Morphology and Phylogenetic Positions of Two Novel Gogorevia Species (Bacillariophyta) from the Han River, South Korea
Abstract
1. Introduction
2. Results
2.1. Species Description
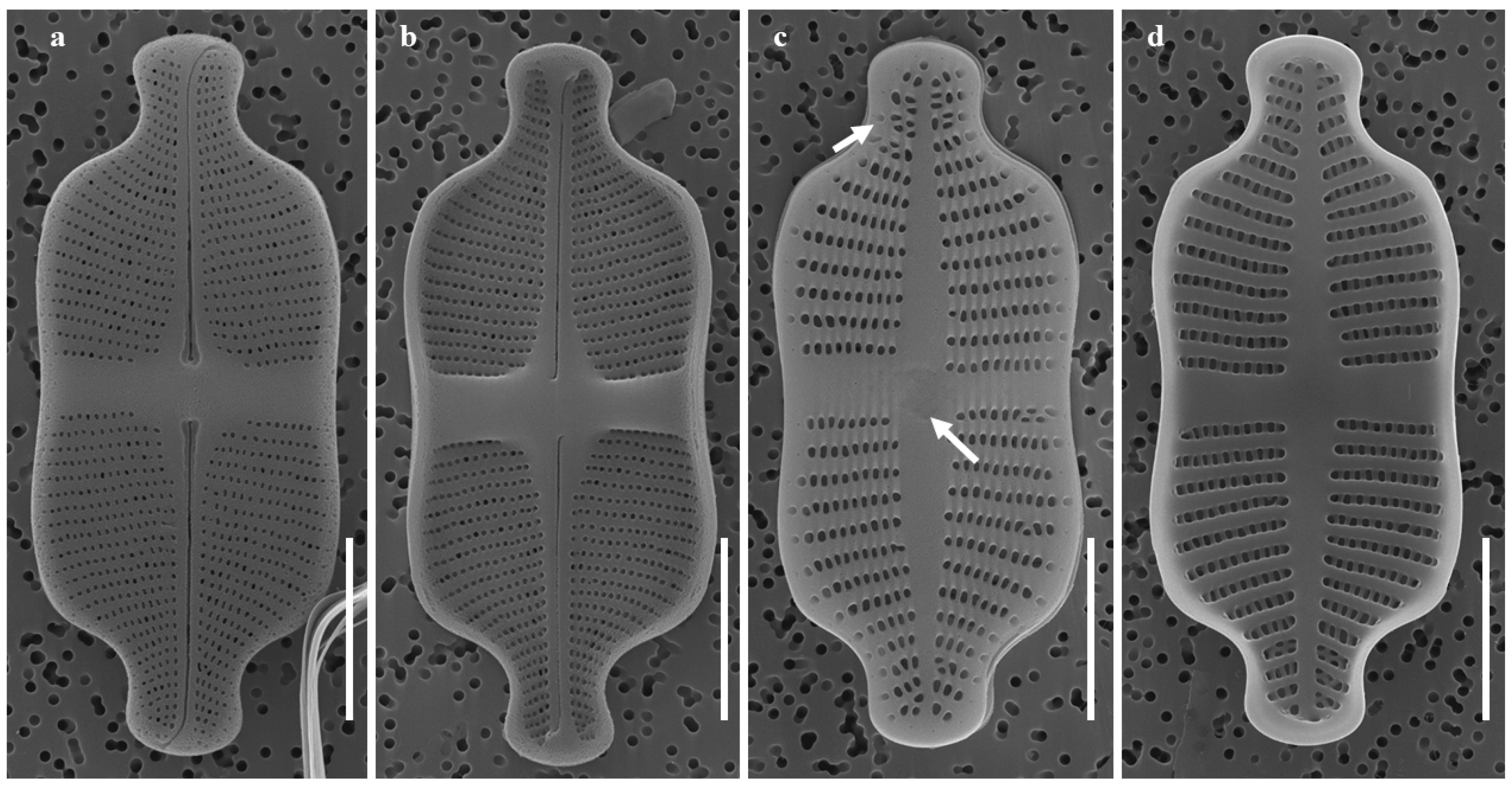
2.1.1. Taxonomic Characteristics of G. contracta W. Wang and B.H. Kim sp. nov. (LM, Figure 1; SEM, Figure 2)
- Description:
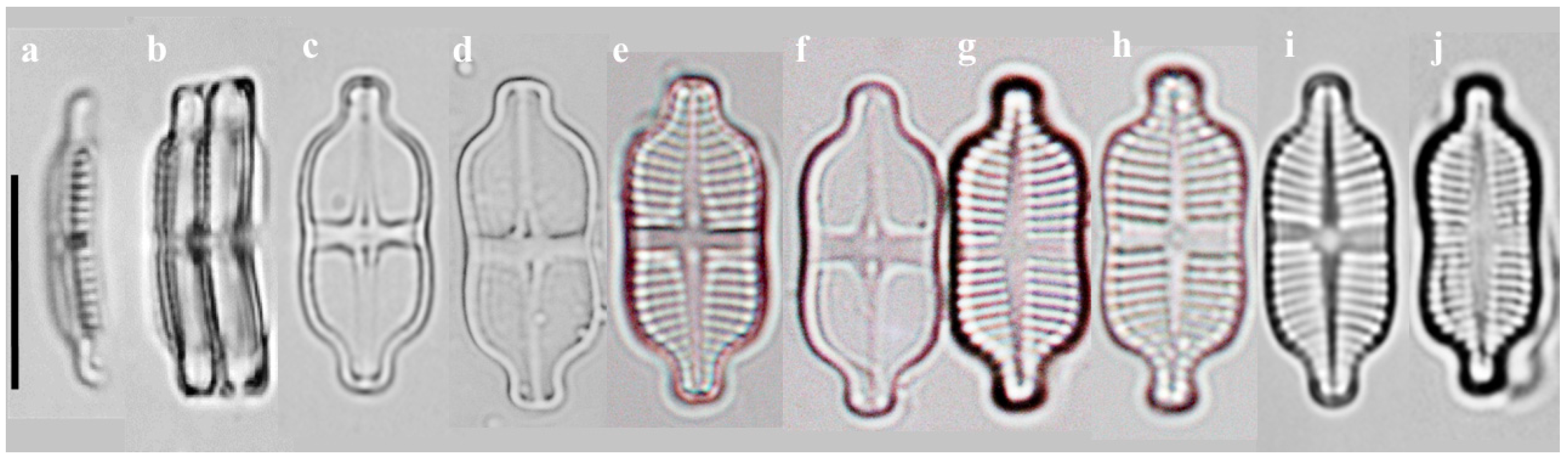
- SEM observations:

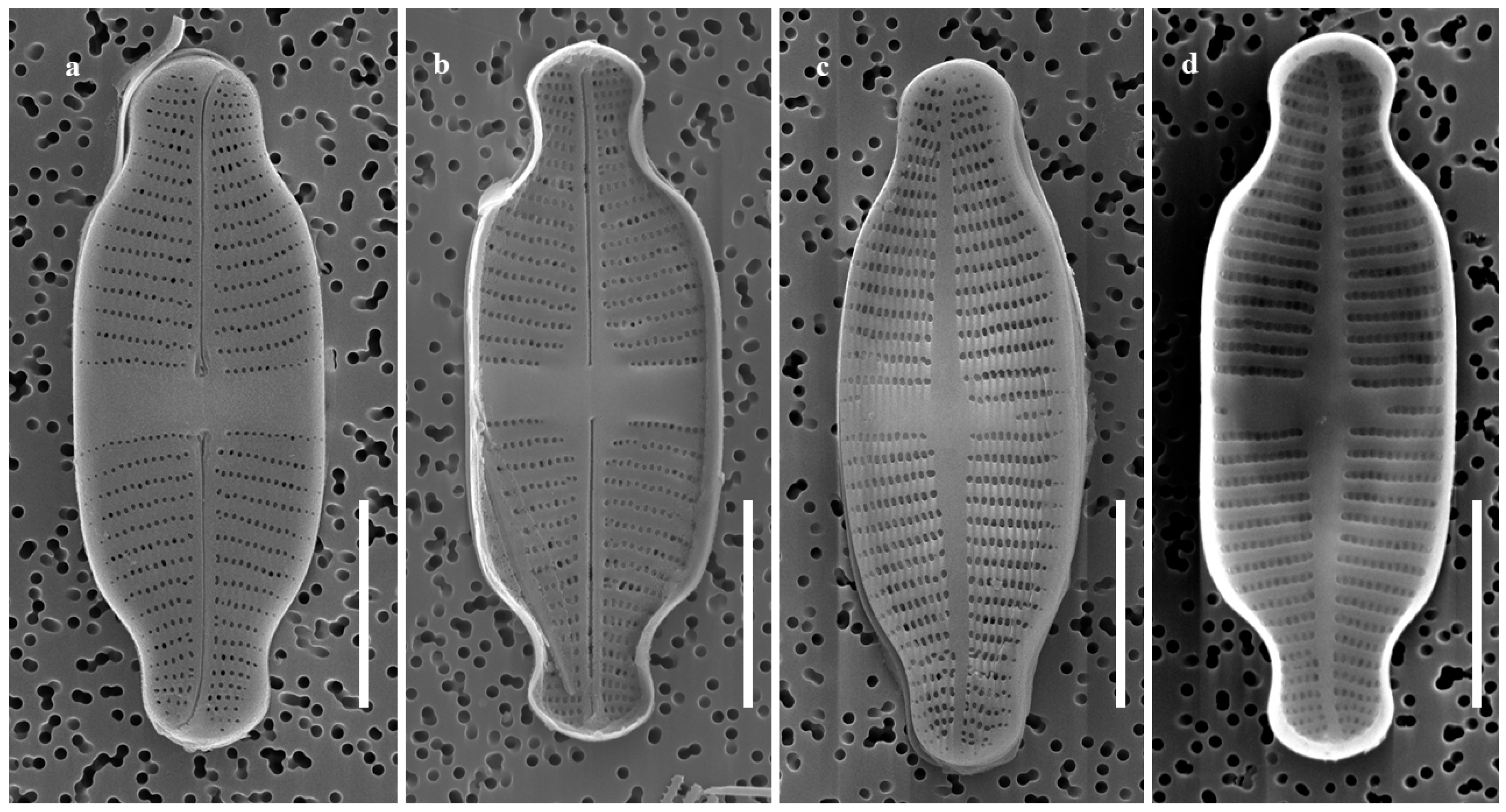
2.1.2. Taxonomic Characteristics of G. recticentralis W. Wang and B.H. Kim sp. nov. (LM, Figure 3; SEM, Figure 4)
- Description:
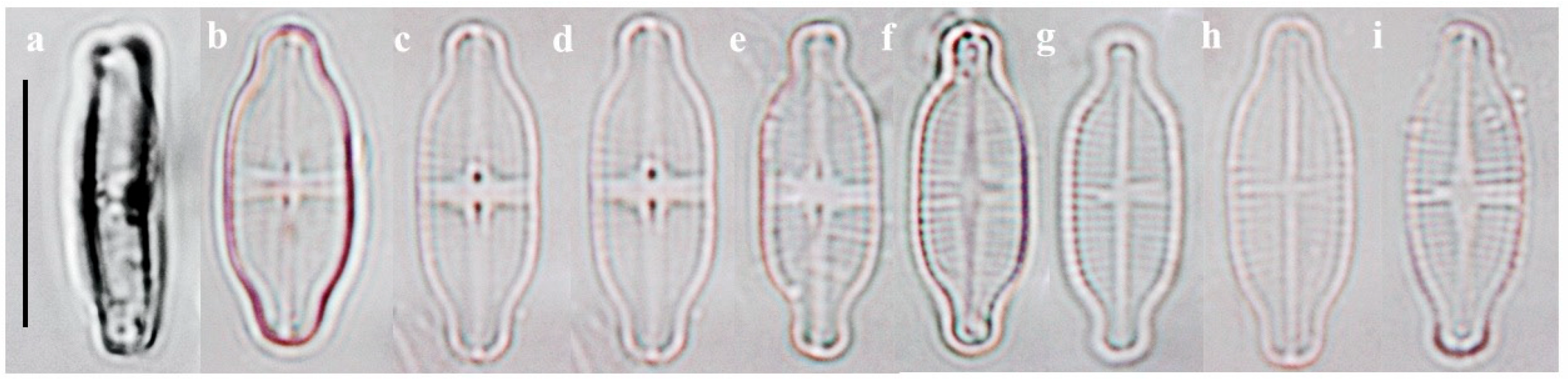
- SEM observations:
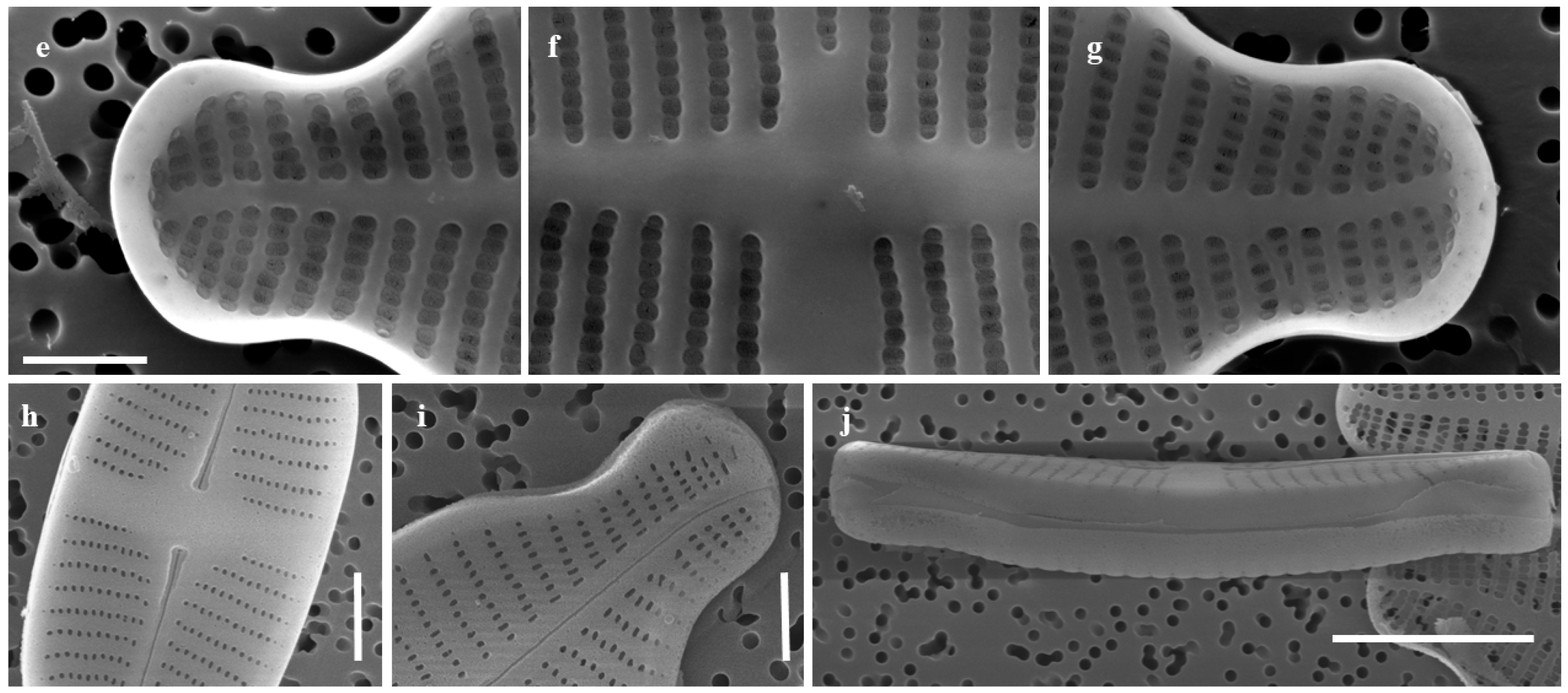
2.2. Comparative Morphology
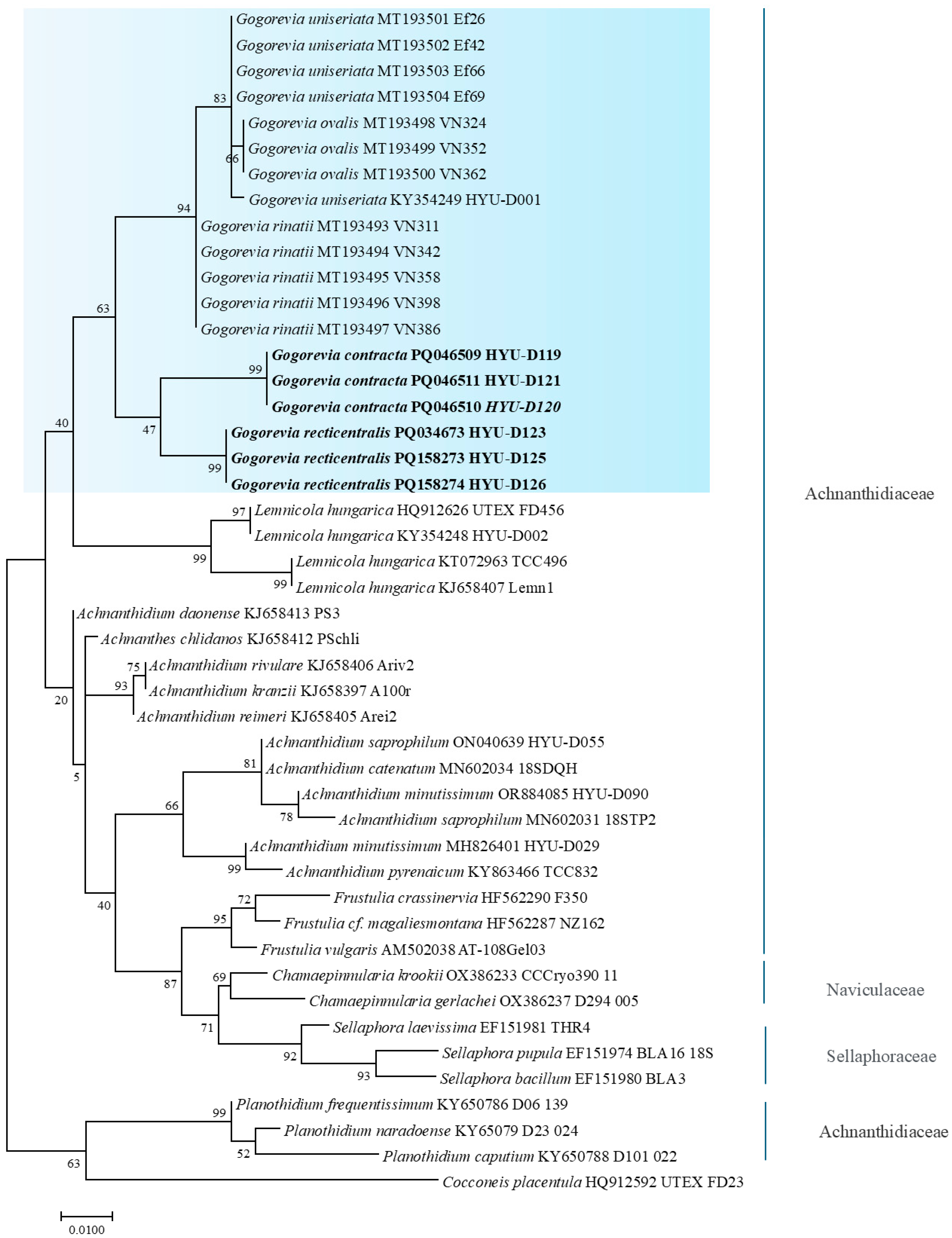
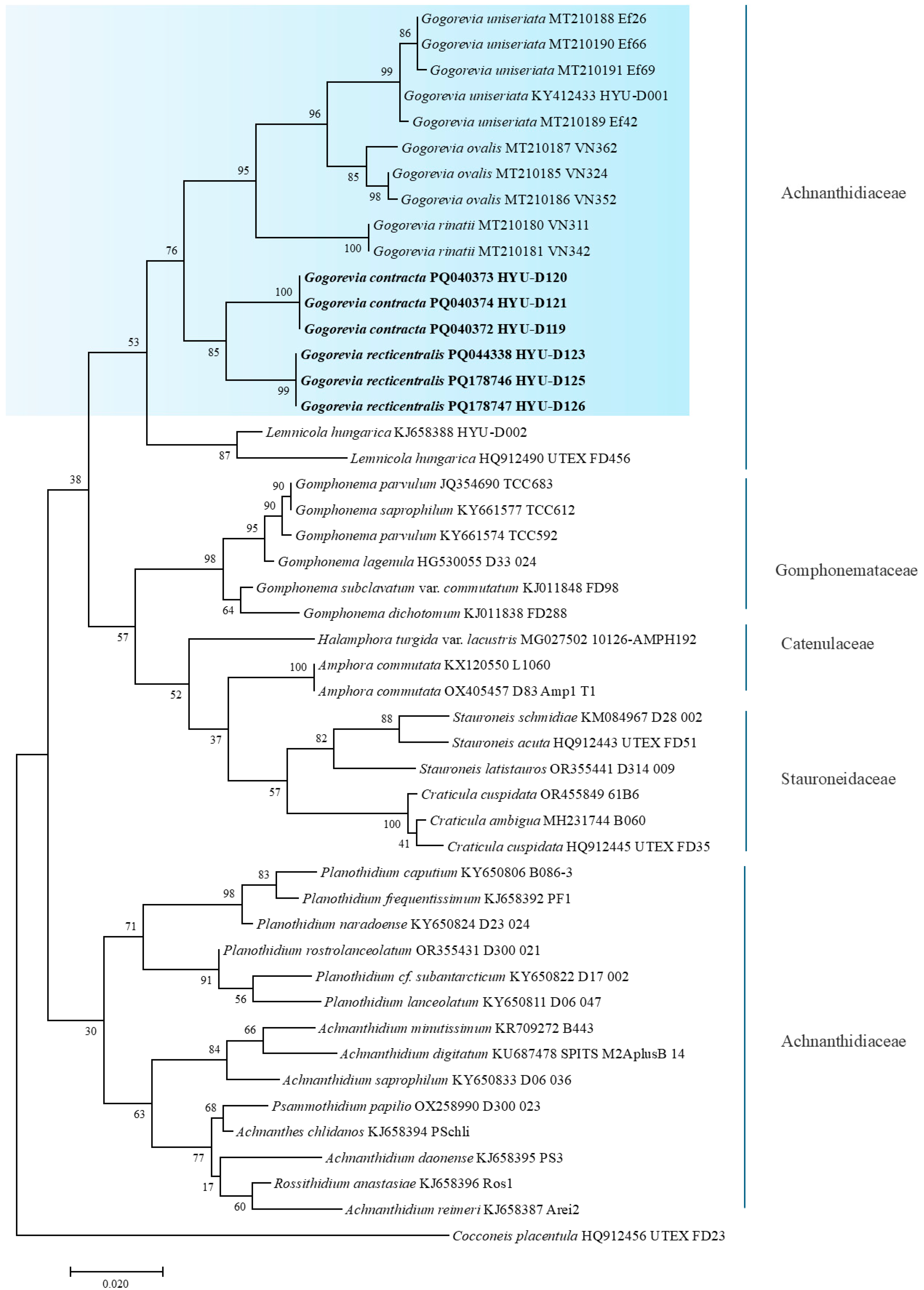
