Photosynthetic Characterization of Oil Palm (Elaeis guineensis Jacq.) Seedlings During Late In Vitro Development and Acclimatization
Abstract
1. Introduction
2. Results
2.1. Gas Exchange
2.2. Light and CO2 Response Curve Analyses
2.3. Chlorophyll Fluorescence
2.4. Comparison Between In Vitro- and Seed-Derived Seedlings in the Nursery Stage
3. Discussion
4. Materials and Methods
4.1. Location
4.2. Plant Material
4.3. Gas Exchange Measurements
4.4. Light and CO2 Curves
- Amax is the maximum photosynthetic rate;
- K is the saturation constant for light (equal to ½ PPFD);
- Rd is the dark respiration rate.
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nugroho, Y.A.; Sumertajaya, I.M.; Wiendi, N.M.A.; Toruan-Mathius, N. Estimation of genetic parameters for in vitro culture traits and selection best progenies for tenera oil palm tissue culture. Energy Procedia 2014, 47, 316–322. [Google Scholar] [CrossRef]
- Corrêa, T.R.; Motoike, S.Y.; Coser, S.M.; da Silveira, G.; de Resende, M.D.V.; Chia, G.S. Estimation of genetic parameters for in vitro oil palm characteristics (Elaeis guineensis Jacq.) and selection of genotypes for cloning capacity and oil yield. Ind. Crops Prod. 2015, 77, 1033–1038. [Google Scholar] [CrossRef]
- Soh, A.C.; Wong, G.; Tan, C.C.; Chew, P.S.; Chong, S.; Ho, Y.W.; Wong, C.K.; Choo, C.N. Commercial-scale propagation and planting of elite oil palm clones: Research and development towards realization. J. Oil Palm Res. 2011, 23, 936–952. [Google Scholar]
- Ruffoni, B.; Savona, M. Physiological and biochemical analysis of growth abnormalities associated with plant tissue culture. Hortic. Environ. Biotechnol. 2013, 54, 191–205. [Google Scholar] [CrossRef]
- Chandra, S.; Bandopadhyay, R. Acclimatization of tissue cultured plantlets: From laboratory to land. Biotechnol. Lett. 2010, 32, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Kiferle, C.; Lucchesini, M.; Maggini, R.; Pardossi, A.; Mensuali-Sodi, A. In vitro culture of sweet basil: Gas exchanges, growth, and rosmarinic acid production. Biol. Plant. 2014, 58, 601–610. [Google Scholar] [CrossRef]
