Enhancing Salt Tolerance in Tomato Plants Through PEG6000 Seed Priming: Inducing Antioxidant Activity and Mitigating Oxidative Stress
Abstract
1. Introduction
2. Results
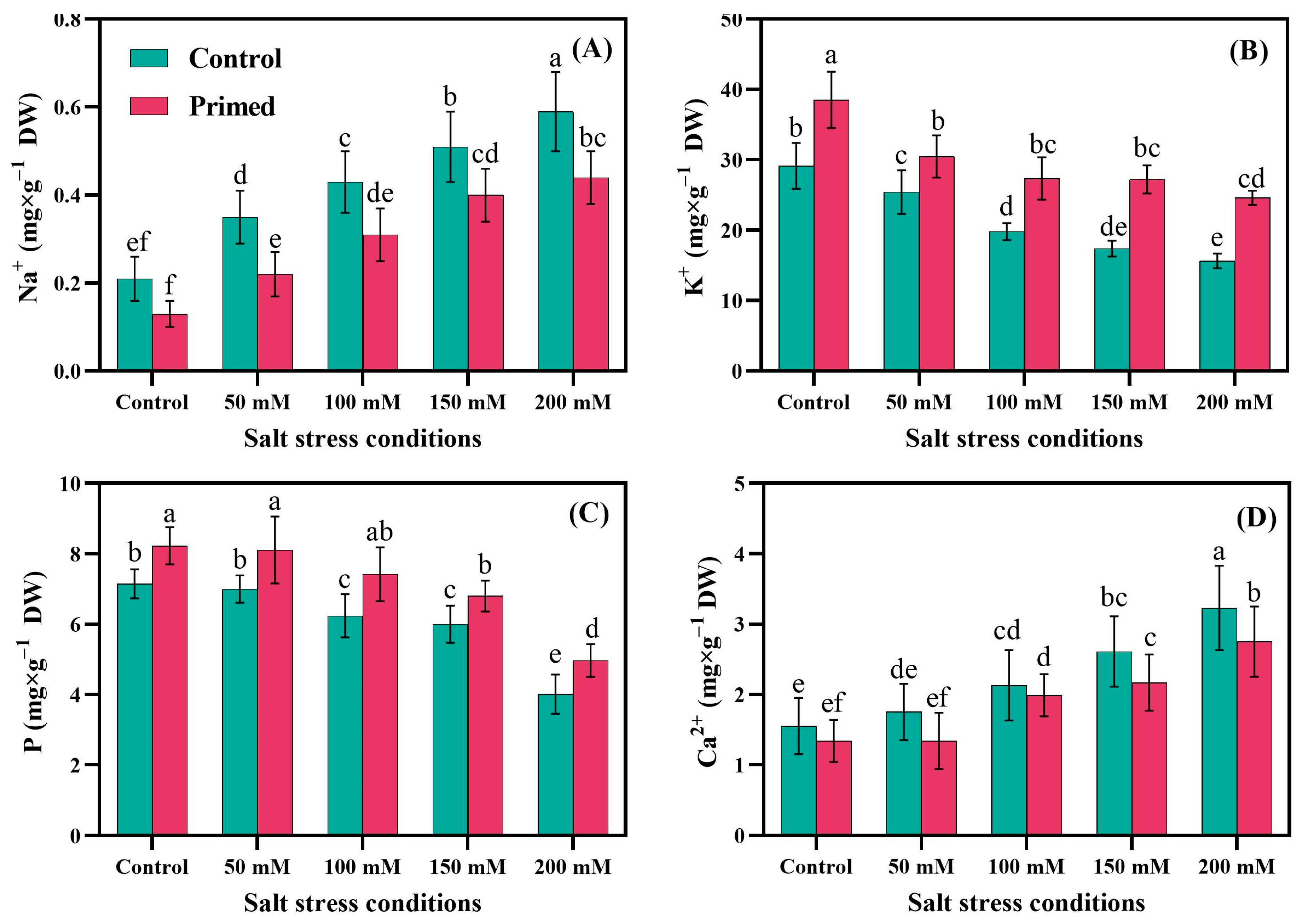
2.1. Leaf Nutrient Analysis
2.2. Fruit Quality Parameters
2.3. Cell Damage Indicators
2.4. Antioxidant Enzymes and Redox Regulation
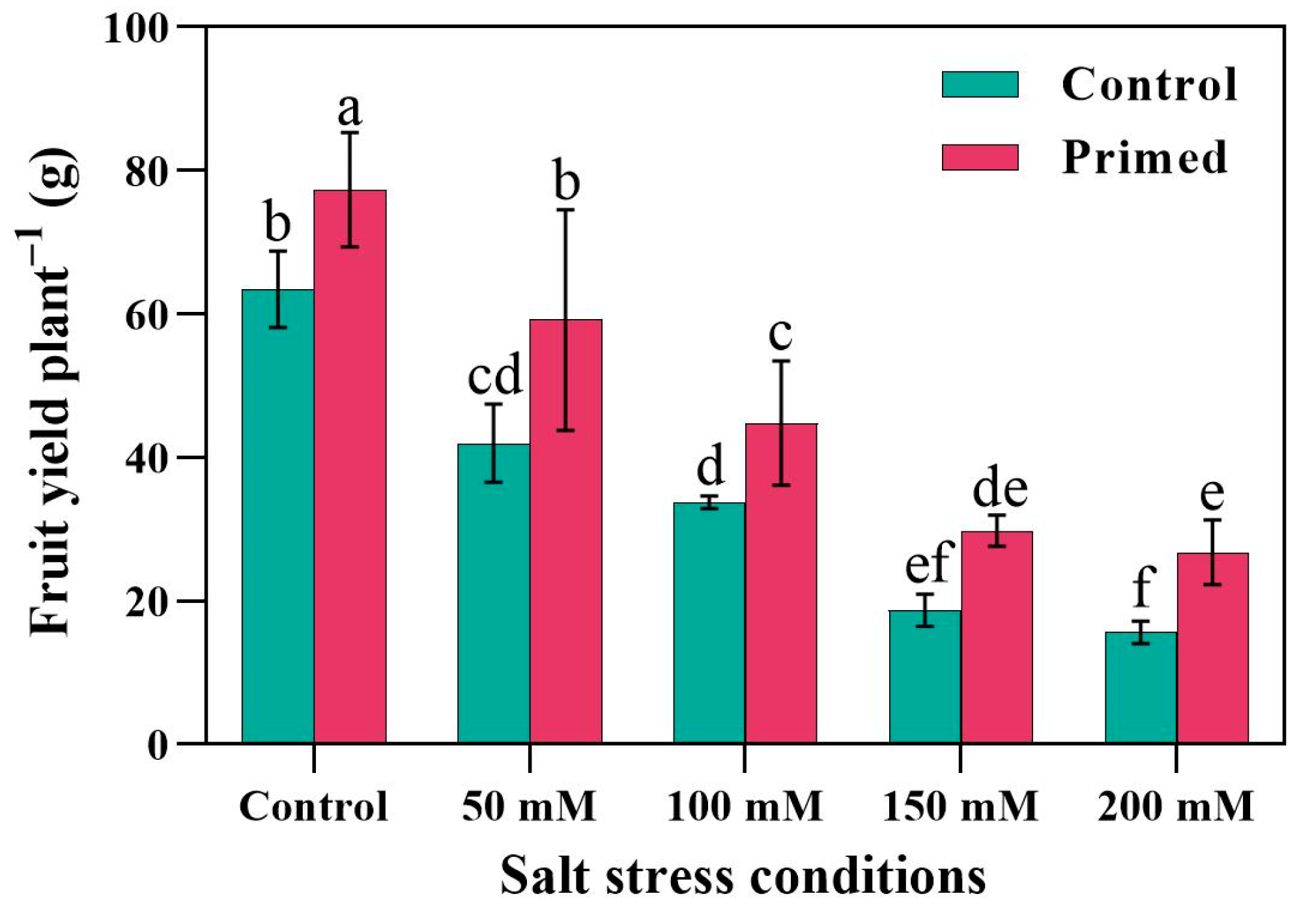
2.5. Fruit Yield
3. Discussion
3.1. Nutrient Composition in Leaf
3.2. Fruit Quality Parameters
3.3. Cell Damage Indicators
3.4. Antioxidant Enzyme Activity
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Determination of Antioxidant Components
4.3. Quantification of ABA in Shoot and Root Cells
4.4. Determination of Reactive Oxygen Species (ROS)
4.5. Determination of Stress Indicators
4.6. Determination of Water Potential in Plant Tissues
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- González-García, Y.; López-Vargas, E.R.; Pérez-Álvarez, M.; Cadenas-Pliego, G.; Benavides-Mendoza, A.; Valdés-Reyna, J.; Pérez-Labrada, F.; Juárez-Maldonado, A. Seed Priming with Carbon Nanomaterials Improves the Bioactive Compounds of Tomato Plants under Saline Stress. Plants 2022, 11, 1984. [Google Scholar] [CrossRef] [PubMed]
- Tommonaro, G.; Abbamondi, G.R.; Nicolaus, B.; Poli, A.; D’Angelo, C.; Iodice, C.; De Prisco, R. Productivity and Nutritional Trait Improvements of Different Tomatoes Cultivated with Effective Microorganisms Technology. Agric. 2021, 11, 112. [Google Scholar] [CrossRef]
- Malik, A.; Mor, V.S.; Tokas, J.; Punia, H.; Malik, S.; Malik, K.; Sangwan, S.; Tomar, S.; Singh, P.; Singh, N.; et al. Biostimulant-Treated Seedlings under Sustainable Agriculture: A Global Perspective Facing Climate Change. Agronomy 2021, 11, 14. [Google Scholar] [CrossRef]
- Murshed, R.; Lopez-Lauri, F.; Sallanon, H. Effect of Salt Stress on Tomato Fruit Antioxidant Systems Depends on Fruit Development Stage. Physiol. Mol. Biol. Plants 2014, 20, 15–29. [Google Scholar] [CrossRef]
- Biswas, S.; Seal, P.; Majumder, B.; Biswas, A.K. Efficacy of Seed Priming Strategies for Enhancing Salinity Tolerance in Plants: An Overview of the Progress and Achievements. Plant Stress 2023, 9, 100186. [Google Scholar] [CrossRef]
- Habibi, N.; Aryan, S.; Amin, M.W.; Sanada, A.; Terada, N.; Koshio, K. Potential Benefits of Seed Priming under Salt Stress Conditions on Physiological, and Biochemical Attributes of Micro-Tom Tomato Plants. Plants 2023, 12, 2187. [Google Scholar] [CrossRef] [PubMed]
- Aloui, H.; Souguir, M.; Latique, S.; Hannachi, C. Germination and Growth in Control and Primed Seeds of Pepper as Affected by Salt Stress. Cercet. Agron. Mold. 2014, XLVII, 83–95. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hossain, M.A.; Hossain, K.F.B.; Sikder, M.T.; Shammi, M.; Rasheduzzaman, M.; Hossain, M.A.; Alam, A.M.; Uddin, M.K. Effects of NaCl-Salinity on Tomato (Lycopersicon Esculentum Mill.) Plants in a Pot Experiment. Open Agric. 2018, 3, 578–585. [Google Scholar] [CrossRef]
- Habibi, N.; Tayobong, R.R.P.; Naoki, P.; Atsushi, T.; Kaihei, S. Novel Insights into Seed Priming for Tomato Plants: Restoring Root Vitality in the Face of Salt Stress. Hortic. Environ. Biotechnol. 2024, 66, 361–380. [Google Scholar] [CrossRef]
- Bacha, H.; Tekaya, M.; Drine, S.; Guasmi, F.; Touil, L.; Enneb, H.; Triki, T.; Cheour, F.; Ferchichi, A. Impact of Salt Stress on Morpho-Physiological and Biochemical Parameters of Solanum lycopersicum Cv. Microtom Leaves. S. Afr. J. Bot. 2017, 108, 364–369. [Google Scholar] [CrossRef]
