Qualitative and Quantitative Differences in Osmolytes Accumulation and Antioxidant Activities in Response to Water Deficit in Four Mediterranean Limonium Species
Abstract
:1. Introduction
2. Results
2.1. Substrate Moisture at the End of Treatments
2.2. Effects of Drought on Plant Growth and Photosynthetic Pigments Levels
2.3. Ion Accumulation
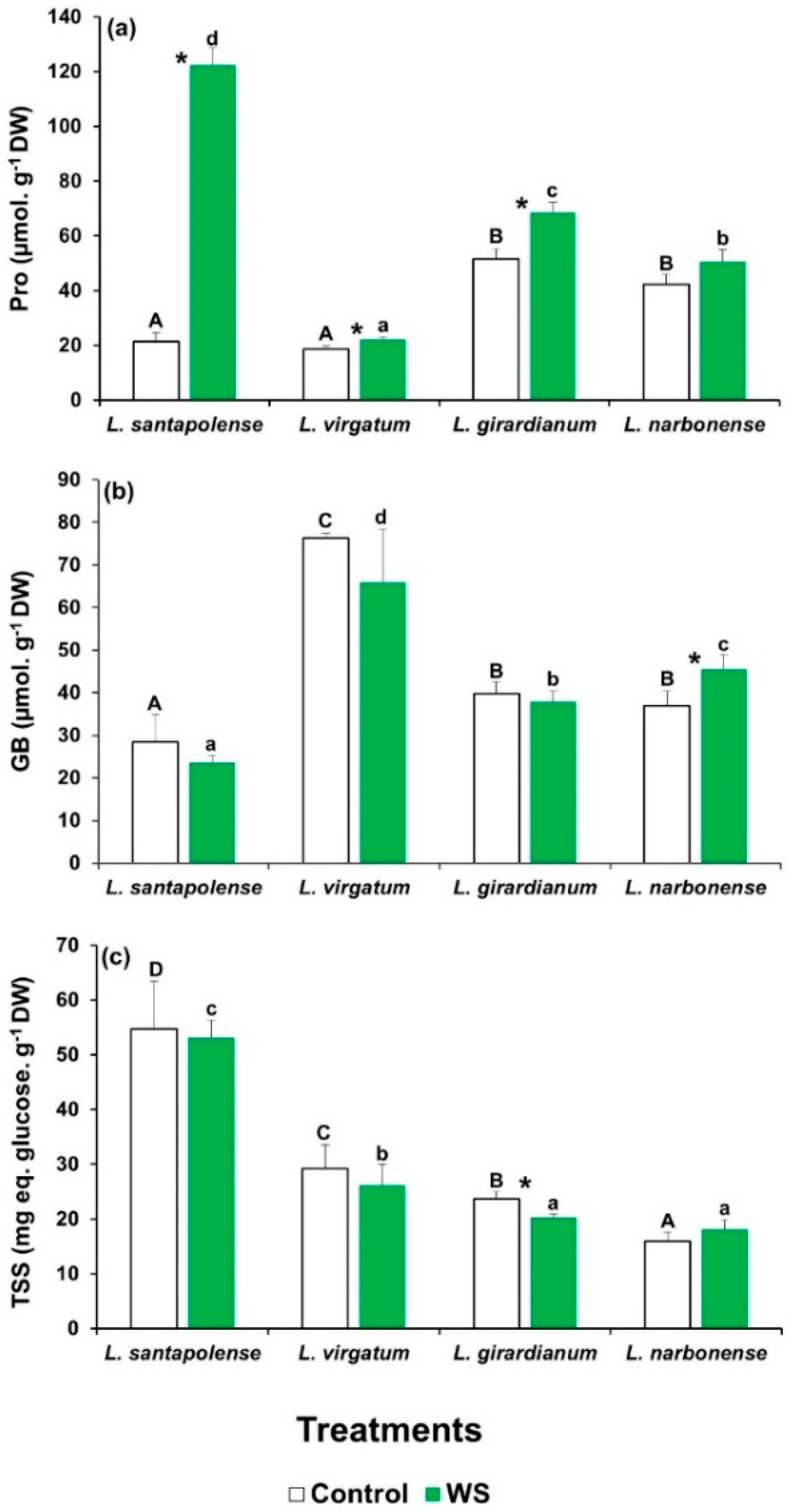
2.4. Water Stress-Induced Osmolyte Accumulation
2.5. Oxidative Stress and Activation of Antioxidant Systems
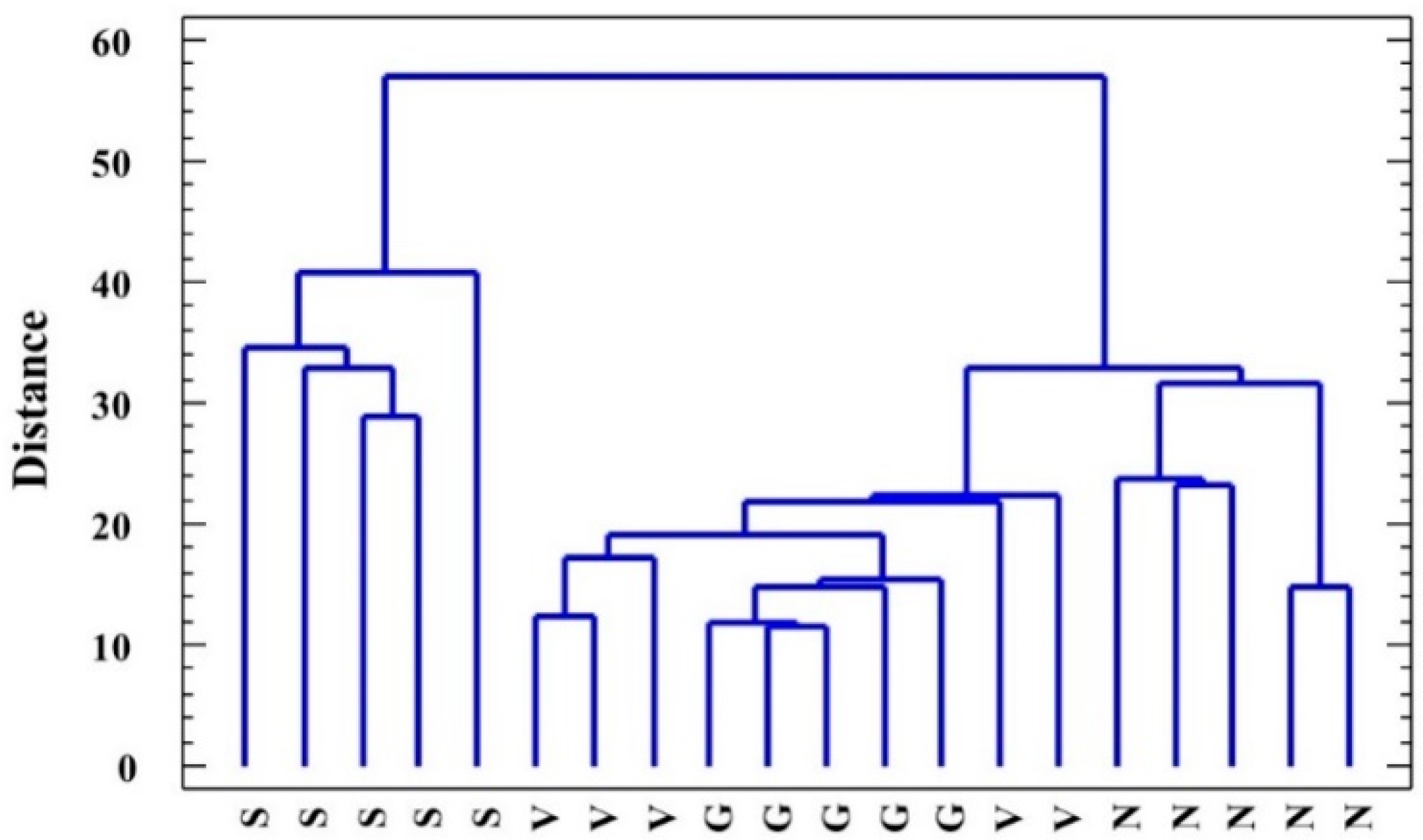
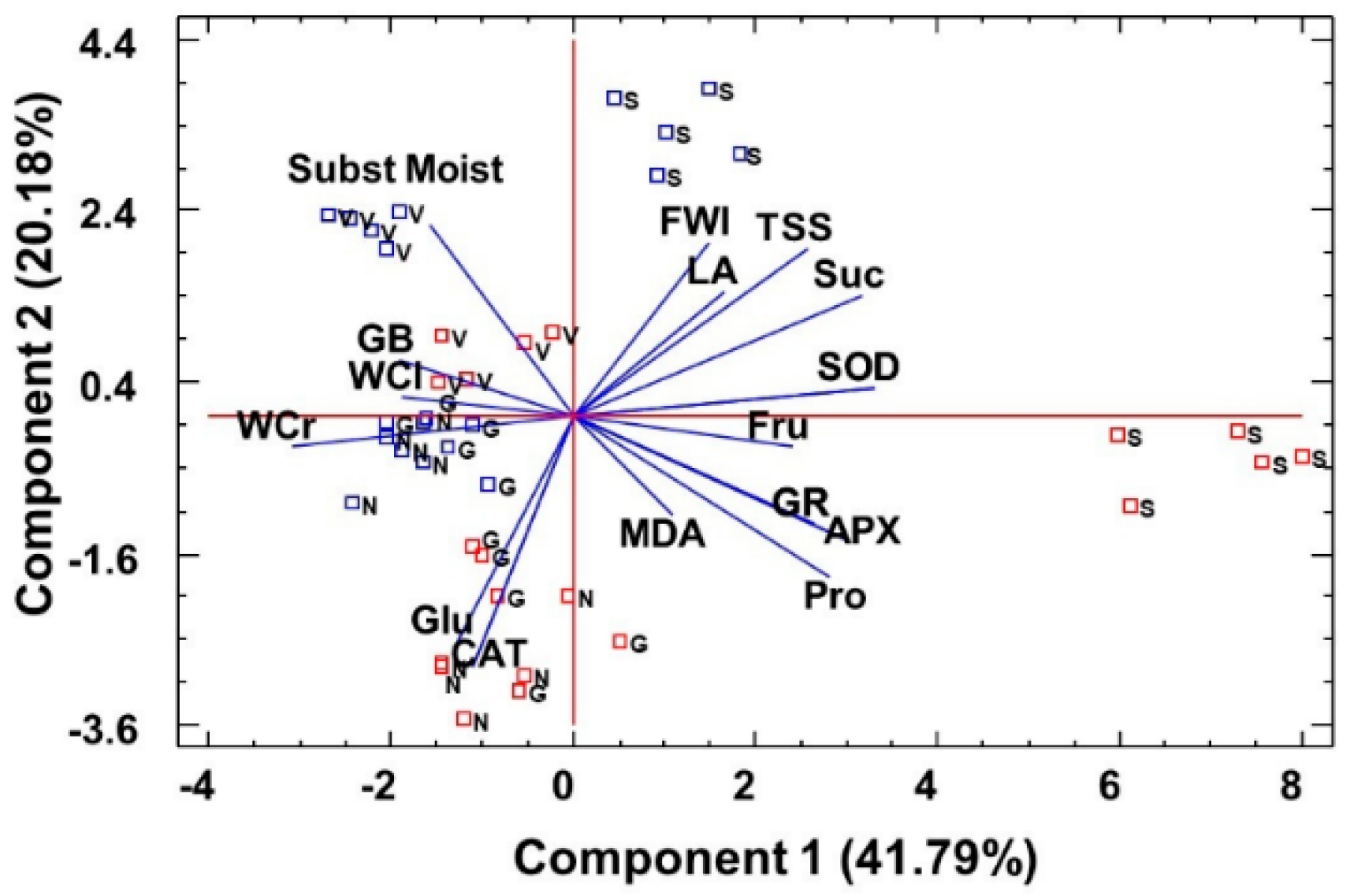
2.6. Statistical Analysis of Data: Factorial ANOVA, Clustering of Species, and Principal Component Analysis
3. Discussion
4. Materials and Methods
4.1. Sampling Sites and Seed Sampling
4.2. Plant Growth, Drought Treatments, and Sampling of Plant Material
4.3. Substrate Analysis
4.4. Photosynthetic Pigments
4.5. Ion Content Measurements
4.6. Osmolyte Quantification
4.7. HPLC Analysis of Soluble Carbohydrates
4.8. MDA, H2O2, DPPH, and Non-Enzymatic Antioxidants
4.9. Enzymatic Antioxidant Activities
4.10. Statistics Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lionello, P. The Climate of the Mediterranean Region, from the Past to the Future; Elsevier: Amsterdam, The Netherlands, 2012; p. 592. ISBN 978-012-416-042-2. [Google Scholar]
- Hoerling, M.; Eischeid, J.; Perlwitz, J.; Quan, X.; Zhang, T.; Pegion, P. On the increased frequency of Mediterranean drought. J. Clim. 2015, 25, 2146–2161. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Van Breusegem, F.; Dat, J.F. Reactive oxygen species in plant cell death. Plant Physiol. 2006, 141, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Mafakheri, A.; Siosemardeh, A.; Bahramnejad, B.; Struik, P.C.; Sohrabi, Y. Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Austr. J. Crop Sci. 2010, 4, 580–585. [Google Scholar]