- Kozai, T. Photoautotrophic micropropagation—Environmental control for promoting photosynthesis. Propag. Ornam. Plants 2010, 10, 188–204. [Google Scholar]
- Martins, J.P.R.; Verdoodt, V.; Pasqual, M.; De Proft, M. Impacts of photoautotrophic and photomixotrophic conditions on in vitro propagated Billbergia zebrina (Bromeliaceae). Plant Cell Tiss. Org. Cult. 2015, 123, 121–132. [Google Scholar] [CrossRef]
- Chaari-Rkhis, A.; Maalej, M.; Chelli-Chaabouni, A.; Fki, L.; Drira, N. Photosynthesis parameters during acclimatization of in vitro-grown olive plantlets. Photosynthetica 2015, 53, 613–616. [Google Scholar] [CrossRef]
- Seon, J.H.; Cui, Y.Y.; Kozai, T.; Paek, K.Y. Influence of in vitro growth conditions on photosynthetic competence and survival rate of Rehmannia glutinosa plantlets during acclimatization period. Plant Cell Tiss. Org. Cult. 2000, 61, 135–142. [Google Scholar] [CrossRef]
- Carvalho, L.C.; Leonor Osório, M.; Manuela Chaves, M.; Amâncio, S. Chlorophyll fluorescence as an indicator of photosynthetic functioning of in vitro grapevine and chestnut plantlets under ex vitro acclimatization. Plant Cell Tiss. Org. Cult. 2001, 67, 271–280. [Google Scholar] [CrossRef]
- Asmar, S.A.; Castro, E.M.; Pasqual, M.; Pereira, F.J.; Soares, J.D.R. Changes in leaf anatomy and photosynthesis of micropropagated banana plantlets under different silicon sources. Sci. Hort. 2013, 161, 328–332. [Google Scholar] [CrossRef]
- Bag, N.; Chandra, S.; Palni, L.M.S.; Nandi, S.K. Micropropagation of Dev-ringal [Thamnocalamus spathiflorus (Trin.) Munro]—A temperate bamboo, and comparison between in vitro propagated plants and seedlings. Plant Sci. 2000, 156, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Pospísilová, J.; Synková, H.; Haisel, D.; Semorádová, S. Acclimation of Plantlets to Ex Vitro Conditions: Effects of Air Humidity, Irradiance, CO2 Concentration and Abscisic Acid (a Review). Acta Hortic. 2007, 748, 29–38. [Google Scholar] [CrossRef]
- Hazarika, B.N. Acclimatization of tissue-cultures plants. Curr. Sci. 2003, 85, 1704–1712. [Google Scholar]
- Weckx, S.; Inzé, D.; Maene, L. Tissue culture of oil palm: Finding the balance between mass propagation and somaclonal variation. Front. Plant Sci. 2019, 10, 722. [Google Scholar] [CrossRef]
