Identification of Superior Alleles for Seedling Stage Salt Tolerance in the USDA Rice Mini-Core Collection
Abstract
:1. Introduction
2. Results
2.1. Phenotypic Variation for Seedling Salt Tolerance Traits in USDA Rice Mini-Core
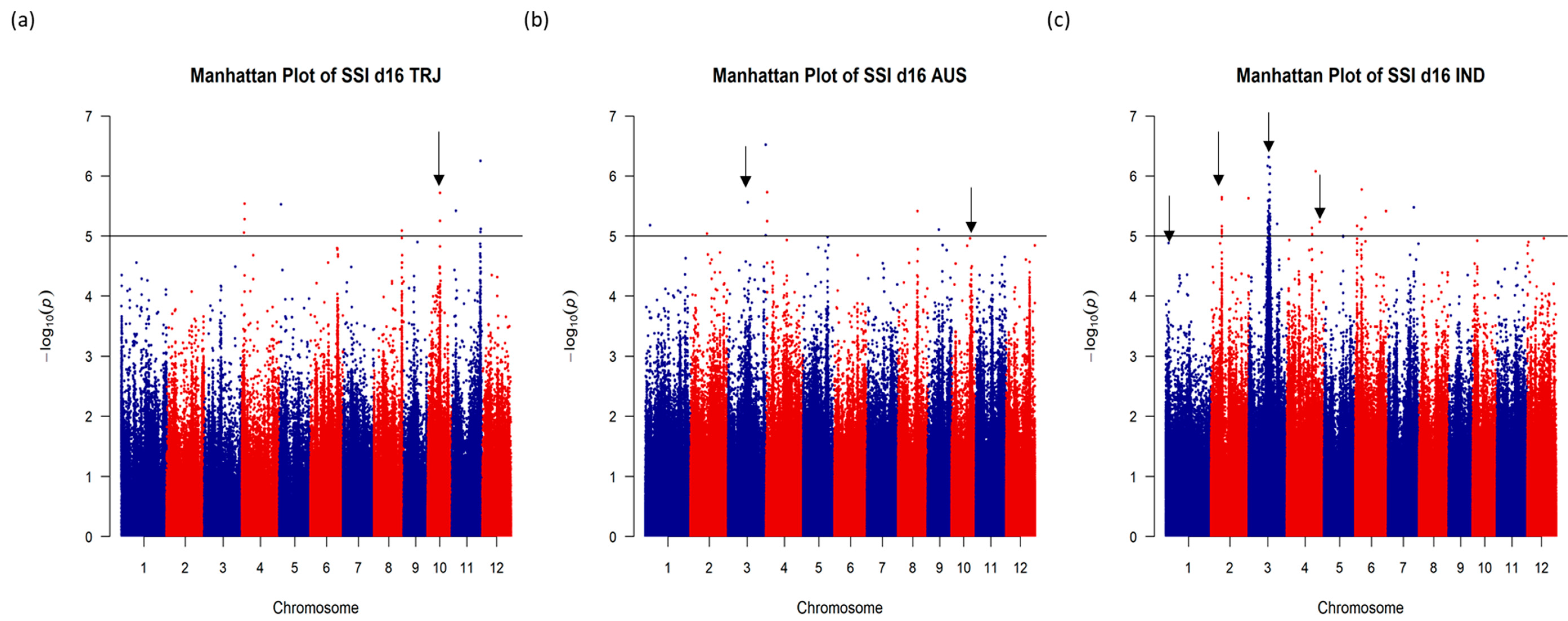
2.2. Identification of SNPs and QTLs Associated with Early Vigor and Tolerance for Salt Injury under Salt Stress
3. Discussion
4. Materials and Methods
4.1. Plant Material, Growth Conditions, Salt Stress Treatment
4.2. Phenotypic Data Collection, Processing, and Analysis
- Growth in plant height under 10 days of salt stress (Δ PHT-d10): PHT_d10 – PHT_d0
- Growth in plant height under 16 days of salt stress (Δ PHT-d16): PHT_d16 – PHT_d0
- Green leaf number (GLN) produced under 14 days salt stress (Δ green leaf#): GLN_d14 – GLN_d0
- Total biomass after 16 days of salt stress (TB): SB_d16 + RB_d16
4.3. Genome-Wide Association Mapping, Identification of Single-Nucleotide Polymorphisms (SNPs) and Candidate Genes
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McNally, K.L.; Childs, K.L.; Bohnert, R.; Davidson, R.M.; Zhao, K.; Ulat, V.J.; Zeller, G.; Clark, R.M.; Hoen, D.R.; Bureau, T.E.; et al. Genomewide SNP variation reveals relationships among landraces and modern varieties of rice. Proc. Natl. Acad. Sci. USA 2009, 106, 12273–12278. [Google Scholar] [CrossRef] [Green Version]
- Nigatu, G.; Hansen, J.; Childs, N.; Seeley, R. Sub-Saharan Africa Is Projected to Be the Leader in Global Rice Imports; Amber Waves: 2017, United States Department of Agriculture, Economic Research Service. Available online: https://www.ers.usda.gov/amber-waves/2017/october/sub-saharan-africa-is-projected-to-be-the-leader-in-global-rice-imports/ (accessed on 30 September 2019).