- Li, Z.; Wakao, S.; Fischer, B.B.; Niyogi, K.K. Sensing and responding to excess light. Annu. Rev. Plant Biol. 2009, 60, 239–260. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S.; Bose, J.; Fuglsang, A.; Pottosin, I. On a quest for stress tolerance genes: Membrane transporters in sensing and adapting to hostile soils. J. Exp. Bot. 2016, 67, 1015–1031. [Google Scholar] [CrossRef]
- Schachtman, D.P.; Goodger, J.Q.D. Chemical root to shoot signaling under drought. Trends Plant Sci. 2008, 13, 281–287. [Google Scholar] [CrossRef]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef]
- Martínez, J.P.; Ledent, J.F.; Bajji, M.; Kinet, J.M.; Lutts, S. Effect of water stress on growth, Na+ and K+ accumulation and water use efficiency in relation to osmotic adjustment in two populations of Atriplex halimus L. Plant Growth Reg. 2003, 41, 63–73. [Google Scholar] [CrossRef]
- Xi, J.J.; Chen, H.Y.; Bai, W.P.; Yang, R.C.; Yang, P.Z.; Chen, J.; Hu, T.M.; Wang, S.M. Sodium-related adaptations to drought: New insights from the xerophyte plant Zygophyllum xanthoxylum. Front. Plant Sci. 2018, 9, 1678. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012. [Google Scholar] [CrossRef]
- Kar, R.K. Plant responses to water stress: Role of reactive oxygen species. Plant Signal Behav. 2011, 6, 1741–1745. [Google Scholar] [CrossRef] [PubMed]
- Golldack, D.; Li, C.; Harikrishnan, M.; Probst, N. Tolerance to drought and salt stress in plants: Unraveling the signaling networks. Front. Plant Sci. 2014, 5, 151. [Google Scholar] [CrossRef] [PubMed]
- Erben, M. Limonium Mill. In Flora Ibérica; Castroviejo, S., Aedo, C., Cirujano, S., Laínz, M., Montserrat, P., Morales, R., Muñoz Garmendia, F., Navarro, C., Paiva, J., Soriano, C., Eds.; Editorial CSIC: Madrid, Spain, 2013; Volume 3, pp. 2–14. [Google Scholar]
- Gagneul, D.; Aïnouche, A.; Duhazé, C.; Lugan, R.; Larher, F.R.; Bouchereau, A. A reassessment of the function of the so-called compatible solutes in the halophytic Plumbaginaceae Limonium latifolium. Plant Physiol. 2007, 144, 1598–1611. [Google Scholar] [CrossRef]
