Plant Functional Traits Better Explain the Global Latitudinal Patterns of Leaf Insect Herbivory than Climatic Factors
Abstract
1. Introduction
2. Materials and Methods
2.1. Leaf Insect Herbivory Data
2.2. Climate and Soil Data
2.3. Plant Functional Traits Data
2.4. Statistical Analysis
3. Results
3.1. Global Latitudinal Patterns of Herbivory
3.2. Impact of Climate on the Global Latitudinal Patterns of Herbivory
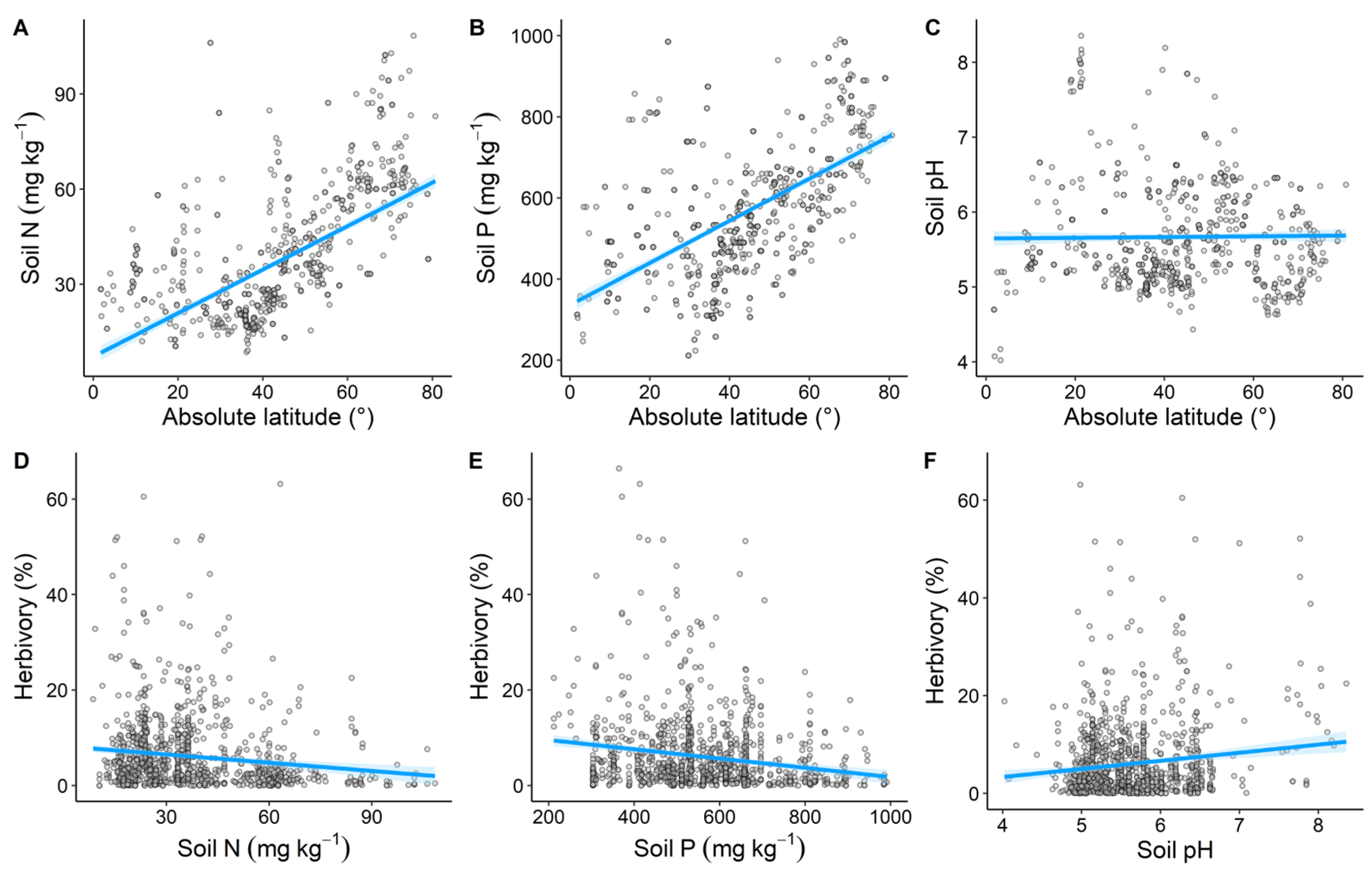
3.3. Impact of Soil Nutrients on the Global Latitudinal Patterns of Herbivory
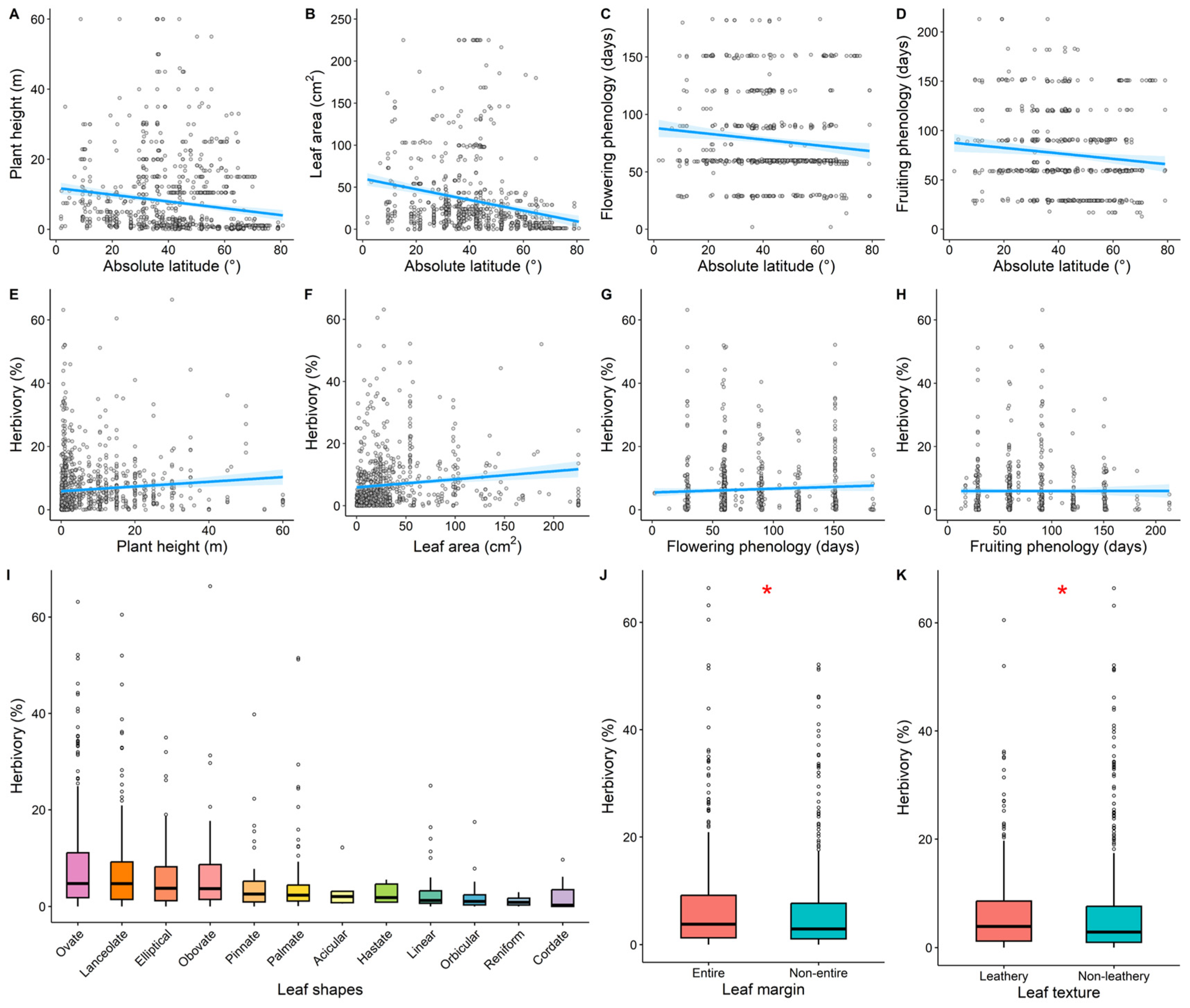
3.4. Impact of Plant Functional Traits on the Global Latitudinal Patterns of Herbivory
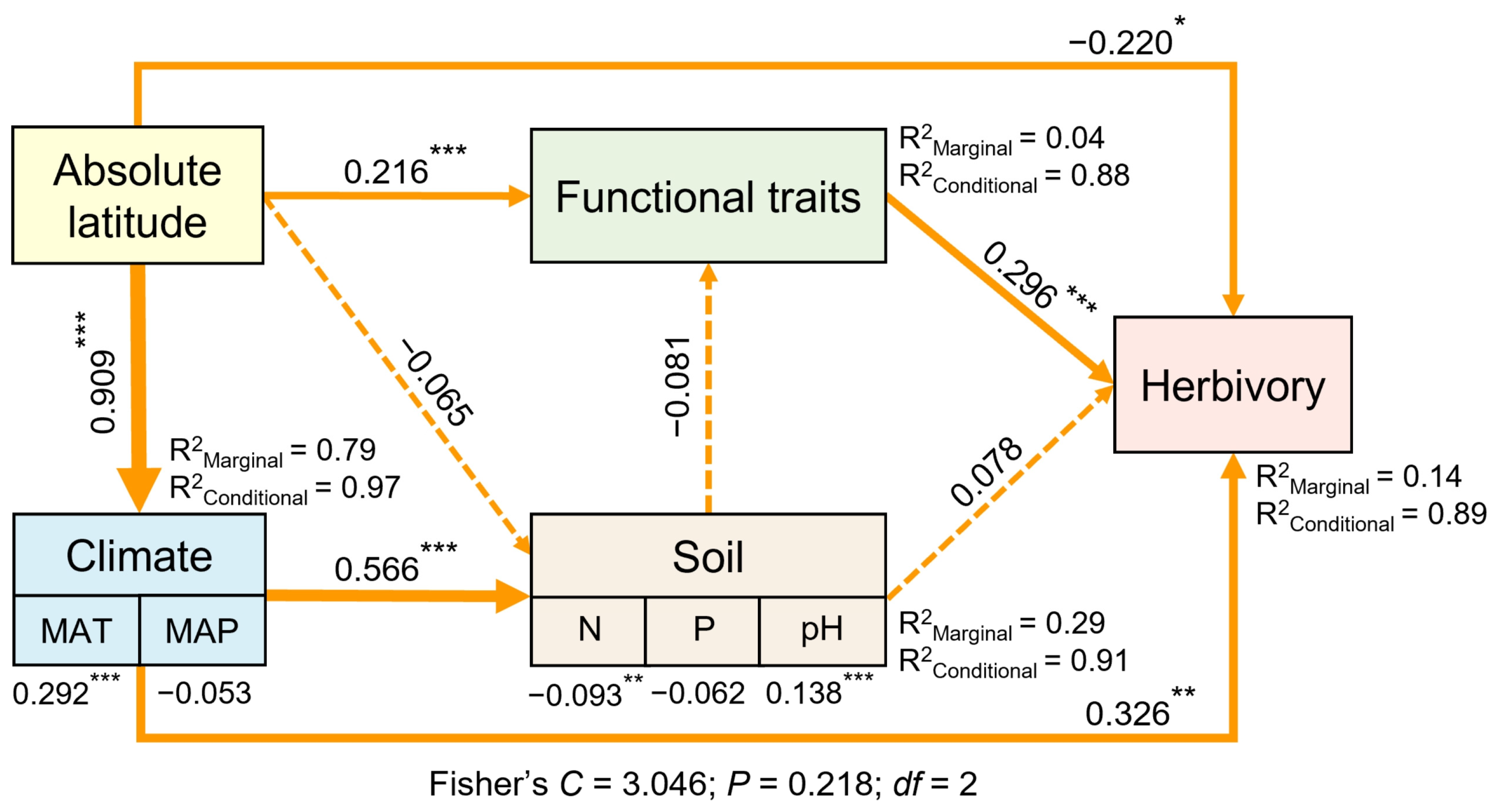
3.5. Relative Importance of Climate, Soil Nutrients, and Plant Functional Traits in Predicting Latitudinal Variation in Herbivory
4. Discussion
4.1. Global Latitudinal Patterns of Herbivory and Their Influencing Factors
4.2. Effects of Climatic Factors on Latitudinal Variation in Herbivory
4.3. Effects of Soil Nutrient Factors on Latitudinal Variation in Herbivory
4.4. Effects of Plant Functional Traits on Latitudinal Variation in Herbivory
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Borer, E.T.; Seabloom, E.W.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Lind, E.M.; Adler, P.B.; Alberti, J.; Anderson, T.M.; Bakker, J.D.; et al. Herbivores and Nutrients Control Grassland Plant Diversity via Light Limitation. Nature 2014, 508, 517–520. [Google Scholar] [CrossRef] [PubMed]
- De Long, J.R.; Heinen, R.; Hannula, S.E.; Jongen, R.; Steinauer, K.; Bezemer, T.M. Plant-Litter-Soil Feedbacks in Common Grass Species Are Slightly Negative and Only Marginally Modified by Litter Exposed to Insect Herbivory. Plant Soil 2023, 485, 227–244. [Google Scholar] [CrossRef]
- Tang, H.; Zhu, X.; Zhong, Y.; Li, Y.; Luo, W.; Liu, H.; Descombes, P.; Gange, A.C.; Chu, C. Global Latitudinal Patterns in Leaf Herbivory Are Related to Variation in Climate, Rather than Phytochemicals or Mycorrhizal Types. Natl. Sci. Rev. 2023, 10, nwad236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xu, G.-R.; Zhang, Y.-X.; Zhang, W.-F.; Cao, M. Canopy Height, Rather than Neighborhood Effects, Shapes Leaf Herbivory in a Tropical Rainforest. Ecology 2023, 104, e4028. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, M.V.; Stekolshchikov, A.V.; Söderman, G.; Labina, E.S.; Zverev, V.; Zvereva, E.L. Sap-Feeding Insects on Forest Trees along Latitudinal Gradients in Northern Europe: A Climate-Driven Patterns. Glob. Chang. Biol. 2015, 21, 106–116. [Google Scholar] [CrossRef]
- Anstett, D.N.; Nunes, K.A.; Baskett, C.; Kotanen, P.M. Sources of Controversy Surrounding Latitudinal Patterns in Herbivory and Defense. Trends Ecol. Evol. 2016, 31, 789–802. [Google Scholar] [CrossRef]
