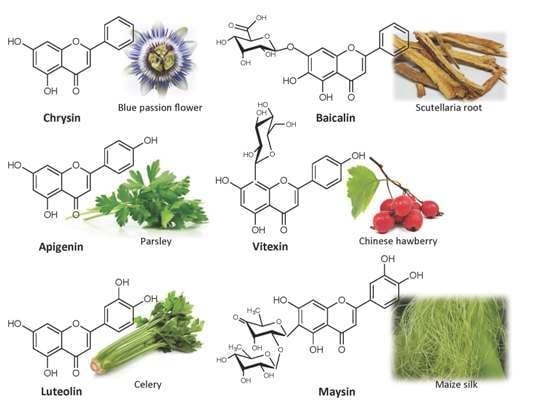
Flavones: From Biosynthesis to Health Benefits
Abstract
:1. Introduction
2. Flavone Biosynthesis: Multiple and Specialized Biosynthesis Pathways Result in Flavone Chemical Diversity
2.1. Multiple Enzymes Can Form the Flavone Backbone
2.1.1. Biosynthesis of Flavone Precursors
2.1.2. Flavone Synthase I (FNSI) Class
2.1.3. Flavone Synthase II (FNSII) Class
2.1.4. 2-Hydroxyflavanones as Flavone Precursors
2.1.5. Evolutionary Relationships between Flavone Synthase II (FNSII), Flavanone-2-Hydroxylase (F2H), and Isoflavone Synthase (IFS) in the CYP93 Subfamily
2.2. Biosynthesis of O- and C-glycosyl Flavones
2.2.1. Backbone or Decoration First?
2.2.2. O- and C-glycosyl Transferases
2.2.3. Evidence for Dehydratase Activity
2.3. Dedicated Biosynthetic Pathway for 4′ Deoxyflavones (B-Ring Deoxyflavonoids)
2.4. Other Flavone Modifications
3. Biological Activities of Flavones
3.1. Biological Activities of Flavones in Plants
3.1.1. Abiotic Protection
3.1.2. Biotic Protection
3.1.3. Plant Development
3.2. Molecular Interactions of Flavones with Other Molecules
3.2.1. Interactions with Lipids: Flavone-Membrane Interaction
3.2.2. Interactions with Nucleic Acids
3.2.3. Interactions with Proteins
3.3. Major Flavone Health Benefits
4. Conclusions and Future Prospects
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PAL | phenylalanine ammonia-lyase |
| C4H | cinnamic acid 4-hydroxylase |
| CoA | coenzyme A |
| 4CL | p-coumaroyl: CoA ligase |
| 4CL-like | cinnamic acid specific CoA ligase |
| CHS | chalcone synthase |
| CHI | chalcone isomerase |
| FNS | flavone synthase |
| 2-ODD | Fe2+/2-oxoglutarate-dependent dioxygenase |
| F2H | flavanone-2-hydroxylase |
| F3′H | flavanone-3′-hydroxylase |
| F6H | flavanone-6-hydroxylase |
| CYP | cytochrome P450 |
| IFS | isoflavone synthase |
| UGTs | UDP-glycosyltransferases |
| CGT | C-glycosyl transferase |
| OGT | O-glycosyl transferase |
| HID | 2-hydroxyisoflavanone dehydratase |
| OMT | O-methyltransferase |
| FOMT | flavonoid O-methyltransferase |
| RHM | UDP-rhamnose synthase |
References
- Andersen, O.M.; Markham, K.R. Flavonoids: Chemistry, Biochemistry and Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 1–1256. [Google Scholar]
- Schmitz-Hoerner, R.; Weissenbock, G. Contribution of phenolic compounds to the UV-B screening capacity of developing barley primary leaves in relation to DNA damage and repair under elevated UV-B levels. Phytochemistry 2003, 64, 243–255. [Google Scholar] [CrossRef]
- Morimoto, S.; Tateishi, N.; Matsuda, T.; Tanaka, H.; Taura, F.; Furuya, N.; Matsuyama, N.; Shoyama, Y. Novel hydrogen peroxide metabolism in suspension cells of Scutellaria baicalensis Georgi. J. Biol. Chem. 1998, 273, 12606–12611. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, I.; Kissen, R.; Bones, A.M. Phytoalexins in defense against pathogens. Trends Plant Sci. 2012, 17, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Peters, N.K.; Frost, J.W.; Long, S.R. A plant flavone, luteolin, induces expression of Rhizobium meliloti nodulation genes. Science 1986, 233, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, B.R.; Snook, M.E. Effect of corn silk age on flavone content and development of corn earworm (Lepidoptera: Noctuidae) larvae. J. Econ. Entomol. 1995, 88, 1795–1800. [Google Scholar] [CrossRef]
- Hooper, A.M.; Hassanali, A.; Chamberlain, K.; Khan, Z.; Pickett, J.A. New genetic opportunities from legume intercrops for controlling Striga spp. Parasitic weeds. Pest Manag. Sci. 2009, 65, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Mori, M.; Kondo, T. Blue flower color development by anthocyanins: From chemical structure to cell physiology. Nat. Prod. Rep. 2009, 26, 884–915. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.; Lu, F.; Regner, M.; Zhu, Y.; Rencoret, J.; Ralph, S.A.; Zakai, U.I.; Morreel, K.; Boerjan, W.; Ralph, J. Tricin, a flavonoid monomer in monocot lignification. Plant Physiol. 2015, 167, 1284–1295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, L.H.; Jiang, Y.M.; Shi, J.; TomáS-Barberán, F.A.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in food and their health benefits. Plant Foods Hum. Nutr. 2004, 59, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, J.L.; Austin, M.B.; Stewart, C., Jr.; Noel, J.P. Structure and function of enzymes involved in the biosynthesis of phenylpropanoids. Plant Physiol. Biochem. 2008, 46, 356–370. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, J.L.; Jez, J.M.; Bowman, M.E.; Dixon, R.A.; Noel, J.P. Structure of chalcone synthase and the molecular basis of plant polyketide biosynthesis. Nat. Struct. Biol. 1999, 6, 775–784. [Google Scholar] [PubMed]
- Jez, J.M.; Noel, J.P. Mechanism of chalcone synthase. pKa of the catalytic cysteine and the role of the conserved histidine in a plant polyketide synthase. J. Biol. Chem. 2000, 275, 39640–39646. [Google Scholar] [CrossRef] [PubMed]
- Jez, J.M.; Bowman, M.E.; Dixon, R.A.; Noel, J.P. Structure and mechanism of the evolutionarily unique plant enzyme chalcone isomerase. Nat. Struct. Biol. 2000, 7, 786–791. [Google Scholar] [PubMed]
- Ngaki, M.N.; Louie, G.V.; Philippe, R.N.; Manning, G.; Pojer, F.; Bowman, M.E.; Li, L.; Larsen, E.; Wurtele, E.S.; Noel, J.P. Evolution of the chalcone-isomerase fold from fatty-acid binding to stereospecific catalysis. Nature 2012, 485, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Rosado, A.; Hicks, G.R.; Norambuena, L.; Rogachev, I.; Meir, S.; Pourcel, L.; Zouhar, J.; Brown, M.Q.; Boirsdore, M.P.; Puckrin, R.S.; et al. Sortin1-hypersensitive mutants link vacuolar-trafficking defects and flavonoid metabolism in arabidopsis vegetative tissues. Chem. Biol. 2011, 18, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Martens, S.; Mithofer, A. Flavones and flavone synthases. Phytochemistry 2005, 66, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.X.; Han, X.J.; Wu, Y.F.; Lou, H.X. The function and catalysis of 2-oxoglutarate-dependent oxygenases involved in plant flavonoid biosynthesis. Int. J. Mol. Sci. 2014, 15, 1080–1095. [Google Scholar] [CrossRef] [PubMed]
- Martens, S.; Forkmann, G.; Matern, U.; Lukacin, R. Cloning of parsley flavone synthase I. Phytochemistry 2001, 58, 43–46. [Google Scholar] [CrossRef]
