Two SNP Mutations Turned off Seed Shattering in Rice
Abstract
1. Introduction
2. Results
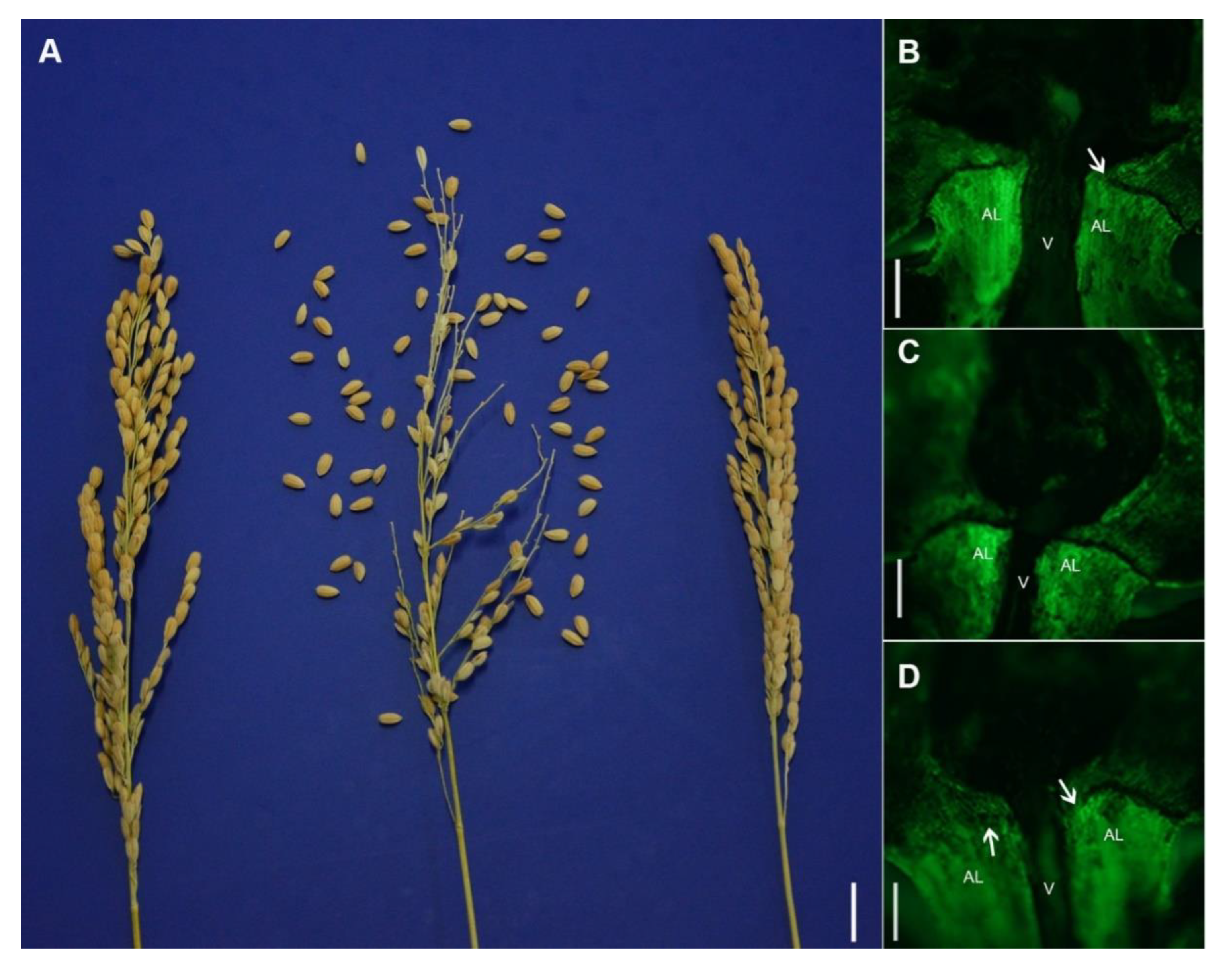
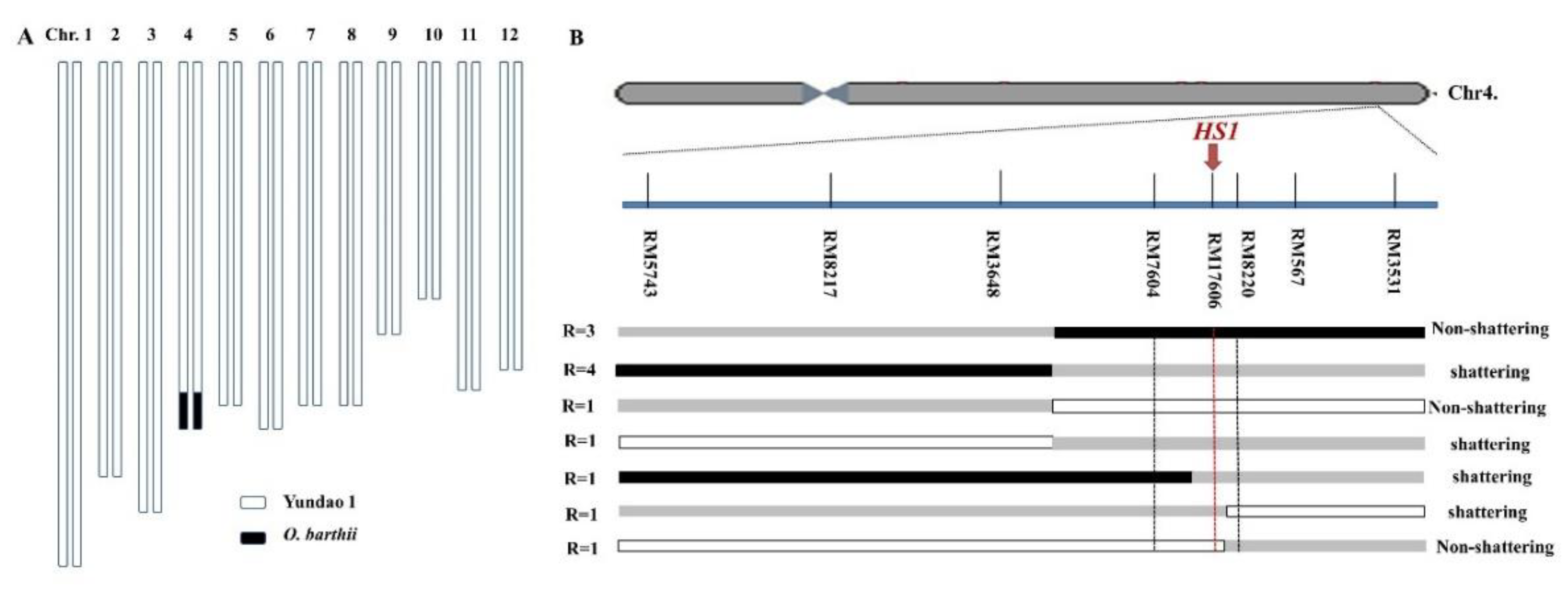
2.1. HS1 Controlled the Hybrid Seed Shattering in Rice
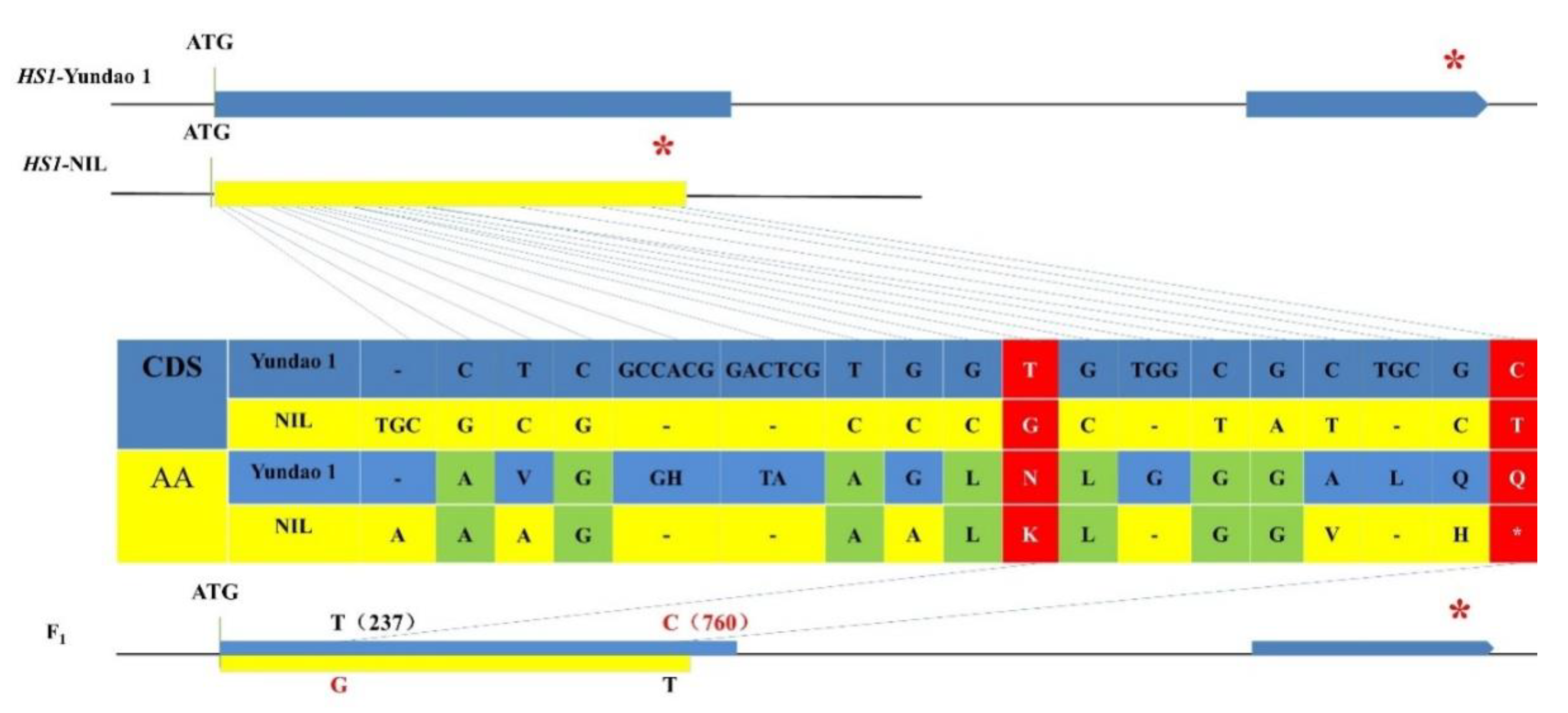
2.2. Dominant Complementation of G Allele at G237T Site and C Allele at C760T Site in HS1 Led to Hybrid Seed Shattering Phenotype
2.3. Two SNP Mutations Turned off Seed Shattering in Rice
3. Discussion
4. Materials and methods
4.1. Plant Materials
4.2. Evaluation of Seed Shattering Rate
4.3. Microscopy
4.4. DNA Extraction and SSR Analysis
4.5. Linkage Analysis
4.6. Sequencing
4.7. Haplotype Analysis of the SH4 Gene
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Ji, H.; Kim, S.-R.; Kim, Y.-H.; Kim, H.; Eun, M.-Y.; Jin, I.-D.; Cha, Y.-S.; Yun, D.-W.; Ahn, B.-O.; Lee, M.C.; et al. Inactivation of the CTD phosphatase-like gene OsCPL1 enhances the development of the abscission layer and seed shattering in rice. Plant J. 2010, 61, 96–106. [Google Scholar] [CrossRef]
- Avik, R.; Debarati, C. Shattering or not shattering: That is the question in domestication of rice (Oryza sativa L.). Genet. Resour. Crop Evol. 2018, 65, 391–395. [Google Scholar]
- Oba, S.; Sumi, N.; Fujimoto, F.; Yasue, T. Association between Grain Shattering Habit and Formation of Abscission Layer Controlled by Grain Shattering gene sh-2 in Rice (Oryza sativa L.). Jpn. J. Crop Sci. 1995, 64, 607–615. [Google Scholar] [CrossRef]