- Al Hassan, M.; Estrelles, E.; Soriano, P.; López-Gresa, M.P.; Bellés, J.M.; Boscaiu, M.; Vicente, O. Unraveling salt tolerance mechanisms in halophytes: A comparative study on four Mediterranean Limonium species with different geographic distribution patterns. Front. Plant Sci. 2017, 8, 1438. [Google Scholar] [CrossRef] [PubMed]
- Galmés, J.; Cifre, J.; Medrano, H.; Flexas, J. Modulation of relative growth rate and its components by water stress in Mediterranean species with different growth forms. Oecologia 2005, 145, 21–31. [Google Scholar] [CrossRef]
- Galmés, J.; Molins, A.; Flexas, J.; Conesa, M.À. Coordination between leaf CO2 difussion and Rubisco properties allow maximizing photosynthetic efficiency in Limonium species. Plant Cell Environ. 2017, 40, 2081–2094. [Google Scholar] [CrossRef]
- Guerin, R.G.; Lowe, A.J. Leaf morphology shift linked to climate change. Ecol. Lett. 2012, 8, 882–886. [Google Scholar] [CrossRef]
- Kumar, D.; Al Hassan, M.; Naranjo, M.A.; Agrawal, V.; Boscaiu, M.; Vicente, O. Effects of salinity and drought on growth, ionic relations, compatible solutes and activation of antioxidant systems in oleander (Nerium oleander L.). PLoS ONE 2017, 12. [Google Scholar] [CrossRef]
- Marček, T.; Hamow, K.Á.; Végh, B.; Janda, T.; Darko, E. Metabolic response to drought in six winter wheat genotypes. PLoS ONE 2019, 19. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.N.; Ferreira-Silva, S.L.; Almeida Viégas, R.; Gomes Silveira, J.A. The role of organic and inorganic solutes in the osmotic adjustment of drought-stressed Jatropha curcas plants. Environ. Exp. Bot. 2010, 69, 279–285. [Google Scholar] [CrossRef]
- Gámez, A.L.; Soba, D.; Zamarreño, Á.M.; García-Mina, J.M.; Aranjuelo, I.; Morales, F. Effect of water stress during grain filling on yield, quality and physiological traits of Illpa and Rainbow quinoa (Chenopodium quinoa Willd.) cultivars. Plants 2019, 8, 173. [Google Scholar] [CrossRef] [PubMed]