- Kang, M.; Jackson, R.B. Salinity of deep groundwater in California: Water quantity, quality, and protection. Proc. Natl. Acad. Sci. USA 2016, 113, 7768–7773. [Google Scholar] [CrossRef] [Green Version]
- Verma, A.K.; Gupta, S.K.; Isaac, R.K. Use of saline water for irrigation in monsoon climate and deep water table regions: Simulation modeling with SWAP. Agric. Water Manag. 2012, 115, 186–193. [Google Scholar] [CrossRef]
- Wassmann, R.; Hien, N.X.; Hoanh, C.T.; Tuong, T.P. Sea level rise affecting the Vietnamese Mekong delta: Water elevation in the flood season and implications for rice production. Clim. Chang. 2004, 66, 89–107. [Google Scholar] [CrossRef]
- Takagi, H.; Tamiru, M.; Abe, A.; Yoshida, K.; Uemura, A.; Yaegashi, H.; Obara, T.; Oikawa, K.; Utsushi, H.; Kanzaki, E.; et al. MutMap accelerates breeding of a salt-tolerant rice cultivar. Nat. Biotechnol. 2015, 33, 445. [Google Scholar] [CrossRef]
- Reba, M.L.; Daniels, M.; Chen, Y.; Sharpley, A.; Bouldin, J.; Teague, T.G.; Daniel, P.; Henry, C.G. A statewide network for monitoring agricultural water quality and water quantity in Arkansas. J. Soil Water Conserv. 2013, 68, 45A–49A. [Google Scholar] [CrossRef] [Green Version]
- Aguilar, M.; Fernández-Ramírez, J.L.; Aguilar-Blanes, M.; Ortiz-Romero, C. Rice sensitivity to saline irrigation in Southern Spain. Agric. Water Manag. 2017, 188, 21–28. [Google Scholar] [CrossRef]
- Grattan, S.R.; Zeng, L.; Shannon, M.C.; Roberts, S.R. Rice is more sensitive to salinity than previously thought. Calif. Agric. 2002, 56, 189–198. [Google Scholar] [CrossRef] [Green Version]
- Hoang, T.M.L.; Tran, T.N.; Nguyen, T.K.T.; Williams, B.; Wurm, P.; Bellairs, S.; Mundree, S. Improvement of salinity stress tolerance in rice: Challenges and opportunities. Agronomy 2016, 6, 54. [Google Scholar] [CrossRef]
- Zeng, L.; Shannon, M.C. Salinity effects on seedling growth and yield components of rice. Crop Sci. 2000, 40, 996–1003. [Google Scholar] [CrossRef]
- Morton, M.J.L.; Awlia, M.; Al-Tamimi, N.; Saade, S.; Pailles, Y.; Negrão, S.; Tester, M. Salt stress under the scalpel—Dissecting the genetics of salt tolerance. Plant J. 2019, 97, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Munns, R. Genes and salt tolerance: Bringing them together. New Phytol. 2005, 167, 645–663. [Google Scholar] [CrossRef] [PubMed]
- Reddy, I.N.B.L.; Kim, B.-K.; Yoon, I.-S.; Kim, K.-H.; Kwon, T.-R. Salt tolerance in rice: Focus on mechanisms and approaches. Rice Sci. 2017, 24, 123–144. [Google Scholar] [CrossRef]
- Li, Y.-F.; Zheng, Y.; Vemireddy, L.R.; Panda, S.K.; Jose, S.; Ranjan, A.; Panda, P.; Govindan, G.; Cui, J.; Wei, K.; et al. Comparative transcriptome and translatome analysis in contrasting rice genotypes reveals differential mRNA translation in salt-tolerant Pokkali under salt stress. BMC Genom. 2018, 19, 935. [Google Scholar] [CrossRef] [PubMed]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Negrão, S.; Courtois, B.; Ahmadi, N.; Abreu, I.; Saibo, N.; Oliveira, M.M. Recent updates on salinity stress in rice: From physiological to molecular responses. Crit. Rev. Plant Sci. 2011, 30, 329–377. [Google Scholar] [CrossRef]
- Yuan, F.; Leng, B.; Wang, B. Progress in studying salt secretion from the salt glands in recretohalophytes: How do plants secrete salt? Front. Plant Sci. 2016, 7, 977. [Google Scholar] [CrossRef]
- Thomson, M.J.; De Ocampo, M.; Egdane, J.; Rahman, M.A.; Sajise, A.G.; Adorada, D.L.; Tumimbang-Raiz, E.; Blumwald, E.; Seraj, Z.I.; Singh, R.K.; et al. Characterizing the Saltol quantitative trait locus for salinity tolerance in rice. Rice 2010, 3, 148–160. [Google Scholar] [CrossRef]
- Bonilla, P.; Dvorak, J.; Mackell, D.; Deal, K.; Gregorio, G. RFLP and SSLP mapping of salinity tolerance genes in chromosome 1 of rice (Oryza sativa L.) using recombinant inbred lines. Philipp. Agric. Sci. 2002, 85, 68–76. [Google Scholar]
- Zhang, G.-Y.; Guo, Y.; Chen, S.-L.; Chen, S.-Y. RFLP tagging of a salt tolerance gene in rice. Plant Sci. 1995, 110, 227–234. [Google Scholar] [CrossRef]
- Cohen, S.P.; Leach, J.E. Abiotic and biotic stresses induce a core transcriptome response in rice. Sci. Rep. 2019, 9, 6273. [Google Scholar] [CrossRef] [PubMed]