2.3. Molecular Analysis
3. Discussion
3.1. Taxonomic and Ecological Implications of G. contracta and G. recticentralis
3.2. Phylogenetic and Taxonomic Considerations for Gogorevia
4. Materials and Methods
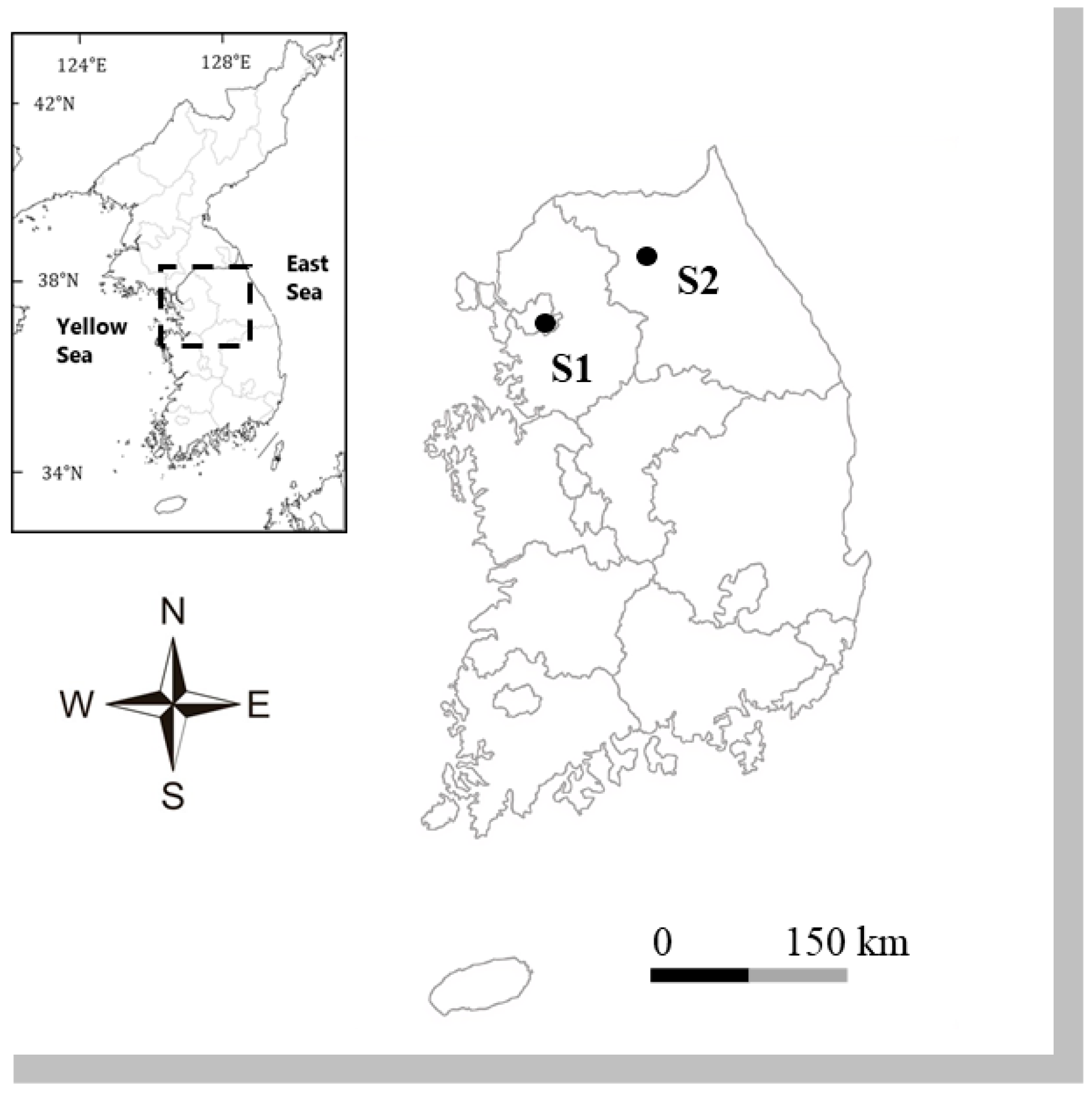
4.1. Sample Collection and Culture
4.2. Morphological Study of Diatoms
4.3. Molecular Study of Diatoms
4.4. Taxonomic Studies on New Species
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LM | Light Microscopy |
| SEM | Scanning Electron Microscopy |
| SSU rRNA | Small Subunit Ribosomal RNA |
| rbcL | Ribulose-1,5-bisphosphate carboxylase/oxygenase large subunit gene |
| PCR | Polymerase Chain Reaction |
| DO | Dissolved Oxygen |
| WT | Water Temperature |
| NTU | Nephelometric Turbidity Unit |
| NCBI | National Center for Biotechnology Information |