- Baskett, C.A.; Schemske, D.W. Latitudinal Patterns of Herbivore Pressure in a Temperate Herb Support the Biotic Interactions Hypothesis. Ecol. Lett. 2018, 21, 578–587. [Google Scholar] [CrossRef]
- Lynn, J.S.; Fridley, J.D.; Vandvik, V. More than What They Eat: Uncoupled Biophysical Constraints Underlie Geographic Patterns of Herbivory. Ecography 2023, 2023, e06114. [Google Scholar] [CrossRef]
- Coley, P.D.; Bryant, J.P.; Chapin, F.S. Resource Availability and Plant Antiherbivore Defense. Science 1985, 230, 895–899. [Google Scholar] [CrossRef]
- Kozlov, M.V.; Lanta, V.; Zverev, V.; Zvereva, E.L. Global Patterns in Background Losses of Woody Plant Foliage to Insects. Glob. Ecol. Biogeogr. 2015, 24, 1126–1135. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Ma, K. Latitudinal Variation in Herbivory: Hemispheric Asymmetries and the Role of Climatic Drivers. J. Ecol. 2016, 104, 1089–1095. [Google Scholar] [CrossRef]
- Zvereva, E.L.; Zverev, V.; Usoltsev, V.A.; Kozlov, M.V. Latitudinal Pattern in Community-Wide Herbivory Does Not Match the Pattern in Herbivory Averaged across Common Plant Species. J. Ecol. 2020, 108, 2511–2520. [Google Scholar] [CrossRef]
- Liu, X.; Lin, Z.; Hu, K.; Wang, X.; Zhang, P.; Xiao, Y.; Zhang, L.; Liu, M. Geographical Variation in Community-Wide Herbivory Matches Patterns of Intraspecific Variation Instead of Species Turnover. Glob. Ecol. Biogeogr. 2023, 32, 1140–1151. [Google Scholar] [CrossRef]
- Gao, J.; Fang, C.; Zhao, B. The Latitudinal Herbivory Hypothesis Revisited: To Be Part Is to Be Whole. Ecol. Evol. 2019, 9, 3681–3688. [Google Scholar] [CrossRef] [PubMed]
- Loughnan, D.; Williams, J.L. Climate and Leaf Traits, Not Latitude, Explain Variation in Plant–Herbivore Interactions across a Species’ Range. J. Ecol. 2019, 107, 913–922. [Google Scholar] [CrossRef]
- Moreira, X.; Abdala-Roberts, L.; Parra-Tabla, V.; Mooney, K.A. Latitudinal Variation in Herbivory: Influences of Climatic Drivers, Herbivore Identity and Natural Enemies. Oikos 2015, 124, 1444–1452. [Google Scholar] [CrossRef]
- Outhwaite, C.L.; McCann, P.; Newbold, T. Agriculture and Climate Change Are Reshaping Insect Biodiversity Worldwide. Nature 2022, 605, 97–102. [Google Scholar] [CrossRef]
- Kozlov, M.V.; Zverev, V.; Sandner, T.M.; van Nieukerken, E.J.; Zvereva, E.L. Poleward Increase in Feeding Efficiency of Leafminer Stigmella Lapponica (Lepidoptera: Nepticulidae) in a Latitudinal Gradient Crossing a Boreal Forest Zone. Insect Sci. 2023, 30, 857–866. [Google Scholar] [CrossRef]
- Faltýnek Fric, Z.; Rindoš, M.; Konvička, M. Phenology Responses of Temperate Butterflies to Latitude Depend on Ecological Traits. Ecol. Lett. 2020, 23, 172–180. [Google Scholar] [CrossRef]
