Silicon-Mediated Modulation of Olive Leaf Phytochemistry: Genotype-Specific and Stress-Dependent Responses
Abstract
:1. Introduction
2. Results
2.1. Local Effects of Foliar Silicon Application
2.1.1. Phytochemical Responses in Locally Treated Leaves Throughout the Experiment
2.1.2. Principal Component Analysis (PCA)
2.1.3. Partial Least Squares Discriminant Analyses (PLS-DAs)
2.1.4. Silicon and Mineral Nutrients Content in Locally Treated Leaves Throughout the Experiment
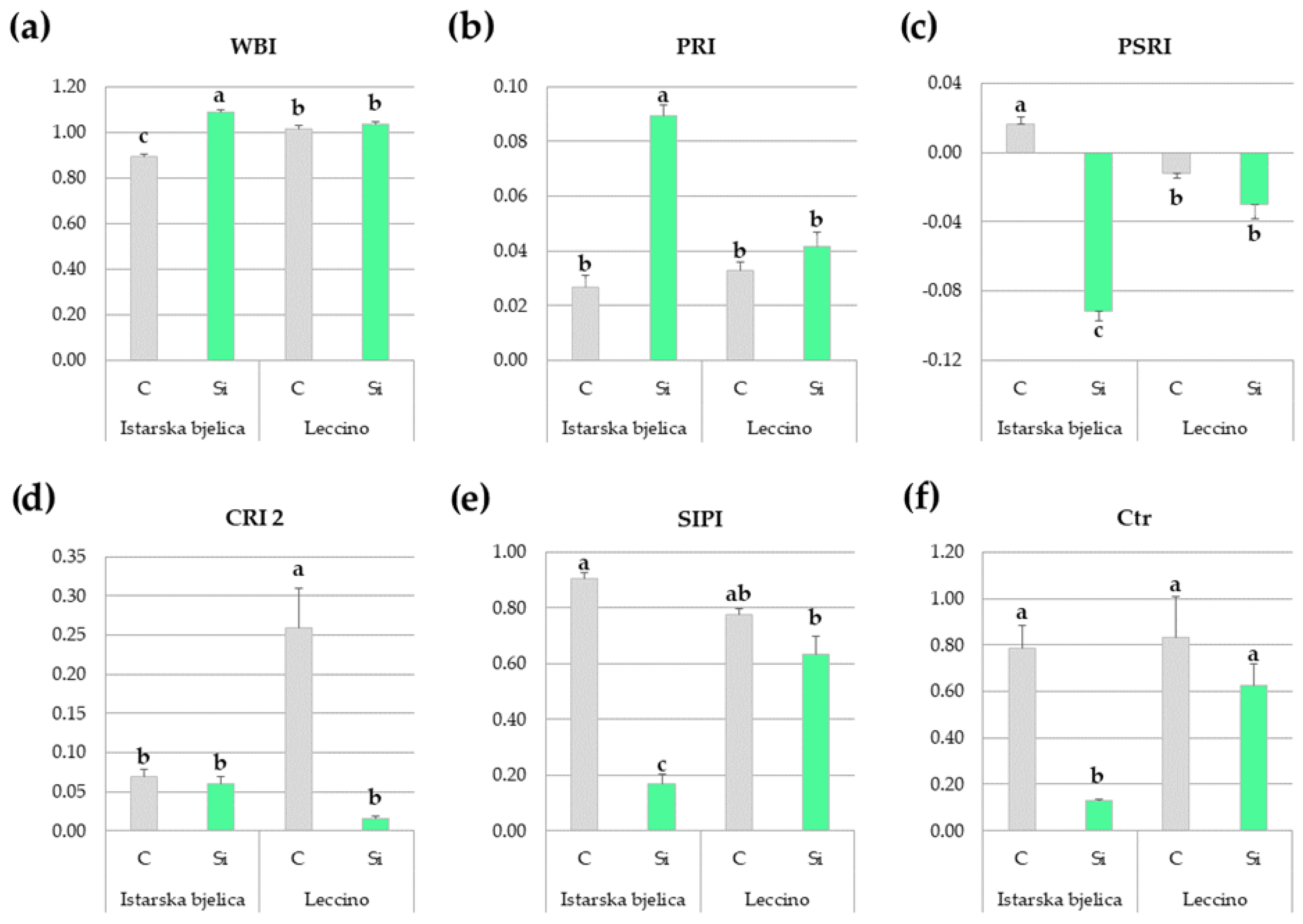
2.1.5. Spectral Reflectance Indices of Locally Treated Leaves Throughout the Experiment
2.2. Systemic Effects of Foliar Silicon Application
2.2.1. Phytochemical Responses of Juvenile, Untreated Leaves
2.2.2. Silicon and Mineral Nutrients Content in Juvenile, Untreated Leaves
3. Discussion
3.1. Research Highlights
3.2. Short-Term Effects of Foliar-Applied Si
3.3. Long-Term Effects of Foliar-Applied Si
3.4. Silicon’s Systemic Effects
4. Materials and Methods
4.1. Experimental Set-Up and Foliar Treatments
4.2. Soil Properties
4.3. Environmental Variables
4.4. Cultivar Characterization
4.5. Leaf Spectral Reflectance Measurements
4.6. Plant Material Sampling and Preparation
4.7. Chemicals
4.8. Extraction of Olive Leaf Secondary Metabolites
4.9. Determination of Total Phenolic Content (TPC)
4.10. Identification and Quantification of Olive Leaf Secondary Metabolites by High-Performance Liquid Chromatography (HPLC)
4.11. Elemental Analyisis
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Markhali, F.S.; Teixeira, J.A.; Rocha, C.M.R. Olive Tree Leaves—A Source of Valuable Active Compounds. Processes 2020, 8, 1177. [Google Scholar] [CrossRef]
- Dixon, R.A.; Jazz Dickinson, A. A Century of Studying Plant Secondary Metabolism—From “What?” to “Where, How, and Why?”. Plant Physiol. 2024, 195, 48–66. [Google Scholar] [CrossRef] [PubMed]
- Nicolì, F.; Negro, C.; Vergine, M.; Aprile, A.; Nutricati, E.; Sabella, E.; Miceli, A.; Luvisi, A.; De Bellis, L. Evaluation of Phytochemical and Antioxidant Properties of 15 Italian Olea europaea L. Cultivar Leaves. Molecules 2019, 24, 1998. [Google Scholar] [CrossRef] [PubMed]
- Talhaoui, N.; Gómez-Caravaca, A.M.; Roldán, C.; León, L.; De la Rosa, R.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Chemometric Analysis for the Evaluation of Phenolic Patterns in Olive Leaves from Six Cultivars at Different Growth Stages. J. Agric. Food Chem. 2015, 63, 1722–1729. [Google Scholar] [CrossRef] [PubMed]
- Kourti, M.; Zoi, S.; Fotios, T.; Stathopoulos, P.; Koutra, C.; Alexios, L.S.; Demetrios, K. The Bioactivity of a Hydroxytyrosol-Enriched Extract Originated After Direct Hydrolysis of Olive Leaves from Greek Cultivars. Molecules 2024, 29, 299. [Google Scholar] [CrossRef]
- Benavente-García, O.; Castillo, J.; Lorente, J.; Ortuño, A.; Del Rio, J.A. Antioxidant Activity of Phenolics Extracted from Olea europaea L. Leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Goulas, V.; Papoti, V.T.; Exarchou, V.; Tsimidou, M.Z.; Gerothanassis, I.P. Contribution of Flavonoids to the Overall Radical Scavenging Activity of Olive (Olea europaea L.) Leaf Polar Extracts. J. Agric. Food Chem. 2010, 58, 3303–3308. [Google Scholar] [CrossRef]
- Piccini, C.; Cantini, C.; Cai, G.; Diana, S.; Romi, M.; Dias, M.C. Chemical Profiling of Two Italian Olea europaea (L.) Varieties Subjected to UV-B Stress. Plants 2022, 11, 680. [Google Scholar] [CrossRef]
- Cetinkaya, H.; Koc, M.; Kulak, M. Monitoring of Mineral and Polyphenol Content in Olive Leaves under Drought Conditions: Application Chemometric Techniques. Ind. Crops Prod. 2016, 88, 78–84. [Google Scholar] [CrossRef]
