ind. 2020, 46, 28–35. [Google Scholar]
- Xia, B.H.; Zhao, J.; Gong, P.; Han, X.B.; Peng, Y.L.; Liu, K.; Wang, C.Q.; Ding, Y.Q.; Du, B.H. Optimal Fermentation Conditions for Bacillus amyloliquefaciens DSYZ Based on Response Surface Methodology. J. Shandong Agric. Univ. (Nat. Sci. Ed.) 2021, 52, 241–246. [Google Scholar]
- Song, K.D.; Li, Z.J.; Han, K.C.; Cui, M.Y.; Zhou, M.M. Characteristics of traditional dough fermentation starter (Jiaozi) made from different size corn flour. Food Sci. Technol. 2019, 44, 185–189. [Google Scholar] [CrossRef]
- Zhou, X.P.; Shu, C.H.; Teng, K.; Gan, Z.D.; Xiao, Q.M.; Chen, w.; Li, X.H.; Liu, T.B. Identification and Fermentation Optimization of Antagonistic Bacillus amyloliquefaciens Xe01. Chin. Tob. Sci. 2020, 41, 58–67. [Google Scholar] [CrossRef]
- Xie, L.J.; Xiao, L.; Wu, S.D.; Cheng, W. Optimization of Fermentation Conditions for Endophytic Bacillus amyloliquefaciens L-4-3. Hunan Agric. Sci. 2022, 9, 10–13+21. [Google Scholar] [CrossRef]
- Zhang, W.; Wei, L.; Xu, R.; Lin, G.; Xin, H.; Lv, Z.; Qian, H.; Shi, H. Evaluation of the Antibacterial Material Production in the Fermentation of Bacillus amyloliquefaciens-9 from Whitespotted Bamboo Shark (Chiloscyllium plagiosum). Mar. Drugs 2020, 18, 119. [Google Scholar] [CrossRef] [PubMed]
- Wiggins, E.H.; Mann, P.F.E.; Trevelyan, W.E.; Harrison, J.S. Effect of pH on the synthesis of cell carbohydrate during fermentation by Baker’s yeast. Biochim. Biophys. Acta 1952, 8, 537–542. [Google Scholar] [CrossRef]
- Gibbons, W.R.; Westby, C.A. Effects of inoculum size on solid-phase fermentation of fodder beets for fuel ethanol production. Appl. Envion. Microbiol. 1986, 52, 960–962. [Google Scholar] [CrossRef]
- Yang, Q.H. Optimization of culture medium and fermentation conditions of Bacillus amyloliquefaciens CQN-2 strain from fishery sources. J. Fish. Res. 2020, 42, 339–347. [Google Scholar] [CrossRef]
- Xiong, H.; Li, Y.; Cai, Y.; Cao, Y.; Wang, Y. Isolation of Bacillus amyloliquefaciens JK6 and identification of its lipopeptides surfactin for suppressing tomato bacterial wilt. RSC Adv. 2015, 5, 82042–82049. [Google Scholar] [CrossRef]
- Wei, D.P.; Ye, J.R.; Liang, M.J. Spray drying processes of Bacillus valeriana YH-18. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2020, 44, 209–214. [Google Scholar]
- Wu, G.; Liu, Y.; Xu, Y.; Zhang, G.; Shen, Q.; Zhang, R. Exploring Elicitors of the Beneficial Rhizobacterium Bacillus amyloliquefaciens SQR9 to Induce Plant Systemic Resistance and Their Interactions with Plant Signaling Pathways. Mol. Plant-Microbe Interact. 2018, 31, 560–567. [Google Scholar] [CrossRef]
- Ma, X.; Huang, Y.; Cheng, J.; Wang, W. Microencapsulation of Bacillus subtilis and its control of Rhizoctonia solani damping-off of tomato. Chin. J. Pestic. Sci. 2015, 17, 462–468. [Google Scholar]
- Ma, D. Optimization of Solid-state Fermentation Process and Development of Biocontrol Agent from Streptomyces microflavus. ResearchGate 2018, AMYa-008. [Google Scholar] [CrossRef]
- Wu, D.L.; Wang, Z.G.; Mei, L.; Xue, X.H. Optimization of process parameters for the preparation of microcapsules of lactic acid bacteria by vacuum low-temperature spray drying method. Jiangsu Agric. Sci. 2015, 43, 367–370. [Google Scholar] [CrossRef]