- Rival, A.; Beulé, T.; Lavergne, D.; Nato, A.; Havaux, M.; Puard, M. Development of photosynthetic characteristics in oil palm during in vitro micropropagation. J. Plant Physiol. 1997, 150, 520–527. [Google Scholar] [CrossRef]
- Sáez, P.L.; Bravo, L.A.; Sáez, K.L.; Sánchez-Olate, M.; Latsague, M.I.; Ríos, D.G. Photosynthetic and leaf anatomical characteristics of Castanea sativa: A comparison between in vitro and nursery plants. Biol. Plant. 2012, 56, 15–24. [Google Scholar] [CrossRef]
- Zanderluce, G.L.; Bezerra, K.M.G.; Scherwinski-pereira, J.E. Adaptability and leaf anatomical features in oil palm seedlings produced by embryo rescue and pre-germinated seeds. Braz. J. Plant Physiol. 2010, 22, 209–215. [Google Scholar] [CrossRef]
- Gadea, P.; Chinchilla, C.; Rodríguez, W. Oil palm compact clones: A preliminary study on some physiological and anatomical changes during acclimatization of ramets. ASD Oil Palm Pap. 2012, 37, 1–9. [Google Scholar]
- Tezara, W.; Martínez, D.; Rengifo, E.; Herrera, A. Photosynthetic responses of the tropical spiny shrub Lycium nodosum (Solanaceae) to drought, soil salinity and saline spray. Ann. Bot. 2003, 92, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Sima, B.D.; Desjardins, Y.; Van Quy, L. Sucrose enhances phosphoenolpyruvate carboxylase activity of in vitro Solanum tuberosum L. under non-limiting nitrogen conditions. In Vitr. Cell. Dev. Biol.-Plant 2001, 37, 480–489. [Google Scholar] [CrossRef]
- Ayub, R.A.; Dos Santos, J.N.; Zanlorensi, L.A.; Da Silva, D.M.; De Carvalho, T.C.; Grimaldi, F. Sucrose concentration and volume of liquid medium on the in vitro growth and development of blackberry cv. Tupy in temporary immersion systems. Cienc. E Agrotecnologia 2019, 43, e007219. [Google Scholar] [CrossRef]
- Pinheiro, M.V.M.; Ríos-Ríos, A.M.; da Cruz, A.C.F.; Rocha, D.I.; Orbes, M.Y.; Saldanha, C.W.; Batista, D.S.; de Carvalho, A.C.P.P.; Otoni, W.C. CO2 enrichment alters morphophysiology and improves growth and acclimatization in Etlingera Elatior (Jack) R.M. Smith micropropagated plants. Rev. Bras. Botanica 2021, 44, 799–809. [Google Scholar] [CrossRef]