- Montenegro, S.; Álvarez-Rivera, G.; Mendiola, J.A.; Ibáñez, E.; Cifuentes, A. Extraction and Mass Spectrometric Characterization of Terpenes Recovered from Olive Leaves Using a New Adsorbent-Assisted Supercritical CO2 Process. Foods 2021, 10, 1301. [Google Scholar] [CrossRef]
- Araújo, M.; Prada, J.; Mariz-Ponte, N.; Santos, C.; Pereira, A.J.; Silva, A.M.S.; Dias, M.C.P. Antioxidant Adjustments of Olive Trees (Olea europaea) under Field Stress Conditions. Plants 2021, 10, 684. [Google Scholar] [CrossRef] [PubMed]
- Stefi, A.L.; Vassilacopoulou, D.; Routsi, E.; Stathopoulos, P.; Argyropoulou, A.; Skaltsounis, A.; Christodoulakis, N.S. The Combined Environmental Stress on the Leaves of Olea europaea L. and the Relief Mechanism through Biosynthesis of Certain Secondary Metabolites. J. Plant Growth Regul. 2020, 40, 1044–1059. [Google Scholar] [CrossRef]
- Martínez-Navarro, M.E.; Cebrián-Tarancón, C.; Salinas, M.R.; Alonso, G.L. Evolution of Oleuropein and Other Bioactive Compounds in Arbequina Olive Leaves under Different Agronomic Conditions. Horticulturae 2022, 8, 530. [Google Scholar] [CrossRef]
- Özcan, M.M.; Matthäus, B.A. Review: Benefit and Bioactive Properties of Olive (Olea europaea L.) Leaves. Eur. Food Res. Technol. 2016, 243, 89–99. [Google Scholar] [CrossRef]
- Kulak, M.; Cetinkaya, H. A Systematic Review: Polyphenol Contents in Stressed-Olive Trees and Its Fruit Oil; InTech eBooks: Closter, NJ, USA, 2018. [Google Scholar] [CrossRef]
- Pasković, I.; Lukić, I.; Žurga, P.; Majetić Germek, V.; Brkljača, M.; Koprivnjak, O.; Major, N.; Grozić, K.; Franić, M.; Ban, D.; et al. Temporal Variation of Phenolic and Mineral Composition in Olive Leaves Is Cultivar Dependent. Plants 2020, 9, 1099. [Google Scholar] [CrossRef]
- Lukić, I.; Pasković, I.; Žurga, P.; Majetić Germek, V.; Brkljača, M.; Marcelić, Š.; Ban, D.; Grozić, K.; Lukić, M.; Užila, Z.; et al. Determination of the Variability of Biophenols and Mineral Nutrients in Olive Leaves with Respect to Cultivar, Collection Period and Geographical Location for Their Targeted and Well-Timed Exploitation. Plants 2020, 9, 1667. [Google Scholar] [CrossRef]
- Palm, E.R.; Salzano, A.M.; Vergine, M.; Negro, C.; Nissim, W.G.; Sabbatini, L.; Balestrini, R.; Concetta, M.; Fortunato, S.; Gohari, G.; et al. Response to Salinity Stress in Four Olea europaea L. Genotypes: A Multidisciplinary Approach. Environ. Exp. Bot. 2024, 218, 105586. [Google Scholar] [CrossRef]
- Parri, S.; Cai, G.; Romi, M.; Cantini, C.; Pinto, D.C.G.A.; Silva, A.M.S.; Dias, M.C.P. Comparative Metabolomics of Leaves and Stems of Three Italian Olive Cultivars under Drought Stress. Front. Plant Sci. 2024, 15, 1408731. [Google Scholar] [CrossRef] [PubMed]
- Colzi, I.; Marone, E.; Luti, S.; Pazzagli, L.; Mancuso, S.; Taiti, C. Metabolic Responses in Leaves of 15 Italian Olive Cultivars in Correspondence to Variable Climatic Elements. Plants 2023, 12, 1953. [Google Scholar] [CrossRef]
- Shen, S.; Zhan, C.; Yang, C.; Fernie, A.R.; Luo, J. Metabolomics-Centered Mining of Plant Metabolic Diversity and Function: Past Decade and Future Perspectives. Mol. Plant 2022, 16, 43–63. [Google Scholar] [CrossRef]
- Lorini, A.; Camargo Aranha, B.; da Fonseca Antunes, B.; Murowaniecki Otero, D.; Jacques, A.C.; Zambiazi, R.C. Metabolic Profile of Olive Leaves of Different Cultivars and Collection Times. Food Chem. 2020, 345, 128758. [Google Scholar] [CrossRef] [PubMed]
- Tadić, J.; Dumičić, G.; Veršić Bratinčević, M.; Vitko, S.; Liber, Z.; Brkanac, S.R. Comparative Analysis of Cultivated and Wild Olive Genotypes to Salinity and Drought Stress. Front. Plant Sci. 2024, 15, 1423761. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Jeong, B.R. Silicon (Si): Review and Future Prospects on the Action Mechanisms in Alleviating Biotic and Abiotic Stresses in Plants. Ecotoxicol. Environ. Saf. 2018, 147, 881–896. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Bhat, J.A.; Siddiqui, M.H.; Rinklebe, J.; Ahmad, P. Integration of Silicon and Secondary Metabolites in Plants: A Significant Association in Stress Tolerance. J. Exp. Bot. 2020, 71, 6758–6774. [Google Scholar] [CrossRef]