- Gebhardt, Y.H.; Witte, S.; Steuber, H.; Matern, U.; Martens, S. Evolution of flavone synthase I from parsley flavanone 3β-hydroxylase by site-directed mutagenesis. Plant Physiol. 2007, 144, 1442–1454. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, Y.; Witte, S.; Forkmann, G.; Lukacin, R.; Matern, U.; Martens, S. Molecular evolution of flavonoid dioxygenases in the family Apiaceae. Phytochemistry 2005, 66, 1273–1284. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Kim, J.H.; Kim, B.G.; Lim, Y.; Ahn, J.H. Characterization of flavone synthase I from rice. BMB Rep. 2008, 41, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Han, X.J.; Wu, Y.F.; Gao, S.; Yu, H.N.; Xu, R.X.; Lou, H.X.; Cheng, A.X. Functional characterization of a Plagiochasma appendiculatum flavone synthase I showing flavanone 2-hydroxylase activity. FEBS Lett. 2014, 588, 2307–2314. [Google Scholar] [CrossRef] [PubMed]
- Falcone Ferreyra, M.L.; Emiliani, J.; Rodriguez, E.J.; Campos-Bermudez, V.A.; Grotewold, E.; Casati, P. The identification of maize and arabidopsis type I flavone synthases links flavones with hormones and biotic interactions. Plant Physiol. 2015, 169, 1090–1107. [Google Scholar] [CrossRef] [PubMed]
- Zeilmaker, T.; Ludwig, N.R.; Elberse, J.; Seidl, M.F.; Berke, L.; Van Doorn, A.; Schuurink, R.C.; Snel, B.; Van den Ackerveken, G. DOWNY MILDEW RESISTANT 6 and DMR6-LIKE OXYGENASE 1 are partially redundant but distinct suppressors of immunity in Arabidopsis. Plant J. 2015, 81, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, M.; Huibers, R.P.; Elberse, J.; Van den Ackerveken, G. Arabidopsis DMR6 encodes a putative 2OG-Fe(II) oxygenase that is defense-associated but required for susceptibility to downy mildew. Plant J. 2008, 54, 785–793. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Mou, Z. Salicylic acid and its function in plant immunity. J. Integr. Plant Biol. 2011, 53, 412–428. [Google Scholar] [CrossRef] [PubMed]
- Akashi, T.; Fukuchi-Mizutani, M.; Aoki, T.; Ueyama, Y.; Yonekura-Sakakibara, K.; Tanaka, Y.; Kusumi, T.; Ayabe, S. Molecular cloning and biochemical characterization of a novel cytochrome p450, flavone synthase II, that catalyzes direct conversion of flavanones to flavones. Plant Cell Physiol. 1999, 40, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Fliegmann, J.; Furtwangler, K.; Malterer, G.; Cantarello, C.; Schuler, G.; Ebel, J.; Mithofer, A. Flavone synthase II (CYP93B16) from soybean (Glycine max L.). Phytochemistry 2010, 71, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.Y.; Zhu, F.Y.; Chan, W.L.; Liu, H.; Lo, C. Cytochrome P450 93G1 is a flavone synthase II that channels flavanones to the biosynthesis of tricin O-linked conjugates in rice. Plant Physiol. 2014, 165, 1315–1327. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, X.C.; Liu, Y.; Du, H.; Shu, Q.Y.; Su, S.; Wang, L.J.; Li, S.S.; Wang, L.S. Flavone synthases from Lonicera japonica and L. macranthoides reveal differential flavone accumulation. Sci. Rep. 2016, 6, 19245. [Google Scholar] [CrossRef] [PubMed]
- Akashi, T.; Aoki, T.; Ayabe, S. Identification of a cytochrome P450 cDNA encoding (2S)-flavanone 2-hydroxylase of licorice (Glycyrrhiza echinata L.; Fabaceae) which represents licodione synthase and flavone synthase II. FEBS Lett. 1998, 431, 287–290. [Google Scholar] [CrossRef]
- Zhang, J.; Subramanian, S.; Zhang, Y.; Yu, O. Flavone synthases from Medicago truncatula are flavanone-2-hydroxylases and are important for nodulation. Plant Physiol. 2007, 144, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Chu, H.; Wang, M.; Chu, I.K.; Lo, C. Identification of flavone phytoalexins and a pathogen-inducible flavone synthase II gene (SbFNSII) in sorghum. J. Exp. Bot. 2010, 61, 983–994. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Chu, H.; Chu, I.K.; Lo, C. CYP93G2 is a flavanone 2-hydroxylase required for C-glycosylflavone biosynthesis in rice. Plant Physiol. 2010, 154, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Morohashi, K.; Casas, M.I.; Falcone Ferreyra, M.L.; Mejia-Guerra, M.K.; Pourcel, L.; Yilmaz, A.; Feller, A.; Carvalho, B.; Emiliani, J.; Rodriguez, E.; et al. A genome-wide regulatory framework identifies maize pericarp color1 controlled genes. Plant Cell 2012, 24, 2745–2764. [Google Scholar] [CrossRef] [PubMed]
- Martens, S.; Forkmann, G. Cloning and expression of flavone synthase II from Gerbera hybrids. Plant J. 1999, 20, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Brkljacic, J.; Grotewold, E.; Scholl, R.; Mockler, T.; Garvin, D.F.; Vain, P.; Brutnell, T.; Sibout, R.; Bevan, M.; Budak, H.; et al. Brachypodium as a model for the grasses: Today and the future. Plant Physiol. 2011, 157, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Kitada, C.; Gong, Z.; Tanaka, Y.; Yamazaki, M.; Saito, K. Differential expression of two cytochrome P450s involved in the biosynthesis of flavones and anthocyanins in chemo-varietal forms of Perilla frutescens. Plant Cell Physiol. 2001, 42, 1338–1344. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhang, Y.; Wang, G.; Hill, L.; Weng, J.-K.; Chen, X.-Y.; Xue, H.; Martin, C. A specialized flavone biosynthetic pathway has evolved in the medicinal plant, Scutellaria baicalensis. Sci. Adv. 2016, 2. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.; Yu, O.; Lau, S.M.; O’Keefe, D.P.; Odell, J.; Fader, G.; McGonigle, B. Identification and expression of isoflavone synthase, the key enzyme for biosynthesis of isoflavones in legumes. Nat. Biotechnol. 2000, 18, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Lapcik, O. Isoflavonoids in non-leguminous taxa: A rarity or a rule? Phytochemistry 2007, 68, 2909–2916. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Ayabe, S. Multiple mutagenesis of P450 isoflavonoid synthase reveals a key active-site residue. Biochem. Biophys. Res. Commun. 2005, 330, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Huang, Y.; Tang, Y. Genetic and metabolic engineering of isoflavonoid biosynthesis. Appl. Microbiol. Biotechnol. 2010, 86, 1293–1312. [Google Scholar] [CrossRef] [PubMed]