- Huggins, T.D.; Chen, M.H.; Fjellstrom, R.G.; Jackson, A.K.; McClung, A.M.; Edwards, J.D. Association analysis of three diverse rice (Oryza sativa L.) germplasm collections for loci regulating grain quality traits. Plant Genome 2019, 12, 170085. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, X.; Vieira, F.G.; Xiao, Y.; Li, Z.; Wang, J.; Nielsen, R.; Chu, C. The power of inbreeding: NGS-based GWAS of rice reveals convergent evolution during rice domestication. Mol. Plant 2016, 9, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Mauleon, R.; Hu, Z.; Chebotarov, D.; Tai, S.; Wu, Z.; Li, M.; Zheng, T.; Fuentes, R.R.; Zhang, F.; et al. Genomic variation in 3010 diverse accessions of Asian cultivated rice. Nature 2018, 557, 43–49. [Google Scholar] [CrossRef]
- Rana, M.M.; Takamatsu, T.; Baslam, M.; Kaneko, K.; Itoh, K.; Harada, N.; Sugiyama, T.; Ohnishi, T.; Kinoshita, T.; Takagi, H.; et al. Salt Tolerance Improvement in Rice through Efficient SNP Marker-Assisted Selection Coupled with Speed-Breeding. Int. J. Mol. Sci. 2019, 20, 2585. [Google Scholar] [CrossRef]
- Singh, A.K.; Ansari, M.W.; Pareek, A.; Singla-Pareek, S.L. Raising salinity tolerant rice: Recent progress and future perspectives. Physiol. Mol. Biol. Plants 2008, 14, 137–154. [Google Scholar] [CrossRef]
- McCouch, S.R.; Wright, M.H.; Tung, C.W.; Maron, L.G.; McNally, K.L.; Fitzgerald, M.; Singh, N.; DeClerck, G.; Agosto-Perez, F.; Korniliev, P.; et al. Open access resources for genome-wide association mapping in rice. Nat. Commun. 2016, 7, 10532. [Google Scholar] [CrossRef]
- Al-Tamimi, N.; Brien, C.; Oakey, H.; Berger, B.; Saade, S.; Ho, Y.S.; Schmöckel, S.M.; Tester, M.; Negrão, S. Salinity tolerance loci revealed in rice using high-throughput non-invasive phenotyping. Nat. Commun. 2016, 7, 13342. [Google Scholar] [CrossRef] [Green Version]
- Patishtan, J.; Hartley, T.N.; Fonseca de Carvalho, R.; Maathuis, F.J.M. Genome-wide association studies to identify rice salt-tolerance markers. Plant Cell Environ. 2018, 41, 970–982. [Google Scholar] [CrossRef]
- Kim, T.-S.; He, Q.; Kim, K.-W.; Yoon, M.-Y.; Ra, W.-H.; Li, F.P.; Tong, W.; Yu, J.; Oo, W.H.; Choi, B.; et al. Genome-wide resequencing of KRICE_CORE reveals their potential for future breeding, as well as functional and evolutionary studies in the post-genomic era. BMC Genom. 2016, 17, 408. [Google Scholar] [CrossRef]
- Ahmadi, N.; Negrão, S.; Katsantonis, D.; Frouin, J.; Ploux, J.; Letourmy, P.; Droc, G.; Babo, P.; Trindade, H.; Bruschi, G.; et al. Targeted association analysis identified japonica rice varieties achieving Na+/K+ homeostasis without the allelic make-up of the salt tolerant indica variety Nona Bokra. Theor. Appl. Genet. 2011, 123, 881–895. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, D.; Wang, M.; Sun, J.; Qi, Y.; Li, J.; Wei, X.; Han, L.; Qiu, Z.; Tang, S.; et al. A core collection and mini core collection of Oryza sativa L. in China. Theor. Appl. Genet. 2011, 122, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, K.K.; Singh, A.; Pattnaik, S.; Sandhu, M.; Kaur, S.; Jain, S.; Tiwari, S.; Mehrotra, S.; Anumalla, M.; Samal, R.; et al. Identification of a diverse mini-core panel of Indian rice germplasm based on genotyping using microsatellite markers. Plant Breed. 2015, 134, 164–171. [Google Scholar] [CrossRef]
- Agrama, H.A.; Yan, W.; Lee, F.; Fjellstrom, R.; Chen, M.-H.; Jia, M.; McClung, A. Genetic assessment of a mini-core subset developed from the USDA rice genebank. Crop Sci. 2009, 49, 1336–1346. [Google Scholar] [CrossRef]
- Yan, W.; Rutger, J.N.; Bryant, R.J.; Bockelman, H.E.; Fjellstrom, R.G.; Chen, M.-H.; Tai, T.H.; McClung, A.M. Development and evaluation of a core subset of the USDA rice germplasm collection. Crop Sci. 2007, 47, 869–878. [Google Scholar] [CrossRef]
- Li, X.; Yan, W.; Agrama, H.; Hu, B.; Jia, L.; Jia, M.; Jackson, A.; Moldenhauer, K.; McClung, A.; Wu, D. Genotypic and phenotypic characterization of genetic differentiation and diversity in the USDA rice mini-core collection. Genetica 2010, 138, 1221–1230. [Google Scholar] [CrossRef]
- Li, X.; Yan, W.; Agrama, H.; Jia, L.; Shen, X.; Jackson, A.; Moldenhauer, K.; Yeater, K.; McClung, A.; Wu, D. Mapping QTLs for improving grain yield using the USDA rice mini-core collection. Planta 2011, 234, 347–361. [Google Scholar] [CrossRef]
- Li, X.; Yan, W.; Agrama, H.; Jia, L.; Jackson, A.; Moldenhauer, K.; Yeater, K.; McClung, A.; Wu, D. Unraveling the complex trait of harvest index with association mapping in rice (Oryza sativa L.). PLoS ONE 2012, 7, e29350. [Google Scholar] [CrossRef]