- Kaveh, H.; Nemati, H.; Farsi, M.; Vatandoost Jartoodeh, S. How Salinity Affect Germination and Emergence of Tomato Lines. J. Biol. Environ. Sci. 2011, 5, 159–163. [Google Scholar]
- Zhang, Z.; Chen, Y.; Wang, C.; Wang, P.; Tao, F. Future Extreme Temperature and Its Impact on Rice Yield in China. Int. J. Climatol. 2017, 37, 4814–4827. [Google Scholar] [CrossRef]
- Pérez-López, U.; Robredo, A.; Lacuesta, M.; Mena-Petite, A.; Muñoz-Rueda, A. The Impact of Salt Stress on the Water Status of Barley Plants Is Partially Mitigated by Elevated CO2. Environ. Exp. Bot. 2009, 66, 463–470. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, W.Y.; Yun, D.J. A New Insight of Salt Stress Signaling in Plant. Mol. Cells 2016, 39, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, M.; Waheed, A.; Wahab, A.; Majeed, M.; Nazim, M.; Liu, Y.H.; Li, L.; Li, W.J. Soil Salinity and Drought Tolerance: An Evaluation of Plant Growth, Productivity, Microbial Diversity, and Amelioration Strategies. Plant Stress 2024, 11, 100319. [Google Scholar] [CrossRef]
- Kesawat, M.S.; Satheesh, N.; Kherawat, B.S.; Kumar, A.; Kim, H.U.; Chung, S.M.; Kumar, M. Regulation of Reactive Oxygen Species during Salt Stress in Plants and Their Crosstalk with Other Signaling Molecules—Current Perspectives and Future Directions. Plants 2023, 12, 864. [Google Scholar] [CrossRef]
- Fu, H.; Yang, Y. How Plants Tolerate Salt Stress. Curr. Issues Mol. Biol. 2023, 45, 5914–5934. [Google Scholar] [CrossRef]
- Alam, M.S.; Tester, M.; Fiene, G.; Mousa, M.A.A. Early Growth Stage Characterization and the Biochemical Responses for Salinity Stress in Tomato. Plants 2021, 10, 712. [Google Scholar] [CrossRef]
- Ali, M.M.; Javed, T.; Mauro, R.P.; Shabbir, R.; Afzal, I.; Yousef, A.F. Effect of Seed Priming with Potassium Nitrate on the Performance of Tomato. Agricultural 2020, 10, 498. [Google Scholar] [CrossRef]
- Biswas, S.; Rasal-Monir, M.; Islam, M.; Modak, S.; Humayun Kabir, M. Induction of Salt Tolerance in Tomato Through Seed Priming. Plant 2019, 7, 47. [Google Scholar] [CrossRef]
- Hajer, A.S.; Malibari, A.A.; Al-Zahrani, H.S.; Almaghrabi, O.A. Responses of Three Tomato Cultivars to Sea Water Salinity 1. Effect of Salinity on the Seedling Growth. Afr. J. Biotechnol. 2006, 5, 855–861. [Google Scholar]
- Kumar, S.; Singh, T.B.; Agnihotri, R.K.; Chaturvedi, P. Comparative Effect of NaCl and PEG on Physiological and Biochemical Attributes during Vegetative Stage of Tomato CARAS. Res. Jr. Agril. Sci. 2021, 12, 955–961. [Google Scholar]
- Wang, B.; Wang, J.; Yang, T.; Wang, J.; Dai, Q.; Zhang, F.; Xi, R.; Yu, Q.; Li, N. The Transcriptional Regulatory Network of Hormones and Genes under Salt Stress in Tomato Plants (Solanum lycopersicum L.). Front. Plant Sci. 2023, 14, 1115593. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, A.; Amjad, M.; Jahangir, M.M.; Khan, S.M.; Cui, H.; Hu, J. Induction of Salt Tolerance in Tomato (Lycopersicon Esculentum Mill.) Seeds through Sand Priming. Aust. J. Crop Sci. 2012, 6, 1199–1203. [Google Scholar]
- Theerakulpisut, P.; Kanawapee, N.; Panwong, B. Seed Priming Alleviated Salt Stress Effects on Rice Seedlings by Improving Na+/K+ and Maintaining Membrane Integrity. Int. J. Plant Biol. 2016, 7, 53–58. [Google Scholar] [CrossRef]
- Garcia, D.; Zhao, S.; Arif, S.; Zhao, Y.; Ming, L.C.; Huang, D. Seed Priming Technology as a Key Strategy to Increase Crop Plant Production under Adverse Environmental Conditions. J. Agric. Hortic. Res. 2022, 5, 27–46. [Google Scholar] [CrossRef]