- Van der Weijde, T.; Huxley, L.M.; Hawkins, S.; Sembiring, E.H.; Farrar, K.; Dolstra, O.; Visser, R.G.; Trindade, L.M. Impact of drought stress on growth and quality of miscanthus for biofuel production. GCB Bioenergy 2017, 9, 770–782. [Google Scholar] [CrossRef]
- Tabot, P.T.; Adams, J.B. Salt secretion, proline accumulation and increased branching confer tolerance to drought and salinity in the endemic halophyte Limonium linifolium. S. Afr. J. Bot. 2014, 94, 64–73. [Google Scholar] [CrossRef]
- Hanson, D.A.; Rathinasabapathi, B.; Chamberlin, B.; Gage, D.A. Comparative physiological evidence that β-alanin betaine and choline-O-sulfate act as compatible osmolytes in halophytic Limonium species. Plant Physiol. 1991, 97, 1199–1205. [Google Scholar] [CrossRef]
- Rhodes, D.; Hanson, A.D. Quaternary ammonium and tertiary sulfonium compounds in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1993, 44, 357–384. [Google Scholar] [CrossRef]
- Murakeözy, E.P.; Nagy, Z.; Duhazé, C.; Bouchereau, A.; Tuba, Z. Seasonal changes in the levels of compatible osmolytes in three halophytic species of inland saline vegetation in Hungary. J. Plant Physiol. 2003, 160, 395–401. [Google Scholar] [CrossRef]
- Gil, R.; Lull, C.; Boscaiu, M.; Bautista, I.; Lidón, A.; Vicente, O. Soluble carbohydrates as osmolytes in several halophytes from a Mediterranean salt marsh. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 2011, 39, 9–17. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef]
- Sofo, A.; Scopa, A.; Nuzzaci, M.; Vitti, A. Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. Int. J. Mol. Sci. 2015, 16, 13561–13578. [Google Scholar] [CrossRef] [PubMed]
- Del Río, L.A.; Palma, J.M.; Sandalio, L.M.; Corpas, F.J.; Pastori, G.M.; Bueno, P.; López-Huertas, E. Peroxisomes as a source of superoxide and hydrogen peroxide in stressed plants. Biochem. Soc. Trans. 1996, 24, 434–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sagar, B.; Kedare, B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar]