- Saad, F. Silicon-Mediated Modulations of Genes and Secondary Metabolites in Plants; Elsevier eBooks: Amsterdam, The Netherlands, 2022; pp. 77–90. [Google Scholar] [CrossRef]
- Song, X.P.; Verma, K.K.; Tian, D.D.; Zhang, X.Q.; Liang, Y.J.; Huang, X.; Li, C.N.; Li, Y.R. Exploration of Silicon Functions to Integrate with Biotic Stress Tolerance and Crop Improvement. Biol. Res. 2021, 54, 19. [Google Scholar] [CrossRef]
- Gulzar, N.; Lone, R.; Al-Sadi, A.M.; Azeez, A. Interactive Role of Silicon and Phenolics in Biotic Stress Regulation in Plants and Expression of Phenylpropanoid Pathway Genes. In Plant Phenolics in Biotic Stress Management; Lone, R., Khan, S., Mohammed Al-Sadi, A., Eds.; Springer: Singapore, 2024. [Google Scholar] [CrossRef]
- Cooke, J.; Leishman, M.R. Tradeoffs between Foliar Silicon and Carbonbased Defences: Evidence from Vegetation Communities of Contrasting Soil Types. Oikos 2012, 121, 2052–2060. [Google Scholar] [CrossRef]
- Frew, A.; Powell, J.R.; Sallam, N.; Allsopp, P.G.; Johnson, S.N. Trade-Offs between Silicon and Phenolic Defenses May Explain Enhanced Performance of Root Herbivores on Phenolic-Rich Plants. J. Chem. Ecol. 2016, 42, 768–771. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Rahman, M.M.; Ansary, M.M.U.; Keya, S.S.; Abdelrahman, M.; Miah, M.G.; Phan Tran, L.-S. Silicon in Mitigation of Abiotic Stress-Induced Oxidative Damage in Plants. Crit. Rev. Biotechnol. 2021, 41, 918–934. [Google Scholar] [CrossRef] [PubMed]
- Shetty, R.; Fretté, X.; Jensen, B.; Shetty, N.P.; Jensen, J.D.; Jørgensen, H.J.L.; Newman, M.-A.; Christensen, L.P. Silicon-Induced Changes in Antifungal Phenolic Acids, Flavonoids, and Key Phenylpropanoid Pathway Genes during the Interaction between Miniature Roses and the Biotrophic Pathogen Podosphaera Pannosa. Plant Physiol. 2011, 157, 2194–2205. [Google Scholar] [CrossRef]
- Rahman, A.; Wallis, C.M.; Uddin, W. Silicon-Induced Systemic Defense Responses in Perennial Ryegrass against Infection by Magnaporthe Oryzae. Phytopathology 2015, 105, 748–757. [Google Scholar] [CrossRef]
- Khan, I.; Awan, S.A.; Rizwan, M.; Brestič, M.; Xie, W. Silicon: An Essential Element for Plant Nutrition and Phytohormones Signaling Mechanism under Stressful Conditions. Plant Growth Regul. 2022, 100, 301–319. [Google Scholar] [CrossRef]
- Chain, F.; Côté-Beaulieu, C.; Belzile, F.; Menzies, J.G.; Bélanger, R.R. A Comprehensive Transcriptomic Analysis of the Effect of Silicon on Wheat Plants under Control and Pathogen Stress Conditions. Mol. Plant-Microbe Interact. 2009, 22, 1323–1330. [Google Scholar] [CrossRef]
- Hao, L.; Shi, S.; Guo, H.; Zhang, J.; Li, P.; Feng, Y. Transcriptome Analysis Reveals Differentially Expressed MYB Transcription Factors Associated with Silicon Response in Wheat. Sci. Rep. 2021, 11, 4330. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Song, Z.; Su, Q.; Wei, Z.-H.; Li, W.; Jiang, Z.-X.; Tian, P.; Wang, Z.; Yang, X.; Yang, M.; et al. Transcriptomic and Metabolomic Reveals Silicon Enhances Adaptation of Rice under Dry Cultivation by Improving Flavonoid Biosynthesis, Osmoregulation, and Photosynthesis. Front. Plant Sci. 2022, 13, 967537. [Google Scholar] [CrossRef]
- Luyckx, M.; Hausman, J.-F.; Lutts, S.; Guerriero, G. Silicon and Plants: Current Knowledge and Technological Perspectives. Front. Plant Sci. 2017, 8, 411. [Google Scholar] [CrossRef]