- Guo, X.; Zhi, W.; Feng, Y.; Zhou, G.; Zhu, G. Seed Priming Improved Salt-Stressed Sorghum Growth by Enhancing Antioxidative Defense. PLoS ONE 2022, 17, e0263036. [Google Scholar] [CrossRef] [PubMed]
- Mirabi, E.; Hasanabadi, M. Effect of Seed Priming on Some Characteristic of Seedling and Seed Vigor of Tomato (Lycopersicun esculentum). J. Adv. Lab. Res. Biol. 2012, III, 1–5. [Google Scholar]
- Iqbal, M.; Ashraf, M. Seed Treatment with Auxins Modulates Growth and Ion Partitioning in Salt-Stressed Wheat Plants. J. Integr. Plant Biol. 2007, 49, 1003–1015. [Google Scholar] [CrossRef]
- Maggio, A.; Raimondi, G.; Martino, A.; Pascale, S. De Salt Stress Response in Tomato beyond the Salinity Tolerance Threshold. Environ. Exp. Bot. 2007, 59, 276–282. [Google Scholar] [CrossRef]
- Hameed, A.; Hussain, S.; Nisar, F.; Rasheed, A.; Shah, S.Z. Seed Priming as an Effective Technique for Enhancing Salinity Tolerance in Plants: Mechanistic Insights and Prospects for Saline Agriculture with a Special Emphasis on Halophytes. Seeds 2025, 4, 14. [Google Scholar] [CrossRef]
- Chen, K.; Arora, R. Priming Memory Invokes Seed Stress-Tolerance. Environ. Exp. Bot. 2013, 94, 33–45. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.; Tran, L.S.P. Impacts of Priming with Silicon on the Growth and Tolerance of Maize Plants to Alkaline Stress. Front. Plant Sci. 2016, 7, 243. [Google Scholar] [CrossRef]
- Dai, L.Y.; De Zhu, H.; De Yin, K.; Du, J.D.; Zhang, Y.X. Seed Priming Mitigates the Effects of Saline-Alkali Stress in Soybean Seedlings. Chil. J. Agric. Res. 2017, 77, 118–125. [Google Scholar] [CrossRef]
- Pradhan, N.; Prakash, P.; Tiwari, S.K. Osmopriming of Tomato Genotypes with Polyethylene Glycol 6000 Induces Tolerance to Salinity Stress Osmopriming of Tomato Genotypes with Polyethylene Glycol 6000 Induces. Trends Biosci. 2015, 7, 4412–4417. [Google Scholar]
- Mitra, D.; Mondal, R.; Khoshru, B.; Shadangi, S.; Das Mohapatra, P.K.; Panneerselvam, P. Rhizobacteria Mediated Seed Bio-Priming Triggers the Resistance and Plant Growth for Sustainable Crop Production. Curr. Res. Microb. Sci. 2021, 2, 100071. [Google Scholar] [CrossRef]
- Parra, M.; Albacete, A.; Martínez-Andújar, C.; Pérez-Alfocea, F. Increasing Plant Vigour and Tomato Fruit Yield under Salinity by Inducing Plant Adaptation at the Earliest Seedling Stage. Environ. Exp. Bot. 2007, 60, 77–85. [Google Scholar] [CrossRef]
- Kaya, M.D.; Ergin, N.; Harmancı, P.; Kulan, E.G. Seed Priming as a Method of Preservation and Restoration of Sunflower Seeds. OCL—Oilseeds Fats Crop. Lipids 2024, 31, 4. [Google Scholar] [CrossRef]
- Ali, S.; Ullah, S.; Khan, M.N.; Khan, W.M.; Razak, S.A.; Wahab, S.; Hafeez, A.; Khan Bangash, S.A.; Poczai, P. The Effects of Osmosis and Thermo-Priming on Salinity Stress Tolerance in Vigna radiata L. Sustainability 2022, 14, 12924. [Google Scholar] [CrossRef]
- Ishtiaq, M.; Mazhar, M.W.; Maqbool, M.; Hussain, T.; Hussain, S.A.; Casini, R.; Abd-ElGawad, A.M.; Elansary, H.O. Seed Priming with the Selenium Nanoparticles Maintains the Redox Status in the Water Stressed Tomato Plants by Modulating the Antioxidant Defense Enzymes. Plants 2023, 12, 1556. [Google Scholar] [CrossRef]
- Moradi, L.; Siosemardeh, A. Combination of Seed Priming and Nutrient Foliar Application Improved Physiological Attributes, Grain Yield, and Biofortification of Rainfed Wheat. Front. Plant Sci. 2023, 14, 1287677. [Google Scholar] [CrossRef] [PubMed]