- Li, T.; Holst, T.; Michelsen, A.; Rinnan, R. Amplification of Plant Volatile Defence against Insect Herbivory in a Warming Arctic Tundra. Nat. Plants 2019, 5, 568–574. [Google Scholar] [CrossRef]
- Joswig, J.S.; Wirth, C.; Schuman, M.C.; Kattge, J.; Reu, B.; Wright, I.J.; Sippel, S.D.; Rüger, N.; Richter, R.; Schaepman, M.E.; et al. Climatic and Soil Factors Explain the Two-Dimensional Spectrum of Global Plant Trait Variation. Nat. Ecol. Evol. 2022, 6, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Hamann, E.; Blevins, C.; Franks, S.J.; Jameel, M.I.; Anderson, J.T. Climate Change Alters Plant–Herbivore Interactions. New Phytol. 2021, 229, 1894–1910. [Google Scholar] [CrossRef] [PubMed]
- Saupe, E.E.; Myers, C.E.; Townsend Peterson, A.; Soberón, J.; Singarayer, J.; Valdes, P.; Qiao, H. Spatio-Temporal Climate Change Contributes to Latitudinal Diversity Gradients. Nat. Ecol. Evol. 2019, 3, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Kooyers, N.J.; Blackman, B.K.; Holeski, L.M. Optimal Defense Theory Explains Deviations from Latitudinal Herbivory Defense Hypothesis. Ecology 2017, 98, 1036–1048. [Google Scholar] [CrossRef]
- Baskett, C.A.; Schroeder, L.; Weber, M.G.; Schemske, D.W. Multiple Metrics of Latitudinal Patterns in Insect Pollination and Herbivory for a Tropical-Temperate Congener Pair. Ecol. Monogr. 2020, 90, e01397. [Google Scholar] [CrossRef]
- Valdés-Correcher, E.; Moreira, X.; Augusto, L.; Barbaro, L.; Bouget, C.; Bouriaud, O.; Branco, M.; Centenaro, G.; Csóka, G.; Damestoy, T.; et al. Search for Top-down and Bottom-up Drivers of Latitudinal Trends in Insect Herbivory in Oak Trees in Europe. Glob. Ecol. Biogeogr. 2021, 30, 651–665. [Google Scholar] [CrossRef]
- Salazar, D.; Marquis, R.J. Herbivore Pressure Increases toward the Equator. Proc. Natl. Acad. Sci. USA 2012, 109, 12616–12620. [Google Scholar] [CrossRef]
- Bale, J.S.; Masters, G.J.; Hodkinson, I.D.; Awmack, C.; Bezemer, T.M.; Brown, V.K.; Butterfield, J.; Buse, A.; Coulson, J.C.; Farrar, J.; et al. Herbivory in Global Climate Change Research: Direct Effects of Rising Temperature on Insect Herbivores. Glob. Chang. Biol. 2002, 8, 1–16. [Google Scholar] [CrossRef]
- Rasmann, S.; Pellissier, L.; Defossez, E.; Jactel, H.; Kunstler, G. Climate-Driven Change in Plant–Insect Interactions along Elevation Gradients. Funct. Ecol. 2014, 28, 46–54. [Google Scholar] [CrossRef]
- Roslin, T.; Hardwick, B.; Novotny, V.; Petry, W.K.; Andrew, N.R.; Asmus, A.; Barrio, I.C.; Basset, Y.; Boesing, A.L.; Bonebrake, T.C.; et al. Higher Predation Risk for Insect Prey at Low Latitudes and Elevations. Science 2017, 356, 742–744. [Google Scholar] [CrossRef]
- Kent, D.R.; Lynn, J.S.; Pennings, S.C.; Souza, L.A.; Smith, M.D.; Rudgers, J.A. Weak Latitudinal Gradients in Insect Herbivory for Dominant Rangeland Grasses of North America. Ecol. Evol. 2020, 10, 6385–6394. [Google Scholar] [CrossRef] [PubMed]