- Konishi, S.; Izawa, T.; Lin, S.Y.; Ebana, K.; Fukuta, Y.; Sasaki, T.; Yano, M.; Huang, X.; Hansen, N.; Tsuji, N. An SNP Caused Loss of Seed Shattering During Rice Domestication. Science 2006, 312, 1392–1396. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, A.; Sang, T. Rice domestication by reducing shattering. Science 2008, 311, 1936–1939. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Griffith, M.E.; Li, X.; Zhu, Z.; Tan, L.; Fu, Y.; Zhang, W.; Wang, X.; Xie, D.; Sun, C. Origin of seed shattering in rice (Oryza sativa L.). Planta 2007, 226, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Liu, X.; Wang, M.; Meyer, R.S.; Luo, X.; Ndjiondjop, M.-N.; Tan, L.; Zhang, J.; Wu, J.; Cai, H.; et al. A single-nucleotide polymorphism causes smaller grain size and loss of seed shattering during African rice domestication. Nat. Plants 2017, 3, 17064. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lu, D.; Li, C.; Luo, J.; Zhu, B.-F.; Zhu, J.; Shangguan, Y.; Wang, Z.; Sang, T.; Zhou, B.; et al. Genetic control of seed shattering in rice by the APETALA2 transcription factor shattering abortion1. Plant Cell 2012, 24, 1034–1048. [Google Scholar] [CrossRef]
- Yoon, J.; Cho, L.-H.; Kim, S.L.; Choi, H.; Koh, H.-J.; An, G. The BEL1-type homeobox gene SH5 induces seed shattering by enhancing abscission-zone development and inhibiting lignin biosynthesis. Plant J. 2014, 79, 717–728. [Google Scholar] [CrossRef]
- Sun, P.; Zhang, W.; Wang, Y.; He, Q.; Shu, F.; Liu, H.; Wang, J.; Wang, J.; Yuan, L.; Deng, H. OsGRF4 controls grain shape, panicle length and seed shattering in rice. J. Integr. Plant Biol. 2016, 58, 836–847. [Google Scholar] [CrossRef]