- Bryant, R.; Proctor, A.; Hawkridge, M.; Jackson, A.; Yeater, K.; Counce, P.; Yan, W.; McClung, A.; Fjellstrom, R. Genetic variation and association mapping of silica concentration in rice hulls using a germplasm collection. Genetica 2011, 139, 1383–1398. [Google Scholar] [CrossRef]
- Bryant, R.J.; Jackson, A.K.; Yeater, K.M.; Yan, W.G.; McClung, A.M.; Fjellstrom, R.G. Genetic variation and association mapping of protein concentration in brown rice using a diverse rice germplasm collection. Cereal Chem. 2013, 90, 445–452. [Google Scholar] [CrossRef]
- Li, K.; Bao, J.; Corke, H.; Sun, M. Association Analysis of Markers Derived from Starch Biosynthesis Related Genes with Starch Physicochemical Properties in the USDA Rice Mini-Core Collection. Front. Plant Sci. 2017, 8, 424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, L.; Yan, W.; Agrama, H.A.; Yeater, K.; Li, X.; Hu, B.; Moldenhauer, K.; McClung, A.; Wu, D. Searching for germplasm resistant to sheath blight from the USDA rice core collection. Crop Sci. 2011, 51, 1507–1517. [Google Scholar] [CrossRef]
- Jia, L.; Yan, W.; Zhu, C.; Agrama, H.A.; Jackson, A.; Yeater, K.; Li, X.; Huang, B.; Hu, B.; McClung, A.; et al. Allelic analysis of sheath blight resistance with association mapping in rice. PLoS ONE 2012, 7, e32703. [Google Scholar] [CrossRef] [PubMed]
- Schläppi, M.R.; Jackson, A.K.; Eizenga, G.C.; Wang, A.; Chu, C.; Shi, Y.; Shimoyama, N.; Boykin, D.L. Assessment of five chilling tolerance traits and GWAS mapping in rice using the USDA mini-core collection. Front. Plant Sci. 2017, 8, 957. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.D.; Haritha, G.; Sunitha, T.; Krishnamurthy, S.L.; Divya, B.; Padmavathi, G.; Ram, T.; Sarla, N. Haplotyping of rice genotypes using simple sequence repeat markers associated with salt tolerance. Rice Sci. 2016, 23, 317–325. [Google Scholar] [CrossRef]
- Yamamoto, A.; Sawada, H.; Shim, I.S.; Usui, K.; Fujihara, S. Effect of salt stress on physiological response and leaf polyamine content in NERICA rice seedlings. Plant Soil Environ. 2011, 57, 571–576. [Google Scholar] [CrossRef] [Green Version]
- Alexandrov, N.; Tai, S.; Wang, W.; Mansueto, L.; Palis, K.; Fuentes, R.R.; Ulat, V.J.; Chebotarov, D.; Zhang, G.; Li, Z.; et al. SNP-Seek database of SNPs derived from 3000 rice genomes. Nucleic Acids Res. 2015, 43, D1023–D1027. [Google Scholar] [CrossRef]
- Zhao, H.; Yao, W.; Ouyang, Y.; Yang, W.; Wang, G.; Lian, X.; Xing, Y.; Chen, L.; Xie, W. RiceVarMap: A comprehensive database of rice genomic variations. Nucleic Acids Res. 2014, 43, D1018–D1022. [Google Scholar] [CrossRef]
- Duan, Y.-B.; Li, J.; Qin, R.-Y.; Xu, R.-F.; Li, H.; Yang, Y.-C.; Ma, H.; Li, L.; Wei, P.-C.; Yang, J.-B. Identification of a regulatory element responsible for salt induction of rice OsRAV2 through ex situ and in situ promoter analysis. Plant Mol. Biol. 2016, 90, 49–62. [Google Scholar] [CrossRef]
- Kong, W.; Zhong, H.; Deng, X.; Gautam, M.; Gong, Z.; Zhang, Y.; Zhao, G.; Liu, C.; Li, Y. Evolutionary Analysis of GH3 Genes in Six Oryza Species/Subspecies and Their Expression under Salinity Stress in Oryza sativa ssp. japonica. Plants 2019, 8, 30. [Google Scholar] [CrossRef]
- Yadav, D.K.; Islam, S.M.S.; Tuteja, N. Rice heterotrimeric G-protein gamma subunits (RGG1 and RGG2) are differentially regulated under abiotic stress. Plant Signal. Behav. 2012, 7, 733–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, Q.; Zheng, C.; Sun, S.; Amjad, H.; Liang, K.; Lin, W. The role of plant cation/proton antiporter gene family in salt tolerance. Biol. Plant. 2018, 62, 617–629. [Google Scholar] [CrossRef]
- Xu, F.; Fang, J.; Ou, S.; Gao, S.; Zhang, F.; Du, L.; Xiao, Y.; Wang, H.; Sun, X.; Chu, J.; et al. Variations in CYP78A13 coding region influence grain size and yield in rice. Plant Cell Environ. 2015, 38, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Jiang, W.; Tang, S.; Wan, J.; Long, Q.; Pan, X.; Shi, Q.; Wu, Z. Xanthine Dehydrogenase Involves in the Response of Photosystem and Reactive Oxygen Metabolism to Drought Stress in Rice. Russ. J. Plant Physiol. 2018, 65, 404–411. [Google Scholar] [CrossRef]
- Gu, J.-F.; Qiu, M.; Yang, J.-C. Enhanced tolerance to drought in transgenic rice plants overexpressing C4 photosynthesis enzymes. Crop J. 2013, 1, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, G.K.; Jwa, N.-S.; Shibato, J.; Han, O.; Iwahashi, H.; Rakwal, R. Diverse environmental cues transiently regulate OsOPR1 of the “octadecanoid pathway” revealing its importance in rice defense/stress and development. Biochem. Biophys. Res. Commun. 2003, 310, 1073–1082. [Google Scholar] [CrossRef]