- Coskun, D.; Deshmukh, R.; Sonah, H.; Menzies, J.G.; Reynolds, O.; Ma, J.F.; Kronzucker, H.J.; Bélanger, R.R. The Controversies of Silicon’s Role in Plant Biology. New Phytol. 2018, 221, 67–85. [Google Scholar] [CrossRef] [PubMed]
- Frew, A.; Weston, L.A.; Reynolds, O.L.; Gurr, G.M. The Role of Silicon in Plant Biology: A Paradigm Shift in Research Approach. Ann. Bot. 2018, 121, 1265–1273. [Google Scholar] [CrossRef]
- Hodson, M.J.; White, P.J.; Mead, A.; Broadley, M.R. Phylogenetic Variation in the Silicon Composition of Plants. Ann. Bot. 2005, 96, 1027–1046. [Google Scholar] [CrossRef]
- Hassan, I.F.; Ajaj, R.; Gaballah, M.S.; Ogbaga, C.C.; Kalaji, H.M.; Hatterman-Valenti, H.M.; Alam-Eldein, S.M. Foliar Application of Nano-Silicon Improves the Physiological and Biochemical Characteristics of “Kalamata” Olive Subjected to Deficit Irrigation in a Semi-Arid Climate. Plants 2022, 11, 1561. [Google Scholar] [CrossRef]
- Nascimento-Silva, K.; Benlloch-González, M.; Fernández-Escobar, R. Silicon Nutrition in Young Olive Plants: Effect of Dose, Application Method, and Cultivar. Hortscience 2022, 57, 1534–1539. [Google Scholar] [CrossRef]
- Martos-García, I.; Fernández-Escobar, R.; Benlloch-González, M. Silicon Is a Non-Essential Element but Promotes Growth in Olive Plants. Sci. Hortic. 2024, 323, 112541. [Google Scholar] [CrossRef]
- Martos-García, I.; Fernández-Escobar, R.; Benlloch-González, M. Silicon Affects Growth and Nitrogen Uptake of Young Olive Plants. HortScience 2024, 60, 1–4. [Google Scholar] [CrossRef]
- Pasković, I.; Franić, M.; Polić Pasković, M.; Talhaoui, N.; Marcelić, Š.; Lukić, I.; Fredotović, Ž.; Žurga, P.; Major, N.; Ban, S.G.; et al. Silicon Foliar Fertilisation Ameliorates Olive Leaves Polyphenolic Compounds Levels and Elevates Its Potential towards Different Cancer Cells. Appl. Sci. 2024, 14, 4669. [Google Scholar] [CrossRef]
- Borghini, F.; Tamasi, G.; Loiselle, S.A.; Baglioni, M.; Ferrari, S.; Bisozzi, F.; Costantini, S.; Tozzi, C.; Riccaboni, A.; Rossi, C. Phenolic Profiles in Olive Leaves from Different Cultivars in Tuscany and Their Use as a Marker of Varietal and Geographical Origin on a Small Scale. Molecules 2024, 29, 3617. [Google Scholar] [CrossRef]
- Petridis, A.; Therios, I.; Samouris, G.; Koundouras, S.; Giannakoula, A. Effect of Water Deficit on Leaf Phenolic Composition, Gas Exchange, Oxidative Damage and Antioxidant Activity of Four Greek Olive (Olea europaea L.) Cultivars. Plant Physiol. Biochem. 2012, 60, 1–11. [Google Scholar] [CrossRef]
- Mechri, B.; Tekaya, M.; Hammami, M.; Chehab, H. Root Verbascoside and Oleuropein Are Potential Indicators of Drought Resistance in Olive Trees (Olea europaea L.). Plant Physiol. Biochem. 2019, 141, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Polić Pasković, M.; Herak Ćustić, M.; Lukić, I.; Marcelić, Š.; Žurga, P.; Vidović, N.; Major, N.; Goreta Ban, S.; Pecina, M.; Ražov, J.; et al. Foliar Nutrition Strategies for Enhancing Phenolic and Amino Acid Content in Olive Leaves. Plants 2024, 13, 3514. [Google Scholar] [CrossRef]
- Cetinkaya, H.; Kulak, M. Relationship Between Total Phenolic, Total Flavonoid and Oleuropein in Different Aged Olive (Olea europaea L.) Cultivar Leaves. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 81–85. [Google Scholar] [CrossRef]
- Guerriero, G.; Hausman, J.-F.; Legay, S. Silicon and the Plant Extracellular Matrix. Front. Plant Sci. 2016, 7, 463. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Huynh, W.Q.; Kronzucker, H.J. The Role of Silicon in Higher Plants under Salinity and Drought Stress. Front. Plant Sci. 2016, 7, 1072. [Google Scholar] [CrossRef]