- Massad, T.J.; Richards, L.A.; Philbin, C.; Yamaguchi, L.F.; Kato, M.J.; Jeffrey, C.S.; Oliveira Jr, C.; Ochsenrider, K.; de Moraes, M.M.; Tepe, E.J.; et al. The Chemical Ecology of Tropical Forest Diversity: Environmental Variation, Chemical Similarity, Herbivory, and Richness. Ecology 2022, 103, e3762. [Google Scholar] [CrossRef]
- Schrijvers-Gonlag, M.; Skarpe, C.; Andreassen, H.P. Influence of Light Availability and Soil Productivity on Insect Herbivory on Bilberry (Vaccinium Myrtillus L.) Leaves Following Mammalian Herbivory. PLoS ONE 2020, 15, e0230509. [Google Scholar] [CrossRef] [PubMed]
- de la Peña, E.; Baeten, L.; Steel, H.; Viaene, N.; De Sutter, N.; De Schrijver, A.; Verheyen, K. Beyond Plant–Soil Feedbacks: Mechanisms Driving Plant Community Shifts Due to Land-Use Legacies in Post-Agricultural Forests. Funct. Ecol. 2016, 30, 1073–1085. [Google Scholar] [CrossRef]
- Züst, T.; Agrawal, A.A. Trade-Offs Between Plant Growth and Defense Against Insect Herbivory: An Emerging Mechanistic Synthesis. Annu. Rev. Plant Biol. 2017, 68, 513–534. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.E.; Craine, J.M.; Lany, N.K.; Jonard, M.; Ollinger, S.V.; Groffman, P.M.; Fulweiler, R.W.; Angerer, J.; Read, Q.D.; Reich, P.B.; et al. Evidence, Causes, and Consequences of Declining Nitrogen Availability in Terrestrial Ecosystems. Science 2022, 376, eabh3767. [Google Scholar] [CrossRef]
- Graff, P.; Gundel, P.E.; Salvat, A.; Cristos, D.; Chaneton, E.J. Protection Offered by Leaf Fungal Endophytes to an Invasive Species against Native Herbivores Depends on Soil Nutrients. J. Ecol. 2020, 108, 1592–1604. [Google Scholar] [CrossRef]
- Hartemink, A.E.; Barrow, N.J. Soil pH—Nutrient Relationships: The Diagram. Plant Soil 2023, 486, 209–215. [Google Scholar] [CrossRef]
- Jia, S.; Yang, X.; Castagneyrol, B.; Yang, L.; Yin, Q.; He, C.; Yang, Z.; Zhu, Y.; Hao, Z. Neighbouring Tree Effects on Leaf Herbivory: Insect Specialisation Matters More than Host Plant Leaf Traits. J. Ecol. 2024, 112, 189–199. [Google Scholar] [CrossRef]
- Li, W.; Chen, Y.; Shen, Y.; Lu, Y.; Yu, S. Plant Trait Differences and Soil Moisture Jointly Affect Insect Herbivory on Seedling Young Leaves in a Subtropical Forest. For. Ecol. Manag. 2021, 482, 118878. [Google Scholar] [CrossRef]
- Massey, F.P.; Massey, K.; Press, M.C.; Hartley, S.E. Neighbourhood Composition Determines Growth, Architecture and Herbivory in Tropical Rain Forest Tree Seedlings. J. Ecol. 2006, 94, 646–655. [Google Scholar] [CrossRef]
- Brown, V.K.; Lawton, J.H.; Grubb, P.J.; Chaloner, W.G.; Harper, J.L.; Lawton, J.H. Herbivory and the Evolution of Leaf Size and Shape. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1997, 333, 265–272. [Google Scholar]