- Gil, R.; Bautista, I.; Boscaiu, M.; Lidón, A.; Wankhade, S.; Sánchez, H.; Llinares, J.; Vicente, O. Responses of five Mediterranean halophytes to seasonal changes in environmental conditions. AoB PLANTS 2014, 6. [Google Scholar] [CrossRef]
- Bose, J.; Rodrigo-Moreno, A.; Shabala, S. ROS homeostasis in halophytes in the context of salinity stress tolerance. J. Exp. Bot. 2014, 65, 1241–1257. [Google Scholar] [CrossRef]
- Fini, A.; Brunetti, C.; Di Ferdinando, M.; Ferrini, F.; Tattini, M. Stress-induced flavonoid biosynthesis and the antioxidant machinery of plants. Plant Signal. Behav. 2011, 6, 709–711. [Google Scholar] [CrossRef]
- Souid, A.; Bellani, L.; Magné, C.; Zorrig, W.; Smaoui, A.; Abdelly, C.; Longo, V.; Ben Hamed, K. Physiological and antioxidant responses of the sabkha biotope halophyte Limonium delicatulum to seasonal changes in environmental conditions. Plant Physiol. Biochem. 2018, 123, 180–191. [Google Scholar] [CrossRef]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef]
- Caverzan, A.; Casassola, A.; Brammer, S.P. Antioxidant responses of wheat plants under stress. Genet. Mol. Biol. 2016, 39. [Google Scholar] [CrossRef]
- Gunes, A.; Pilbeam, D.J.; Inal, A.; Bagci, E.G.; Coban, S. Influence of silicon on antioxidant mechanisms and lipid peroxidation in chickpea (Cicer arietinum L.) cultivars under drought stress. J. Plant Interact. 2007, 2, 105–113. [Google Scholar] [CrossRef]
- Hameed, A.; Gulzar, S.; Aziz, I.; Hussain, T.; Gul, B.; Khan, M.A. Effects of salinity and ascorbic acid on growth, water status and antioxidant system in a perennial halophyte. AoB Plants 2015, 7. [Google Scholar] [CrossRef] [Green Version]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Califor. Agr. Exp. Stat. 1950, 32, 347. [Google Scholar]
- Rasband, W.S. ImageJ; US National Institutes of Health: Bethesda, MD, USA, 1997–2012. Available online: http://rsb.info.nih.gov/ij/ (accessed on 22 January 2019).