| KCTC | Korean Collection for Type Cultures |
| A.N. | Accession Number |
| ITS | Internal Transcribed Spacer |
| COI | Cytochrome c Oxidase subunit I |
References
- Kociolek, J.P.; Williams, D.M.; Stepanek, J.; Liu, Q.; Liu, Y.; You, Q.M.; Karthick, B.; Kulikovskiy, M. Rampant homoplasy and adaptive radiation in pennate diatoms. Plant Ecol. Evol. 2019, 152, 131–141. [Google Scholar] [CrossRef]
- Medlin, L.K.; Kaczmarska, I. Evolution of the diatoms: V. Morphological and cytological support for the major clades and taxonomic revision. Phycologia 2004, 43, 245–270. [Google Scholar] [CrossRef]
- Nakov, T.; Beaulieu, J.M.; Alverson, A.J. Accelerated diversification is related to life history and locomotion in a hyperdiverse lineage of microbial eukaryotes (Diatoms, Bacillariophyta). New Phytol. 2018, 219, 462–473. [Google Scholar] [CrossRef]
- Kulikovskiy, M.; Maltsev, Y.; Glushchenko, A.; Kuznetsova, I.; Kapustin, D.; Gusev, E.; Lange-Bertalot, H.; Genkal, S.; Kociolek, J.P. Gogorevia, a new monoraphid diatom genus for Achnanthes exigua and allied taxa (Achnanthidiaceae) described on the basis of an integrated molecular and morphological approach? J. Phycol. 2020, 56, 1601–1613. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, P.; Kim, H.-K.; Lee, H.; Han, M.-S.; Kim, B.-H. Lemnicola hungarica (Bacillariophyceae) and the new monoraphid diatom Lemnicola uniseriata sp. nov. (Bacillariophyceae) from South Korea. Diatom Res. 2018, 33, 69–87. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. 2024. Available online: https://www.algaebase.org (accessed on 19 October 2024).