- Schuldt, A.; Bruelheide, H.; Durka, W.; Eichenberg, D.; Fischer, M.; Kröber, W.; Härdtle, W.; Ma, K.; Michalski, S.G.; Palm, W.-U.; et al. Plant Traits Affecting Herbivory on Tree Recruits in Highly Diverse Subtropical Forests. Ecol. Lett. 2012, 15, 732–739. [Google Scholar] [CrossRef]
- Zettlemoyer, M.A. Leaf Traits Mediate Herbivory across a Nitrogen Gradient Differently in Extirpated vs. Extant Prairie Species. Oecologia 2022, 198, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Krimmel, B.; Pearse, I.S. Tolerance and Phenological Avoidance of Herbivory in Tarweed Species. Ecology 2016, 97, 1357–1363. [Google Scholar] [CrossRef]
- Pilson, D. Herbivory and Natural Selection on Flowering Phenology in Wild Sunflower, Helianthus Annuus. Oecologia 2000, 122, 72–82. [Google Scholar] [CrossRef]
- Cárdenas, R.E.; Valencia, R.; Kraft, N.J.B.; Argoti, A.; Dangles, O. Plant Traits Predict Inter- and Intraspecific Variation in Susceptibility to Herbivory in a Hyperdiverse Neotropical Rain Forest Tree Community. J. Ecol. 2014, 102, 939–952. [Google Scholar] [CrossRef]
- Kurokawa, H.; Oguro, M.; Takayanagi, S.; Aiba, M.; Shibata, R.; Mimura, M.; Yoshimaru, H.; Nakashizuka, T. Plant Characteristics Drive Ontogenetic Changes in Herbivory Damage in a Temperate Forest. J. Ecol. 2022, 110, 2772–2784. [Google Scholar] [CrossRef]
- Wang, B.; Tian, C.; Liang, Y. Leaf Traits-Mediated Effects of Tree Diversity on Insect Herbivory on Populus Laurifolia in a Riparian Forest Ecosystem. For. Ecol. Manag. 2022, 504, 119777. [Google Scholar] [CrossRef]
- Kozlov, M.V.; Lanta, V.; Zverev, V.; Zvereva, E.L. Background Losses of Woody Plant Foliage to Insects Show Variable Relationships with Plant Functional Traits across the Globe. J. Ecol. 2015, 103, 1519–1528. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Ma, K. The Association of Leaf Lifespan and Background Insect Herbivory at the Interspecific Level. Ecology 2017, 98, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Mendes, G.M.; Silveira, F.A.O.; Oliveira, C.; Dáttilo, W.; Guevara, R.; Ruiz-Guerra, B.; Boaventura, M.G.; Sershen; Ramdhani, S.; Phartyal, S.S.; et al. How Much Leaf Area Do Insects Eat? A Data Set of Insect Herbivory Sampled Globally with a Standardized Protocol. Ecology 2021, 102, e03301. [Google Scholar] [CrossRef]
- Shi, P.; Ratkowsky, D.A.; Li, Y.; Zhang, L.; Lin, S.; Gielis, J. A General Leaf Area Geometric Formula Exists for Plants—Evidence from the Simplified Gielis Equation. Forests 2018, 9, 714. [Google Scholar] [CrossRef]
- He, J.; Reddy, G.V.P.; Liu, M.; Shi, P. A General Formula for Calculating Surface Area of the Similarly Shaped Leaves: Evidence from Six Magnoliaceae Species. Glob. Ecol. Conserv. 2020, 23, e01129. [Google Scholar] [CrossRef]