- Nowakowska, K.; Pińkowska, A.; Siedlecka, E.; Pacholczak, A. The effect of cytokinins on shoot proliferation, biochemical changes and genetic stability of Rhododendron ‘Kazimierz Odnowiciel’ in the in vitro cultures. Plant Cell Tiss. Org. Cult. 2022, 149, 675–684. [Google Scholar] [CrossRef]
- Hdider, C.; Desjardins, Y. Changes in ribulose-1,5-bisphosphate carboxylase/oxygenase and phosphoenolpyruvate carboxylase activities and CO2 fixation during the rooting of strawberry shoots in vitro. Can. J. Plant Sci. 1994, 74, 827–831. [Google Scholar] [CrossRef]
- Estrada-Luna, A.A.; Davies, J.T.; Egilla, J.N. Physiological changes and growth of micropropagated chile ancho pepper plantlets during acclimatization and post-acclimatization. Plant Cell Tiss. Org. Cult. 2001, 66, 17–24. [Google Scholar] [CrossRef]
- Talavera, C.; Contreras, F.; Espadas, F.; Fuentes, G.; Santamaría, J.M. Cultivating in vitro coconut palms (Cocos nucifera) under glasshouse conditions with natural light, improves in vitro photosynthesis nursery survival and growth. Plant Cell Tiss. Org. Cult. 2005, 83, 287–292. [Google Scholar] [CrossRef]
- Aragón, C.E.; Escalona, M.; Capote, I.; Pina, D.; Cejas, I.; Rodriguez, R.; Jesus Cañal, M.; Sandoval, J.; Roels, S.; Debergh, P.; et al. Photosynthesis and carbon metabolism in plantain (Musa AAB) plantlets growing in temporary immersion bioreactors and during ex vitro acclimatization. In Vitr. Cell. Dev. Biol.-Plant 2005, 41, 550–554. [Google Scholar] [CrossRef]
- Yue, D.; Gosselin, A.; Desjardins, Y. Reexamination of the Photosynthetic Capacity of Invitro-Cultured Strawberry Plantlets. J. Am. Soc. Hort. Sci. 1993, 118, 419–424. [Google Scholar] [CrossRef]
- Pospíšilová, J.; Haisel, D.; Synková, H.; Baťková-Spoustová, P. Improvement of ex vitro transfer of tobacco plantlets by addition of abscisic acid to the last subculture. Biol. Plant. 2009, 53, 617–624. [Google Scholar] [CrossRef]
- Dias, G.M.d.G.; Soares, J.D.r.R.; Pasqual, M.; Silva, R.A.L.; Rodrigues, L.C.d.A.; Pereira, F.J.; de Castro, E.M. Photosynthesis and leaf Anatomy of Anthurium cv. Rubi plantlets cultured in vitro under different silicon (Si) concentrations. Aust. J. Crop Sci. 2014, 8, 1160–1167. [Google Scholar]