- Farid, A.; Malinovsky, F.G.; Veit, C.; Schoberer, J.; Zipfel, C.; Strasser, R. Specialized Roles of the Conserved Subunit OST3/6 of the Oligosaccharyltransferase Complex in Innate Immunity and Tolerance to Abiotic Stresses. Plant Physiol. 2013, 162, 24–38. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Zou, Y.; Hu, J.; Ding, Y. Genome-wide analysis of the rice PPR gene family and their expression profiles under different stress treatments. BMC Genom. 2018, 19, 720. [Google Scholar] [CrossRef]
- Li, L.; Xu, X.; Chen, C.; Shen, Z. Genome-Wide Characterization and Expression Analysis of the Germin-Like Protein Family in Rice and Arabidopsis. Int. J. Mol. Sci. 2016, 17, 1622. [Google Scholar] [CrossRef]
- Rashid, M.; Guangyuan, H.; Guangxiao, Y.; Hussain, J.; Xu, Y. AP2/ERF Transcription Factor in Rice: Genome-Wide Canvas and Syntenic Relationships between Monocots and Eudicots. Evol. Bioinform. 2012, 8, EBO-S9369. [Google Scholar] [CrossRef]
- Kim, Y.-O.; Kang, H.; Ahn, S.-J. Overexpression of phytochelatin synthase AtPCS2 enhances salt tolerance in Arabidopsis thaliana. J. Plant Physiol. 2019, 240, 153011. [Google Scholar] [CrossRef] [PubMed]
- Sahi, C.; Singh, A.; Kumar, K.; Blumwald, E.; Grover, A. Salt stress response in rice: Genetics, molecular biology, and comparative genomics. Funct. Integr. Genomics 2006, 6, 263–284. [Google Scholar] [CrossRef] [PubMed]
- Paleari, L.; Movedi, E.; Confalonieri, R. Trait-based model development to support breeding programs. A case study for salt tolerance and rice. Sci. Rep. 2017, 7, 4352. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Böhm, J.; Messerer, M.; Müller, H.M.; Scholz-Starke, J.; Gradogna, A.; Scherzer, S.; Maierhofer, T.; Bazihizina, N.; Zhang, H.; Stigloher, C.; et al. Understanding the molecular basis of salt sequestration in epidermal bladder cells of Chenopodium quinoa. Curr. Biol. 2018, 28, 3075–3085. [Google Scholar] [CrossRef]
- Wu, H.; Shabala, L.; Zhou, M.; Su, N.; Wu, Q.; Ul-Haq, T.; Zhu, J.; Mancuso, S.; Azzarello, E.; Shabala, S. Root vacuolar Na+ sequestration but not exclusion from uptake correlates with barley salt tolerance. Plant J. 2019, 100, 55–67. [Google Scholar] [CrossRef]
- Lee, K.-S.; Choi, W.-Y.; Ko, J.-C.; Kim, T.-S.; Gregorio, G.B. Salinity tolerance of japonica and indica rice (Oryza sativa L.) at the seedling stage. Planta 2003, 216, 1043–1046. [Google Scholar] [CrossRef]
- De Leon, T.B.; Linscombe, S.; Subudhi, P.K. Molecular dissection of seedling salinity tolerance in rice (Oryza sativa L.) using a high-density GBS-based SNP linkage map. Rice 2016, 9, 52. [Google Scholar] [CrossRef]
- Shi, Y.; Gao, L.; Wu, Z.; Zhang, X.; Wang, M.; Zhang, C.; Zhang, F.; Zhou, Y.; Li, Z. Genome-wide association study of salt tolerance at the seed germination stage in rice. BMC Plant Biol. 2017, 17, 92. [Google Scholar] [CrossRef]
- Swain, D.M.; Sahoo, R.K.; Srivastava, V.K.; Tripathy, B.C.; Tuteja, R.; Tuteja, N. Function of heterotrimeric G-protein γ subunit RGG1 in providing salinity stress tolerance in rice by elevating detoxification of ROS. Planta 2017, 245, 367–383. [Google Scholar] [CrossRef]
- Blary, A.; Jenczewski, E. Manipulation of crossover frequency and distribution for plant breeding. Theor. Appl. Genet. 2019, 132, 575–592. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.; Mi, X.-F.; Shan, J.-X.; Li, X.-M.; Xu, J.-L.; Lin, H.-X. The QTL GNP1 encodes GA20ox1, which increases grain number and yield by increasing cytokinin activity in rice panicle meristems. PLoS Genet. 2016, 12, e1006386. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.M.; Manosalva, P.M.; Snelling, J.; Bruce, M.; Leung, H.; Leach, J.E. Rice Germin-Like Proteins: Allelic diversity and relationships to early stress responses. Rice 2010, 3, 43–55. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, A.; Mithra, S.V.A.; Krishnamurthy, S.L.; Parida, S.K.; Jain, S.; Tiwari, K.K.; Kumar, P.; Rao, A.R.; Sharma, S.K.; et al. Genome-wide association mapping of salinity tolerance in rice (Oryza sativa). DNA Res. 2015, 22, 133–145. [Google Scholar] [CrossRef]
- Wang, H.; Lee, A.-R.; Park, S.-Y.; Jin, S.-H.; Lee, J.; Ham, T.-H.; Park, Y.; Zhao, W.-G.; Kwon, S.-W. Genome-wide association study reveals candidate genes related to low temperature tolerance in rice (Oryza sativa) during germination. 3 Biotech 2018, 8, 235. [Google Scholar] [CrossRef]