- Abhinandan, K.; Skori, L.; Stanic, M.; Hickerson, N.M.N.; Jamshed, M.; Samuel, M.A. Abiotic Stress Signaling in Wheat—An Inclusive Overview of Hormonal Interactions during Abiotic Stress Responses in Wheat. Front. Plant Sci. 2018, 9, 734. [Google Scholar] [CrossRef] [PubMed]
- Jakab, G.; Cottier, V.; Toquin, V.; Rigoli, G.; Zimmerli, L.; Métraux, J.-P.; Mauch-Mani, B. β-Aminobutyric Acid-Induced Resistance in Plants. Eur. J. Plant Pathol. 2001, 107, 29–37. [Google Scholar] [CrossRef]
- Jisha, K.C.; Puthur, J.T. Seed Priming with BABA (β-Amino Butyric Acid): A Cost-Effective Method of Abiotic Stress Tolerance in Vigna radiata (L.) Wilczek. Protoplasma 2016, 253, 277–289. [Google Scholar] [CrossRef]
- Wargent, J.J.; Pickup, D.A.; Paul, N.D.; Roberts, M.R. Reduction of Photosynthetic Sensitivity in Response to Abiotic Stress in Tomato Is Mediated by a New Generation Plant Activator. BMC Plant Biol. 2013, 13, 108. [Google Scholar] [CrossRef]
- Rhaman, M.S.; Imran, S.; Rauf, F.; Khatun, M.; Baskin, C.C.; Murata, Y.; Hasanuzzaman, M. Seed Priming with Phytohormones: An Effective Approach for the Mitigation of Abiotic Stress. Plants 2021, 10, 37. [Google Scholar] [CrossRef]
- Lei, C.; Bagavathiannan, M.; Wang, H.; Sharpe, S.M.; Meng, W.; Yu, J. Osmopriming with Polyethylene Glycol (PEG) for Abiotic Stress Tolerance in Germinating Crop Seeds: A Review. Agronomy 2021, 11, 2194. [Google Scholar] [CrossRef]
- El Moukhtari, A.; Cabassa-Hourton, C.; Farissi, M.; Savouré, A. How Does Proline Treatment Promote Salt Stress Tolerance During Crop Plant Development? Front. Plant Sci. 2020, 11, 1127. [Google Scholar] [CrossRef]
- Wang, R.; Li, C.; Zeng, L.; Liu, L.; Xi, J.; Li, J. Polyethylene Glycol Priming Enhances the Seed Germination and Seedling Growth of Scutellaria Baicalensis Georgi under Salt Stress. Plants 2024, 13, 565. [Google Scholar] [CrossRef]
- Nowicki, M.; Nowakowska, M.; Nowak, K.; Szczechura, W.; Kaminski, P. Seed Priming and Abiotic Stress Tolerance in Carrot: Unraveling the Mechanisms of Improved Germination. PLoS ONE 2025, 20, e0318753. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, E.; Yao, M.; Xue, D.; Zhao, N.; Zhou, Y.; Li, B.; Wang, K.; Miao, Y.; Gu, C.; et al. PEG-6000 Priming Improves Aged Soybean Seed Vigor via Carbon Metabolism, ROS Scavenging, Hormone Signaling, and Lignin Synthesis Regulation. Agronomy 2023, 13, 3021. [Google Scholar] [CrossRef]
- Almakas, A.; Elrys, A.S.; Desoky, E.-S.M.; Al-Shuraym, L.A.; Alhag, S.K.; Alshaharni, M.O.; Alnadari, F.; NanNan, Z.; Farooq, Z.; El-Tarabily, K.A.; et al. Enhancing Soybean Germination and Vigor under Water Stress: The Efficacy of Bio-Priming with Sodium Carboxymethyl Cellulose and Gum Arabic. Front. Plant Sci. 2025, 15, 1475148. [Google Scholar] [CrossRef]
- Hamidian, M.; Kazemeini, S.A.; Movahhedi Dehnavi, M.; Ramezanian, A.; Mottaghi Jahromie, M.R.; Farsijani, P.; Iranshahi, R.; Mohebi, P.; Fereshteh Hekmat, M.; Hassani, M.; et al. Individual and Combined Exogenous Application of Melatonin and Methyl Jasmonate Confer Salinity Stress Tolerance in Tomato by Enhancing Antioxidants Defense System. Sci. Hortic. 2025, 342, 114040. [Google Scholar] [CrossRef]
- Khan, M.; Hussain, A.; Yun, B.-W.; Mun, B.-G. Melatonin: The Multifaceted Molecule in Plant Growth and Defense. Int. J. Mol. Sci. 2024, 25, 6799. [Google Scholar] [CrossRef]
- Samanta, S.; Roychoudhury, A. Crosstalk of Melatonin with Major Phytohormones and Growth Regulators in Mediating Abiotic Stress Tolerance in Plants. S. Afr. J. Bot. 2023, 163, 201–216. [Google Scholar] [CrossRef]