- Akashi, T.; Aoki, T.; Ayabe, S. Cloning and functional expression of a cytochrome P450 cDNA encoding 2-hydroxyisoflavanone synthase involved in biosynthesis of the isoflavonoid skeleton in licorice. Plant Physiol. 1999, 121, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Kinoshita, K.; Akashi, T.; Aoki, T.; Ayabe, S. Key amino acid residues required for aryl migration catalysed by the cytochrome P450 2-hydroxyisoflavanone synthase. Plant J. 2002, 31, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Hofer, B. Recent developments in the enzymatic O-glycosylation of flavonoids. Appl. Microbiol. Biotechnol. 2016, 100, 4269–4281. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Cruz, M.; Snook, M.; McMullen, M.D. The genetic basis of C-glycosyl flavone B-ring modification in maize (Zea mays L.) silks. Genome 2003, 46, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Kraut, L.; Mues, R.; Sim-Sim, M. Acylated flavone and glycerol glucosides from two Frullania species. Phytochemistry 1993, 34, 211–218. [Google Scholar] [CrossRef]
- Schoeneborn, R.; Mues, R. Flavone di-C-glycosides from Plagiochila jamesonii and Plagiochasma rupestre. Phytochemistry 1993, 34, 1143–1145. [Google Scholar] [CrossRef]
- Imperato, F. 3,6,8-tri-C-xylosylapigenin from Asplenium viviparum. Phytochemistry 1993, 33, 729–730. [Google Scholar] [CrossRef]
- Wallace, J.W. Chemotaxonomy of the Hymenophyllaceae. II. C-glycosylflavones and flavone-O-glycosides of Trichomanes S.L. Am. J. Bot. 1996, 83, 1304–1308. [Google Scholar]
- Webby, R.F.; Markham, K.R. Isoswertiajaponin 2″-O-β-arabinopyranoside and other flavone-C-glycosides from the antarctic grass Deschampsia antarctica. Phytochemistry 1994, 36, 1323–1326. [Google Scholar] [CrossRef]
- Norbaek, R.; Aaboer, D.B.; Bleeg, I.S.; Christensen, B.T.; Kondo, T.; Brandt, K. Flavone C-glycoside, phenolic acid, and nitrogen contents in leaves of barley subject to organic fertilization treatments. J. Agric. Food Chem. 2003, 51, 809–813. [Google Scholar] [PubMed]
- Norbaek, R.; Brandt, K.; Kondo, T. Identification of flavone C-glycosides including a new flavonoid chromophore from barley leaves (Hordeum vulgare L.) by improved NMR techniques. J. Agric. Food Chem. 2000, 48, 1703–1707. [Google Scholar] [CrossRef] [PubMed]
- Snook, M.E.; Widstrom, N.W.; Wiseman, B.R.; Gueldner, R.C.; Wilson, R.L.; Himmelsbach, D.S.; Harwood, J.S.; Costello, C.E. New flavone C-glycosides from corn (Zea mays L.) for the control of the corn earworm (Helicoverpa zea). In Bioregulators for Crop Protection and Pest Control; American Chemical Society: Washington, DC, USA, 1994; Volume 557, pp. 122–135. [Google Scholar]
- Snook, M.E.; Gueldner, R.C.; Widstrom, N.W.; Wiseman, B.R.; Himmelsbach, D.S.; Harwood, J.S.; Costello, C.E. Levels of maysin and maysin analogs in silks of maize germplasm. J. Agric. Food Chem. 1993, 41, 1481–1485. [Google Scholar] [CrossRef]
- Suzuki, R.; Okada, Y.; Okuyama, T. Two flavone C-glycosides from the style of Zea mays with glycation inhibitory activity. J. Nat. Prod. 2003, 66, 564–565. [Google Scholar] [CrossRef] [PubMed]
- Afifi, F.U.; Khalil, E.; Abdalla, S. Effect of isoorientin isolated from Arum palaestinum on uterine smooth muscle of rats and guinea pigs. J. Ethnopharmacol. 1999, 65, 173–177. [Google Scholar] [CrossRef]
- Ali, Z.; Ahmad, V.U.; Ali, M.S.; Iqbal, F.; Zahid, M.; Alam, N. Two new C-glycosylflavones from Silene conoidea. Nat. Prod. Lett. 1999, 13, 121–129. [Google Scholar] [CrossRef]
- Marchart, E.; Kopp, B. Capillary electrophoretic separation and quantification of flavone-O- and C-glycosides in Achillea setacea W. et K. J. Chromatogr. B 2003, 792, 363–368. [Google Scholar] [CrossRef]
- Krauze-Baranowska, M.; Cisowski, W. Flavone C-glycosides from Bryonia alba and B. dioica. Phytochemistry 1995, 39, 727–729. [Google Scholar] [CrossRef]
- Maatooq, G.T.; El-Sharkawy, S.H.; Afifi, M.S.; Rosazza, J.P.N. C-p-hydroxybenzoylglycoflavones from Citrullus colocynthis. Phytochemistry 1997, 44, 187–190. [Google Scholar] [CrossRef]
- Abou-Zaid, M.M.; Lombardo, D.A.; Kite, G.C.; Grayer, R.J.; Veitch, N.C. Acylated flavone C-glycosides from Cucumis sativus. Phytochemistry 2001, 58, 167–172. [Google Scholar] [CrossRef]
- Shih-Hsien, K.; Ming-Hong, Y.; Mei-Ing, C.; Chun-Nan, L. A flavone C-glycoside and an aromatic glucoside from Gentiana species. Phytochemistry 1996, 41, 309–312. [Google Scholar] [CrossRef]
- Latte, K.P.; Ferreira, D.; Venkatraman, M.S.; Kolodziej, H. O-Galloyl-C-glycosylflavones from Pelargonium reniforme. Phytochemistry 2002, 59, 419–424. [Google Scholar] [CrossRef]
- Takagi, S.; Yamaki, M.; Inoue, K. Flavone di-C-glycosides from Scutellaria baicalensis. Phytochemistry 1981, 20, 2443–2444. [Google Scholar] [CrossRef]
- Ma, C.-M.; Nakamura, N.; Hattori, M. Saponins and C-glycosyl flavones from the seeds of Abrus precatorius. Chem. Pharm. Bull. 1998, 46, 982–987. [Google Scholar] [CrossRef]
- Liu, M.; Liu, Q.; Liu, Y.-L.; Hou, C.-Y.; Mabry, T.J. An acylated flavone C-glycoside from Glycyrrhiza eurycarpa. Phytochemistry 1994, 36, 1089–1090. [Google Scholar] [CrossRef]
- Kamel, M.S. Flavone C-glycosides from Lupinus hartwegii. Phytochemistry 2003, 63, 449–452. [Google Scholar] [CrossRef]
- Lobstein, A.; Weniger, B.; Um, B.H.; Steinmetz, M.; Declercq, L.; Anton, R. 4″-hydroxymaysin and cassiaoccidentalin b, two unusual C-glycosylflavones from Mimosa pudica (Mimosaceae). Biochem. Syst. Ecol. 2002, 30, 375–377. [Google Scholar] [CrossRef]
- Williams, C.A.; Toscano De Brito, A.L.; Harborne, J.B.; Eagles, J.; Waterman, P.G. Methylated C-glycosylflavones as taxonomic markers in orchids of the subtribe Ornithocephalinae. Phytochemistry 1994, 37, 1045–1053. [Google Scholar] [CrossRef]
- Voirin, B.; Sportouch, M.; Raymond, O.; Jay, M.; Bayet, C.; Dangles, O.; El Hajji, H. Separation of flavone C-glycosides and qualitative analysis of Passiflora incarnata L. by capillary zone electrophoresis. Phytochem. Anal. 2000, 11, 90–98. [Google Scholar] [CrossRef]
- Kumar, J.K.; Rao, M.S.; Rao, P.S.; Tóth, G.; Balázs, B.; Duddeck, H. Flavone glycosides from Polygala telephioides and Polygala arvensis. Nat. Prod. Lett. 1999, 14, 35–38. [Google Scholar] [CrossRef]