- Wang, Q.X.; You, Q.M. Atlas of Common Diatoms in the Lower Yangtze River; Science Press: Beijing, China, 2024; 111p. (In Chinese) [Google Scholar]
- Ponader, K.C.; Potapova, M.G. Diatoms from the genus Achnanthidium in flowing waters of the Appalachian Mountains (North America): Ecology, distribution and taxonomic notes. Limnologica 2007, 37, 227–241. [Google Scholar] [CrossRef]
- Miao, M.; Li, Z.; Hwang, E.-A.; Kim, H.-K.; Lee, H.; Kim, B.-H. Two new benthic diatoms of the genus Achnanthidium (Bacillariophyceae) from the Hangang River, Korea. Diversity 2020, 12, 285. [Google Scholar] [CrossRef]
- Tan, L.; Wang, P.; Cho, I.-H.; Hwang, E.-A.; Lee, H.; Kim, B.-H. Morphology and phylogenetic position of three new raphid diatoms (Bacillariophyceae) from Hangang River, South Korea. Phytotaxa 2020, 442, 153–182. [Google Scholar] [CrossRef]
- Hwang, E.-A.; Kim, H.-K.; Cho, I.-H.; Yi, C.; Kim, B.-H. Morphological and molecular studies of three new diatom species from mountain streams in South Korea. Diversity 2022, 14, 790. [Google Scholar] [CrossRef]
- Mann, D.G. The species concept in diatoms. Phycologia 1999, 38, 437–495. [Google Scholar] [CrossRef]
- Borrego-Ramos, M.; Becares, E.; Garcia, P.; Nistal, A.; Blanco, S. Epiphytic diatom-based biomonitoring in Mediterranean ponds: Traditional microscopy versus metabarcoding approaches. Water 2021, 13, 1351. [Google Scholar] [CrossRef]
- Round, F.E.; Crawford, R.M.; Mann, D.G. The Diatoms: Biology and Morphology of the Genera; Cambridge University Press: New York, NY, USA, 1990; 747p. [Google Scholar]
- Bruder, K.; Medlin, L.K. Molecular assessment of phylogenetic relationships in selected species/genera in the navicu loid diatoms (Bacillariophyta). I. The genus Placoneis. Nova Hedwig. 2007, 85, 331–352. [Google Scholar] [CrossRef]
- Medlin, L.K.; Kooistra, W.H.; Gersonde, R.; Wellbrock, U. Evolution of the diatoms (Bacillariophyta). II. Nuclear-encoded small-subunit rRNA sequence comparisons confirm a paraphyletic origin for the centric diatoms. Mol. Biol. Evol. 1996, 13, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.G.; Simpson, G.E.; Sluiman, H.J.; Moller, M. rbcL gene tree of diatoms: A second large data-set for phylogenetic reconstruction. Phycologia 2001, 40, 1–2. [Google Scholar]
- Guo, L.; Sui, Z.; Zhang, S.; Ren, Y.; Liu, Y. Comparison of potential diatom ‘barcode’ genes (the 18S rRNA gene and ITS, COI, rbcL) and their effectiveness in discriminating and determining species taxonomy in the Bacillariophyta. Int. J. Syst. Evol. Microbiol. 2015, 65 Pt 4, 1369–1380. [Google Scholar] [CrossRef] [PubMed]
- Morales, E.A.; Siver, P.A.; Trainor, F.R. Identification of diatoms during ecological assessments: Comparison between light and scanning electron microscopy. Proc. Acad. Nat. Sci. Phila. 2001, 151, 29–37. [Google Scholar] [CrossRef]
- Plinski, M.; Witkowski, A. Okrzemki–Bacillariophyta (Diatoms) (with the English key for the identification to the genus) Cz. 4/2: Okrzemki pierzaste (Fragilariophyceae, Eunotiophyceae, Achnanthales) Part two: Pennate diatoms-I). In Flora Zatoki Gdanskiej i Wód Przyleglych (Baltyk Poludniowy); Wydawnictwo Uniwersytetu Gdańskiego: Sopot, Poland, 2011; pp. 1–167. [Google Scholar]
- Taylor, J.C.; Cocquyt, C.; Karthick, B.; Van de Vijver, B. Analysis of the type of Achnnathes exigua Grunow (Bacillariophyta) with the description of a new Antarctic diatom species. Fottea 2014, 14, 43–51. [Google Scholar] [CrossRef]
- Bahls, L.; Boynton, B.; Johnston, B. Atlas of diatoms (Bacillariophyta) from diverse habitats in remote regions of western Canada. PhytoKeys 2018, 105, 1–186. [Google Scholar] [CrossRef]
- Spaulding, S.A.; Potapova, M.G.; Bishop, I.W.; Lee, S.S.; Gasperak, T.S.; Jovanoska, E.; Furey, P.C.; Edlund, M.B. Diatoms.org: Supporting taxonomists, connecting communities. Diatom Res. 2021, 36, 291–304. [Google Scholar] [CrossRef]
- Medlin, L.K.; Cooper, A.; Hill, C.; Wrieden, S.; Wellbrock, U. Phylogenetic position of the Chromista plastids based on small subunit rRNA coding regions. Curr. Genet. 1995, 28, 560–565. [Google Scholar] [CrossRef]
- Alverson, A.J. Molecular systematics and the diatom species. Protist 2008, 159, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Sorhannus, U. Diatom phylogenetics inferred based on direct optimization of nuclear-encoded SSU rRNA sequences. Cladistics 2004, 20, 487–497. [Google Scholar] [CrossRef]
- Lange-Bertalot, H.; Kulbs, K.; Lauser, T.; Norpel-Schempp, M.; Willmann, M. Diatom taxa introduced by Georg Krasske: Documentation and revision. Iconogr. Diatomol. 1996, 3, 1–358. [Google Scholar]
- Sato, S.; Mann, D.G.; Matsumoto, S.; Medlin, L.K. Pseudostriatella (Bacillariophyta): A description of a new araphid diatom genus based on observations of frustule and auxospore structure and 18S rDNA phylogeny. Phycologia 2008, 47, 371–391. [Google Scholar] [CrossRef]
- Beakes, G.W.; Canter, H.M.; Jaworski, G.H.M. Zoospore ultrastructure of Zygorhizidium affluens and Zygorhizidium planktonicum, two chytrids parasitizing the diatom Asterionella formosa. Can. J. Bot.-Rev. Can. Bot. 1988, 66, 1054–1067. [Google Scholar] [CrossRef]
- Schoeman, F.R.; Archibald, R.E.M. The Diatom Flora of Southern Africa; National Institute for Water Research, Council for Scientific and Industrial Research: Pretoria, South Africa, 1976. [Google Scholar]
- Ki, J.S.; Cho, S.Y.; Katano, T.; Jung, S.W.; Lee, J.; Park, B.S.; Kang, S.H.; Han, M.S. Comprehensive comparisons of three pennate diatoms, Diatoma tenue, Fragilaria vaucheriae, and Navicula pelliculosa, isolated from summer Arctic reservoirs (Svalbard 79¡Æ N), by fine-scale morphology and nuclear 18S ribosomal DNA. Polar Biol. 2009, 32, 147–159. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Nyati, S.; Bhattacharya, D.; Werth, S.; Honegger, R. Phylogenetic analysis of LSU and SSU rDNA group I introns of lichen photobionts associated with the genera Xanthoria and Xanthomendoza (Teloschistaceae, lichenized Ascomycetes). J. Phycol. 2013, 49, 147–159. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef]