- Sairam, R.K.; Srivastava, G.C.; Agarwal, S.; Meena, R.C. Differences in Antioxidant Activity in Response to Salinity Stress in Tolerant and Susceptible Wheat Genotypes. Biol. Plant. 2005, 49, 85–91. [Google Scholar] [CrossRef]
- Zhu, Z.; Wei, G.; Li, J.; Qian, Q.; Yu, J. Silicon Alleviates Salt Stress and Increases Antioxidant Enzymes Activity in Leaves of Salt-Stressed Cucumber (Cucumis sativus L.). Plant Sci. 2004, 167, 527–533. [Google Scholar] [CrossRef]
- Gangur, V.; Gonipeta, B.; Kim, E.; Parvataneni, S. Mechanism of Walnut Induced Anaphylaxis-like Shock Reaction in Mice (175.1). Physiol. Plant. 1994, 92, 696–711. [Google Scholar] [CrossRef]
- Borsani, O.; Valpuesta, V.; Botella, M.A. Developing Salt Tolerant Plants in a New Century: A Molecular Biology Approach. Plant Cell. Tissue Organ Cult. 2003, 73, 101–115. [Google Scholar] [CrossRef]
- Mittova, V.; Guy, M.; Tal, M.; Volokita, M. Salinity Up-Regulates the Antioxidative System in Root Mitochondria and Peroxisomes of the Wild Salt-Tolerant Tomato Species Lycopersicon Pennellii. J. Exp. Bot. 2004, 55, 1105–1113. [Google Scholar] [CrossRef]
- Roșca, M.; Mihalache, G.; Stoleru, V. Tomato Responses to Salinity Stress: From Morphological Traits to Genetic Changes. Front. Plant Sci. 2023, 14, 1118383. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Takahashi, Y.; Berberich, T.; Imai, A.; Miyazaki, A.; Takahashi, T.; Michael, A.; Kusano, T. The Polyamine Spermine Protects against High Salt Stress in Arabidopsis Thaliana. FEBS Lett. 2006, 580, 6783–6788. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Wang, X.-S.; Guo, H.-D.; Bai, S.-Y.; Khan, A.; Wang, X.-M.; Gao, Y.-M.; Li, J.-S. Tomato Salt Tolerance Mechanisms and Their Potential Applications for Fighting Salinity: A Review. Front. Plant Sci. 2022, 13, 949541. [Google Scholar] [CrossRef]
- Parvin, K.; Nahar, K.; Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Mohsin, S.M.; Fujita, M. Exogenous Vanillic Acid Enhances Salt Tolerance of Tomato: Insight into Plant Antioxidant Defense and Glyoxalase Systems. Plant Physiol. Biochem. 2020, 150, 109–120. [Google Scholar] [CrossRef]
- Miransari, M. Use of Microbes for the Alleviation of Soil Stresses; Springer: New York, NY, USA, 2014; Volume 1, ISBN 9781461494669. [Google Scholar]
- Raziq, A.; Wang, Y.; Mohi Ud Din, A.; Sun, J.; Shu, S.; Guo, S. A Comprehensive Evaluation of Salt Tolerance in Tomato (Var. Ailsa Craig): Responses of Physiological and Transcriptional Changes in RBOH’s and ABA Biosynthesis and Signalling Genes. Int. J. Mol. Sci. 2022, 23, 1603. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.; Olorunwa, O.J.; Horgan, T.E.; Wilson, J.; Barickman, T.C.; Li, T.; Bheemanahalli, R. Seed Priming Attenuates the Impact of Salt Stress and Enhances Lettuce Yields. J. Agric. Food Res. 2024, 15, 100947. [Google Scholar] [CrossRef]
- Ahmad, P.; Abdel Latef, A.A.; Abd-Allah, E.F.; Hashem, A.; Sarwat, M.; Anjum, N.A.; Gucel, S. Calcium and Potassium Supplementation Enhanced Growth, Osmolyte Secondary Metabolite Production, and Enzymatic Antioxidant Machinery in Cadmium-Exposed Chickpea (Cicer arietinum L.). Front. Plant Sci. 2016, 7, 513. [Google Scholar] [CrossRef]
- Carvalho, L.C.; Vidigal, P.; Amâncio, S. Oxidative Stress Homeostasis in Grapevine (Vitis vinifera L.). Front. Environ. Sci. 2015, 3, 20. [Google Scholar] [CrossRef]
- Aydın, A. The Growth, Leaf Antioxidant Enzymes and Amino Acid Content of Tomato as Affected by Grafting on Wild Tomato Rootstocks 1 (S. pimpinellifolium and S. habrochaites) Under Salt Stress. Sci. Hortic. 2024, 325, 112679. [Google Scholar] [CrossRef]