- Zou, J.H.; Yang, J.; Zhou, L. Acylated flavone C-glycosides from Trollius ledebouri. J. Nat. Prod. 2004, 67, 664–667. [Google Scholar] [CrossRef] [PubMed]
- Ferreres, F.; Silva, B.M.; Andrade, P.B.; Seabra, R.M.; Ferreira, M.A. Approach to the study of C-glycosyl flavones by ion trap HPLC-PAD-ESI/MS/MS: Application to seeds of quince (Cydonia oblonga). Phytochem. Anal. 2003, 14, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Yamamoto, K.; Morimitsu, Y.; Osawa, T. Isolation of C-glucosylflavone from lemon peel and antioxidative activity of flavonoid compounds in lemon fruit. J. Agric. Food Chem. 1997, 45, 4619–4623. [Google Scholar] [CrossRef]
- Carnat, A.P.; Carnat, A.; Fraisse, D.; Lamaison, J.L.; Heitz, A.; Wylde, R.; Teulade, J.C. Violarvensin, a new flavone di-C-glycoside from Viola arvensis. J. Nat. Prod. 1998, 61, 272–274. [Google Scholar] [CrossRef] [PubMed]
- Brazier-Hicks, M.; Evans, K.M.; Gershater, M.C.; Puschmann, H.; Steel, P.G.; Edwards, R. The C-glycosylation of flavonoids in cereals. J. Biol. Chem. 2009, 284, 17926–17934. [Google Scholar] [CrossRef] [PubMed]
- Ikezawa, N.; Iwasa, K.; Sato, F. Molecular cloning and characterization of CYP80G2, a cytochrome P450 that catalyzes an intramolecular C-C phenol coupling of (S)-reticuline in magnoflorine biosynthesis, from cultured Coptis japonica cells. J. Biol. Chem. 2008, 283, 8810–8821. [Google Scholar] [CrossRef] [PubMed]
- Gesell, A.; Rolf, M.; Ziegler, J.; Diaz Chavez, M.L.; Huang, F.-C.; Kutchan, T.M. CYP719B1 is salutaridine synthase, the C-C phenol-coupling enzyme of morphine biosynthesis in opium poppy. J. Biol. Chem. 2009, 284, 24432–24442. [Google Scholar] [CrossRef] [PubMed]
- Ilari, A.; Franceschini, S.; Bonamore, A.; Arenghi, F.; Botta, B.; Macone, A.; Pasquo, A.; Bellucci, L.; Boffi, A. Structural basis of enzymatic (S)-norcoclaurine biosynthesis. J. Biol. Chem. 2009, 284, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, P.; Usera, A.R.; O’Connor, S.E. Biocatalytic asymmetric formation of tetrahydro-β-carbolines. Tetrahedron Lett. 2010, 51, 4400–4402. [Google Scholar] [CrossRef] [PubMed]
- Seisser, B.; Zinkl, R.; Gruber, K.; Kaufmann, F.; Hafner, A.; Kroutil, W. Cutting long syntheses short: Access to non-natural tyrosine derivatives employing an engineered tyrosine phenol lyase. Adv. Synth. Catal. 2010, 352, 731–736. [Google Scholar] [CrossRef]
- Schrittwieser, J.H.; Resch, V.; Sattler, J.H.; Lienhart, W.-D.; Durchschein, K.; Winkler, A.; Gruber, K.; Macheroux, P.; Kroutil, W. Biocatalytic enantioselective oxidative C-C coupling by aerobic C-H activation. Angew. Chem. 2011, 50, 1068–1071. [Google Scholar] [CrossRef] [PubMed]
- Sagui, F.; Chirivì, C.; Fontana, G.; Nicotra, S.; Passarella, D.; Riva, S.; Danieli, B. Laccase-catalyzed coupling of catharanthine and vindoline: An efficient approach to the bisindole alkaloid anhydrovinblastine. Tetrahedron 2009, 65, 312–317. [Google Scholar] [CrossRef]
- Lee, C.C.; Hu, Y.; Ribbe, M.W. Vanadium nitrogenase reduces CO. Science 2010. [Google Scholar] [CrossRef] [PubMed]
- Bock, K.W. The udp-glycosyltransferase (UGT) superfamily expressed in humans, insects and plants: Animal-plant arms-race and co-evolution. Biochem. Pharmacol. 2016, 99, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Falcone Ferreyra, M.L.; Rodriguez, E.; Casas, M.I.; Labadie, G.; Grotewold, E.; Casati, P. Identification of a bifunctional maize C- and O-glucosyltransferase. J. Biol. Chem. 2013, 288, 31678–31688. [Google Scholar] [CrossRef] [PubMed]
- Nagatomo, Y.; Usui, S.; Ito, T.; Kato, A.; Shimosaka, M.; Taguchi, G. Purification, molecular cloning and functional characterization of flavonoid C-glucosyltransferases from Fagopyrum esculentum M. (Buckwheat) cotyledon. Plant J. 2014, 80, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Hirade, Y.; Kotoku, N.; Terasaka, K.; Saijo-Hamano, Y.; Fukumoto, A.; Mizukami, H. Identification and functional analysis of 2-hydroxyflavanone C-glucosyltransferase in soybean (Glycine max). FEBS Lett. 2015, 589, 1778–1786. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Nishizaki, Y.; Yamada, E.; Tatsuzawa, F.; Nakatsuka, T.; Takahashi, H.; Nishihara, M. Identification of the glucosyltransferase that mediates direct flavone C-glucosylation in Gentiana triflora. FEBS Lett. 2015, 589, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Chen, R.; Wang, R.; Li, J.; Xie, K.; Bian, C.; Sun, L.; Zhang, X.; Liu, J.; Yang, L.; et al. Probing the catalytic promiscuity of a regio- and stereospecific c-glycosyltransferase from Mangifera indica. Angew. Chem. 2015, 54, 12678–12682. [Google Scholar] [CrossRef] [PubMed]
- Waiss, A.C.; Chan, B.G.; Elliger, C.A.; Wiseman, B.R.; McMillian, W.W.; Widstrom, N.W.; Zuber, M.S.; Keaster, A.J. Maysin, a flavone glycoside from corn silks with antibiotic activity toward corn earworm. J. Econ. Entomol. 1979, 72, 256–258. [Google Scholar] [CrossRef]
- Elliger, C.A.; Chan, B.G.; Waiss, A.C.; Lundin, R.E.; Haddon, W.F. C-glycosylflavones from Zea mays that inhibit insect development. Phytochemistry 1980, 19, 293–297. [Google Scholar] [CrossRef]
- Wiseman, B.R.; Snook, M.E.; Isenhour, D.J.; Mihm, J.A.; Widstrom, N.W. Relationship between growth of corn earworm and fall armyworm larvae (Lepidoptera: Noctuidae) and maysin concentration in corn silks. J. Econ. Entomol. 1992, 85, 2473–2477. [Google Scholar] [CrossRef]
- Wiseman, B.R.; Widstrom, N.W.; McMillian, W.W.; Waiss, A.C. Relationship between maysin concentration in corn silk and corn earworm (Lepidoptera: Noctuidae) growth. J. Econ. Entomol. 1985, 78, 423–427. [Google Scholar] [CrossRef]
- Casas, M.I.; Falcone-Ferreyra, M.L.; Jiang, N.; Mejia-Guerra, M.K.; Rodriguez, E.J.; Wilson, T.; Engelmeier, J.; Casati, P.; Grotewold, E. Identification and characterization of maize salmon silks genes involved in insecticidal maysin biosynthesis. Plant Cell 2016. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.G. The Inheritance of Salmon Silk Color in Maize; Cornell University: Ithaca, NY, USA, 1921. [Google Scholar]