- Yang, H.J.; Park, H.K.; Park, S.J.; Hwang, S.J.; Kim, B.H.; Kim, H.S.; Lee, J.H.; Kim, Y.J.; Lee, H.Y. Ecological Guidebook of Korean Diatoms; Ministry of Environment & National Institute of Environmental Research: Incheon, Republic of Korea; Jeonghaengsa Press: Seoul, Republic of Korea, 2011. [Google Scholar]
- Kim, Y.J.; Won, D.H.; Lee, M.H.; Kim, H.S.; Choi, J.S.; Lee, J.H.; Kim, B.H.; Kim, H.K.; Kim, G.H. Picture Book and Ecology of Epilithic Diatoms in Korean Estuary; Ministry of Environment & National Institute of Environmental Research: Incheon, Republic of Korea; Dongjin Press: Seoul, Republic of Korea, 2021. [Google Scholar]
| G. contracta | G. recticentralis | G. exilis | G. rinatii | G. uniseriata | G. rostellata | G.australexiguum | |
|---|---|---|---|---|---|---|---|
| Length (μm) | 14.26–16.15 | 13.04–14.65 | 5–17 | 11.5–15.0 | 5.5–8.0 | 15.1–19.8 | 14.7–18.8 |
| Width (μm) | 5.52–8.28 | 4.86–5.89 | 4.5–6.2 | 5–6 | 3.0–3.5 | 6.4–7.2 | 6.3–7.5 |
| L/W ratio | 1.75–2.74 | 2.45–2.85 | N.d. | N.d. | 1.5–2.5 | N.d. | N.d. |
| Valve | Elongated, somewhat rectangular shape with rounded ends; middle part slightly constricted | Capitate, sides parallel | Linear–elliptic to elliptic–lanceolate with narrowly capitate, subcapitate, rostrate, or subrostrate apices | Lanceolate to elliptical with pro-tracted, short rostrate, with broadly rounded apices | Frustule slightly bent, raphe valve concave | Linear–elliptic with narrow subcapitate apices | Linear with parallel, clearly undulating margins and protracted, distinctly rostrate apices |
| Striation | Radiate on both valves | From apices to center, microradiate to parallel on raphe valve but parallel to microradiation on rapheless valve | Radiate on both valves, but almost parallel at the apices | Uniseriate, fine, radiate throughout, more strongly radiate near apices; sometimes striae become biseriate near the axial area | Radiate | Nearly parallel on the rapheless valve center but otherwise radiate, more so near apices; more closely spaced near apices; strongly radiate throughout and a bit more closely spaced near apices | Radiate throughout, more strongly radiate near the apices on raphe valve; parallel, becoming radiate towards the apices on rapheless valve |
| Areolae | Areolae on rapheless valve are noticeably larger than on rapheless; elongated to rounded | Elongated to rounded shape, occluded by individual hymenes | Areolae on rapheless valve round or transapically elongated externally, apically elongated internally | Not resolved in LM; elongated to rounded shape; occluded by individual hymenes | Uniseriate | N.d. | Not discernible in LM; small, rounded, becoming larger towards valve margins covered by cribrate structures internally |
| Raphe valve | |||||||
| Raphe | Straight, drop-shaped central raphe pores; central nodule is unidirectional on outer side but turns to different side on the inner side; terminal raphe endings deflected and turn to opposite side | Straight, drop-shaped proximal endings; distal endings are deflected and turned in opposite directions and terminate at the edge of the valve margin | Straight, deflected to opposite sides near the apices; terminal raphe fissures strongly curved to opposite sides; external proximal raphe ends are simple, located in slight “pinhole” depressions; central raphe ends curve toward opposite sides internally | Straight, drop-shaped proximal raphe endings | Straight, tear-drop-shaped central raphe pores accompanied by ridges and grooves and turn in the opposite direction internally | Straight; the terminal fissures appear to curve in opposite directions; the proximal raphe ends are expanded in the shape of teardrops | Straight with straight, clearly expanded proximal raphe endings; distal raphe fissures deflected to opposite sides, terminating in droplike pores, almost invisible in LM |
| Axial area | Very narrow, linear, opening rather abruptly to the central area; raised on the inner side | Very narrow, linear, opening rather abruptly to the central area | Narrow, slightly sigmoid | Very narrow, linear, opening rather abruptly to the central area | Helictoglossae raised and turn to opposite directions | Narrow, widens abruptly into a transverse fascia | Narrow, opening rather abruptly to the central area; terminating on small helictoglossae |
| Central area | Raised central stauros on the inner side; bow-tie-shaped | Roughly symmetrical, narrow, rectangular-to-wedge-shaped fascia; shortened striae area are absent; forming a raised stauros on the internal side | Distinct fascia, often slightly wider on one side | More or less symmetrical, narrow, rectangular-to wedge-shaped fascia reaching the valve margins; shortened striae in the central area absent; forming a raised stauros internally | Wide, slightly asymmetric, bow-tie-shaped stauros | Bow-tie-shaped, occasionally bordered by a few shortened striae | Rather broad, rectangular, weakly asymmetrical fascia, lacking any shortened striae bordering the central area |
| Density of striae (/10 μm) | 35–40 | 25–28 | 24–34 | 26.0–32.5 | 30–35 | 30–32 | 28–30 |
| Striae | Uniseriate | At the valve ends, striae sometimes become biseriate near the axial area | N.d. | Uniseriate, almost parallel, becoming more radiate toward the apices | Striae near the central stauros are shorter than the other striae | N.d. | Narrower than the virgae, uniseriate |
| Density of areolae (/10 μm) | 50–60 | 65–70 | N.d. | 70 | N.d. | N.d. | N.d. |
| Rapheless valve | |||||||