- Zheng, X.; Niklas, K.J.; Ratkowsky, D.A.; Jiao, Y.; Ding, H.; Shi, P. Comparison of Leaf Shape between a Photinia Hybrid and One of Its Parents. Plants 2022, 11, 2370. [Google Scholar] [CrossRef]
- Lai, J.; Zou, Y.; Zhang, J.; Peres-Neto, P.R. Generalizing Hierarchical and Variation Partitioning in Multiple Regression and Canonical Analyses Using the rdacca.hp R Package. Methods Ecol. Evol. 2022, 13, 782–788. [Google Scholar] [CrossRef]
- Wan, N.-F.; Zheng, X.-R.; Fu, L.-W.; Kiær, L.P.; Zhang, Z.; Chaplin-Kramer, R.; Dainese, M.; Tan, J.; Qiu, S.-Y.; Hu, Y.-Q.; et al. Global Synthesis of Effects of Plant Species Diversity on Trophic Groups and Interactions. Nat. Plants 2020, 6, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Jiang, P.; Chase, J.M.; Liu, X. Global Insect Herbivory and Its Response to Climate Change. Curr. Biol. 2024, 34, 2558–2569.e3. [Google Scholar] [CrossRef]
- van Huis, A.; Gasco, L. Insects as Feed for Livestock Production. Science 2023, 379, 138–139. [Google Scholar] [CrossRef]
- Harvey, J.A.; Tougeron, K.; Gols, R.; Heinen, R.; Abarca, M.; Abram, P.K.; Basset, Y.; Berg, M.; Boggs, C.; Brodeur, J.; et al. Scientists’ Warning on Climate Change and Insects. Ecol. Monogr. 2023, 93, e1553. [Google Scholar] [CrossRef]
- Wei, B.; Zhang, D.; Wang, G.; Liu, Y.; Li, Q.; Zheng, Z.; Yang, G.; Peng, Y.; Niu, K.; Yang, Y. Experimental Warming Altered Plant Functional Traits and Their Coordination in a Permafrost Ecosystem. New Phytol. 2023, 240, 1802–1816. [Google Scholar] [CrossRef] [PubMed]
- Becerra, J.X. On the Factors That Promote the Diversity of Herbivorous Insects and Plants in Tropical Forests. Proc. Natl. Acad. Sci. USA 2015, 112, 6098–6103. [Google Scholar] [CrossRef]
- Li, Y.; Schmid, B.; Schuldt, A.; Li, S.; Wang, M.-Q.; Fornoff, F.; Staab, M.; Guo, P.-F.; Anttonen, P.; Chesters, D.; et al. Multitrophic Arthropod Diversity Mediates Tree Diversity Effects on Primary Productivity. Nat. Ecol. Evol. 2023, 7, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Johansson, F.; Orizaola, G.; Nilsson-Örtman, V. Temperate Insects with Narrow Seasonal Activity Periods Can Be as Vulnerable to Climate Change as Tropical Insect Species. Sci. Rep. 2020, 10, 8822. [Google Scholar] [CrossRef]
- Lim, J.Y.; Fine, P.V.A.; Mittelbach, G.G. Assessing the Latitudinal Gradient in Herbivory. Glob. Ecol. Biogeogr. 2015, 24, 1106–1112. [Google Scholar] [CrossRef]
- Zvereva, E.L.; Kozlov, M.V. Meta-Analysis of Elevational Changes in the Intensity of Trophic Interactions: Similarities and Dissimilarities with Latitudinal Patterns. Ecol. Lett. 2022, 25, 2076–2087. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Hui, D.; Liu, H.; Wang, F.; Yao, K.; Lu, H.; Ren, H.; Wang, J. Responses of Plant Water Uptake Sources to Altered Precipitation Patterns in a Tropical Secondary Forest. Agric. For. Meteorol. 2024, 355, 110138. [Google Scholar] [CrossRef]