- Hazarika, B.N. Morpho-physiological disorders in in vitro culture of plants. Sci. Hortic. 2006, 108, 105–120. [Google Scholar] [CrossRef]
- Tisarum, R.; Samphumphung, T.; Theerawitaya, C.; Prommee, W.; Cha-um, S. In vitro photoautotrophic acclimatization, direct transplantation and ex vitro adaptation of rubber tree (Hevea brasiliensis). Plant Cell Tiss. Org. Cult. 2018, 133, 215–223. [Google Scholar] [CrossRef]
- Tezara, W.; Domínguez, T.S.T.; Loyaga, D.W.; Ortiz, R.N.; Chila, V.H.R.; Ortega, M.J.B. Photosynthetic activity of oil palm (Elaeis guineensis) and interspecific hybrid genotypes (Elaeis oleifera × Elaeis guineensis), and response of hybrids to water deficit. Sci. Hort. 2021, 287, 110263. [Google Scholar] [CrossRef]
- Cheah, S.S.; Teh, C.B.S. Parameterization of the Farquhar-von Caemmerer-Berry C3 photosynthesis model for oil palm. Photosynthetica 2020, 58, 769–779. [Google Scholar] [CrossRef]
- Alvarez, C.; Sáez, P.; Sáez, K.; Sánchez-Olate, M.; Ríos, D. Effects of light and ventilation on physiological parameters during in vitro acclimatization of Gevuina avellana mol. Plant Cell Tiss. Org. Cult. 2012, 110, 93–101. [Google Scholar] [CrossRef]
- Cha-um, S.; Ulziibat, B.; Kirdmanee, C. Effects of temperature and relative humidity during in vitro acclimatization, on physiological changes and growth characters of Phalaenopsis adapted to in vivo. Aust. J. Crop Sci. 2010, 4, 750–756. [Google Scholar]
- Grzelak, M.; Pacholczak, A.; Nowakowska, K. Challenges and insights in the acclimatization step of micropropagated woody plants. Plant Cell Tissue Organ Cult. 2024, 159, 72. [Google Scholar] [CrossRef]
- Lakho, M.A.; Jatoi, M.A.; Solangi, N.; Abul-Soad, A.A.; Qazi, M.A.; Abdi, G. Optimizing in vitro nutrient and ex vitro soil mediums-driven responses for multiplication, rooting, and acclimatization of pineapple. Sci. Rep. 2023, 13, 1275. [Google Scholar] [CrossRef]