| Axial area | Lanceolate, fusiform, narrow | Lanceolate, narrows; turn slightly in the opposite direction at the end | Narrow and slightly sigmoid | Slightly raised internally above the surface | Sigmoid | Wider and expands gradually to merge with an asymmetric, irregular transverse central area | Narrow, opening rather abruptly to the central area |
| Density of striae (/10 μm) | 15–22.5 | 25–28 | 20–25 | 25–30 | 30–35 | 24–28 | 23–24 |
| Striae | Most uniseriate, become irregularly biseriate near the central and apices area | Most uniseriate, become irregularly biseriate near the central and apices area | N.d. | N.d. | N.d. | N.d. | Uniseriate; striae occasionally biseriate near the apices |
| Density of areolae (/10 μm) | 40–50 | 65–70 | N.d. | 70 | N.d. | N.d. | N.d. |
| Central area | Asymmetric, one side has one more stria than the other side; rectangular; irregular markings sometimes present in the central area | Asymmetrical wedge-shaped, on one side formed by shortened striae; slightly raised above the surface internally | Small, transapically rectangular, often asymmetric | Asymmetrical wedge-shaped, on one side formed by shortened striae; slightly raised above the surface internally | A reduced central area, with one or two lines of short striae on one side of stauros | N.d. | Narrow, rectangular, asymmetrical fascia, lacking any shortened striae bordering the central area; irregular markings (shallow depressions) sometimes present in the central area |
| References | This study | This study | [20,21] | [4] | [4,5] | [4,21,22] | [21] |
| Species | Strains | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Gogorevia constricta | PQ046509 | 0.009 | 0.010 | 0.010 | 0.010 | 0.010 | 0.010 | 0.012 | 0.010 | 0.010 | 0.013 | 0.012 | 0.013 | 0.014 | 0.011 | 0.012 | 0.012 | 0.010 | 0.012 | 0.010 | |
| 2 | Gogorevia recticentralis | PQ034673 | 0.033 | 0.009 | 0.009 | 0.009 | 0.009 | 0.009 | 0.011 | 0.010 | 0.010 | 0.013 | 0.012 | 0.013 | 0.014 | 0.012 | 0.013 | 0.014 | 0.010 | 0.013 | 0.010 | |
| 3 | Gogorevia uniseriata | KY354249 | 0.045 | 0.033 | 0.002 | 0.005 | 0.003 | 0.011 | 0.012 | 0.011 | 0.011 | 0.013 | 0.012 | 0.012 | 0.013 | 0.013 | 0.012 | 0.014 | 0.011 | 0.012 | 0.010 | |
| 4 | Gogorevia uniseriata | MT193501 | 0.048 | 0.036 | 0.002 | 0.004 | 0.002 | 0.010 | 0.013 | 0.011 | 0.011 | 0.012 | 0.012 | 0.011 | 0.013 | 0.012 | 0.012 | 0.014 | 0.011 | 0.012 | 0.010 | |
| 5 | Gogorevia rinatii | MT193493 | 0.046 | 0.033 | 0.009 | 0.007 | 0.005 | 0.010 | 0.012 | 0.010 | 0.010 | 0.012 | 0.011 | 0.012 | 0.013 | 0.012 | 0.012 | 0.014 | 0.010 | 0.012 | 0.009 | |
| 6 | Gogorevia ovalis | MT193498 | 0.050 | 0.038 | 0.005 | 0.002 | 0.009 | 0.010 | 0.013 | 0.010 | 0.011 | 0.012 | 0.012 | 0.011 | 0.013 | 0.013 | 0.011 | 0.014 | 0.011 | 0.011 | 0.009 | |
| 7 | Achnanthes chlidanos | KJ658412 | 0.038 | 0.036 | 0.045 | 0.043 | 0.038 | 0.041 | 0.009 | 0.005 | 0.006 | 0.011 | 0.008 | 0.011 | 0.011 | 0.009 | 0.008 | 0.011 | 0.006 | 0.008 | 0.003 | |
| 8 | Lemnicola hungarica | KY354248 | 0.060 | 0.048 | 0.060 | 0.063 | 0.055 | 0.065 | 0.038 | 0.010 | 0.010 | 0.013 | 0.012 | 0.012 | 0.013 | 0.012 | 0.011 | 0.014 | 0.010 | 0.011 | 0.009 | |
| 9 | Achnanthidium reimeri | KJ658405 | 0.041 | 0.041 | 0.050 | 0.048 | 0.043 | 0.045 | 0.012 | 0.045 | 0.002 | 0.011 | 0.009 | 0.010 | 0.011 | 0.009 | 0.009 | 0.011 | 0.002 | 0.009 | 0.005 | |
| 10 | Achnanthidium rivulare | KJ658406 | 0.043 | 0.043 | 0.053 | 0.050 | 0.046 | 0.048 | 0.014 | 0.048 | 0.002 | 0.011 | 0.009 | 0.011 | 0.012 | 0.009 | 0.008 | 0.011 | 0.000 | 0.008 | 0.006 | |
| 11 | Planothidium naradoense | KY65079 | 0.076 | 0.071 | 0.068 | 0.065 | 0.066 | 0.065 | 0.055 | 0.076 | 0.055 | 0.058 | 0.012 | 0.004 | 0.008 | 0.012 | 0.011 | 0.013 | 0.011 | 0.011 | 0.011 | |
| 12 | Achnanthidium minutissimum | MH826401 | 0.055 | 0.063 | 0.065 | 0.063 | 0.058 | 0.060 | 0.031 | 0.060 | 0.038 | 0.036 | 0.065 | 0.012 | 0.012 | 0.010 | 0.008 | 0.012 | 0.009 | 0.008 | 0.008 | |
| 13 | Planothidium frequentissimum | KY650786 | 0.071 | 0.071 | 0.060 | 0.058 | 0.058 | 0.058 | 0.055 | 0.073 | 0.050 | 0.053 | 0.009 | 0.060 | 0.008 | 0.012 | 0.011 | 0.013 | 0.011 | 0.011 | 0.010 | |
| 14 | Planothidium caputium | KY650788 | 0.086 | 0.073 | 0.071 | 0.068 | 0.068 | 0.068 | 0.055 | 0.076 | 0.058 | 0.060 | 0.029 | 0.070 | 0.028 | 0.013 | 0.011 | 0.014 | 0.012 | 0.011 | 0.011 | |
| 15 | Chamaepinnularia krookii | OX386233 | 0.055 | 0.060 | 0.068 | 0.065 | 0.063 | 0.068 | 0.038 | 0.066 | 0.038 | 0.041 | 0.065 | 0.046 | 0.063 | 0.076 | 0.009 | 0.010 | 0.009 | 0.009 | 0.009 | |
| 16 | Achnanthidium saprophilum | ON040639 | 0.058 | 0.068 | 0.060 | 0.058 | 0.058 | 0.055 | 0.036 | 0.053 | 0.036 | 0.033 | 0.055 | 0.026 | 0.053 | 0.060 | 0.041 | 0.012 | 0.008 | 0.000 | 0.008 | |
| 17 | Sellaphora pupula | EF151974 | 0.065 | 0.083 | 0.086 | 0.083 | 0.081 | 0.086 | 0.053 | 0.083 | 0.053 | 0.055 | 0.081 | 0.066 | 0.078 | 0.091 | 0.043 | 0.063 | 0.011 | 0.012 | 0.011 | |
| 18 | Achnanthidium kranzii | KJ658397 | 0.043 | 0.043 | 0.053 | 0.050 | 0.046 | 0.048 | 0.014 | 0.048 | 0.002 | 0.000 | 0.058 | 0.036 | 0.053 | 0.060 | 0.041 | 0.033 | 0.055 | 0.008 | 0.006 | |
| 19 | Achnanthidium catenatum | MN602034 | 0.058 | 0.068 | 0.060 | 0.058 | 0.058 | 0.055 | 0.036 | 0.053 | 0.036 | 0.033 | 0.055 | 0.026 | 0.053 | 0.060 | 0.041 | 0.000 | 0.063 | 0.033 | 0.008 | |
| 20 | Achnanthidium daonense | KJ658413 | 0.038 | 0.041 | 0.041 | 0.038 | 0.033 | 0.036 | 0.005 | 0.038 | 0.012 | 0.014 | 0.058 | 0.028 | 0.050 | 0.055 | 0.038 | 0.033 | 0.053 | 0.014 | 0.033 |
| Species | Strains | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Gogorevia constricta | PQ040372 | 0.007 | 0.013 | 0.012 | 0.012 | 0.011 | 0.010 | 0.011 | 0.012 | 0.012 | 0.012 | 0.012 | 0.013 | 0.013 | 0.012 | 0.013 | 0.012 | 0.013 | 0.012 | 0.013 | |
| 2 | Gogorevia recticentralis | PQ044338 | 0.031 | 0.012 | 0.012 | 0.011 | 0.011 | 0.010 | 0.011 | 0.012 | 0.011 | 0.012 | 0.011 | 0.013 | 0.013 | 0.012 | 0.013 | 0.012 | 0.012 | 0.012 | 0.013 | |