- Alayafi, A.H.; Al-Solaimani, S.G.M.; Abd El-Wahed, M.H.; Alghabari, F.M.; Sabagh, A.E. Silicon Supplementation Enhances Productivity, Water Use Efficiency and Salinity Tolerance in Maize. Front. Plant Sci. 2022, 13, 953451. [Google Scholar] [CrossRef]
- Verma, K.K.; Song, X.-P.; Verma, C.L.; Chen, Z.-L.; Rajput, V.D.; Wu, K.-C.; Liao, F.; Chen, G.-L.; Li, Y.-R. Functional Relationship between Photosynthetic Leaf Gas Exchange in Response to Silicon Application and Water Stress Mitigation in Sugarcane. Biol. Res. 2021, 54, 15. [Google Scholar] [CrossRef] [PubMed]
- Detmann, K.C.; Araújo, W.L.; Martins, S.C.V.; Sanglard, L.M.V.P.; Reis, J.V.; Detmann, E.; Rodrigues, F.Á.; Nunes-Nesi, A.; Fernie, A.R.; DaMatta, F.M. Silicon Nutrition Increases Grain Yield, Which, in Turn, Exerts a Feed-Forward Stimulation of Photosynthetic Rates via Enhanced Mesophyll Conductance and Alters Primary Metabolism in Rice. New Phytol. 2012, 196, 752–762. [Google Scholar] [CrossRef]
- Rastogi, A.; Yadav, S.; Hussain, S.; Kataria, S.; Hajihashemi, S.; Kumari, P.; Yang, X.; Brestic, M. Does Silicon Really Matter for the Photosynthetic Machinery in Plants…? Plant Physiol. Biochem. 2021, 169, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Bosabalidis, A.M.; Kofidis, G. Comparative Effects of Drought Stress on Leaf Anatomy of Two Olive Cultivars. Plant Sci. 2002, 163, 375–379. [Google Scholar] [CrossRef]
- Bacelar, E.A.; Correia, C.M.; Moutinho-Pereira, J.M.; Goncalves, B.C.; Lopes, J.I.; Torres-Pereira, J.M.G. Sclerophylly and Leaf Anatomical Traits of Five Field-Grown Olive Cultivars Growing under Drought Conditions. Tree Physiol. 2004, 24, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Sun, D.; Wang, C.; Qin, H.; Ding, H.; Li, Y.; Guo, T. Silicon Application Alleviates Drought Stress in Wheat Through Transcriptional Regulation of Multiple Antioxidant Defense Pathways. J. Plant Growth Regul. 2016, 35, 1–10. [Google Scholar] [CrossRef]
- Exley, C. Silicon in Life: Whither Biological Silicification? In Progress in Molecular and Subcellular Biology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 173–184. [Google Scholar] [CrossRef]
- Mougiou, N.; Trikka, F.; Trantas, E.; Ververidis, F.; Makris, A.; Argiriou, A.; Vlachonasios, K.E. Expression of Hydroxytyrosol and Oleuropein Biosynthetic Genes Are Correlated with Metabolite Accumulation during Fruit Development in Olive, Olea europaea, Cv. Koroneiki. Plant Physiol. Biochem. 2018, 128, 41–49. [Google Scholar] [CrossRef]
- Vriet, C.; Hennig, L.; Laloi, C. Stress-Induced Chromatin Changes in Plants: Of Memories, Metabolites and Crop Improvement. Cell. Mol. Life Sci. 2015, 72, 1261–1273. [Google Scholar] [CrossRef]
- Nedukha, O.; Zolotareva, O.; Netsvetov, M. Phenotypic variability of epidermis structure and silicon inclusions in the leaves of Quercus robur in the Feofaniya Park. Plant Introd. 2023, 97/98, 18–32. [Google Scholar] [CrossRef]
- Hartley, S.E.; Fitt, R.N.; McLarnon, E.L.; Wade, R.N. Defending the Leaf Surface: Intra- and Inter-Specific Differences in Silicon Deposition in Grasses in Response to Damage and Silicon Supply. Front. Plant Sci. 2015, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, S.S.; Motesharezadeh, B.; Moshiri, F.; Hosseini, H.M.; Alikhani, H.A. Silicon Utilization Efficiency of Different Wheat Cultivars in a Calcareous Soil. Silicon 2019, 11, 2159–2168. [Google Scholar] [CrossRef]
- Tubana, B.S.; Babu, T.; Datnoff, L.E. A Review of Silicon in Soils and Plants and Its Role in US Agriculture. Soil Sci. 2016, 181, 393–411. [Google Scholar] [CrossRef]
- Sheng, H.; Lei, Y.; Wei, J.; Yang, Z.; Peng, L.; Li, W.; Liu, Y. Analogy of Silicon and Boron in Plant Nutrition. Front. Plant Sci. 2024, 15, 1353706. [Google Scholar] [CrossRef]