- Liu, F.; Li, Y.Y.; Chen, W.; Cao, C.X.; Wen, S.H.; Rao, B.; Huang, D.Y. Optimization of Fermentation Medium for Spore Production by Bacillus velezensis YC11 through Response-Surface Methodology. Chin. J. Biol. Control 2023, 39, 915–923. [Google Scholar] [CrossRef]
- Gong, J.H.; Wang, J. Introduction to the method of enumerating viable bacteria by dilution-coated plate method. Teach. Biol. 2018, 43, 70–71. [Google Scholar]
- Hu, S.; Mei, L.H.; Yao, S.J. Optimization of nattokinase liquid fermentation by response surface methodology. Food Ferment. Ind. 2003, 1, 13–17. [Google Scholar]
- Ma, H.Z.; Yan, M.; Li, L.; Yang, Z.P.; Zhang, J.X.; Meng, Q.X.; Shi, X.Y. Optimization of Fermentation Parameters of Bacillus velezensis C44 Based on Response Surface Methodology. J. Shanxi Agric. Sci. 2023, 51, 1088–1097. [Google Scholar]
- Liu, J.; Zhang, C.Z.; Zhao, H. Optimization of medium and fermentation conditions of Bacillus velezensis P9 resistant to Fusarium oxysporum. China Brew. 2023, 42, 157–162. [Google Scholar]
- Shi, H.M.; Ye, J.R.; Wang, M.; Lu, L.X.; Shi, J.W. Optimizing spore-producing medium and culture conditions of Bacillus velezensis strain YH-18 by response surface methodology. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2023, 47, 209–218. [Google Scholar]
- Wang, X.; Gao, J.; Gao, Y.; Zhang, L.; Xu, C.; Li, Q.; Li, L.; Xue, J. Analysis of surfactant production by Bacillus cereus GX7 and optimization of fermentation conditions. Colloids Surf. B Biointerfaces 2024, 233, 113629. [Google Scholar] [CrossRef]
- Xiao, H.Q.; Li, Y.Z.; Zhao, M.M.; Lin, Q.L.; Liu, J.; Zhou, Q.; Jiang, M.J. Optimization of Spray Drying Parameters for the Probiotic Powder Preparation of Bacillus subtilis Prob 1822. Sci. Technol. Food Ind. 2020, 41, 114–120. [Google Scholar] [CrossRef]





| Source of Variance | Coefficient Estimate | Stdized Effec | Sum of Squares | Degrees of Freedom | Mean Square | F | p | Contribution/% | Inference | Importance Ranking |
|---|---|---|---|---|---|---|---|---|---|---|
| Model | 10,964.00 | 8 | 1370.50 | 25.69 | 0.0110 | * | ||||
| A: yeast extract concentration | 8.28 | 16.57 | 823.20 | 1 | 823.20 | 15.43 | 0.0294 | 7.37 | 5 | |
| B: yeast powder concentration | 16.36 | 32.73 | 3212.78 | 1 | 3212.78 | 60.21 | 0.0045 | 28.77 | 1 | |
| C: magnesium sulfate concentration | −13.45 | −26.89 | 2169.49 | 1 | 2169.49 | 40.66 | 0.0078 | 19.43 | 3 | |
| D: initial pH | 4.59 | 9.18 | 252.73 | 1 | 252.73 | 4.74 | 0.1178 | 2.26 | 7 | |
| E: inoculum volume | 4.15 | 8.29 | 206.26 | 1 | 206.26 | 3.87 | 0.1440 | 1.85 | 8 | |
| F: shaking bottle time | 15.28 | 30.56 | 2802.66 | 1 | 2802.66 | 52.53 | 0.0054 | 25.10 | 2 | |
| G: shaking bottle temperature | −7.07 | −14.15 | 600.24 | 1 | 600.24 | 11.25 | 0.0439 | 5.38 | 6 | |
| H: loading volume | 8.64 | 17.29 | 896.66 | 1 | 896.66 | 16.80 | 0.0263 | 8.03 | 4 |
| Treatment No. | Yeast Powder Concentration/g/L | Shaking Time/h | Magnesium Sulfate Concentration/g/L | Number of Bacteria/ (×109 cfu·mL−1) |
|---|---|---|---|---|
| 1 | 8 | 24 | 3 | 2.22 |
| 2 | 9 | 26 | 4 | 3.02 |
| 3 | 10 | 28 | 5 | 3.16 |
| 4 | 11 | 30 | 6 | 3.5 |
| 5 | 12 | 32 | 7 | 4.05 |
| 6 | 13 | 34 | 8 | 3.27 |
| Source | Sum of Squares | Degrees of Freedom | Mean Square | F | p | Inference |
|---|---|---|---|---|---|---|
| Model | 9.48 | 9 | 1.05 | 11.69 | 0.0019 | ** |