| 3 | Gogorevia uniseriata | MT210188 | 0.070 | 0.064 | 0.003 | 0.008 | 0.011 | 0.012 | 0.014 | 0.013 | 0.014 | 0.014 | 0.014 | 0.014 | 0.013 | 0.014 | 0.016 | 0.014 | 0.015 | 0.015 | 0.015 | |
| 4 | Gogorevia uniseriata | KY412433 | 0.066 | 0.068 | 0.004 | 0.007 | 0.011 | 0.011 | 0.014 | 0.013 | 0.014 | 0.014 | 0.015 | 0.014 | 0.012 | 0.014 | 0.016 | 0.014 | 0.015 | 0.015 | 0.014 | |
| 5 | Gogorevia ovalis | MT210186 | 0.062 | 0.060 | 0.033 | 0.029 | 0.010 | 0.011 | 0.014 | 0.013 | 0.014 | 0.015 | 0.014 | 0.014 | 0.013 | 0.013 | 0.015 | 0.014 | 0.016 | 0.014 | 0.014 | |
| 6 | Gogorevia rinatii | MT210180 | 0.061 | 0.064 | 0.058 | 0.054 | 0.056 | 0.010 | 0.013 | 0.012 | 0.013 | 0.014 | 0.013 | 0.013 | 0.012 | 0.012 | 0.014 | 0.013 | 0.015 | 0.014 | 0.013 | |
| 7 | Lemnicola hungarica | KJ658388 | 0.053 | 0.047 | 0.068 | 0.064 | 0.064 | 0.059 | 0.010 | 0.011 | 0.010 | 0.011 | 0.011 | 0.013 | 0.013 | 0.011 | 0.012 | 0.012 | 0.012 | 0.012 | 0.011 | |
| 8 | Psammothidium papilio | OX258990 | 0.068 | 0.072 | 0.089 | 0.084 | 0.091 | 0.084 | 0.059 | 0.010 | 0.004 | 0.008 | 0.010 | 0.012 | 0.012 | 0.014 | 0.013 | 0.012 | 0.010 | 0.012 | 0.011 | |
| 9 | Planothidium naradoense | KY650824 | 0.070 | 0.072 | 0.083 | 0.078 | 0.081 | 0.072 | 0.068 | 0.055 | 0.010 | 0.011 | 0.012 | 0.009 | 0.008 | 0.012 | 0.013 | 0.013 | 0.012 | 0.012 | 0.006 | |
| 10 | Achnanthes chlidanos | KJ658394 | 0.072 | 0.072 | 0.089 | 0.085 | 0.091 | 0.087 | 0.055 | 0.011 | 0.049 | 0.007 | 0.009 | 0.011 | 0.012 | 0.014 | 0.013 | 0.012 | 0.010 | 0.012 | 0.011 | |
| 11 | Achnanthidium daonense | KJ658395 | 0.078 | 0.078 | 0.093 | 0.098 | 0.104 | 0.095 | 0.068 | 0.031 | 0.063 | 0.027 | 0.009 | 0.013 | 0.012 | 0.014 | 0.014 | 0.012 | 0.010 | 0.013 | 0.012 | |
| 12 | Achnanthidium saprophilum | KY650833 | 0.074 | 0.057 | 0.091 | 0.095 | 0.091 | 0.084 | 0.065 | 0.049 | 0.068 | 0.045 | 0.045 | 0.013 | 0.013 | 0.014 | 0.014 | 0.013 | 0.007 | 0.013 | 0.012 | |
| 13 | Planothidium cf. subantarcticum | KY650822 | 0.078 | 0.072 | 0.089 | 0.085 | 0.089 | 0.089 | 0.078 | 0.070 | 0.047 | 0.063 | 0.080 | 0.074 | 0.007 | 0.014 | 0.013 | 0.012 | 0.012 | 0.013 | 0.010 | |
| 14 | Planothidium lanceolatum | KY650811 | 0.072 | 0.070 | 0.078 | 0.074 | 0.083 | 0.074 | 0.078 | 0.070 | 0.039 | 0.068 | 0.076 | 0.076 | 0.027 | 0.014 | 0.014 | 0.013 | 0.013 | 0.012 | 0.010 | |
| 15 | Stauroneis schmidiae | KM084967 | 0.070 | 0.074 | 0.089 | 0.085 | 0.083 | 0.078 | 0.063 | 0.089 | 0.074 | 0.086 | 0.086 | 0.089 | 0.087 | 0.084 | 0.011 | 0.012 | 0.013 | 0.012 | 0.012 | |
| 16 | Halamphora turgida var. lacustris | MG027502 | 0.082 | 0.072 | 0.106 | 0.102 | 0.095 | 0.093 | 0.076 | 0.082 | 0.076 | 0.078 | 0.093 | 0.084 | 0.078 | 0.087 | 0.057 | 0.011 | 0.013 | 0.011 | 0.013 | |
| 17 | Amphora commutata | KX120550 | 0.078 | 0.066 | 0.085 | 0.089 | 0.089 | 0.085 | 0.074 | 0.080 | 0.076 | 0.076 | 0.074 | 0.074 | 0.074 | 0.080 | 0.066 | 0.055 | 0.013 | 0.013 | 0.013 | |
| 18 | Achnanthidium minutissimum | KR709272 | 0.080 | 0.068 | 0.093 | 0.097 | 0.102 | 0.095 | 0.072 | 0.051 | 0.065 | 0.051 | 0.055 | 0.029 | 0.070 | 0.076 | 0.082 | 0.080 | 0.076 | 0.013 | 0.012 | |
| 19 | Gomphonema parvulum | JQ354690 | 0.074 | 0.072 | 0.095 | 0.091 | 0.091 | 0.089 | 0.078 | 0.074 | 0.074 | 0.074 | 0.091 | 0.080 | 0.074 | 0.076 | 0.068 | 0.061 | 0.074 | 0.076 | 0.012 | |
| 20 | Planothidium caputium | KY650806 | 0.080 | 0.076 | 0.091 | 0.087 | 0.091 | 0.082 | 0.068 | 0.065 | 0.017 | 0.059 | 0.074 | 0.070 | 0.053 | 0.051 | 0.080 | 0.078 | 0.083 | 0.065 | 0.072 |
| Species | Strain Number | Sample Locality | Collection of Date | Coordinates | Temperature (°C) | pH | DO (mg/L) | Conductivity (µs/cm) | Turbidity (NTU) | GenBank A.N. (rbcL) | GenBank A.N. (SSU) | KCTC A.N. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gogorevia contracta | HYU-D119 | Yangjaecheon(S1) | 31 January 2024 | 37°29′13.56″ N, 127°3′20.84″ E | 10.69 | 7.66 | 13.26 | 478 | 7.0 | PQ040372 | PQ046509 | AG61355 |
| HYU-D120 | Yangjaecheon(S1) | 31 January 2024 | 37°29′13.56″ N, 127°3′20.84″ E | 10.69 | 7.66 | 13.26 | 478 | 7.0 | PQ040373 | PQ046510 | AG61356 | |
| HYU-D121 | Yangjaecheon(S1) | 31 January 2024 | 37°29′13.56″ N, 127°3′20.84″ E | 10.69 | 7.66 | 13.26 | 478 | 7.0 | PQ040374 | PQ046511 | AG61357 | |
| Gogorevia recticentralis | HYU-D123 | Godugyo(S2) | 9 August 2023 | 37°51′16.04″ N, 127°44′52.09″ E | 23.74 | 7.25 | 9.42 | 156 | 10.5 | PQ044338 | PQ034673 | AG61359 |
| HYU-D125 | Godugyo(S2) | 9 August 2023 | 37°51′16.04″ N, 127°44′52.09″ E | 23.74 | 7.25 | 9.42 | 156 | 10.5 | PQ178746 | PQ158273 | AG61361 | |
| HYU-D126 | Godugyo(S2) | 9 August 2023 | 37°51′16.04″ N, 127°44′52.09″ E | 23.74 | 7.25 | 9.42 | 156 | 10.5 | PQ178747 | PQ158274 | AG61362 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Li, Y.; Han, B.-H.; Hwang, S.-O.; Kim, B.-H. Morphology and Phylogenetic Positions of Two Novel Gogorevia Species (Bacillariophyta) from the Han River, South Korea. Plants 2025, 14, 1272. https://doi.org/10.3390/plants14091272
Wang W, Li Y, Han B-H, Hwang S-O, Kim B-H. Morphology and Phylogenetic Positions of Two Novel Gogorevia Species (Bacillariophyta) from the Han River, South Korea. Plants. 2025; 14(9):1272. https://doi.org/10.3390/plants14091272
Chicago/Turabian StyleWang, Weihan, Yuyao Li, Byeong-Hun Han, Su-Ok Hwang, and Baik-Ho Kim. 2025. "Morphology and Phylogenetic Positions of Two Novel Gogorevia Species (Bacillariophyta) from the Han River, South Korea" Plants 14, no. 9: 1272. https://doi.org/10.3390/plants14091272
APA StyleWang, W., Li, Y., Han, B.-H., Hwang, S.-O., & Kim, B.-H. (2025). Morphology and Phylogenetic Positions of Two Novel Gogorevia Species (Bacillariophyta) from the Han River, South Korea. Plants, 14(9), 1272. https://doi.org/10.3390/plants14091272