- Gregorio, G.B.; Senadhira, D.; Mendoza, R.D. Screening Rice for Salinity Tolerance; IRRI Discussion Paper Series No. 22; International Rice Research Institute (IRRI): Manila, Philippines, 1997; p. 30. [Google Scholar]
- Negrão, S.; Schmöckel, S.M.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef]
- Eizenga, G.C.; Jia, M.H.; Jackson, A.K.; Boykin, D.L.; Ali, M.L.; Shakiba, E.; Tran, N.T.; McCouch, S.R.; Edwards, J.D. Validation of yield component traits identified by genome-wide association mapping in a tropical japonica × tropical japonica rice biparental mapping population. Plant Genome 2019, 12, 180021. [Google Scholar] [CrossRef]
- Bradbury, P.J.; Zhang, Z.; Kroon, D.E.; Casstevens, T.M.; Ramdoss, Y.; Buckler, E.S. TASSEL: Software for association mapping of complex traits in diverse samples. Bioinformatics 2007, 23, 2633–2635. [Google Scholar] [CrossRef]
- Zhang, Z.; Ersoz, E.; Lai, C.-Q.; Todhunter, R.J.; Tiwari, H.K.; Gore, M.A.; Bradbury, P.J.; Yu, J.; Arnett, D.K.; Ordovas, J.M.; et al. Mixed linear model approach adapted for genome-wide association studies. Nat. Genet. 2010, 42, 355. [Google Scholar] [CrossRef]
- Turner, S.D. Qqman: An R package for visualizing GWAS results using Q-Q and manhattan plots. Biorxiv 2014, 005165. [Google Scholar] [CrossRef]
- Kawahara, Y.; De la Bastide, M.; Hamilton, J.P.; Kanamori, H.; McCombie, W.R.; Ouyang, S.; Schwartz, D.C.; Tanaka, T.; Wu, J.; Zhou, S.; et al. Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data. Rice 2013, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Sakai, H.; Lee, S.S.; Tanaka, T.; Numa, H.; Kim, J.; Kawahara, Y.; Wakimoto, H.; Yang, C.-C.; Iwamoto, M.; Abe, T.; et al. Rice Annotation Project Database (RAP-DB): An Integrative and Interactive Database for Rice Genomics. Plant Cell Physiol. 2013, 54, e6. [Google Scholar] [CrossRef] [PubMed]
- Mansueto, L.; Fuentes, R.R.; Borja, F.N.; Detras, J.; Abriol-Santos, J.M.; Chebotarov, D.; Sanciangco, M.; Palis, K.; Copetti, D.; Poliakov, A.; et al. Rice SNP-seek database update: New SNPs, indels, and queries. Nucleic Acids Res. 2017, 45, D1075–D1081. [Google Scholar] [CrossRef] [PubMed]
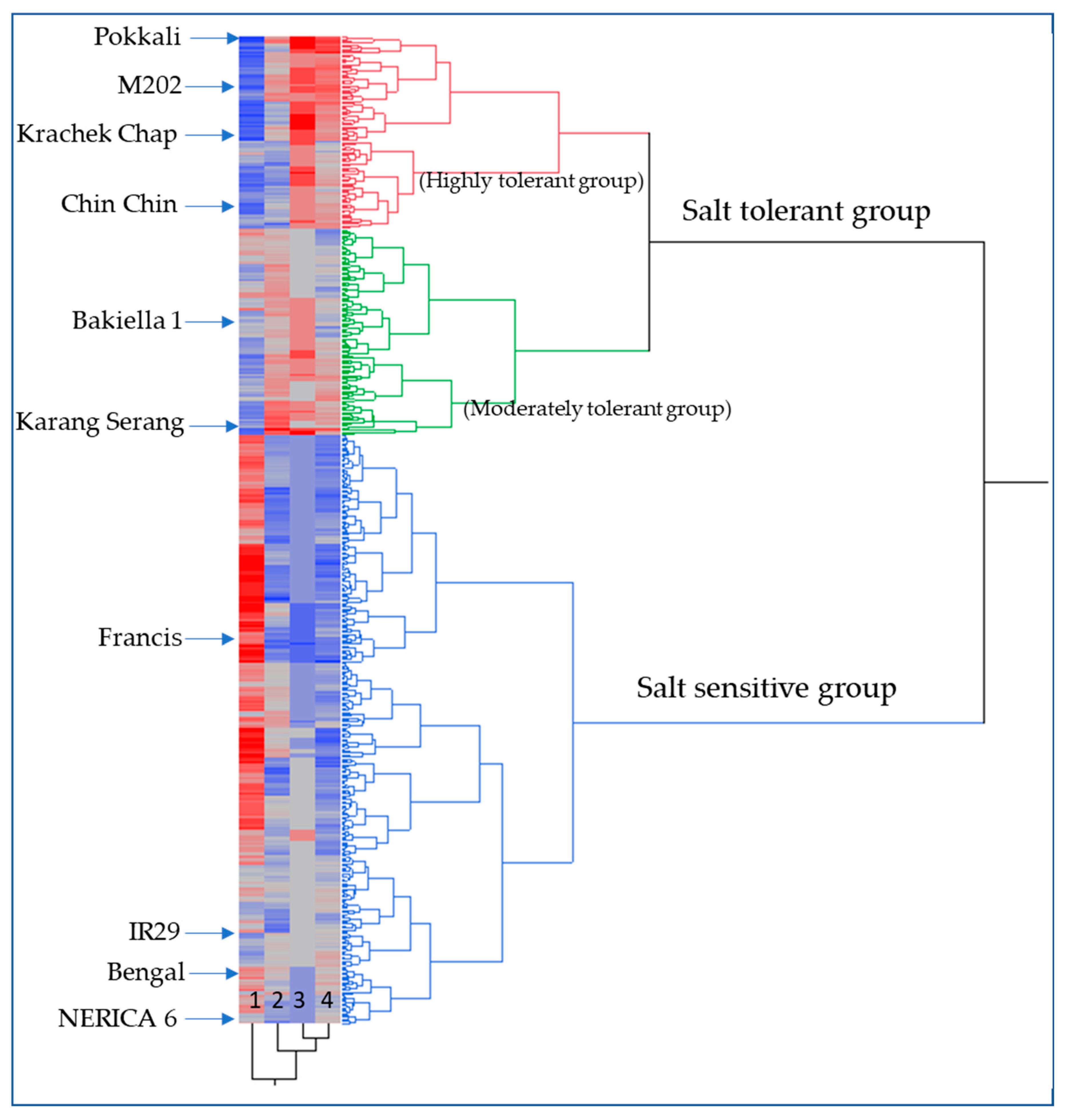

| Classification | Genotype | SSI Score-d10 | SSI Score-d16 | Δ PHT-d10 (cm) | Δ PHT-d16 (cm) | Δ Green Leaf#-d14 | Total Biomass Plant−1 (g) | Shoot Biomass Plant−1 (g) | Root Biomass Plant−1 (g) |
|---|---|---|---|---|---|---|---|---|---|
| I | Sensitive checks | 5.45 a | 7.53 a | 8.25 c | 8.29 d | −2.97 d | 0.16 d | 0.13 d | 0.03 d |
| Tolerant checks | 1.38 d | 2.32 d | 22.44 a | 29.43 a | 0.64 a | 1.30 a | 1.08 a | 0.22 a | |
| URMC * | 4.61 b | 6.39 b | 12.98 b | 14.07 b | −2.06 c | 0.28 c | 0.23 c | 0.05 c | |
| Vietnamese lines * | 3.52 c | 4.94 c | 10.45 c | 12.06 c | −1.24 b | 0.35 b | 0.29 b | 0.07 b | |
| II * | AUS | 4.46 B | 6.50 B | 12.96 A | 13.94 A | −2.08 A,B | 0.27 B | 0.22 B | 0.05 B |
| IND | 3.95 C | 5.48 C | 12.61 A | 14.00 A | −1.76 A | 0.34 A | 0.27 A | 0.07 A | |
| TEJ | 3.20 C | 4.93 B,C | 14.32 A | 15.45 A | −1.33 A,B | 0.44 A | 0.36 A | 0.08 A | |
| TRJ | 5.63 A | 7.55 A | 12.04 A | 12.89 A | −2.24 B | 0.20 C | 0.16 C | 0.04 C | |
| ALL δ | 4.43 ± 0.07 | 6.15 ± 0.09 | 12.57 ± 0.20 | 13.75 ± 0.23 | −1.93 ± 0.06 | 0.29 ± 0.01 | 0.24 ± 0.01 | 0.06 ± 0.001 |
| Genotype | Min/Max | SSI Score-d10 | SSI Score-d16 | Δ PHT-d10 (cm) | Δ PHT-d16 (cm) | Δ Green Leaf#-d14 | Total Biomass Plant−1 (g) | Shoot Biomass Plant−1 (g) | Root Biomass Plant−1 (g) |
|---|---|---|---|---|---|---|---|---|---|
| Sensitive check | Min | 3.80 | 5.20 | 2.60 | 2.60 | −4.00 | 0.11 | 0.01 | 0.01 |
| Max | 7.40 | 9.00 | 11.30 | 11.30 | −2.00 | 0.27 | 0.21 | 0.06 | |
| Tolerant check | Min | 1.00 | 1.00 | 6.00 | 8.80 | −1.00 | 0.42 | 0.35 | 0.07 |
| Max | 3.80 | 4.60 | 30.20 | 47.70 | 2.00 | 1.99 | 1.66 | 0.33 | |
| URMC | Min | 1.00 | 1.80 | 3.60 | 4.00 | −4.00 | 0.03 | 0.03 | 0.01 |
| Max | 9.00 | 9.00 | 27.70 | 30.40 | 1.00 | 0.99 | 0.81 | 0.18 | |
| Vietnamese lines | Min | 1.40 | 2.00 | 0.00 | 4.40 | −5.00 | 0.14 | 0.11 | 0.02 |
| Max | 6.60 | 8.20 | 18.50 | 20.70 | 1.00 | 0.81 | 0.67 | 0.15 | |
| AUS * | Min | 1.30 | 2.00 | 5.00 | 5.00 | −4.00 | 0.09 | 0.07 | 0.02 |
| Max | 7.40 | 9.00 | 20.50 | 25.90 | 1.00 | 0.81 | 0.66 | 0.14 | |
| IND * | Min | 1.00 | 1.80 | 0.00 | 4.00 | −5.00 | 0.12 | 0.09 | 0.02 |
| Max | 7.40 | 9.00 | 27.70 | 30.40 | 1.00 | 0.99 | 0.81 | 0.18 | |
| TEJ * | Min | 1.60 | 2.00 | 11.50 | 11.50 | −3.00 | 0.21 | 0.17 | 0.04 |