- Yoon, J.; Cho, L.-H.; Antt, H.W.; Koh, H.-J.; An, G. KNOX Protein OSH15 Induces Grain Shattering by Repressing Lignin Biosynthesis Genes. Plant Physiol. 2017, 174, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Wu, W.; Wang, M.; Meyer, R.S.; Ndjiondjop, M.-N.; Tan, L.; Zhou, H.; Zhang, J.; Fu, Y.; Cai, H.; et al. Genetic control of seed shattering during African rice domestication. Nat. Plants 2018, 4, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Crawford, G.W.; Jiang, L.; Chen, X. Rice Domestication Revealed by Reduced Shattering of Archaeological rice from the Lower Yangtze valley. Sci. Rep. 2016, 6, 28136. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, R.; Thanh, P.T.; Nimura, N.; Htun, T.M.; Yamasaki, M.; Ishii, T. Allelic interaction at seed-shattering loci in the genetic backgrounds of wild and cultivated rice species. Genes Genet. Syst. 2010, 85, 265–271. [Google Scholar] [CrossRef]
- Wang, M.; Yu, Y.; Haberer, G.; Marri, P.R.; Fan, C.; Goicoechea, J.L.; Zuccolo, A.; Song, X.; Kudrna, D.; Ammiraju, J.S.S.; et al. The genome sequence of African rice (Oryza glaberrima) and evidence for independent domestication. Nat. Genet. 2014, 46, 982–988. [Google Scholar] [CrossRef]
- Huang, X.; Kurata, N.; Wei, X.; Wang, Z.-X.; Wang, A.; Zhao, Q.; Zhao, Y.; Liu, K.; Lu, H.; Li, W.; et al. A map of rice genome variation reveals the origin of cultivated rice. Nature 2012, 490, 497–501. [Google Scholar] [CrossRef]
- Khush, G.S. Origin, dispersal, cultivation and variation of rice. Oryza Mol. Plant 1997, 35, 25–34. [Google Scholar] [CrossRef]
- Brar, D.; Khush, G. Alien introgression in rice. Plant Mol. Biol. 1997, 35, 35–47. [Google Scholar] [CrossRef]