- Pérez-Labrada, F.; López-Vargas, E.R.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Benavides-Mendoza, A.; Juárez-Maldonado, A. Responses of Tomato Plants under Saline Stress To. Plants 2019, 8, 151. [Google Scholar] [CrossRef]
- Borromeo, I.; Domenici, F.; Del Gallo, M.; Forni, C. Role of Polyamines in the Response to Salt Stress of Tomato. Plants 2023, 12, 1855. [Google Scholar] [CrossRef]
- Li, S.; Cui, L.; Zhang, Y.; Wang, Y.; Mao, P. The Variation Tendency of Polyamines Forms and Components of Polyamine Metabolism in Zoysiagrass (Zoysia japonica Steud.) to Salt Stress with Exogenous Spermidine Application. Front. Physiol. 2017, 8, 208. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Shi, Y.; Zhang, Z.; Zou, Z.; Zhang, H.; Zhao, J. Effect of Exogenous Spermidine on Polyamine Content and Metabolism in Tomato Exposed to Salinity-Alkalinity Mixed Stress. Plant Physiol. Biochem. 2012, 57, 200–209. [Google Scholar] [CrossRef]
- Jahan, M.S.; Shu, S.; Wang, Y.; Chen, Z.; He, M.; Tao, M.; Sun, J.; Guo, S. Melatonin Alleviates Heat-Induced Damage of Tomato Seedlings by Balancing Redox Homeostasis and Modulating Polyamine and Nitric Oxide Biosynthesis. BMC Plant Biol. 2019, 19, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Marco, F.; Alcázar, R.; Tiburcio, A.F.; Carrasco, P. Interactions between Polyamines and Abiotic Stress Pathway Responses Unraveled by Transcriptome Analysis of Polyamine Overproducers. Omi. A J. Integr. Biol. 2011, 15, 775–781. [Google Scholar] [CrossRef]
- Raza, M.A.; Saeed, A.; Munir, H.; Ziaf, K.; Shakeel, A.; Saeed, N.; Munawar, A.; Rehman, F. Screening of Tomato Genotypes for Salinity Tolerance Based on Early Growth Attributes and Leaf Inorganic Osmolytes. Arch. Agron. Soil Sci. 2017, 63, 501–512. [Google Scholar] [CrossRef]
- Khalifa, N.S. Protein Expression after NaCl Treatment in Two Tomato Cultivars Differing in Salt Tolerance. Acta Biol. Cracoviensia Ser. Bot. 2012, 54, 79–86. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of Plant Responses to Salt Stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef]
- Michel, B.E.; Kaufmann, M.R. The Osmotic Potential of Polyethylene Glycol 6000. Plant Physiol. 1973, 51, 914–916. [Google Scholar] [CrossRef]
- Habibi, N.; Sediqui, N.; Terada, N.; Sanada, A.; Koshio, K. Effects of Salinity on Growth, Physiological and Biochemical Responses of Tomato. J. ISSAAS 2021, 27, 14–28. [Google Scholar]
- Han, Y.; Jiang, J.; Liu, H.; Ma, Q.; Xu, W.; Xu, Y.; Xu, Z.; Chong, K. Overexpression of OsSIN, Encoding a Novel Small Protein, Causes Short Internodes in Oryza Sativa. Plant Sci. 2005, 169, 487–495. [Google Scholar] [CrossRef]
- Gutteridge, J.M.C. Superoxide Dismutases. Int. J. Radiat. Biol. 1983, 44, 316–317. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.L.; Reid, D.M. Leaf Senescence and Lipid Peroxidation: Effects of Some Phytohormones, and Scavengers of Free Radicals and Singlet Oxygen. Physiol. Plant. 1982, 56, 453–457. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen Peroxide Is Scavenged by Ascorbate-Specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Griffith, O.W.; Meister, A. Origin and Turnover of Mitochondrial Glutathione. Proc. Natl. Acad. Sci. USA 1985, 82, 4668–4672. [Google Scholar] [CrossRef]
- Kranner, I.; Birtić, S.; Anderson, K.M.; Pritchard, H.W. Glutathione Half-Cell Reduction Potential: A Universal Stress Marker and Modulator of Programmed Cell Death? Free Radic. Biol. Med. 2006, 40, 2155–2165. [Google Scholar] [CrossRef]