| Max | 4.60 | 7.20 | 17.10 | 19.10 | 1.00 | 0.71 | 0.58 | 0.13 | |
| TRJ * | Min | 3.00 | 3.20 | 4.70 | 4.70 | −4.00 | 0.03 | 0.03 | 0.01 |
| Max | 9.00 | 9.00 | 25.50 | 30.40 | 0.00 | 0.48 | 0.39 | 0.09 | |
| ALL * | Min | 1.00 | 1.80 | 0.00 | 4.00 | −5.00 | 0.03 | 0.03 | 0.01 |
| Max | 9.00 | 9.00 | 27.70 | 30.40 | 1.00 | 0.99 | 0.81 | 0.18 |
| SSI Score-d10 | SSI Score-d16 | Δ PHT-d10 | Δ PHT-d16 | Δ Green Leaf#-d14 | Total Biomass | Shoot Biomass | Root Biomass | |
|---|---|---|---|---|---|---|---|---|
| SSI score-d10 | 1 | |||||||
| SSI score-d16 | 0.9041 * | 1 | ||||||
| Δ PHT-d10 | −0.4270 * | −0.4309 * | 1 | |||||
| Δ PHT-d16 | −0.5366 * | −0.5708 * | 0.9367 * | 1 | ||||
| Δ green leaf#-d14 | −0.7985 * | −0.8577 * | 0.4030 * | 0.5393 * | 1 | |||
| Total biomass | −0.7218 * | −0.7495 * | 0.5838 * | 0.7177 * | 0.7170 * | 1 | ||
| Shoot biomass | −0.7177 * | −0.7417 * | 0.5885 * | 0.7191 * | 0.7112 * | 0.9991 * | 1 | |
| Root biomass | −0.7267 * | −0.7696 * | 0.5447 * | 0.6918 * | 0.7286 * | 0.9809 * | 0.9722 * | 1 |
| SNP ID | Chr. | Position (bp) | Ref. Allele | Alt. Allele | Sub-Pop with Alt. Allele Associated with Traits | Traits Associated with the Alt. Allele | Frequencies for the Ref. Allele | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TRJ | TEJ | ARO | AUS | IND | |||||||
| S1_2594296 | 1 | 2,594,296 | G | C | IND | SSI score, Δ green leaf#, biomass | 95.7% | 50.2% | 85.9% | 64.1% | 79.7% |
| S1_33398135 | 1 | 33,398,135 | C | T | IND | Δ PHT | 100.0% | 99.9% | 100.0% | 98.9% | 43.2% |
| S2_2053382 | 2 | 2,053,382 | A | C | IND | SSI score, Δ PHT, Δ green leaf#, biomass | 99.9% | 100.0% | 100.0% | 99.2% | 65.7% |
| S3_17374343 | 3 | 17,374,343 | C | T | AUS, IND | SSI score, Δ green leaf#, biomass | 97.2% | 99.7% | 19.8% | 91.4% | 10.3% |
| S3_21582523 | 3 | 21,582,523 | C | T | AUS, IND | SSI score, Δ green leaf#, biomass | 90.1% | 98.0% | 5.2% | 41.3% | 44.2% |
| S3_36149293 | 3 | 36,149,293 | A | T | AUS | SSI score, Δ green leaf#, biomass | 99.8% | 100.0% | 100.0% | 57.2% | 98.4% |
| S4_31361839 | 4 | 31,361,839 | G | A | IND | SSI score, Δ green leaf#, biomass | 2.6% | 81.1% | 1.0% | 0.7% | 26.5% |
| S10_11743519 | 10 | 11,743,519 | C | T | TRJ | SSI score, Δ PHT, Δ green leaf#, biomass | 77.2% | 58.1% | 100.0% | 100.0% | 99.6% |
| S10_18801757 | 10 | 18,801,757 | A | C | AUS, IND | SSI score, Δ PHT, Δ green leaf#, biomass | 96.6% | 50.2% | 95.8% | 63.9% | 86.1% |
| SNP | Sub-Pop | Traits | Prob>|t| | Rsquare | Alternate Allele Effect |
|---|---|---|---|---|---|
| 1_2594296 | IND | d10 SSI score | 0.0007 | 0.32 | −1.247 |
| d16 SSI score | <0.0001 | 0.47 | −2.152 | ||
| Δ green leaf#-d14 | <0.0001 | 0.43 | 1.043 | ||
| Shoot biomass d16 | <0.0001 | 0.45 | 0.126 | ||
| Root biomass d16 | <0.0001 | 0.65 | 0.035 | ||
| 1_33398135 | IND | Δ PHT-d10 | <0.0001 | 0.48 | 3.543 |
| Δ PHT-d16 | <0.0001 | 0.44 | 3.998 | ||
| 2_2053382 | IND | d10 SSI score | 0.0020 | 0.22 | −0.904 |
| d16 SSI score | 0.0002 | 0.30 | −1.659 | ||
| Δ PHT-d10 | 0.0935 | 0.07 | 1.536 | ||
| Δ PHT-d16 | 0.0426 | 0.10 | 2.215 | ||
| Δ green leaf#-d14 | 0.0002 | 0.30 | 0.822 | ||
| Shoot biomass d16 | <0.0001 | 0.39 | 0.118 | ||
| Root biomass d16 | <0.0001 | 0.53 | 0.036 | ||
| 3_17374343 | AUS | d10 SSI score | 0.0110 | 0.28 | 1.200 |
| d16 SSI score | 0.0974 | 0.13 | 1.024 | ||
| Shoot biomass d16 | 0.0196 | 0.24 | −0.079 | ||
| Root biomass d16 | 0.0232 | 0.23 | −0.017 | ||
| IND | d10 SSI score | <0.0001 | 0.31 | 1.708 | |
| d16 SSI score | <0.0001 | 0.32 | 2.422 | ||
| Δ green leaf#-d14 | 0.0001 | 0.29 | −1.117 | ||
| Shoot biomass d16 | 0.0014 | 0.21 | −0.123 | ||
| Root biomass d16 | <0.0001 | 0.31 | −0.038 | ||
| 3_21582523 | AUS | d10 SSI score | 0.0008 | 0.51 | 1.336 |
| d16 SSI score | 0.0052 | 0.40 | 1.509 | ||
| Δ green leaf#-d14 | 0.0200 | 0.29 | −0.649 | ||
| Shoot biomass d16 | 0.0116 | 0.34 | −0.079 | ||
| Root biomass d16 | 0.0042 | 0.41 | −0.018 | ||
| IND | d10 SSI score | 0.0010 | 0.33 | 0.987 | |
| d16 SSI score | 0.0006 | 0.36 | 1.460 | ||
| Δ green leaf#-d14 | 0.0006 | 0.36 | −0.740 | ||
| Shoot biomass d16 | 0.0231 | 0.18 | −0.069 | ||
| Root biomass d16 | 0.0135 | 0.21 | −0.018 | ||
| 3_36149293 | AUS | d10 SSI score | 0.0124 | 0.25 | −0.800 |
| d16 SSI score | 0.0008 | 0.41 | −1.426 | ||
| Δ green leaf#-d14 | 0.0011 | 0.39 | 0.686 | ||
| Shoot biomass d16 | 0.0331 | 0.19 | 0.051 | ||
| Root biomass d16 | 0.0089 | 0.27 | 0.013 | ||
| 4_31361839 | IND | d10 SSI score | 0.0196 | 0.40 | 1.113 |