- Sanchez, P.; Wing, R.; Brar, D. Genetics and Genomics of Rice; Zhang, Q., Wing, R.A., Eds.; Springer: New York, NY, USA, 2013; pp. 9–25. [Google Scholar]
- Vaughan, D.A.; Lu, B.-R.; Tomooka, N. The evolving story of rice evolution. Plant Sci. 2008, 174, 394–408. [Google Scholar] [CrossRef]
- Edwards, K.; Johnstone, C.; Thompson, C. A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res. 1991, 19, 1349. [Google Scholar] [CrossRef]
- McCouch, S.; Teytelman, L.; Xu, Y.; Lobos, K.; Clare, K.; Walton, M.; Fu, B.; Maghirang, R.; Li, Z.; Xing, Y.; et al. Development and mapping of 2240 new SSR markers for rice (Oryza sativa L.) (supplement). DNA Res. 2002, 9, 257–279. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, Q.; Feng, Q.; Lu, H.; Li, Y.; Wang, A.; Tian, Q.; Zhan, Q.; Lu, Y.; Zhang, L.; Huang, T.; et al. Pan-genome analysis highlights the extent of genomic variation in cultivated and wild rice. Nat. Genet. 2018, 50, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhao, Y.; Wei, X.; Li, C.; Wang, A.; Zhao, Q.; Li, W.; Guo, Y.; Deng, L.; Zhu, C.; et al. Genome-wide association study of flowering time and grain yield traits in a worldwide collection of rice germplasm. Nat. Genet. 2011, 44, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Thurber, C.S.; Reagon, M.; Gross, B.L.; Olsen, K.M.; Jia, Y.; Caicedo, A.L. Molecular evolution of shattering loci in U.S. weedy rice. Mol. Ecol. 2010, 19, 3271–3284. [Google Scholar] [CrossRef]
| Species | G237T | C760T | No. of Accessions (Varieties) | Phenotype |
|---|---|---|---|---|