- Logan, B.A.; Demmig-Adams, B.; Adams, W.W.; Grace, S.C. Antioxidants and Xanthophyll Cycle-Dependent Energy Dissipation in Cucurbita Pepo L. and Vinca Major L. Acclimated to Four Growth PPFDs in the Field. J. Exp. Bot. 1998, 49, 1869–1879. [Google Scholar] [CrossRef]
- Walker-Simmons, M. ABA Levels and Sensitivity in Developing Wheat Embryos of Sprouting Resistant and Susceptible Cultivars. Plant Physiol. 1987, 84, 61–66. [Google Scholar] [CrossRef]
- Weiler, E.W. Radioimmunoassays for the Differential and Direct Analysis of Free and Conjugated Abscisic Acid in Plant Extracts. Planta 1980, 148, 262–272. [Google Scholar] [CrossRef]
- Norman, S.M.; Poling, S.M.; Maier, V.P. An Indirect Enzyme-Linked Immunosorbent Assay for (+)-Abscisic Acid in Citrus, Ricinus, and Xanthium Leaves. J. Agric. Food Chem. 1988, 36, 225–231. [Google Scholar] [CrossRef]
- Gharsallah, C.; Fakhfakh, H.; Grubb, D.; Gorsane, F. Effect of Salt Stress on Ion Concentration, Proline Content, Antioxidant Enzyme Activities and Gene Expression in Tomato Cultivars. AoB Plants 2016, 8, plw055. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, Y.; Zhao, X.; Jin, X.; Hou, L.; Shi, Y.; Ahammed, G.J. Silicon Compensates Phosphorus Deficit-Induced Growth Inhibition by Improving Photosynthetic Capacity, Antioxidant Potential, and Nutrient Homeostasis in Tomato. Agronomy 2019, 9, 733. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, M.J.; Forney, F.C.P.K.R. Improving the Thiobarbituric Acid-Reactive-Substances Assay for Estimating Lipid Peroxidation in Plant Tissues Containing Anthocyanin and Other Interfering Compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Taulavuori, E.; Hellströ, E.-K.; Taulavuori, K.; Laine, K. Comparison of Two Methods Used to Analyse Lipid Peroxidation from Vaccinium Myrtillus (L.) during Snow Removal, Reacclimation and Cold Acclimation. J. Experimetnal Bot. 2001, 52, 2375–2380. [Google Scholar] [CrossRef] [PubMed]
- Oho, K.; Habibi, N.; Marie, T.; Silva, B.; Terada, N.; Sanada, A.; Shinohara, T.; Gemma, H.; Koshio, K. Elucidation of physicochemical changes in fruit development of “sabara” jaboticaba (Plinia cauliflora (Mart.) Kausel). J. ISSAAS 2022, 28, 34–50. [Google Scholar]
- Morgan, J.A. Interaction of Water Supply and N in Wheat. Plant Physiol. 1984, 76, 112–117. [Google Scholar] [CrossRef]



| Treatment | ABAL (µg g−1 Protein) | ABAR (µg g−1 Protein) | MDAL (nmol g−1 DW) | ProlineL (µmol g−1 FW) | ELL (%) |
|---|---|---|---|---|---|
| S0P0 | 62.34 ± 4.33 d | 68.39 ± 3.76 cd | 3.69 ± 0.46 e | 2.89 ± 0.16 ef | 29.95 ± 3.16 f |
| S0P1 | 79.77 ± 7.71 a | 84.16 ± 4.92 a | 2.27 ± 0.14 f | 2.34 ± 0.11 f | 28.89 ± 2.75 f |
| S1P0 | 51.73 ± 2.09 e | 59.93 ± 2.18 e | 6.77 ± 0.64 d | 4.58 ± 0.29 de | 38.77 ± 4.32 c |
| S1P1 | 69.16 ± 6.78 b | 75.70 ± 3.75 b | 4.35 ± 0.37 e | 2.45 ± 0.13 f | 35.12 ± 4.72 de |
| S2P0 | 45.95 ± 3.15 f | 49.74 ± 2.80 f | 9.15 ± 1.21 c | 5.13 ± 0.41 bc | 39.98 ± 5.12 c |
| S2P1 | 63.39 ± 4.22 cd | 66.51 ± 2.64 d | 7.18 ± 0.95 d | 4.01 ± 0.12 f | 35.68 ± 4.48 de |
| S3P0 | 34.26 ± 2.11 h | 35.86 ± 1.66 g | 13.66 ± 1.76 b | 6.12 ± 0.25 b | 43.15 ± 7.43 b |
| S3P1 | 51.67 ± 3.96 e | 52.30 ± 4.87 f | 9.42 ± 1.32 c | 3.78 ± 0.13 cd | 38.25 ± 4.86 cd |
| S4P0 | 21.36 ± 1.60 i | 20.59 ± 1.33 h | 21.05 ± 3.14 a | 8.41 ± 0.72 a | 47.76 ± 6.74 a |
| S4P1 | 38.8 ± 3.75 gh | 36.70 ± 3.90 g | 15.77 ± 2.28 b | 5.46 ± 0.32 de | 43.49 ± 5.23 b |
| p value | |||||
| S | *** | *** | *** | *** | *** |
| P | *** | *** | *** | *** | * |
| S × P | *** | *** | *** | *** | ** |