| d16 SSI score | 0.0151 | 0.43 | 1.654 | ||
| Δ green leaf#-d14 | 0.0171 | 0.42 | −0.816 | ||
| Shoot biomass d16 | 0.0003 | 0.71 | −0.164 | ||
| Root biomass d16 | 0.0004 | 0.69 | −0.040 | ||
| 10_11743519 | TRJ | d10 SSI score | <0.0001 | 0.58 | −1.248 |
| d16 SSI score | <0.0001 | 0.66 | −1.603 | ||
| Δ PHT-d10 | 0.0416 | 0.21 | 2.016 | ||
| Δ PHT-d16 | 0.0086 | 0.33 | 3.237 | ||
| Δ green leaf#-d14 | 0.0004 | 0.51 | 0.710 | ||
| Shoot biomass d16 | 0.0007 | 0.48 | 0.077 | ||
| Root biomass d16 | 0.0033 | 0.39 | 0.015 | ||
| 10_18801757 | AUS | d10 SSI score | 0.0001 | 0.46 | −1.185 |
| d16 SSI score | <0.0001 | 0.53 | −1.647 | ||
| Δ green leaf#-d14 | <0.0001 | 0.46 | 0.819 | ||
| Shoot biomass d16 | 0.0103 | 0.24 | 0.048 | ||
| Root biomass d16 | 0.0028 | 0.30 | 0.013 | ||
| IND | d10 SSI score | 0.0122 | 0.13 | −0.817 | |
| d16 SSI score | 0.0007 | 0.22 | −1.579 | ||
| Δ PHT-d16 | 0.0918 | 0.06 | 1.948 | ||
| Δ green leaf#-d14 | 0.0013 | 0.20 | 0.723 | ||
| Shoot biomass d16 | 0.0006 | 0.23 | 0.100 | ||
| Root biomass d16 | 0.0005 | 0.23 | 0.026 |
| SNP | MSU7 Locus ID | Position | Gene Name | Putative Function | Reference |
|---|---|---|---|---|---|
| S1_2594296 | LOC_Os01g04800 | Chr01:2202264 - 2203860 | AP2/EREBP129 | Transcriptional reprogramming during salt stress | [50] |
| S1_33398135 | LOC_Os01g57610 | Chr01:33308448 - 33311391 | OsGH3.1 | Auxin-responsive role in salinity tolerance | [51] |
| S2_2053382 | LOC_Os02g04520 | Chr02:2007232 - 2009961 | G-protein γ subunit | Signal transducer during salt stress | [52] |
| LOC_Os02g04630 | Chr02:2070492 - 2075369 | Vacuolar cation/proton exchanger | Protects primary cell mechanisms mediated by ion homeostasis | [53] | |
| S3_17374343 (and S3_21582523) | LOC_Os03g30420 | Chr03:17340415 - 17340601 | GL3.2 | Cytochrome P450, stress tolerance | [22,54] |
| LOC_Os03g31550 | Chr03:17985563 - 17998498 | Aldehyde oxidase putative | Abiotic stress response | [22,55] | |
| LOC_Os03g31750 | Chr03:18153143 - 18160127 | Pyruvate orthophosphate dikinase (PPDK) | Improved plant physiology under abiotic stress | [56] | |
| LOC_Os03g32314 | Chr03:18485606 - 18488371 | AOC1, allene oxide cyclase | Response to salt and other abiotic stresses | [57] | |
| LOC_Os03g33590 | Chr03:19197601 - 19204090 | Interferon-related developmental regulator | Salt sensitivity in Arabidopsis | [22] | |
| LOC_Os03g36730 | Chr03:20363507 - 20370047 | OST3/OST6 family protein | Hypersensitivity to osmotic and salt stress | [58] | |
| LOC_Os03g37260 | Chr03:20650253 - 20653294 | Pentatricopeptide repeat | Post-transcriptional gene regulation under abiotic stresses | [59] | |
| S3_36149293 | LOC_Os03g63970 | Chr03:36152044 - 36152517 | GA20OX1, gibberellin 20 oxidase 1 | Growth and development, downregulated under abiotic stress | [22] |
| S4_31361839 | LOC_Os04g52720 | Chr04:31392876 - 31395185 | GLP4-1, germin-like protein 4-1 | Salt and osmotic stress tolerance | [60] |
| S10_11743519 | LOC_Os10g22600 | Chr10:11731592 - 11732917 | ERF51, ethylene response factor 51 | Osmotic stress tolerance | [61] |
| S10_18801757 | LOC_Os10g35150 | Chr10:18754181 - 18758096 | Expressed protein | Downregulated during abiotic stress | [22] |
| LOC_Os10g35460 | Chr10:18976009 - 18979110 | Phytochelatin synthase | Regulation of osmolytes, Na+/K+ ratio, sequestration in the vacuole for cellular redox homeostasis | [22,62] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rohila, J.S.; Edwards, J.D.; Tran, G.D.; Jackson, A.K.; McClung, A.M. Identification of Superior Alleles for Seedling Stage Salt Tolerance in the USDA Rice Mini-Core Collection. Plants 2019, 8, 472. https://doi.org/10.3390/plants8110472
Rohila JS, Edwards JD, Tran GD, Jackson AK, McClung AM. Identification of Superior Alleles for Seedling Stage Salt Tolerance in the USDA Rice Mini-Core Collection. Plants. 2019; 8(11):472. https://doi.org/10.3390/plants8110472
Chicago/Turabian StyleRohila, Jai S., Jeremy D. Edwards, Gioi D. Tran, Aaron K. Jackson, and Anna M. McClung. 2019. "Identification of Superior Alleles for Seedling Stage Salt Tolerance in the USDA Rice Mini-Core Collection" Plants 8, no. 11: 472. https://doi.org/10.3390/plants8110472
APA StyleRohila, J. S., Edwards, J. D., Tran, G. D., Jackson, A. K., & McClung, A. M. (2019). Identification of Superior Alleles for Seedling Stage Salt Tolerance in the USDA Rice Mini-Core Collection. Plants, 8(11), 472. https://doi.org/10.3390/plants8110472