- Guo, C.; Liu, L.; Sun, H.; Wang, N.; Zhang, K.; Zhang, Y.; Zhu, J.; Li, A.; Bai, Z.; Liu, X.; et al. Predicting Fv/Fm and evaluating cotton drought tolerance using hyperspectral and 1D-CNN. Front. Plant Sci. 2022, 13, 1007150. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Méndez, Y.D.; Romero, H.M. Fitting of photosynthetic response curves to photosynthetically active radiation in oil palm. Agron. Colomb. 2017, 35, 323–329. [Google Scholar] [CrossRef]
- Lu, X.; Sun, P.; Liu, R.; Wang, C.; Tong, L.; Tahir, M.M.; Ma, X.; Bao, J.; Zhang, D.; Wang, M.; et al. In vitro slow-growth conservation, acclimatization, and genetic stability of virus-free apple plants. Hortic. Adv. 2024, 2, 30. [Google Scholar] [CrossRef]
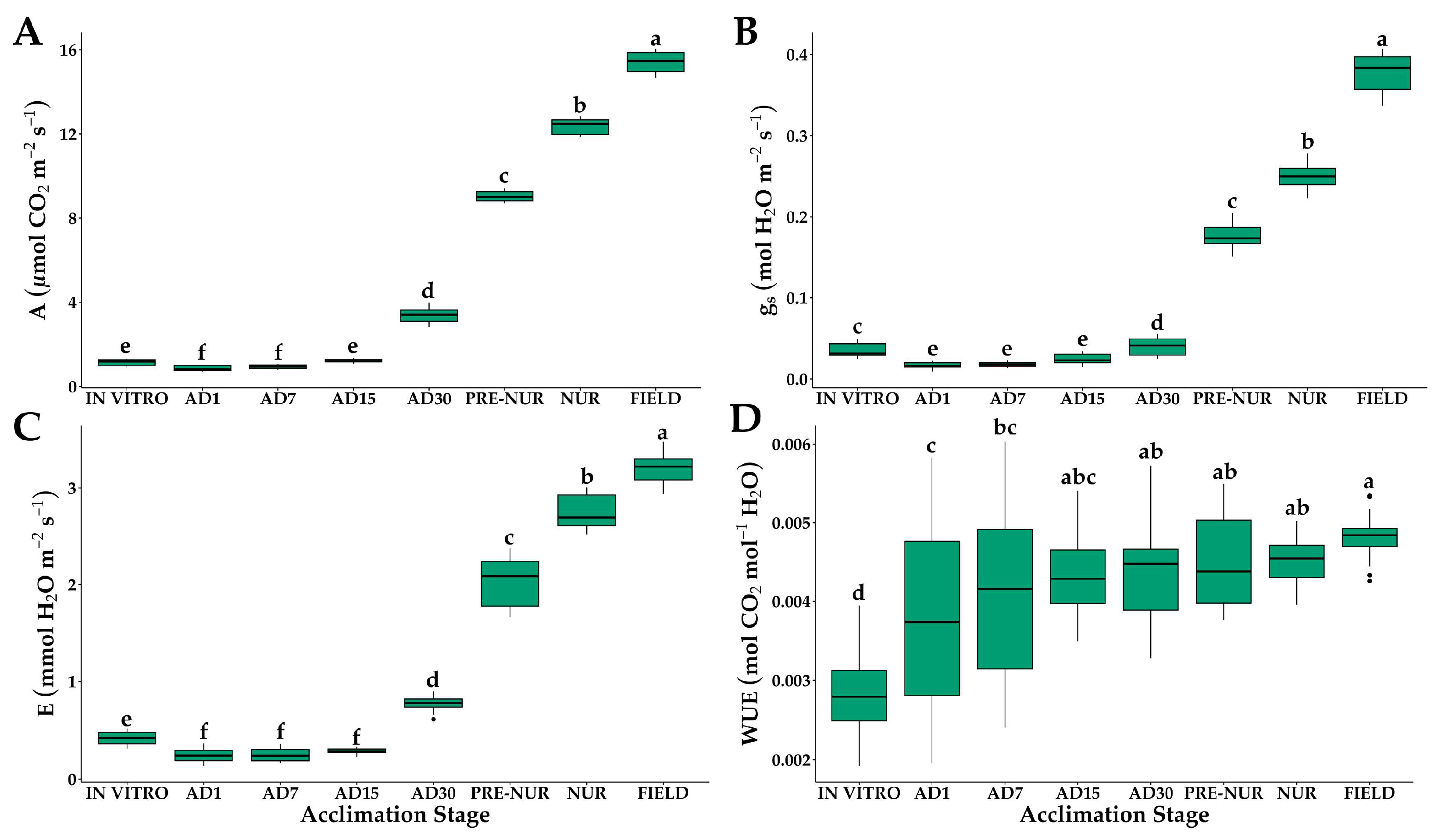
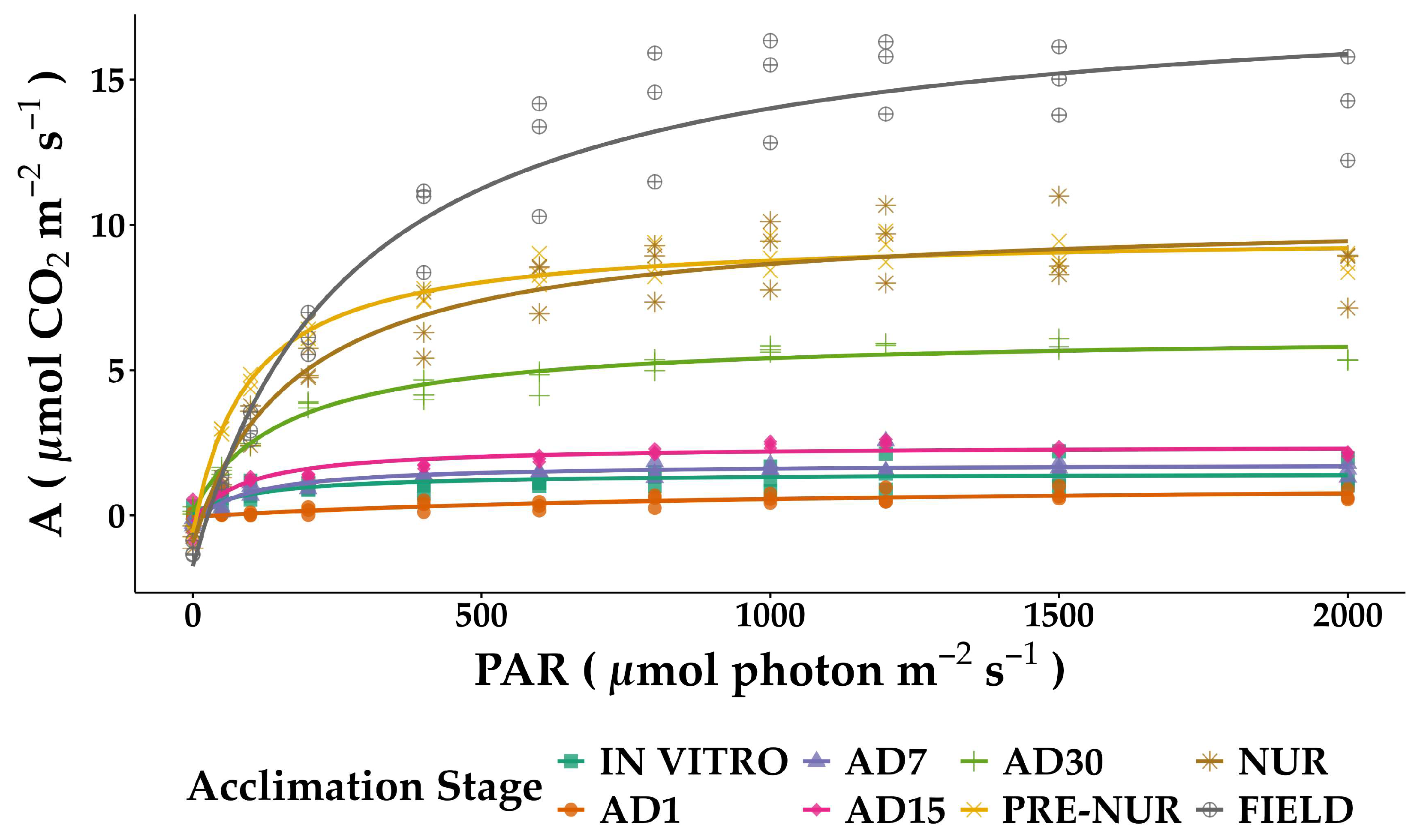


| Acclimation Stage | Maximum Photosynthesis (µmol CO2 m−² s−¹) | Saturation Constant (μmol photon m−2 s−1) | Dark Respiration Rate (μmol CO2 m−2 s−1) | Light Compensation Point (μmol photon m−2 s−1) | Quantum Yield of CO2 (mol CO2 mol−1 photon) |
|---|---|---|---|---|---|
| IN VITRO | 1.42 ± 0.12 | 104.18 ± 16.84 | −0.04 ± 0.11 | 2.64 | 0.0081 |
| AD1 | 1.17 ± 0.14 | 904.70 ± 148.28 | −0.05 ± 0.04 | 39.95 | 0.0009 |
| AD7 | 2.05 ± 0.12 | 93.17 ± 10.07 | −0.27 ±0.11 | 14.01 | 0.0118 |
| AD15 | 2.64 ± 0.22 | 86.93 ± 13.36 | −0.23 ± 0.21 | 8.28 | 0.0156 |
| AD30 | 6.11 ± 0.31 | 160.33 ± 3.24 | −0.15 ± 0.28 | 3.74 | 0.0229 |
| PRE-NURSERY | 10.25 ± 0.27 | 95.02 ± 4.67 | −0.59 ± 0.25 | 5.80 | 0.0517 |
| NURSERY | 11.30 ± 0.54 | 177.43 ± 17.19 | −0.94 ± 0.49 | 16.09 | 0.0399 |
| FIELD | 20.01 ± 0.98 | 269.70 ± 28.84 | −1.75 ± 0.79 | 25.86 | 0.0420 |
| Asat (CO2-Saturated Photosynthetic Rate) (μmol CO2 m−² s−¹) | CE (Carboxylation Efficiency) (mol CO2 m−² s−¹) | Γ (CO2 Compensation Point) (μmol mol−1) | Ls (Relative Stomatal Limitation) (%) | Lm (Mesophyll Limitation) (%) | Vcmax (Maximum Carboxylation Rate of RuBisCO) (μmol m−² s−¹) | Jmax (Maximum Electron Transport Rate) (μmol m−² s−¹) | TPU (Triose Phosphate Utilization Rate) (μmol m−² s−¹) | |
|---|---|---|---|---|---|---|---|---|
| IN VITRO | 8.4 ± 1.1 | 0.062 ± 0.021 | 251.9 ± 28.4 | 66.1 ± 4.7 | 71.8 ± 4.1 | 12.0 ± 1.8 | 35.3 ± 4.8 | 3.0 ± 0.3 |