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Weimberg, R. Solute adjustments in leaves of two species of wheat at two different stages of growth in response to salinity. Physiol. Plant. 1987, 70, 381–388. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Nawaz, K.; Ashraf, M. Exogenous application of glycine betaine modulates activities of antioxidants in maize plants subjected to salt stress. J. Agron. Crop Sci. 2010, 196, 28–37. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Reberd, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Al Hassan, M.; Chaura, J.; López-Gresa, M.P.; Borsai, O.; Daniso, E.; Donat-Torres, M.P.; Mayoral, O.; Vicente, O.; Boscaiu, M. Native-invasive plants vs. halophytes: Stress tolerance mechanisms in two related species. Front. Plant Sci. 2016, 7, 473. [Google Scholar] [CrossRef] [Green Version]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Taulavuori, E.; Hellström, E.-K.; Taulavuori, K.; Laine, K. Comparison of two methods used to analyse lipid peroxidation from Vaccinium myrtillus (L.) during snow removal, reacclimation and cold acclimation. J. Exp. Bot. 2001, 52, 2375–2380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loreto, F.; Velikova, V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol. 2002, 127, 1781–1787. [Google Scholar] [CrossRef]
- Blainski, A.; Lopes, G.C.; Pallazzo De Mello, J.C. Application and analysis of the Folin Ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Beyer, W.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Method. Enzymol. 1984, 105, 121–126. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Connell, J.P.; Mullet, J.E. Pea chloroplast glutathione reductase: Purification and characterization. Plant Physiol. 1986, 82, 351–356. [Google Scholar] [CrossRef] [Green Version]



| Variable | Treatment | Limonium santapolense | Limonium virgatum | Limonium girardianum | Limonium narbonense |
|---|---|---|---|---|---|
| Humidity (%) | C | 45.42 ± 1.43 *A | 45.42 ± 1.25 *A | 45.39 ± 1.43 *A | 43.67 ± 0.71 *A |
| WS | 10.70 ± 1.25 *a | 14.01 ± 1.43 *a | 12.20 ± 1.10 *a | 12.26 ± 0.34 *a |
| Variable | Treatment | L. santapolense | L. virgatum | L. girardianum | L. narbonense |
|---|---|---|---|---|---|
| FWL (g) | C | 5.02 ± 0.42 *B | 2.33 ± 0.47 A | 2.49 ± 0.43 A | 2.05 ± 0.14 *A |
| WS | 3.44 ± 0.12 *b | 2.54 ± 0.66 b | 2.19 ± 0.27 b | 1.47 ± 0.18 *a | |
| LA (cm2) | C | 17.01 ± 1.24 *C | 4.45 ± 0.20 A | 6.06 ± 0.34 AB | 8.60 ± 0.65 B |
| WS | 10.26 ± 0.74 *b | 3.87 ± 0.25 a | 6.27 ± 0.39 a | 6.87 ± 0.63 ab | |
| WCL (%) | C | 84.55 ± 0.26 *A | 87.50 ± 0.46 C | 86.88 ± 0.37 BC | 85.21 ± 0.97 AB |
| WS | 83.61 ± 0.41 *a | 86.44 ± 0.88 bc | 87.95 ± 0.40 c | 84.26 ± 0.76 ab | |
| WCR (%) | C | 61.44 ± 5.50 A | 74.85 ± 1.18 B | 78.82 ± 1.47 B | 80.36 ± 1.01 *B |
| WS | 52,74 ± 5.08 a | 77.68 ± 1.24 b | 73.46 ± 1.93 b | 74.39 ± 0.36 *b | |
| Chl a (mg g−1 DW) | C | 2.37 ± 0.17 AB | 1.74 ± 0.18 A | 2.63 ± 0.13 B | 1.97 ± 0.19 AB |
| WS | 2.27 ± 0.21 ab | 2.18 ± 0.21 ab | 2.75 ± 0.17 b | 1.59 ± 0.13 a | |
| Chl b (mg g−1 DW) | C | 1.11 ± 0.01 A | 1.04 ± 0.06 A | 1.07 ± 0.10 A | 0.99 ± 0.14 A |