- McMullen, M.D.; Kross, H.; Snook, M.E.; Cortes-Cruz, M.; Houchins, K.E.; Musket, T.A.; Coe, E.H., Jr. Salmon silk genes contribute to the elucidation of the flavone pathway in maize (Zea mays L.). J. Hered. 2004, 95, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Hooper, A.M.; Tsanuo, M.K.; Chamberlain, K.; Tittcomb, K.; Scholes, J.; Hassanali, A.; Khan, Z.R.; Pickett, J.A. Isoschaftoside, a C-glycosylflavonoid from Desmodium uncinatum root exudate, is an allelochemical against the development of Striga. Phytochemistry 2010, 71, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Hao, B.; Caulfield, J.C.; Hamilton, M.L.; Pickett, J.A.; Midega, C.A.; Khan, Z.R.; Wang, J.R.; Hooper, A.M. The biosynthesis of allelopathic di-C-glycosylflavones from the roots of Desmodium incanum (G. Mey.) DC. Org. Biomol. Chem. 2015, 13, 11663–11673. [Google Scholar] [CrossRef] [PubMed]
- Akashi, T.; Aoki, T.; Ayabe, S. Molecular and biochemical characterization of 2-hydroxyisoflavanone dehydratase. Involvement of carboxylesterase-like proteins in leguminous isoflavone biosynthesis. Plant Physiol. 2005, 137, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Bonness, M.S.; Liu, M.; Seradge, E.; Dixon, R.A.; Mabry, T.J. Enzymes of B-ring-deoxy flavonoid biosynthesis in elicited cell cultures of “old man” cactus (Cephalocereus senilis). Arch. Biochem. Biophys. 1995, 321, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Berim, A.; Gang, D.R. Methoxylated flavones: Occurrence, importance, biosynthesis. Phytochem. Rev. 2015. [Google Scholar] [CrossRef]
- Berim, A.; Hyatt, D.C.; Gang, D.R. A set of regioselective O-methyltransferases gives rise to the complex pattern of methoxylated flavones in sweet basil. Plant Physiol. 2012, 160, 1052–1069. [Google Scholar] [CrossRef] [PubMed]
- Berim, A.; Gang, D.R. Characterization of two candidate flavone 8-O-methyltransferases suggests the existence of two potential routes to nevadensin in sweet basil. Phytochemistry 2013, 92, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Berim, A.; Kim, M.J.; Gang, D.R. Identification of a unique 2-oxoglutarate-dependent flavone 7-O-demethylase completes the elucidation of the lipophilic flavone network in basil. Plant Cell Physiol. 2015, 56, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Berim, A.; Gang, D.R. The roles of a flavone-6-hydroxylase and 7-O-demethylation in the flavone biosynthetic network of sweet basil. J. Biol. Chem. 2013, 288, 1795–1805. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Kim, B.G.; Chong, Y.; Lim, Y.; Ahn, J.-H. Cation dependent O-methyltransferases from rice. Planta 2007, 227, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.G.; Lee, Y.; Hur, H.G.; Lim, Y.; Ahn, J.H. Flavonoid 3′-O-methyltransferase from rice: cDNA cloning, characterization and functional expression. Phytochemistry 2006, 67, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Monici, M.; Mulinacci, N.; Baglioni, P.; Vincieri, F.F. Flavone photoreactivity. UV-induced reactions in organic solvents and micellar systems. J. Photochem. Photobiol. B 1993, 20, 167–172. [Google Scholar] [CrossRef]
- Casati, P.; Walbot, V. Differential accumulation of maysin and rhamnosylisoorientin in leaves of high-altitude landraces of maize after UV-B exposure. Plant Cell Environ. 2005, 28, 788–799. [Google Scholar] [CrossRef]
- McNally, D.J.; Wurms, K.V.; Labbé, C.; Bélanger, R.R. Synthesis of C-glycosyl flavonoid phytoalexins as a site-specific response to fungal penetration in cucumber. Physiol. Mol. Plant Pathol. 2003, 63, 293–303. [Google Scholar] [CrossRef]
- Mierziak, J.; Wojtasik, W.; Kostyn, K.; Czuj, T.; Szopa, J.; Kulma, A. Crossbreeding of transgenic flax plants overproducing flavonoids and glucosyltransferase results in progeny with improved antifungal and antioxidative properties. Mol. Breed. 2014, 34, 1917–1932. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-M.; Ibrahim, R.K. Tricin-a potential multifunctional nutraceutical. Phytochem. Rev. 2009, 9, 413–424. [Google Scholar] [CrossRef]
- Kong, C.-H.; Xu, X.-H.; Zhang, M.; Zhang, S.-Z. Allelochemical tricin in rice hull and its aurone isomer against rice seedling rot disease. Pest Manag. Sci. 2010, 66, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Mommer, L.; Kirkegaard, J.; van Ruijven, J. Root-root interactions: Towards a rhizosphere framework. Trends Plant Sci. 2016, 21, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Biate, D.L.; Kumari, A.; Annapurna, K.; Kumar, L.V.; Ramadoss, D.; Reddy, K.K.; Naik, S. Legume root exudates: Their role in symbiotic interactions. In Plant Microbes Symbiosis: Applied Facets; Arora, K.N., Ed.; Springer India: New Delhi, India, 2015; pp. 259–271. [Google Scholar]
- Akiyama, K.; Matsuoka, H.; Hayashi, H. Isolation and identification of a phosphate deficiency-induced C-glycosylflavonoid that stimulates arbuscular mycorrhiza formation in melon roots. Mol. Plant Microbe Interact. 2002, 15, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Redmond, J.W.; Batley, M.; Djordjevic, M.A.; Innes, R.W.; Kuempel, P.L.; Rolfe, B.G. Flavones induce expression of nodulation genes in Rhizobium. Nature 1986, 323, 632–635. [Google Scholar] [CrossRef]
- Zhang, J.; Subramanian, S.; Stacey, G.; Yu, O. Flavones and flavonols play distinct critical roles during nodulation of Medicago truncatula by Sinorhizobium meliloti. Plant J. 2009, 57, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, U.A.; Joseph, C.M.; Phillips, D.A. Flavonoids released naturally from alfalfa seeds enhance growth rate of Rhizobium meliloti. Plant Physiol. 1991, 95, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Scervino, J.M.; Ponce, M.A.; Erra-Bassells, R.; Bompadre, J.; Vierheilig, H.; Ocampo, J.A.; Godeas, A. The effect of flavones and flavonols on colonization of tomato plants by arbuscular mycorrhizal fungi of the Genera gigaspora and Glomus. Can. J. Microbiol. 2007, 53, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.P.; Staehelin, C.; Vierheilig, H.; Wiemken, A.; Jabbouri, S.; Broughton, W.J.; Vogeli-Lange, R.; Boller, T. Rhizobial nodulation factors stimulate mycorrhizal colonization of nodulating and nonnodulating soybeans. Plant Physiol. 1995, 108, 1519–1525. [Google Scholar] [PubMed]
- Muzell Trezzi, M.; Vidal, R.A.; Balbinot Junior, A.A.; von Hertwig Bittencourt, H.; da Silva Souza Filho, A.P. Allelopathy: Driving mechanisms governing its activity in agriculture. J. Plant Interact. 2016, 11, 53–60. [Google Scholar] [CrossRef]