- He, X.; Hui, D.; Wang, F.; Deng, Q.; Liu, Z.; Lu, H.; Yao, K.; Ren, H.; Wang, J. Dynamics of Soil and Foliar Phosphorus Fractions in a Secondary Tropical Forest under Altered Seasonal Precipitation Patterns. Plant Soil 2024, 507, 915–937. [Google Scholar] [CrossRef]
- Basset, Y.; Lamarre, G.P.A. Toward a World That Values Insects. Science 2019, 364, 1230–1231. [Google Scholar] [CrossRef]
- Müller, J.; Hothorn, T.; Yuan, Y.; Seibold, S.; Mitesser, O.; Rothacher, J.; Freund, J.; Wild, C.; Wolz, M.; Menzel, A. Weather Explains the Decline and Rise of Insect Biomass over 34 Years. Nature 2024, 628, 349–354. [Google Scholar] [CrossRef]
- Huberty, M.; Steinauer, K.; Heinen, R.; Jongen, R.; Hannula, S.E.; Choi, Y.H.; Bezemer, T.M. Temporal Changes in Plant–Soil Feedback Effects on Microbial Networks, Leaf Metabolomics and Plant–Insect Interactions. J. Ecol. 2022, 110, 1328–1343. [Google Scholar] [CrossRef]
- Ebeling, A.; Strauss, A.T.; Adler, P.B.; Arnillas, C.A.; Barrio, I.C.; Biederman, L.A.; Borer, E.T.; Bugalho, M.N.; Caldeira, M.C.; Cadotte, M.W.; et al. Nutrient Enrichment Increases Invertebrate Herbivory and Pathogen Damage in Grasslands. J. Ecol. 2022, 110, 327–339. [Google Scholar] [CrossRef]
- de Tombeur, F.; Laliberté, E.; Lambers, H.; Faucon, M.-P.; Zemunik, G.; Turner, B.L.; Cornelis, J.-T.; Mahy, G. A Shift from Phenol to Silica-Based Leaf Defences during Long-Term Soil and Ecosystem Development. Ecol. Lett. 2021, 24, 984–995. [Google Scholar] [CrossRef]
- Zaret, M.; Kinkel, L.; Borer, E.T.; Seabloom, E.W. Soil Nutrients Cause Threefold Increase in Pathogen and Herbivore Impacts on Grassland Plant Biomass. J. Ecol. 2023, 111, 1629–1640. [Google Scholar] [CrossRef]
- Deraison, H.; Badenhausser, I.; Börger, L.; Gross, N. Herbivore Effect Traits and Their Impact on Plant Community Biomass: An Experimental Test Using Grasshoppers. Funct. Ecol. 2015, 29, 650–661. [Google Scholar] [CrossRef]
- Walker, T.W.N.; Schrodt, F.; Allard, P.-M.; Defossez, E.; Jassey, V.E.J.; Schuman, M.C.; Alexander, J.M.; Baines, O.; Baldy, V.; Bardgett, R.D.; et al. Leaf Metabolic Traits Reveal Hidden Dimensions of Plant Form and Function. Sci. Adv. 2023, 9, eadi4029. [Google Scholar] [CrossRef]
- Higuchi, Y.; Kawakita, A. Leaf Shape Deters Plant Processing by an Herbivorous Weevil. Nat. Plants 2019, 5, 959–964. [Google Scholar] [CrossRef]
- König, S.; Krauss, J.; Keller, A.; Bofinger, L.; Steffan-Dewenter, I. Phylogenetic Relatedness of Food Plants Reveals Highest Insect Herbivore Specialization at Intermediate Temperatures along a Broad Climatic Gradient. Glob. Chang. Biol. 2022, 28, 4027–4040. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Peter Constabel, C. Tannins in Plant–Herbivore Interactions. Phytochemistry 2011, 72, 1551–1565. [Google Scholar] [CrossRef]