| WS | 1.14 ± 0.25 a | 1.22 ± 0.18 a | 1.03 ± 0.06 a | 0.76 ± 0.09 a | |
| Caro (mg g−1 DW) | C | 1.19 ± 0.07 A | 1.36 ± 0.11 A | 0.97 ± 0.08 A | 1.30 ± 0.21 A |
| WS | 1.02 ± 0.11 a | 1.12 ± 0.09 a | 0.99 ± 0.04 a | 0.93 ± 0.11 a |
| Ion | Treatment | L. santapolense | L. virgatum | L. girardianum | L. narbonense |
|---|---|---|---|---|---|
| Na+ roots | C | 137.22 ± 23.17 *A | 127.99 ± 2.19 A | 119.52 ± 1.68 *A | 174.17 ± 16.09 *A |
| WS | 209.96 ± 42.13 *ab | 116.44 ± 17.76 a | 184.18 ± 11.05 *a | 238.37 ± 22.70 *b | |
| Na+ leaves | C | 450.01 ± 10.61 A | 473.66 ± 27.32 A | 534.93 ± 29.99 A | 552.11 ± 67.78 A |
| WS | 499.65 ± 25.75 b | 426.83 ± 7.46 a | 513.47 ± 22.41 b | 416.29 ± 18.90 a | |
| K+ roots | C | 279.85 ± 35.35 A | 353.33 ± 8.86 AB | 278.78 ± 2.46 A | 382.72 ± 32.19 B |
| WS | 242.79 ± 35.36 a | 300.29 ± 42.01 ab | 326.49 ± 22.23 ab | 406.74 ± 36.69 b | |
| K+ leaves | C | 833.33 ± 20.98 A | 977.25 ± 43.11 A | 974.47 ± 32.05 A | 981.48 ± 97.45 A |
| WS | 839.27 ± 27.83 ab | 897.79 ± 15.08 ab | 1031.71 ± 75.86 b | 823.25 ± 60.43 a | |
| K+/Na+ roots | C | 2.08 ± 0.18 *A | 2.75 ± 0.01 C | 2.33 ± 0.05 *B | 2.20 ± 0.02 *AB |
| WS | 1.30 ± 0.16 *a | 2.60 ± 0.07 c | 1.77 ± 0.03 *b | 1.72 ± 0.09 *b | |
| K+/Na+ leaves | C | 1.85 ± 0.04 A | 2.08 ± 0.26 A | 1.83 ± 0.04 A | 1.80 ± 0.09 A |
| WS | 1.69 ± 0.06 a | 2.11 ± 0.15 b | 1.99 ± 0.06 b | 1.96 ± 0.06 b | |
| Cl− roots | C | 186.77 ± 18.28 *A | 236.95 ± 5.35 A | 203.10 ± 28.32 *A | 355.43 ± 35.59 B |
| WS | 203.10 ± 51.70 *a | 228.49 ± 17.34 a | 299.01 ± 20.26 *ab | 456.98 ± 41.80 b | |
| Cl− leaves | C | 727.78 ± 51.33 A | 787.02 ± 75.02 A | 767.27 ± 32.36 A | 971.50 ± 79.81 A |
| WS | 836.10 ± 47.45 a | 892.80 ± 34.84 a | 856.27 ± 17.34 a | 999.35 ± 82.48 a | |
| Ca2+ roots | C | 13.29 ± 2.03 *B | 4.48 ± 0.64 *A | 6.18 ± 1.20 *A | 14.23 ± 0.94 *B |
| WS | 6.18 ± 2.20 *a | 10.35 ± 1.14 *a | 9.69 ± 0.66 *a | 24.28 ± 2.67 *b | |
| Ca2+ leaves | C | 84.88 ± 7.03 A | 66.74 ± 10.43 A | 61.93 ± 12.40 A | 53.01 ± 12.13 A |
| WS | 97.12 ± 5.66 bc | 68.46 ± 14.90 ab | 117.57 ± 20.63 b | 37.20 ± 1.70 b | |
| Mg2+ roots | C | 64.24 ± 3.36 *C | 40.05 ± 0.36 *A | 50.93 ± 3.01 B | 76.33 ± 3.51 D |
| WS | 50.93 ± 5.51 *a | 57.21 ± 6.28 *a | 64.07 ± 5.07 ab | 78.34 ± 4.52 b | |
| Mg2+ leaves | C | 456.63 ± 29.80 A | 401.16 ± 40.36 A | 486.11 ± 61.29 A | 320.88 ± 34.63 A |
| WS | 538.36 ± 22.53 c | 429.16 ± 49.70 b | 555.07 ± 13.81 c | 260.80 ± 38.60 a |
| Variable | Treatment | L. santapolense | L. virgatum | L. girardianum | L. narbonense |
|---|---|---|---|---|---|
| MDA (nmol g−1 DW) | C | 103.58 ± 16.92 A | 83.93 ± 18.61 A | 149.16 ± 16.74 A | 80.09 ± 7.60 *A |
| WS | 152.59 ± 28.69 a | 138.82 ± 21.224 a | 162.53 ± 17.85 a | 135.28 ± 14.73 *a | |
| H2 O2 (µmol g−1 DW) | C | 17.93 ± 3.29 A | 17.21 ± 2.26 A | 20.86 ± 3.49 A | 26.68 ± 3.24 A |
| WS | 25.25 ± 3.80 b | 12.89 ± 1.28 a | 18.59 ± 0.38 ab | 21.94 ± 1.01 b | |
| DPPH (%) | C | 84.72 ± 6.10 C | 76.94 ± 0.99 BC | 15.28 ± 2.97 A | 52.34 ± 11.27 B |
| WS | 82.60 ± 9.86 b | 62.17 ± 6.90 ab | 34.13 ± 7.97 a | 41.81 ± 5.70 ab | |
| TPC (mg eq. GA g−1 DW) | C | 24.41 ± 4.18 B | 11.50 ± 6.15 A | 6.40 ± 1.31 A | 12.57 ± 3.01 A |