| AD7 | 6.7 ± 1.0 | 0.026 ± 0.001 | 246.6 ± 23.5 | 66.4 ± 3.2 | 76.6 ± 3. 6 | 12.6 ± 1.7 | 29.0 ± 2.5 | 2.6 ± 0.3 |
| AD15 | 6.2 ± 0.3 | 0.030 ± 0.004 | 202.9 ± 16.6 | 55.4 ± 5.3 | 77.8 ± 1.0 | 12.8 ± 1.3 | 34.3 ± 1.5 | 2.5 ± 0.1 |
| AD30 | 10.4 ± 0.3 | 0.061 ± 0.005 | 152.6 ± 9.1 | 38.2 ± 2.8 | 62.4 ± 1.2 | 26.2 ± 2.1 | 53.7 ± 2.5 | 4.0 ± 0.1 |
| PRE-NURSERY | 18.7 ± 0.1 | 0.085 ± 0.009 | 97.7 ± 7.7 | 28.5 ± 2.1 | 32.1 ± 0.5 | 53.3± 4.9 | 87.5 ± 3.3 | 6.7 ± 0.1 |
| NURSERY | 21.1 ± 0.2 | 0.284 ± 0.007 | 153.2 ± 7.2 | 32.0 ± 1.9 | 24.1 ± 0.6 | 53.1 ± 4.5 | 105.8 ± 0.6 | 7.6± 0.1 |
| FIELD | 27.8 ± 0.3 | 0.154 ± 0.021 | 95.5 ± 8.8 | 26.6 ± 0.8 | 0.00 ± 1.1 | 91.0 ± 4.7 | 125.9 ± 1.8 | 9.8± 0.2 |
| Parameter | In Vitro Generated Seedlings | Seed Generated Seedlings |
|---|---|---|
| Photosynthesis (µmol CO2 m−2 s−¹) | 11.20 ± 0.61 b | 11.86 ± 0.72 a |
| Transpiration (mmol H2O m−2 s−¹) | 2.78 ± 0.39 b | 3.62 ± 0.50 a |
| Stomatal conductance (mol H2O m−2 s−¹) | 0.24 ± 0.02 b | 0.31 ± 0.04 a |
| Water Use Efficiency (mole CO2 mol−1 H2O) | 0.0041 ± 0.0006 a | 0.0033 ± 0.0005 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avila-Diazgranados, R.A.; Tezara, W.; Romero, H.M. Photosynthetic Characterization of Oil Palm (Elaeis guineensis Jacq.) Seedlings During Late In Vitro Development and Acclimatization. Plants 2025, 14, 1299. https://doi.org/10.3390/plants14091299
Avila-Diazgranados RA, Tezara W, Romero HM. Photosynthetic Characterization of Oil Palm (Elaeis guineensis Jacq.) Seedlings During Late In Vitro Development and Acclimatization. Plants. 2025; 14(9):1299. https://doi.org/10.3390/plants14091299
Chicago/Turabian StyleAvila-Diazgranados, Rodrigo Andrés, Wilmer Tezara, and Hernán Mauricio Romero. 2025. "Photosynthetic Characterization of Oil Palm (Elaeis guineensis Jacq.) Seedlings During Late In Vitro Development and Acclimatization" Plants 14, no. 9: 1299. https://doi.org/10.3390/plants14091299
APA StyleAvila-Diazgranados, R. A., Tezara, W., & Romero, H. M. (2025). Photosynthetic Characterization of Oil Palm (Elaeis guineensis Jacq.) Seedlings During Late In Vitro Development and Acclimatization. Plants, 14(9), 1299. https://doi.org/10.3390/plants14091299