| O. longistaminata | G | C | 2 | Shattering |
| O. barthii | G | C | 22 | Shattering |
| G | T | 6 | Non-shattering | |
| O. glaberrima | G | C | 8 | Shattering |
| G | T | 111 | Non-shattering | |
| O. glumaepatula | G | C | 2 | Shattering |
| O. meridionalis | G | C | 2 | Shattering |
| O. nivara | G | C | 20 | Shattering |
| T | C | 5 | Non-shattering | |
| O. rufipogon | G | C | 30 | Shattering |
| T | C | 6 | Non-shattering | |
| O. sativa, temperate japonica | T | C | 30 | Non-shattering |
| O. sativa, tropical japonica | T | C | 5 | Non-shattering |
| O. sativa, indica | T | C | 25 | Non-shattering |
| O. sativa, aus | T | C | 5 | Non-shattering |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhou, J.; Yang, Y.; Elgamal, W.H.; Xu, P.; Li, J.; El-Refaee, Y.Z.; Hao, S.; Tao, D. Two SNP Mutations Turned off Seed Shattering in Rice. Plants 2019, 8, 475. https://doi.org/10.3390/plants8110475
Zhang Y, Zhou J, Yang Y, Elgamal WH, Xu P, Li J, El-Refaee YZ, Hao S, Tao D. Two SNP Mutations Turned off Seed Shattering in Rice. Plants. 2019; 8(11):475. https://doi.org/10.3390/plants8110475
Chicago/Turabian StyleZhang, Yu, Jiawu Zhou, Ying Yang, Walid Hassan Elgamal, Peng Xu, Jing Li, Yasser Z. El-Refaee, Suding Hao, and Dayun Tao. 2019. "Two SNP Mutations Turned off Seed Shattering in Rice" Plants 8, no. 11: 475. https://doi.org/10.3390/plants8110475
APA StyleZhang, Y., Zhou, J., Yang, Y., Elgamal, W. H., Xu, P., Li, J., El-Refaee, Y. Z., Hao, S., & Tao, D. (2019). Two SNP Mutations Turned off Seed Shattering in Rice. Plants, 8(11), 475. https://doi.org/10.3390/plants8110475