- Kong, C.H.; Zhao, H.; Xu, X.H.; Wang, P.; Gu, Y. Activity and allelopathy of soil of flavone O-glycosides from rice. J. Agric. Food Chem. 2007, 55, 6007–6012. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Xu, X.; Zhou, B.; Hu, F.; Zhang, C.; Zhang, M. Two compounds from allelopathic rice accession and their inhibitory activity on weeds and fungal pathogens. Phytochemistry 2004, 65, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Liang, W.; Xu, X.; Hu, F.; Wang, P.; Jiang, Y. Release and activity of allelochemicals from allelopathic rice seedlings. J. Agric. Food Chem. 2004, 52, 2861–2865. [Google Scholar] [CrossRef] [PubMed]
- Agrell, J.; Oleszek, W.; Stochmal, A.; Olsen, M.; Anderson, P. Herbivore-induced responses in alfalfa (Medicago sativa). J. Chem. Ecol. 2003, 29, 303–320. [Google Scholar] [CrossRef]
- Grayer, R.J.; Harborne, J.B.; Kimmins, F.M.; Stevenson, P.C.; Wijayagunasekera, H.N.P. Phenolics in Rice Phloem Sap as Sucking Deterrents to the Brown Planthopper, Nilaparvata Lugens; International Society for Horticultural Science (ISHS): Leuven, Belgium, 1994; pp. 691–694. [Google Scholar]
- Adjei-Afriyie, F.; Kim, C.S.; Takemura, M.; Ishikawa, M.; Tebayashi, S.; Horiike, M. Probing stimulants from the rice plant towards the smaller brown planthopper, Laodelphax striatellus (Fallen) (Homoptera: Delphacidae). Z. Naturforsch. C Biol. Sci. 2000, 55, 1038–1043. [Google Scholar] [CrossRef]
- Miles, D.H.; Tunsuwan, K.; Chittawong, V.; Kokpol, U.; Choudhary, M.I.; Clardy, J. Boll weevil antifeedants from Arundo donax. Phytochemistry 1993, 34, 1277–1279. [Google Scholar] [CrossRef]
- Dreyer, D.L.; Jones, K.C. Feeding deterrency of flavonoids and related phenolics towards Schizaphis graminum and Myzus persicae: Aphid feeding deterrents in wheat. Phytochemistry 1981, 20, 2489–2493. [Google Scholar] [CrossRef]
- Ibanez, S.; Gallet, C.; Dommanget, F.; Després, L. Plant chemical defence: A partner control mechanism stabilising plant-seed-eating pollinator mutualisms. BMC Evol. Biol. 2009, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gallet, C.; Ibanez, S.; Zinger, L.; Taravel, F.R.; Trierweiler, M.; Jeacomine, I.; Despres, L. Plant chemical defense induced by a seed-eating pollinator mutualist. J. Chem. Ecol. 2007, 33, 2078–2089. [Google Scholar] [CrossRef] [PubMed]
- Soriano, I.R.; Asenstorfer, R.E.; Schmidt, O.; Riley, I.T. Inducible flavone in oats (Avena sativa) is a novel defense against plant-parasitic nematodes. Phytopathology 2004, 94, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Yabuya, T.; Nakamura, M.; Iwashina, T.; Yamaguchi, M.; Takehara, T. Anthocyanin-flavone copigmentation in bluish purple flowers of Japanese garden iris (Iris ensata Thunb.). Euphytica 1997, 98, 163–167. [Google Scholar] [CrossRef]
- Pan, Y.-Z.; Guan, Y.; Wei, Z.-F.; Peng, X.; Li, T.-T.; Qi, X.-L.; Zu, Y.-G.; Fu, Y.-J. Flavonoid C-glycosides from pigeon pea leaves as color and anthocyanin stabilizing agent in blueberry juice. Ind. Crops Prod. 2014, 58, 142–147. [Google Scholar] [CrossRef]
- Trouillas, P.; Sancho-Garcia, J.C.; De Freitas, V.; Gierschner, J.; Otyepka, M.; Dangles, O. Stabilizing and modulating color by copigmentation: Insights from theory and experiment. Chem. Rev. 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fossen, T.; Rayyan, S.; Holmberg, M.H.; Nimtz, M.; Andersen, O.M. Covalent anthocyanin-flavone dimer from leaves of Oxalis triangularis. Phytochemistry 2007, 68, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Del Río, J.C.; Rencoret, J.; Prinsen, P.; Martínez, Á.T.; Ralph, J.; Gutiérrez, A. Structural characterization of wheat straw lignin as revealed by analytical pyrolysis, 2D-NMR, and reductive cleavage methods. J. Agric. Food Chem. 2012, 60, 5922–5935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, T.-T.; Mao, J.-Z.; Yuan, T.-Q.; Wen, J.-L.; Xu, F. Structural elucidation of the lignins from stems and foliage of Arundo donax Linn. J. Agric. Food Chem. 2013, 61, 5361–5370. [Google Scholar] [CrossRef] [PubMed]
- Rencoret, J.; Ralph, J.; Marques, G.; Gutierrez, A.; Martinez, A.T.; del Rio, J.C. Structural characterization of lignin isolated from coconut (Cocos nucifera) coir fibers. J. Agric. Food Chem. 2013, 61, 2434–2445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Pu, Y.; Yoo, C.G.; Ragauskas, A.J. The occurrence of tricin and its derivatives in plants. Green Chem. 2016, 18, 1439–1454. [Google Scholar] [CrossRef]
- Selvaraj, S.; Krishnaswamy, S.; Devashya, V.; Sethuraman, S.; Krishnan, U.M. Influence of membrane lipid composition on flavonoid-membrane interactions: Implications on their biological activity. Prog. Lipid Res. 2015, 58, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Joshi, A.; Joshi, U.J.; Srivastava, S.; Govil, G. Localization and interaction of hydroxyflavones with lipid bilayer model membranes: A study using DSC and multinuclear NMR. Eur. J. Med. Chem. 2014, 80, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Hendrich, A.B. Flavonoid-membrane interactions: Possible consequences for biological effects of some polyphenolic compounds. Acta Pharmacol. Sin. 2006, 27, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Oteiza, P.I.; Erlejman, A.G.; Verstraeten, S.V.; Keen, C.L.; Fraga, C.G. Flavonoid-membrane interactions: A protective role of flavonoids at the membrane surface? Clin. Dev. Immunol. 2005, 12, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Scheidt, H.A.; Pampel, A.; Nissler, L.; Gebhardt, R.; Huster, D. Investigation of the membrane localization and distribution of flavonoids by high-resolution magic angel spinning NMR spectroscopy. Biochim. Biophys. Acta 2004, 1663, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Janjua, N.K.; Shaheen, A.; Yaqub, A.; Perveen, F.; Sabahat, S.; Mumtaz, M.; Jacob, C.; Ba, L.A.; Mohammed, H.A. Flavonoid-DNA binding studies and thermodynamic parameters. Spectrochim. Acta Mol. Biomol. Spectrosc. 2011, 79, 1600–1604. [Google Scholar] [CrossRef] [PubMed]
- Bible, K.C.; Bible, R.H., Jr.; Kottke, T.J.; Svingen, P.A.; Xu, K.; Pang, Y.P.; Hajdu, E.; Kaufmann, S.H. Flavopiridol binds to duplex DNA. Cancer Res. 2000, 60, 2419–2428. [Google Scholar] [PubMed]