- Pasković, I.; Pecina, M.; Bronić, J.; Perica, S.; Ban, D.; Goreta Ban, S.; Pošćić, F.; Palčić, I.; Herak Ćustić, M. Synthetic Zeolite A as Zinc and Manganese Fertilizer in Calcareous Soil. Commun. Soil Sci. Plant Anal. 2018, 49, 1072–1082. [Google Scholar] [CrossRef]
- Sun, P.; Grignetti, A.; Liu, S.; Casacchia, R.; Salvatori, R.; Pietrini, F.; Loreto, F.; Centritto, M. Associated Changes in Physiological Parameters and Spectral Reflectance Indices in Olive (Olea europaea L.) Leaves in Response to Different Levels of Water Stress. Int. J. Remote Sens. 2008, 29, 1725–1743. [Google Scholar] [CrossRef]
- Penuelas, J.; Pinol, J.; Ogaya, R.; Filella, I. Estimation of Plant Water Concentration by the Reflectance Water Index WI (R900/R970). Int. J. Remote Sens. 1997, 18, 2869–2875. [Google Scholar] [CrossRef]
- Gamon, J.A.; Peñuelas, J.; Field, C.B. A Narrow-Waveband Spectral Index That Tracks Diurnal Changes in Photosynthetic Efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Gitelson, A.A.; Chivkunova, O.B.; Rakitin, V.Y. Non-Destructive Optical Detection of Pigment Changes during Leaf Senescence and Fruit Ripening. Physiol. Plant. 1999, 106, 135–141. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Zur, Y.; Chivkunova, O.B.; Merzlyak, M.N. Assessing Carotenoid Content in Plant Leaves with Reflectance Spectroscopy. Photochem. Photobiol. 2007, 75, 272–281. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Buschmann, C.; Lichtenthaler, H.K. The chlorophyll fluorescence ratio F735/F700 as an accurate measure of the chlorophyll content in plants. Remote Sens. Environ. 1999, 69, 296–302. [Google Scholar] [CrossRef]
- Barnes, J.D.; Balaguer, L.; Manrique, E.; Elvira, S.; Davison, A.W. A reappraisal of the use of DMSO for the extraction and determination of chlorophylls a and b in lichens and higher plants. Environ. Exp. Bot. 1992, 32, 85–100. [Google Scholar] [CrossRef]
- Penuelas, J.; Frederic, B.; Filella, I. Semi-Empirical Indices to Assess Carotenoids/Chlorophyll-a Ratio from Leaf Spectral Reflectance. Photosynthetica 1995, 31, 221–230. [Google Scholar]
- Vogelman, J.E.; Rock, B.N.; Moss, D.M. Red edge spectral measurements from sugar maple leaves. Int. J. Remote Sens. 1993, 14, 1563–1575. [Google Scholar] [CrossRef]
- Carter, G.A. Ratios of leaf reflectances in narrow wavebands as indicators of plant stress. Int. J. Remote Sens. 1994, 15, 697–703. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Pushnik, J.C.; Dobrowski, S.; Ustin, S.L. Steady-state chlorophyll a fluorescence detection from canopy derivative reflectance and double-peak red-edge effects. Remote Sens. Environ. 2003, 84, 283–294. [Google Scholar] [CrossRef]
- Vidović, N.; Pasković, I.; Lukić, I.; Žurga, P.; Majetić Germek, V.; Grozić, K.; Cukrov, M.; Marcelić, Š.; Ban, D.; Talhaoui, N.; et al. Biophenolic Profile Modulations in Olive Tissues as Affected by Manganese Nutrition. Plants 2021, 10, 1724. [Google Scholar] [CrossRef] [PubMed]
- Eastin, E.F. Total nitrogen determining for plant material containing nitrate. Anal. Biochem. 1978, 85, 591–594. [Google Scholar] [CrossRef]
- Mandal, U.K.; Warrington, D.N.; Bhardwaj, A.K.; Bar-Tal, A.; Kautsky, L.; Minz, D.; Levy, G.J. Evaluating Impact of Irrigation Water Quality on a Calcareous Clay Soil Using Principal Component Analysis. Geoderma 2008, 144, 189–197. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. Available online: http://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 11 March 2025).


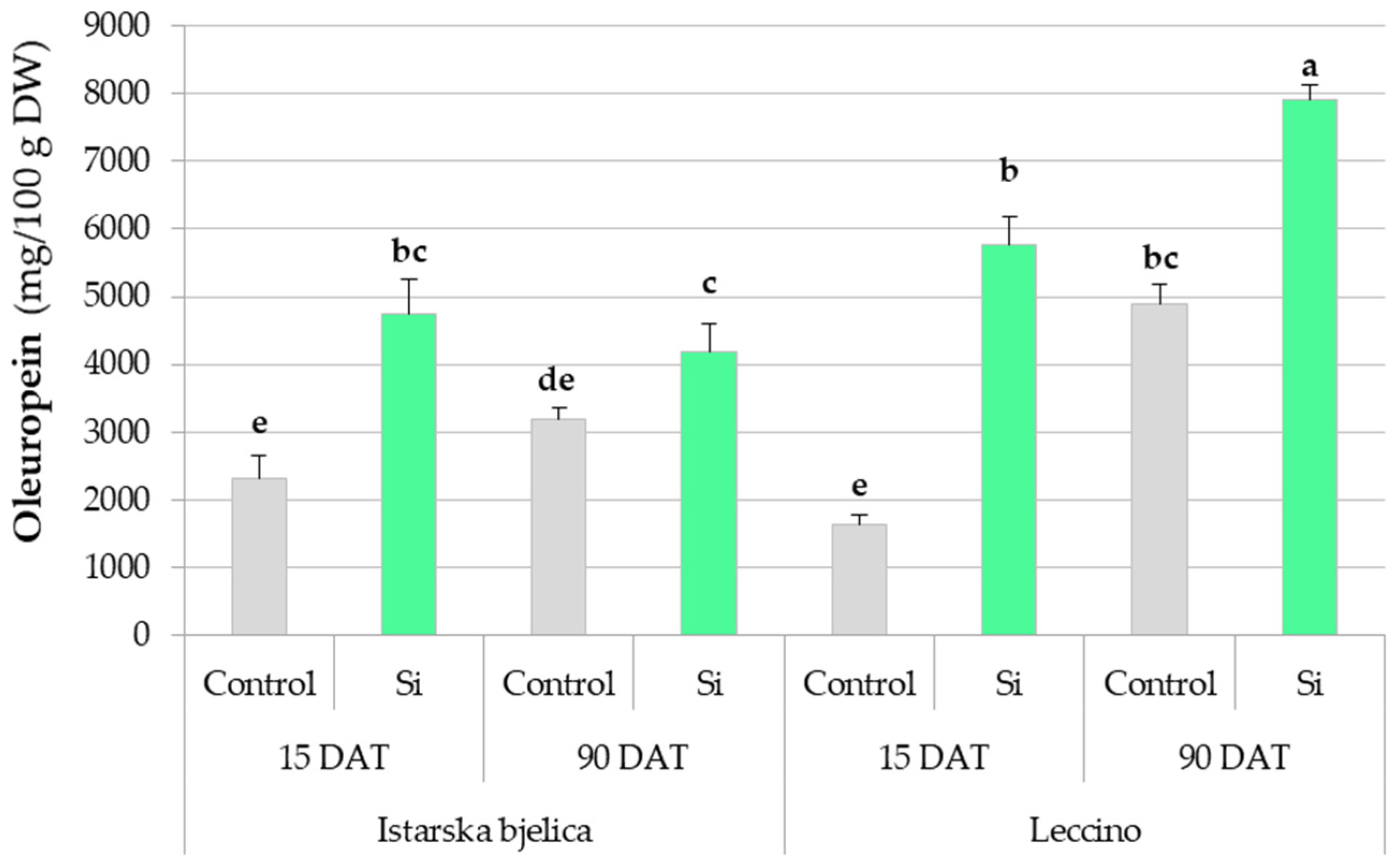

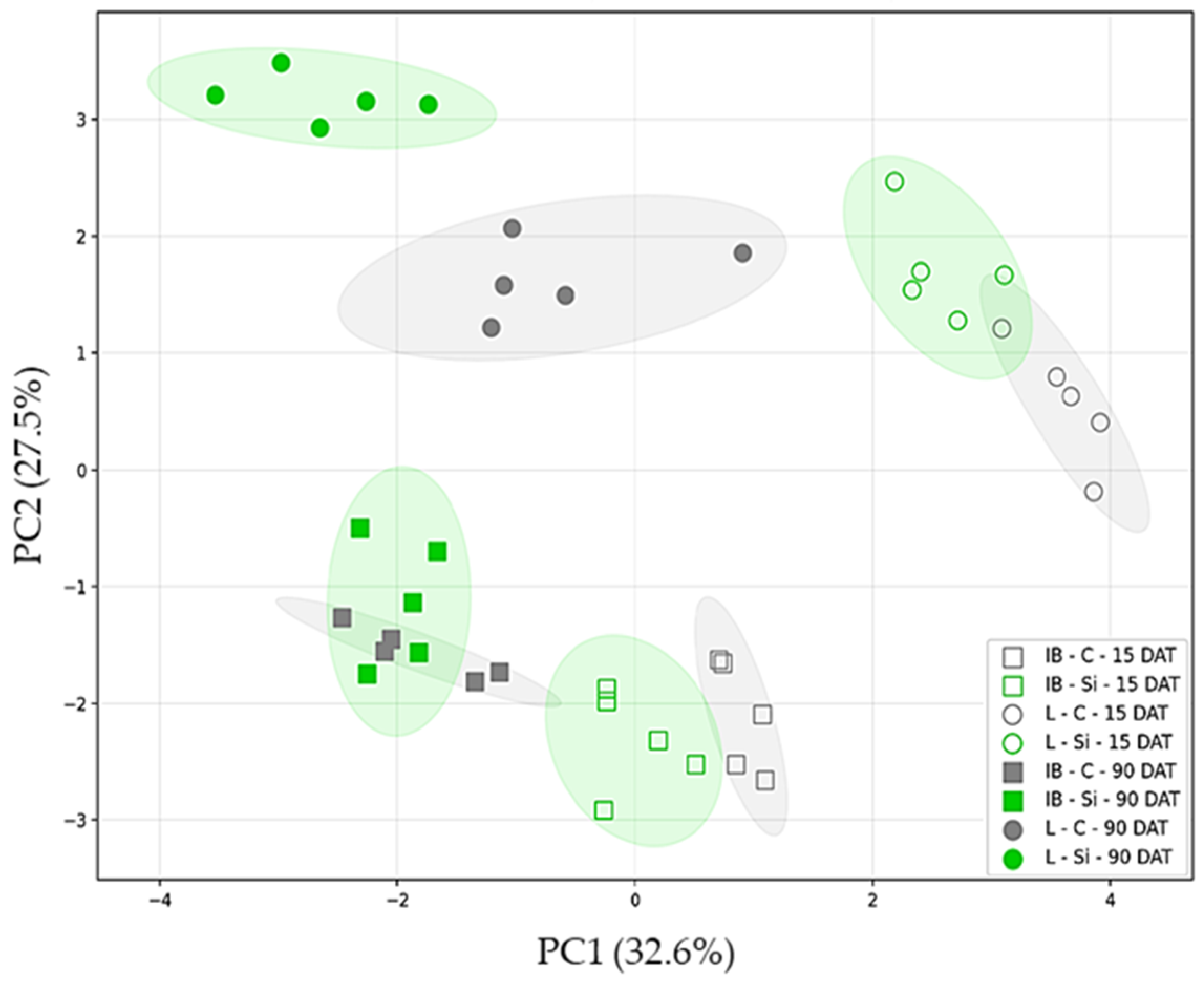
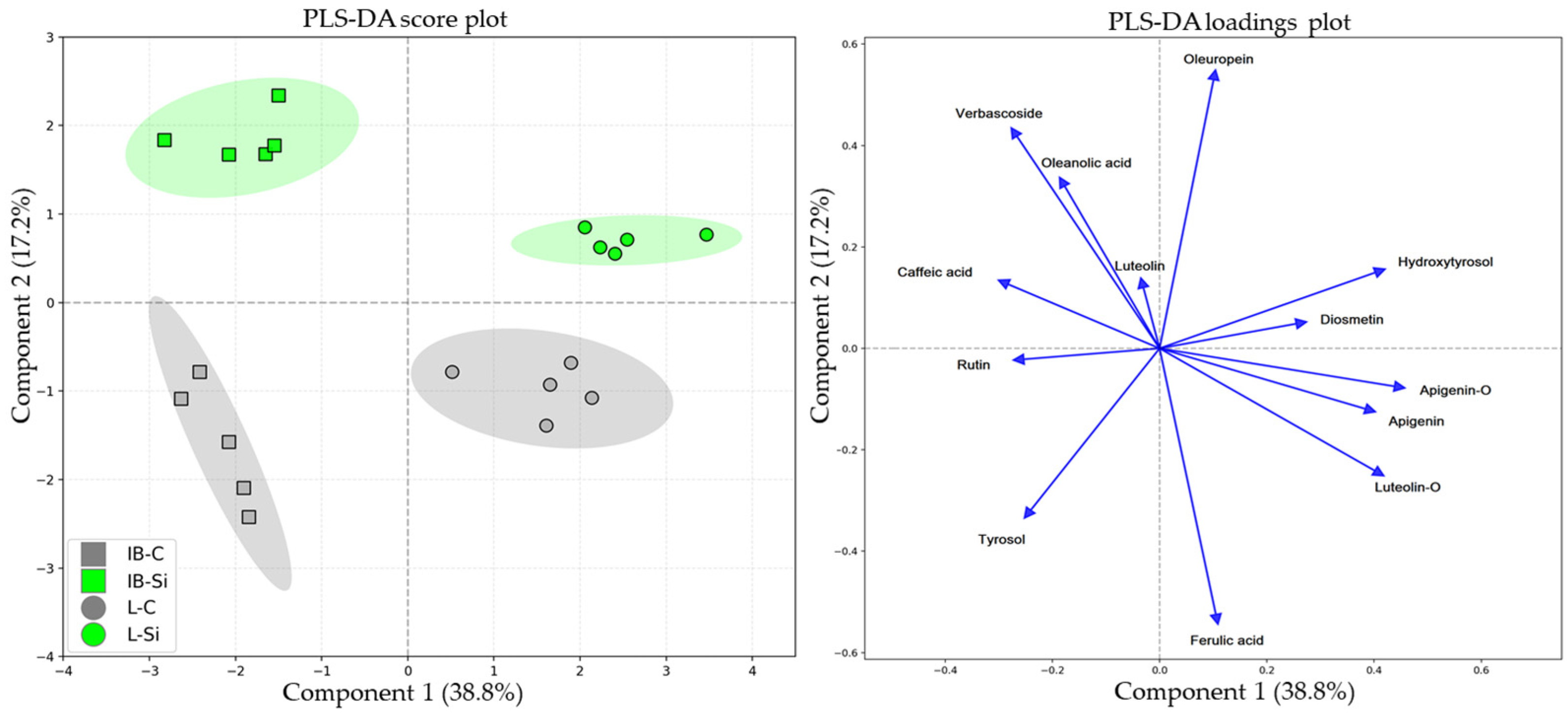
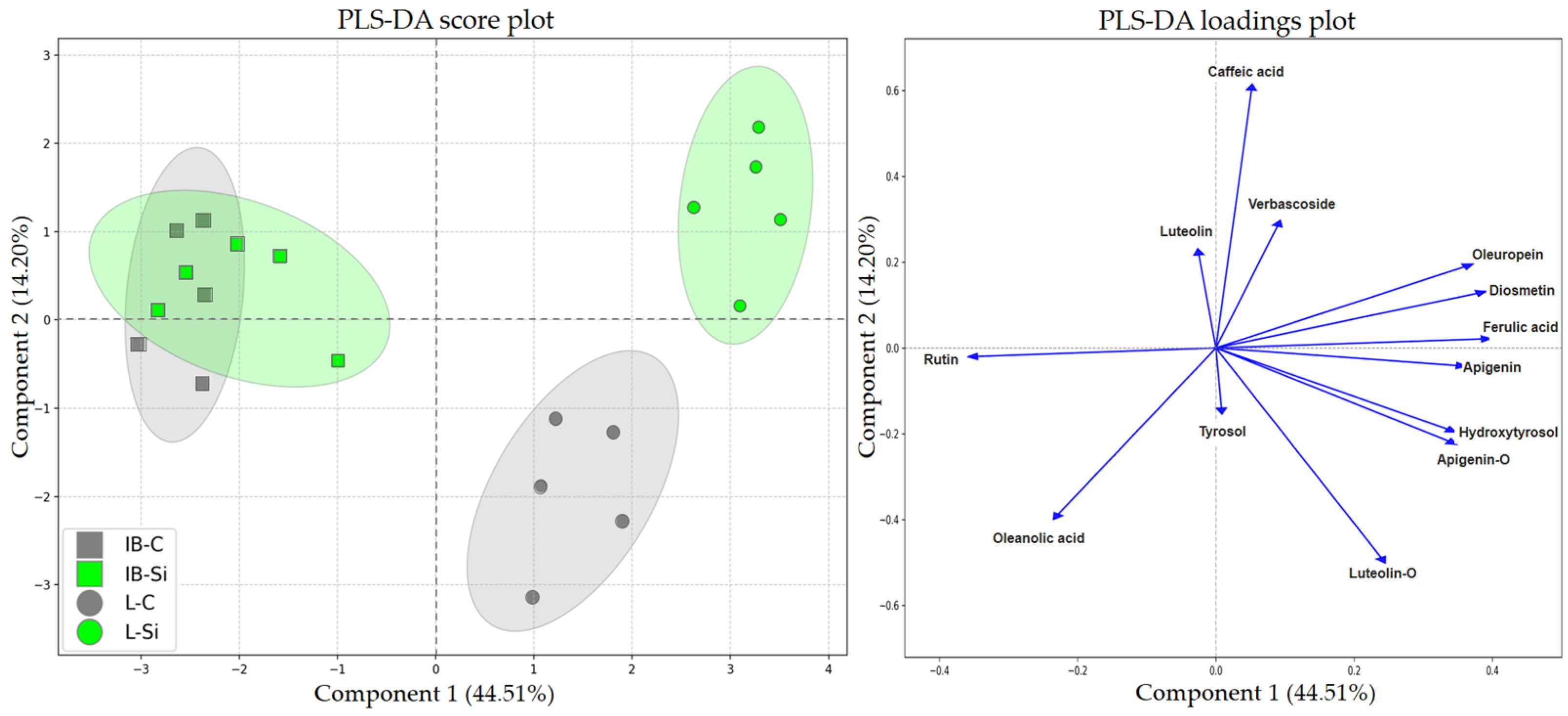