| WS | 23.96 ± 1.81 b | 6.15 ± 0.40 a | 6.56 ± 1.18 a | 9.25 ± 2.77 a | |
| TF (mg eq. C g−1 DW) | C | 1.96 ± 0.37 AB | 1.26 ± 0.16 A | 0.71 ± 0.13 A | 2.95 ± 0.61 B |
| WS | 1.80 ± 0.28 a | 1.00 ± 0.13 a | 1.22 ± 0.18 a | 1.90 ± 0.45 a |
| Growth | Parameter | Treatment (T) | Species (S) | Interaction (T × S) |
| FWl | 2.88 | 10.83 *** | 1.36 | |
| LA | 11.59 ** | 39.65 *** | 2.96 * | |
| WCl | 1.23 | 14.81 *** | 1.45 | |
| WCr | 14.16 *** | 30.38 *** | 4.11 * | |
| Photosynthtetic pigments | Chl a | 0.01 | 10.87 *** | 2.05 |
| Chl b | 0.1 | 1.91 | 0.66 | |
| Caro | 5.97 * | 1.77 | 1.06 | |
| Mono and divalent ions | Na+r | 10.87 ** | 6.06 ** | 1.89 |
| Na+l | 3.01 | 1.91 | 2.96 * | |
| K+r | 1.37 | 133.84 *** | 6.09 ** | |
| K+l | 1.31 | 3.33 * | 1.55 | |
| Cl−r | 8.68 *** | 14.07 *** | 2.90 * | |
| Cl−l | 5.45 * | 3.30 * | 1.33 | |
| Ca2+r | 0.05 | 7.39 *** | 1.37 | |
| Ca2+l | 3.25 | 9.44 **** | 2.80 | |
| Mg2+r | 3.29 | 19.11 *** | 6.44 ** | |
| Mg2+l | 2.08 | 14.55 *** | 1.48 | |
| Compatible solutes | Pro | 172.75 *** | 80.14 *** | 86.56 *** |
| GB | 1.68 | 22.21 *** | 2.81 | |
| TSS | 0.27 | 37.72 *** | 0.23 | |
| Glu | 5.51 * | 39.60 *** | 6.09** | |
| Fru | 25.67 *** | 14.65 *** | 79.13 *** | |
| Suc | 5.35 * | 3.27 * | 1.15 | |
| Oxidative stress markers and antioxidants | MDA | 9.26 ** | 2.40 | 0.50 |
| H2O2 | 0.29 | 4.33 * | 2.29 | |
| Antioxidants | TPC | 2.23 | 27.97 *** | 0.73 |
| TF | 1.05 | 10.06 ** | 2.21 | |
| SOD | 10.84 ** | 28.29 *** | 10.04 *** | |
| CAT | 12.63 * | 73.30 *** | 5.39 ** | |
| APX | 71.35 *** | 21.84 *** | 28.95 *** | |
| GR | 42.53 *** | 16.83 *** | 1.47 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Orenga, S.; Al Hassan, M.; Llinares, J.V.; Lisón, P.; López-Gresa, M.P.; Verdeguer, M.; Vicente, O.; Boscaiu, M. Qualitative and Quantitative Differences in Osmolytes Accumulation and Antioxidant Activities in Response to Water Deficit in Four Mediterranean Limonium Species. Plants 2019, 8, 506. https://doi.org/10.3390/plants8110506
González-Orenga S, Al Hassan M, Llinares JV, Lisón P, López-Gresa MP, Verdeguer M, Vicente O, Boscaiu M. Qualitative and Quantitative Differences in Osmolytes Accumulation and Antioxidant Activities in Response to Water Deficit in Four Mediterranean Limonium Species. Plants. 2019; 8(11):506. https://doi.org/10.3390/plants8110506
Chicago/Turabian StyleGonzález-Orenga, Sara, Mohamad Al Hassan, Josep V. Llinares, Purificación Lisón, M. Pilar López-Gresa, Mercedes Verdeguer, Oscar Vicente, and Monica Boscaiu. 2019. "Qualitative and Quantitative Differences in Osmolytes Accumulation and Antioxidant Activities in Response to Water Deficit in Four Mediterranean Limonium Species" Plants 8, no. 11: 506. https://doi.org/10.3390/plants8110506
APA StyleGonzález-Orenga, S., Al Hassan, M., Llinares, J. V., Lisón, P., López-Gresa, M. P., Verdeguer, M., Vicente, O., & Boscaiu, M. (2019). Qualitative and Quantitative Differences in Osmolytes Accumulation and Antioxidant Activities in Response to Water Deficit in Four Mediterranean Limonium Species. Plants, 8(11), 506. https://doi.org/10.3390/plants8110506