- Ragazzon, P.A.; Iley, J.; Missailidis, S. Structure-activity studies of the binding of the flavonoid scaffold to DNA. Anticancer Res. 2009, 29, 2285–2293. [Google Scholar] [PubMed]
- Zhang, S.; Sun, X.; Kong, R.; Xu, M. Studies on the interaction of apigenin with calf thymus DNA by spectroscopic methods. Spectrochim. Acta Mol. Biomol. Spectrosc. 2014, 136PC, 1666–1670. [Google Scholar] [CrossRef] [PubMed]
- Nafisi, S.; Shadaloi, A.; Feizbakhsh, A.; Tajmir-Riahi, H.A. RNA binding to antioxidant flavonoids. J. Photochem. Photobiol. B 2009, 94, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Bao, W.; Ding, H.; Jang, J.; Zou, G. Binding modes of flavones to human serum albumin: Insights from experimental and computational studies. J. Phys. Chem. B 2010, 114, 12938–12947. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Cao, H.; Wang, Y.; Yamamoto, K.; Wei, X. Structure-affinity relationship of flavones on binding to serum albumins: Effect of hydroxyl groups on ring A. Mol. Nutr. Food Res. 2010, 54, S253–S260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Chen, X.; Guo, J.; Wang, J. Spectroscopic investigation of the interaction between chrysin and bovine serum albumin. J. Mol. Struct. 2009, 921, 346–351. [Google Scholar] [CrossRef]
- Di Pietro, A.; Conseil, G.; Pérez-Victoria, J.M.; Dayan, G.; Baubichon-Cortay, H.; Trompier, D.; Steinfels, E.; Jault, J.M.; de Wet, H.; Maitrejean, M.; et al. Modulation by flavonoids of cell multidrug resistance mediated by P-glycoprotein and related ABC transporters. Cell. Mol. Life Sci. 2014, 59, 307–322. [Google Scholar] [CrossRef]
- Canivenc-Lavier, M.C.; Bentejac, M.; Miller, M.L.; Leclerc, J.; Siess, M.H.; Latruffe, N.; Suschetet, M. Differential effects of nonhydroxylated flavonoids as inducers of cytochrome P450 1A and 2B isozymes in rat liver. Toxicol. Appl. Pharm. 1996, 136, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Reiners, J.J., Jr.; Clift, R.; Mathieu, P. Suppression of cell cycle progression by flavonoids: Dependence on the aryl hydrocarbon receptor. Carcinogenesis 1999, 28, 1561–1566. [Google Scholar] [CrossRef]
- Stresser, D.M.; Turner, S.D.; McNamara, J.; Stocker, P.; Miller, V.P.; Crespi, C.L.; Patten, C.J. A high-throughput screen to identify inhibitors of aromatase (CYP19). Anal. Biochem. 2000, 284, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Hanrahan, J.R.; Chebib, M.; Johnston, G.A.R. Flavonoid modulation of GABAA receptors. Br. J. Pharmacol. 2011, 163, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Huen, M.S.Y.; Leung, J.W.C.; Ng, W.; Lui, W.S.; Chan, M.N.S.; Tze-Fei Wong, J.; Xue, H. 5,7-dihydroxy-6-methoxyflavone, a benzodiazepine site ligand isolated from Scutellaria baicalensis Georgi, with selective antagonistic properties. Biochem. Pharmacol. 2003, 66, 125–132. [Google Scholar] [CrossRef]
- Hui, K.M.; Huen, M.S.; Wang, H.Y.; Zheng, H.; Sigel, E.; Baur, R.; Ren, H.; Li, Z.W.; Wong, J.T.-F.; Xue, H. Anxiolytic effect of wogonin, a benzodiazepine receptor ligand isolated from Scutellaria baicalensis Georgi. Biochem. Pharmacol. 2002, 64, 1415–1424. [Google Scholar] [CrossRef]
- Lin, C.-M.; Chen, C.-S.; Chen, C.-T.; Liang, Y.-C.; Lin, J.-K. Molecular modeling of flavonoids that inhibits xanthine oxidase. Biochem. Biophys. Res. Commun. 2002, 294, 167–172. [Google Scholar] [CrossRef]
- Arango, D.; Morohashi, K.; Yilmaz, A.; Kuramochi, K.; Parihar, A.; Brahimaj, B.; Grotewold, E.; Doseff, A.I. Molecular basis for the action of a dietary flavonoid revealed by the comprehensive identification of apigenin human targets. Proc. Natl. Acad. Sci. USA 2013, 110, E2153–E2162. [Google Scholar] [CrossRef] [PubMed]
- Cos, P.; Ying, L.; Calomme, M.; Hu, J.P.; Cimanga, K.; Van Poel, B.; Pieters, L.; Vlietinck, A.J.; Vanden Berghe, D. Structure-activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J. Nat. Prod. 1998, 61, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Duarte, S.; Arango, D.; Parihar, A.; Hamel, P.; Yasmeen, R.; Doseff, A.I. Apigenin protects endothelial cells from lipopolysaccharide (LPS)-induced inflammation by decreasing caspase-3 activation and modulating mitochondrial function. Int. J. Mol. Sci. 2013, 14, 17664–17679. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Takeda, K.; Kaisho, T. Toll-like receptors: Critical proteins linking innate and acquired immunity. Nat. Immunol. 2001, 2, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Dardick, C.; Ronald, P. Plant and animal pathogen recognition receptors signal through non-RD kinases. PLoS Pathog. 2006, 2, e2. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, C.; Batra, S.; Vargo, M.A.; Voss, O.H.; Gavrilin, M.A.; Wewers, M.D.; Guttridge, D.C.; Grotewold, E.; Doseff, A.I. Apigenin blocks lipopolysaccharide-induced lethality in vivo and proinflammatory cytokines expression by inactivating NF-kappaB through the suppression of p65 phosphorylation. J. Immunol. 2007, 179, 7121–7127. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, H.; Arango, D.; Nicholas, C.; Duarte, S.; Nuovo, G.J.; He, W.; Voss, O.H.; Gonzalez-Mejia, M.E.; Guttridge, D.C.; Grotewold, E.; et al. Dietary apigenin exerts immune-regulatory activity in vivo by reducing NF-kappaB activity, halting leukocyte infiltration and restoring normal metabolic function. Int. J. Mol. Sci. 2016, 17, E323. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.-H.; Lai, C.-S.; Wang, Y.-J.; Ho, C.-T. Acacetin suppressed LPS-induced up-expression of iNOS and COX-2 in murine macrophages and TPA-induced tumor promotion in mice. Biochem. Pharmacol. 2006, 72, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- Hostetler, G.; Riedl, K.; Cardenas, H.; Diosa-Toro, M.; Arango, D.; Schwartz, S.; Doseff, A.I. Flavone deglycosylation increases their anti-inflammatory activity and absorption. Mol. Nutr. Food Res. 2012, 56, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Ku, S.K.; Lee, T.; Bae, J.S. Orientin inhibits HMGB1-induced inflammatory responses in HUVECs and in murine polymicrobial sepsis. Inflammation 2014, 37, 1705–1717. [Google Scholar] [CrossRef] [PubMed]