| Source of Variation | Si (mg/kg DW) | Macronutrients (g/kg DW) | Micronutrients (mg/kg DW) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | P | K | Ca | Mg | S | B | Fe | Mn | Zn | ||||
| Cultivar | |||||||||||||
| Istarska bjelica | 136.69 ± 8.47 | 14.15 ± 0.34 b | 0.81 ± 0.02 | 13.81 ± 0.32 | 9.54 ± 0.24 b | 0.70 ± 0.01 b | 0.88 ± 0.01 b | 8.43 ± 0.18 a | 34.49 ± 20.66 | 40.43 ± 2.04 | 9.22 ± 0.26 b | ||
| Leccino | 137.74 ± 7.42 | 16.02 ± 0.31 a | 0.85 ± 0.02 | 14.33 ± 0.47 | 12.84 ± 0.40 a | 1.01 ± 0.03 a | 1.10 ± 0.02 a | 7.26 ± 0.09 b | 10.55 ± 1.58 | 45.12 ± 1.75 | 14.97 ± 0.33 a | ||
| Treatment | |||||||||||||
| Control | 107.55 ± 4.18 b | 14.82 ± 0.35 | 0.81 ± 0.02 | 14.03 ± 0.42 | 11.26 ± 0.54 | 0.86 ± 0.04 | 1.04 ± 0.03 | 8.04 ± 0.23 a | 32.90 ± 20.71 | 42.36 ± 1.81 | 11.99 ± 0.70 | ||
| Si | 166.88 ± 5.73 a | 15.35 ± 0.40 | 0.84 ± 0.01 | 14.10 ± 0.39 | 11.11 ± 0.39 | 0.85 ± 0.03 | 0.98 ± 0.02 | 7.65 ± 0.10 b | 12.14 ± 1.88 | 43.20 ± 2.10 | 12.20 ± 0.62 | ||
| Sampling time | |||||||||||||
| 15 DAT | 141.75 ± 9.84 | 15.99 ± 0.31 a | 0.90 ± 0.01 a | 15.80 ± 0.25 a | 10.28 ± 0.38 b | 0.89 ± 0.04 a | 1.04 ± 0.03 a | 7.88 ± 0.13 | 31.16 ± 20.79 | 41.51 ± 1.93 | 11.76 ± 0.63 | ||
| 90 DAT | 132.67 ± 5.31 | 14.17 ± 0.35 b | 0.76 ± 0.01 b | 12.33 ± 0.10 b | 12.10 ± 0.48 a | 0.82 ± 0.03 b | 0.95 ± 0.02 b | 7.81 ± 0.22 | 13.89 ± 1.78 | 44.04 ± 1.96 | 12.43 ± 0.69 | ||
| Cv. | n.s. | *** | n.s. | n.s. | *** | *** | *** | *** | n.s. | n.s. | *** | ||
| T | *** | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | * | n.s. | n.s. | n.s. | ||
| ST | n.s. | *** | *** | *** | *** | * | *** | n.s. | n.s. | n.s. | n.s. | ||
| Cv. × T | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | * | ** | n.s. | n.s. | n.s. | ||
| Cv. × ST | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | *** | n.s. | n.s. | n.s. | n.s. | ||
| T × ST | ** | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | ||
| Cv. × T × ST | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | ||
| Source of variation | Total phenolics | Simple phenolic alcohols | Phenolic acids | Terpenoids | |||||
| Hydroxytyrosol | Tyrosol | Caffeic | Ferulic | Verbascoside | Oleanolic acid | ||||
| mg 100 g−1 DW | mg 100 g−1 DW | mg 100 g−1 DW | mg 100 g−1 DW | ||||||
| Cultivar (Cv.) | |||||||||
| Istarska bjelica | 3021.55 ± 136.74 | 15.11 ± 1.17 b | 13.29 ± 0.81 | 5.61 ± 0.31 b | 2.80 ± 0.2 | 12.10 ± 0.90 b | 1430.89 ± 61.45 a | ||
| Leccino | 3409.84 ± 105.01 | 30.01 ± 2.00 a | 14.87 ± 0.61 | 8.18 ± 0.17 a | 2.43 ± 0.14 | 15.04 ± 2.27 a | 1172.68 ± 97.62 b | ||
| Treatment (T) | |||||||||
| Control | 3086.26 ± 99.31 | 20.88 ± 3.06 | 13.41 ± 0.69 | 6.69 ± 0.49 | 2.55 ± 0.21 | 12.17 ± 1.91 b | 1320.41 ± 99.47 | ||
| Si | 3345.13 ± 156.57 | 24.25 ± 2.78 | 14.75 ± 0.77 | 7.09 ± 0.48 | 2.68 ± 0.14 | 14.98 ± 0.86 a | 1283.16 ± 83.89 | ||
| Cv. | n.s. | *** | n.s. | ** | n.s. | * | * | ||
| T | n.s. | n.s. | n.s. | n.s. | n.s. | * | n.s. | ||
| Cv. × T | n.s. | n.s. | n.s. | n.s. | n.s. | ** | n.s. | ||
| Source of variation | Secoiridoids | Flavonoids | |||||||
| Oleuropein | Apigenin | Apigenin-O | Luteolin | Luteolin-O | Rutin | Diosmetin | |||
| mg 100 g−1 DW | mg 100 g−1 DW | ||||||||
| Cultivar (Cv.) | |||||||||
| Istarska bjelica | 1339.05 ± 330.97 | 1.77 ± 0.18 b | 19.83 ± 1.16 b | 8.43 ± 0.78 b | 559.32 ± 24.34 b | 64.54 ± 5.01 a | 4.58 ± 0.25 b | ||
| Leccino | 1421.66 ± 153.54 | 11.10 ± 1.96 a | 77.04 ± 6.9 a | 21.25 ± 3.2 a | 676.31 ± 14.86 a | 51.86 ± 3.58 b | 7.22 ± 0.64 a | ||
| Treatment (T) | |||||||||
| Control | 950.80 ± 73.86 b | 8.51 ± 2.63 a | 53.29 ± 13.12 | 18.16 ± 3.89 a | 621.86 ± 35.38 | 63.76 ± 5.06 a | 6.14 ± 0.85 | ||
| Si | 1809.91 ± 57.26 a | 4.37 ± 0.90 b | 43.58 ± 7.29 | 11.52 ± 1.56 b | 613.77 ± 17.83 | 52.64 ± 3.78 b | 4.45 ± 0.35 | ||