| A: yeast powder concentration | 0.45 | 1 | 0.45 | 5.01 | 0.0602 | ns |
| B: shaking time | 0.02 | 1 | 0.02 | 0.22 | 0.6518 | ns |
| C: magnesium sulfate concentration | 1.05 | 1 | 1.05 | 11.67 | 0.0112 | * |
| AB | 0.09 | 1 | 0.09 | 1.00 | 0.3508 | ns |
| AC | 0.063 | 1 | 0.063 | 0.69 | 0.4323 | ns |
| BC | 0.01 | 1 | 0.01 | 0.11 | 0.7487 | ns |
| A2 | 2.08 | 1 | 2.08 | 23.07 | 0.002 | ** |
| B2 | 0.77 | 1 | 0.77 | 8.54 | 0.0222 | * |
| C2 | 4.23 | 1 | 4.23 | 46.98 | 0.0002 | ** |
| Residual | 0.63 | 7 | 0.09 | |||
| Lack of fit | 0.082 | 3 | 0.027 | 0.2 | 0.891 | ns |
| Pure error | 0.55 | 4 | 0.14 | |||
| Cor total | 10.11 | 16 |
| Treatments | Standard Sample Plot | Sample Number | 2023 Disease Index | 2024 Disease Index |
|---|---|---|---|---|
| Treatment | 1 | 1–30 | 36.60 | 44.00 |
| 2 | 31–60 | 36.60 | 41.30 | |
| 3 | 61–90 | 35.30 | 31.30 | |
| Control | 1 | 91–120 | 58.0 | 64.67 |
| 2 | 121–150 | 64.60 | 70.67 | |
| 3 | 151–180 | 66.50 | 71.61 |
| Trial No. | A: Material/Liquid Ratio | B: Inlet Temperature | C: Inlet Flow Rate | Powder Collection Rate/% |
|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 54.96 |
| 2 | 1 | 2 | 3 | 56.18 |
| 3 | 1 | 3 | 2 | 51.8 |
| 4 | 2 | 1 | 3 | 77.84 |
| 5 | 2 | 2 | 2 | 78.41 |
| 6 | 2 | 3 | 1 | 69.5 |
| 7 | 3 | 1 | 2 | 39.24 |
| 8 | 3 | 2 | 1 | 35.19 |
| 9 | 3 | 3 | 3 | 27.65 |
| K1 | 162.94 | 172.04 | 159.65 | |
| K2 | 225.75 | 169.78 | 169.45 | |
| K3 | 102.08 | 148.95 | 161.67 | |
| R | 41.22 | 7.70 | 3.27 |
| Source | Sum of Squares | df | Mean Square | F | p | Inference |
|---|---|---|---|---|---|---|
| A | 2549.26 | 2 | 1274.63 | 771.61 | 0.001 | ** |
| B | 108.02 | 2 | 54.00 | 32.69 | 0.030 | * |
| C | 17.85 | 2 | 8.925 | 5.40 | 0.156 | ns |
| Error | 3.304 | 2 | 1.652 |
| Factors | Factor A | Factor B | Factor C | Factor D |
|---|---|---|---|---|
| 1 | Skimmed milk powder | 1:20 | 80 °C | 210 mL/h |
| 2 | β-Cyclodextrin | 1:10 | 90 °C | 420 mL/h |
| 3 | Soluble starch | 1:5 | 100 °C | 630 mL/h |
| 4 | Cornstarch | 3:10 | 110 °C | 840 mL/h |
| 5 | Glucose | 120 °C | ||
| 6 | Sucrose |
| Level | Factors | ||
|---|---|---|---|
| Material/Liquid Ratio | Inlet Temperature | Inlet Flow Rate | |
| 1 | 1:20 | 95 | 420 |
| 2 | 1:10 | 100 | 630 |
| 3 | 1:5 | 105 | 840 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Wang, Y.; Pan, M.; Kong, W.; Wang, H.; Tan, J. Optimization of Fermentation Conditions for Bacillus amyloliquefaciens JL54 and Preparation of Powder Through Spray Drying. Plants 2025, 14, 1263. https://doi.org/10.3390/plants14081263
Zhao L, Wang Y, Pan M, Kong W, Wang H, Tan J. Optimization of Fermentation Conditions for Bacillus amyloliquefaciens JL54 and Preparation of Powder Through Spray Drying. Plants. 2025; 14(8):1263. https://doi.org/10.3390/plants14081263
Chicago/Turabian StyleZhao, Leilei, Yanru Wang, Min Pan, Weiliang Kong, Haifeng Wang, and Jiajin Tan. 2025. "Optimization of Fermentation Conditions for Bacillus amyloliquefaciens JL54 and Preparation of Powder Through Spray Drying" Plants 14, no. 8: 1263. https://doi.org/10.3390/plants14081263
APA StyleZhao, L., Wang, Y., Pan, M., Kong, W., Wang, H., & Tan, J. (2025). Optimization of Fermentation Conditions for Bacillus amyloliquefaciens JL54 and Preparation of Powder Through Spray Drying. Plants, 14(8), 1263. https://doi.org/10.3390/plants14081263