- Arango, D.; Diosa-Toro, M.; Rojas-Hernandez, L.S.; Cooperstone, J.L.; Schwartz, S.J.; Mo, X.; Jiang, J.; Schmittgen, T.D.; Doseff, A.I. Dietary apigenin reduces LPS-induced expression of miR-155 restoring immune balance during inflammation. Mol. Nutr. Food Res. 2015, 59, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Gou, J.Y.; Felippes, F.F.; Liu, C.-J.; Weigel, D.; Wang, J.W. Negative regulation of anthocyanin biosynthesis in Arabidopsis by a miR156-targeted SPL transcription factor. Plant Cell 2011, 23, 1512–1522. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Giugliano, D. Diet and inflammation: A link to metabolic and cardiovascular diseases. Eur. Heart J. 2006, 27, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvado, J.; Covas, M.-I.; Corella, D.; Aros, F.; Gomez-Gracia, E.; Ruiz-Gutierrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.M.; Londero, T.M.; Goemann, I.M.; Schaan, B.D. Cardiometabolic effects of cascade trial explained by mediterranean diet. Ann. Intern. Med. 2016, 164, 573–574. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, J.J.; Alblas, J.; van der Pol, S.M.A.; van Tol, E.A.F.; Dijkstra, C.D.; de Vries, H.E. Flavonoids influence monocytic GTPase activity and are protective in experimental allergic encephalitis. J. Exp. Med. 2004, 200, 1667–1672. [Google Scholar] [CrossRef] [PubMed]
- Henkels, K.M.; Frondorf, K.; Gonzalez-Mejia, M.E.; Doseff, A.L.; Gomez-Cambronero, J. IL-8-induced neutrophil chemotaxis is mediated by Janus kinase 3 (JAK3). FEBS Lett. 2011, 585, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Chen, W.K.; Wang, C.-J.; Lin, W.-L.; Tseng, T.H. Apigenin inhibits HGF-promoted invasive growth and metastasis involving blocking PI3K/AKT pathway and beta 4 integrin function in MDA-MB-231 breast cancer cells. Toxicol. Appl. Pharmacol. 2008, 226, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Vargo, M.A.; Voss, O.H.; Poustka, F.; Cardounel, A.J.; Grotewold, E.; Doseff, A.I. Apigenin-induced-apoptosis is mediated by the activation of pkcdelta and caspases in leukemia cells. Biochem. Pharmacol. 2006, 72, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Afaq, F.; Mukhtar, H. Involvement of nuclear factor-kappa B, Bax and Bcl-2 in induction of cell cycle arrest and apoptosis by apigenin in human prostate carcinoma cells. Oncogene 2002, 21, 3727–3738. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Van Dross, R.T.; Abu-Yousif, A.; Morrison, A.R.; Pelling, J.C. Apigenin prevents UVB-induced cyclooxygenase 2 expression: Coupled mRNA stabilization and translational inhibition. Mol. Cell. Biol. 2007, 27, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Arango, D.; Parihar, A.; Villamena, F.A.; Wang, L.; Freitas, M.A.; Grotewold, E.; Doseff, A.I. Apigenin induces DNA damage through the PKCδ-dependent activation of ATM and H2AX causing down-regulation of genes involved in cell cycle control and DNA repair. Biochem. Pharmacol. 2012, 84, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Mejia, M.E.; Voss, O.H.; Murnan, E.J.; Doseff, A.I. Apigenin-induced apoptosis of leukemia cells is mediated by a bimodal and differentially regulated residue-specific phosphorylation of heat-shock protein-27. Cell Death Dis. 2010, 1, e64. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, S.; Kim, S.L.; Choi, J.W.; Seo, J.Y.; Choi, D.J.; Park, Y.I. Corn silk maysin induces apoptotic cell death in PC-3 prostate cancer cells via mitochondria-dependent pathway. Life Sci. 2014, 119, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Kanwal, R.; Shankar, E.; Datt, M.; Chance, M.R.; Fu, P.; MacLennan, G.T.; Gupta, S. Apigenin blocks IKKα activation and suppresses prostate cancer progression. Oncotarget 2015, 6, 31216–31232. [Google Scholar] [PubMed]




| Protein | Accession No. | Species | Function | Ref. |
|---|---|---|---|---|
| CYP93B1 | AB001380 | Glycyrrhiza echinata (Licorice) | F2H | [32] |
| CYP93B2 | AF156976 | Gerbera hybrida | FNSII | [37] |
| CYP93B3 | AB028151 | Antirrhinum majus (Snapdragon) | FNSII | [28] |
| CYP93B4 | AB028152 | Torenia hybrida | FNSII | [38] |
| CYP93B6 | AB045592 | Perilla frutescens var. Crispa | FNSII | [39] |
| CYP93B10 | DQ354373 | Medicago truncatula (Barrelclover) | F2H | [33] |
| CYP93B11 | DQ335809 | Medicago truncatula (Barrelclover) | F2H | [33] |
| CYP93B16 | GU658027 | Glycine max (Soybean) | FNSII | [29] |
| CYP93B24 | KT963453 | Scutellaria baicalensis | FNSII | [40] |
| CYP93B25 | KT963453 | Scutellaria baicalensis | FNSII | [40] |
| CYP93G1 | AK100972 | Oryza sativa (Rice) | FNSII | [30] |
| CYP93G2 | AK099468 | Oryza sativa (Rice) | F2H | [35] |
| CYP93G3 | XP_002461286 | Sorghum bicolor (Sorghum) | F2H | [34] |
| CYP93G5 | GRMZM2G167336 | Zea mays (Maize) | F2H | [36] |
| LjFNSII-1.1 | KU127576 | Lonicera japonica | FNSII | [31] |
| LjFNSII-2.1 | KU127578 | Lonicera japonica | FNSII | [31] |
| LmFNSII-1.1 | KU127580 | Lonicera macranthoides | FNSII | [31] |
| PcFNSI | AY230247 | Petroselinum crispum (Parsley) | FNSI | [19] |
| DcFNSI | AY817675 | Daucus carota (Wild carrot) | FNSI | [21] |
| AgFNSI | AY817676 | Apium graveolens (Celery) | FNSI | [21] |
| CmFNSI | AY817677 | Conium maculatum | FNSI | [21] |
| AcFNSI | DQ683350 | Aethusa cynapium | FNSI | [20] |
| AaFNSI | DQ683352 | Angelica archangelica (Wild celery) | FNSI | [16] |
| CcFNSI | DQ683349 | Cuminum cyminum | FNSI | [16] |
| OsFNSI-1 | NP_922524 | Oryza sativa (Rice) | FNSI | [22] |
| PaFNSI | KJ439220 | Plagiochasma appendiculatum (Liverwort) | FNSI/F2H | [23] |
| ZmFNSI-1 | GRMZM2G099467 | Zea mays (Maize) | FNSI | [24] |
| AtDMR6 | AT5G24530 | Arabidopsis thaliana (Arabidopsis) | FNSI | [24] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, N.; Doseff, A.I.; Grotewold, E. Flavones: From Biosynthesis to Health Benefits. Plants 2016, 5, 27. https://doi.org/10.3390/plants5020027
Jiang N, Doseff AI, Grotewold E. Flavones: From Biosynthesis to Health Benefits. Plants. 2016; 5(2):27. https://doi.org/10.3390/plants5020027
Chicago/Turabian StyleJiang, Nan, Andrea I. Doseff, and Erich Grotewold. 2016. "Flavones: From Biosynthesis to Health Benefits" Plants 5, no. 2: 27. https://doi.org/10.3390/plants5020027
APA StyleJiang, N., Doseff, A. I., & Grotewold, E. (2016). Flavones: From Biosynthesis to Health Benefits. Plants, 5(2), 27. https://doi.org/10.3390/plants5020027