| Treatment | DAO (U mg−1 Protein) | PAO (U mg−1 Protein) | ADC (U mg−1 Protein) |
|---|---|---|---|
| S0P0 | 92.01 ± 5.45 b | 136.66 ± 6.34 b | 125.21 ± 7.61 b |
| S0P1 | 105.90 ± 6.86 a | 150.57 ± 5.22 a | 139.14 ± 4.98 a |
| S1P0 | 73.54 ± 4.32 de | 116.33 ± 4.76 | 105.78 ± 2.34 de |
| S1P1 | 85.48 ± 3.21 c | 130.23 ± 5.12 c | 119.16 ± 4.62 c |
| S2P0 | 55.33 ± 3.18 g | 96.73 ± 3.25 of | 89.67 ± 2.49 f |
| S2P1 | 69.24 ± 2.55 e | 109.90 ± 4.11 d | 103.85 ± 3.85 e |
| S3P0 | 45.01 ± 2.63 h | 77.44 ± 2.65 g | 70.02 ± 1.76 h |
| S3P1 | 58.92 ± 2.11 fg | 91.20 ± 3.78 f | 83.90 ± 1.99 g |
| S4P0 | 26.18 ± 1.05 j | 55.14 ± 1.23 i | 44.25 ± 1.15 j |
| S4P1 | 40.24 ± 1.48 i | 69.72 ± 1.72 h | 60.23 ± 1.77 i |
| p value | |||
| S | *** | ** | ** |
| P | ** | * | * |
| S × P | ** | * | * |
| Treatment | RA (µg g−1·FW) | MSI (%) | LP (mg g−1·FW) | RP (mg g−1·FW) | H2O2 (µmol g−1·FW) | O2•– (µmol g−1·FW) | WP (Mpa) | OP (Mpa) | TP (Mpa) | RWC (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| S0P0 | 26.23 c | 69.20 c | 11.07 c | 1.22 cd | 5.31 h | 3.17 e | −1.93 a | −0.52 ab | 0.81 de | 77.27 ab |
| S0P1 | 37.78 a | 81.17 a | 17.65 a | 2.79 a | 3.25 i | 0.99 f | −1.08 a | −0.26 a | 0.42 e | 84.66 a |
| S1P0 | 23.50 d | 63.80 d | 7.15 e | 0.83 e | 10.82 e | 7.22 d | −3.67 bc | −0.87 bcd | 1.22 bc | 65.99 cd |
| S1P1 | 36.16 a | 76.57 b | 13.41 b | 2.52 b | 7.12 g | 3.81 e | −1.61 a | −0.71 ab | 0.81 cde | 74.41 bc |
| S2P0 | 21.36 e | 44.80 f | 3.22 h | 0.54 f | 16.33 d | 14.85 c | −3.46 bc | −1.07 cde | 1.27 ab | 58.67 def |
| S2P1 | 33.74 b | 56.60 e | 9.02 d | 2.18 bc | 9.04 f | 9.48 e | −2.22 ab | −0.75 bc | 0.84 cde | 68.19 bcd |
| S3P0 | 15.28 f | 26.83 i | 2.29 i | 0.31 g | 25.47 b | 17.33 b | −4.84 de | −1.35 of | 1.44 ab | 51.48 ef |
| S3P1 | 20.15 e | 35.54 g | 4.98 g | 1.13 d | 16.33 d | 12.89 d | −4.21 cd | −1.02 cde | 1.10 bcd | 60.72 de |
| S4P0 | 11.69 g | 15.07 j | 1.21 j | 0.15 h | 34.61 a | 20.61 a | −5.86 e | −1.48 ef | 1.68 a | 38.53 g |
| S4P1 | 15.53 f | 28.12 h | 6.74 f | 1.37 c | 22.63 c | 13.89 cd | −3.62 cd | −1.22 f | 1.38 ab | 50.67 f |
| p value | ||||||||||
| S | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| P | *** | *** | *** | *** | *** | *** | *** | *** | ** | ** |
| S × P | *** | *** | ** | ** | *** | * | * | * | * | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habibi, N.; Aryan, S.; Sediqui, N.; Terada, N.; Sanada, A.; Kamata, A.; Koshio, K. Enhancing Salt Tolerance in Tomato Plants Through PEG6000 Seed Priming: Inducing Antioxidant Activity and Mitigating Oxidative Stress. Plants 2025, 14, 1296. https://doi.org/10.3390/plants14091296
Habibi N, Aryan S, Sediqui N, Terada N, Sanada A, Kamata A, Koshio K. Enhancing Salt Tolerance in Tomato Plants Through PEG6000 Seed Priming: Inducing Antioxidant Activity and Mitigating Oxidative Stress. Plants. 2025; 14(9):1296. https://doi.org/10.3390/plants14091296
Chicago/Turabian StyleHabibi, Nasratullah, Shafiqullah Aryan, Naveedullah Sediqui, Naoki Terada, Atsushi Sanada, Atsushi Kamata, and Kaihei Koshio. 2025. "Enhancing Salt Tolerance in Tomato Plants Through PEG6000 Seed Priming: Inducing Antioxidant Activity and Mitigating Oxidative Stress" Plants 14, no. 9: 1296. https://doi.org/10.3390/plants14091296
APA StyleHabibi, N., Aryan, S., Sediqui, N., Terada, N., Sanada, A., Kamata, A., & Koshio, K. (2025). Enhancing Salt Tolerance in Tomato Plants Through PEG6000 Seed Priming: Inducing Antioxidant Activity and Mitigating Oxidative Stress. Plants, 14(9), 1296. https://doi.org/10.3390/plants14091296