- Caldwell, E.; Read, J.; Sanson, G.D. Which Leaf Mechanical Traits Correlate with Insect Herbivory among Feeding Guilds? Ann. Bot. 2016, 117, 349–361. [Google Scholar] [CrossRef]
- Muiruri, E.W.; Barantal, S.; Iason, G.R.; Salminen, J.-P.; Perez-Fernandez, E.; Koricheva, J. Forest Diversity Effects on Insect Herbivores: Do Leaf Traits Matter? New Phytol. 2019, 221, 2250–2260. [Google Scholar] [CrossRef]
- Givnish, T.J.; Kriebel, R. Causes of Ecological Gradients in Leaf Margin Entirety: Evaluating the Roles of Biomechanics, Hydraulics, Vein Geometry, and Bud Packing. Am. J. Bot. 2017, 104, 354–366. [Google Scholar] [CrossRef]
- Bastida, J.M.; Garrido, J.L.; Cano-Sáez, D.; Perea, A.J.; Pomarede, L.C.; Alcántara, J.M. Effects of Plant Leaf Traits, Abundance and Phylogeny on Differentiation of Herbivorous Insect Assemblages in Mediterranean Mixed Forest. Eur. J. For. Res. 2024, 143, 1149–1164. [Google Scholar] [CrossRef]
- Zhu, F.; Poelman, E.H.; Dicke, M. Insect Herbivore-Associated Organisms Affect Plant Responses to Herbivory. New Phytol. 2014, 204, 315–321. [Google Scholar] [CrossRef]
- Han, T.; Lu, H.; Ren, H.; Wang, J.; Song, G.; Hui, D.; Guo, Q.; Zhu, S. Are Reproductive Traits of Dominant Species Associated with Specific Resource Allocation Strategies during Forest Succession in Southern China? Ecol. Indic. 2019, 102, 538–546. [Google Scholar] [CrossRef]
- Wang, X.; He, Y.; Sedio, B.E.; Jin, L.; Ge, X.; Glomglieng, S.; Cao, M.; Yang, J.; Swenson, N.G.; Yang, J. Phytochemical Diversity Impacts Herbivory in a Tropical Rainforest Tree Community. Ecol. Lett. 2023, 26, 1898–1910. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Y.; Yan, X.; Xu, J.; Jumak, M.; Zhang, R.; Wang, L.; Gao, J. Plant Functional Traits Better Explain the Global Latitudinal Patterns of Leaf Insect Herbivory than Climatic Factors. Plants 2025, 14, 1303. https://doi.org/10.3390/plants14091303
Ji Y, Yan X, Xu J, Jumak M, Zhang R, Wang L, Gao J. Plant Functional Traits Better Explain the Global Latitudinal Patterns of Leaf Insect Herbivory than Climatic Factors. Plants. 2025; 14(9):1303. https://doi.org/10.3390/plants14091303
Chicago/Turabian StyleJi, Yuhui, Xiaoxu Yan, Jiali Xu, Mira Jumak, Ruizhi Zhang, Lan Wang, and Jie Gao. 2025. "Plant Functional Traits Better Explain the Global Latitudinal Patterns of Leaf Insect Herbivory than Climatic Factors" Plants 14, no. 9: 1303. https://doi.org/10.3390/plants14091303
APA StyleJi, Y., Yan, X., Xu, J., Jumak, M., Zhang, R., Wang, L., & Gao, J. (2025). Plant Functional Traits Better Explain the Global Latitudinal Patterns of Leaf Insect Herbivory than Climatic Factors. Plants, 14(9), 1303. https://doi.org/10.3390/plants14091303