| Cv. | n.s. | *** | *** | *** | ** | * | ** | ||
| T | * | * | n.s. | * | n.s. | * | n.s. | ||
| Cv. × T | n.s. | * | * | * | n.s. | n.s. | n.s. | ||
| Source of Variation | Si (mg/kg DW) | Macronutrients (g/kg DW) | Micronutrients (mg/kg DW) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | P | K | Ca | Mg | S | B | Fe | Mn | Zn | ||||
| Cultivar (Cv.) | |||||||||||||
| Istarska bjelica | 59.27 ± 5.3 | 14.22 ± 0.47 b | 0.93 ± 0.01 b | 13.6 ± 0.63 b | 10.02 ± 0.44 b | 0.92 ± 0.03 b | 1.1 ± 0.02 b | 11.67 ± 1.04 a | 12.5 ± 4.99 | 71.7 ± 4.44 | 13.13 ± 0.56 b | ||
| Leccino | 58.77 ± 3.29 | 16.44 ± 0.49 a | 1.12 ± 0.02 a | 15.57 ± 0.38 a | 12.5 ± 0.49 a | 1.08 ± 0.03 a | 1.23 ± 0.03 a | 8.14 ± 0.28 b | 5.5 ± 1.4 | 75.88 ± 2.69 | 17.53 ± 0.74 a | ||
| Treatment (T) | |||||||||||||
| Control | 56.19 ± 5.72 | 15.32 ± 0.23 | 1.03 ± 0.03 | 14.95 ± 0.68 | 11.67 ± 0.74 | 1.02 ± 0.04 | 1.19 ± 0.04 | 11.53 ± 1.06 a | 11.6 ± 4.91 | 74.19 ± 4.38 | 15.49 ± 1.06 | ||
| Si | 61.85 ± 2.2 | 15.34 ± 0.79 | 1.02 ± 0.03 | 14.22 ± 0.47 | 10.85 ± 0.37 | 0.98 ± 0.03 | 1.13 ± 0.03 | 8.28 ± 0.19 b | 6.44 ± 1.96 | 73.39 ± 2.92 | 15.18 ± 0.86 | ||
| Cv. | n.s. | *** | *** | * | ** | ** | * | *** | n.s. | n.s. | ** | ||
| T | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | *** | n.s. | n.s. | n.s. | ||
| Cv. × T | n.s. | *** | n.s. | n.s. | n.s. | n.s. | n.s. | *** | n.s. | n.s. | n.s. | ||
| Abbreviation | Index | Equation | Reference |
|---|---|---|---|
| WBI | Water band index | (R900/R970) | [71] |
| PRI | Photochemical reflectance index | (R531 − R570)/(R531 + R570) | [72] |
| PSRI | Plant senescence reflectance index | (R680 − R500)/R750 | [73] |
| CRI 1 | Carotenoid reflectance index 1 | (1/R510) − (1/R550) | [74] |
| CRI 2 | Carotenoid reflectance index 2 | (1/R510) − (1/R700) | [74] |
| CNDVI | Chlorophyll normalized difference vegetation index | (R750 − R705)/(R750 + R705) | [75] |
| NDVI | Normalized difference vegetation index | (R800 − R680)/(R800+R680) | [75] |
| NPQI | Normalized phaeophytinization index | (R415 − R435)/(R415 + R435) | [76] |
| SIPI | Structure intensive pigment index | (R800 − R445)/(R800 − R680) | [77] |
| VREI | Vogelmann red edge index | (R734 − R747)/(R715 + R720) | [78] |
| Ctr | Carter index | (R695/R420) | [79] |
| ZMI | Zarco-Tejada & Miller index | (R750/R710) | [80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cukrov, M.; Ninkovic, V.; Maslov Bandić, L.; Marcelić, Š.; Palčić, I.; Franić, M.; Žurga, P.; Majetić Germek, V.; Lukić, I.; Lemić, D.; et al. Silicon-Mediated Modulation of Olive Leaf Phytochemistry: Genotype-Specific and Stress-Dependent Responses. Plants 2025, 14, 1282. https://doi.org/10.3390/plants14091282
Cukrov M, Ninkovic V, Maslov Bandić L, Marcelić Š, Palčić I, Franić M, Žurga P, Majetić Germek V, Lukić I, Lemić D, et al. Silicon-Mediated Modulation of Olive Leaf Phytochemistry: Genotype-Specific and Stress-Dependent Responses. Plants. 2025; 14(9):1282. https://doi.org/10.3390/plants14091282
Chicago/Turabian StyleCukrov, Marin, Velemir Ninkovic, Luna Maslov Bandić, Šime Marcelić, Igor Palčić, Mario Franić, Paula Žurga, Valerija Majetić Germek, Igor Lukić, Darija Lemić, and et al. 2025. "Silicon-Mediated Modulation of Olive Leaf Phytochemistry: Genotype-Specific and Stress-Dependent Responses" Plants 14, no. 9: 1282. https://doi.org/10.3390/plants14091282
APA StyleCukrov, M., Ninkovic, V., Maslov Bandić, L., Marcelić, Š., Palčić, I., Franić, M., Žurga, P., Majetić Germek, V., Lukić, I., Lemić, D., & Pasković, I. (2025). Silicon-Mediated Modulation of Olive Leaf Phytochemistry: Genotype-Specific and Stress-Dependent Responses. Plants, 14(9), 1282. https://doi.org/10.3390/plants14091282












