Abstract
Plants respond to abiotic stresses by activating a specific genetic program that supports survival by developing robust adaptive mechanisms. This leads to accelerated senescence and reduced growth, resulting in negative agro-economic impacts on crop productivity. Cytokinins (CKs) customarily regulate various biological processes in plants, including growth and development. In recent years, cytokinins have been implicated in adaptations to osmotic stresses with improved plant growth and yield. Endogenous CK content under osmotic stresses can be enhanced either by transforming plants with a bacterial isopentenyl transferase (IPT) gene under the control of a stress inducible promoter or by exogenous application of synthetic CKs. CKs counteract osmotic stress-induced premature senescence by redistributing soluble sugars and inhibiting the expression of senescence-associated genes. Elevated CK contents under osmotic stress antagonize abscisic acid (ABA) signaling and ABA mediated responses, delay leaf senescence, reduce reactive oxygen species (ROS) damage and lipid peroxidation, improve plant growth, and ameliorate osmotic stress adaptability in plants.
1. Introduction
Osmotic stresses in plants are caused by drought, salinity and high temperature. Drought is a major global problem that impacts 1–3% of all land. This number is predicted to increase to 30% by 2090 [1]. Soil salinity is also a worldwide problem that impacts 397 million hectares of land [2]. Osmotic stress tolerance is a complex process involving a myriad of signaling pathways and results in either upregulation or downregulation of innumerable genes. These genes may either be regulatory (i.e., transcription factors that may further induce some other genes) or functional (i.e., directly involved in the process of stress tolerance). Strategies used to create osmotic stress tolerant transgenic plants involve overexpression of such regulatory or functional genes. The ultimate aim of plants under osmotic stress is to survive with minimal metabolic processes, which results in sluggish growth [3]. Recent findings posit the role of cytokinins (CKs) in mediating cellular responses to drought acclimation [4]. Exogenous application of cytokinins improves plant soluble sugar contents which in turn act as osmolytes to help plants tolerate osmotic stresses [5]. Moreover, CKs play a crucial role in delaying senescence, reducing oxidative damage and upholding plant growth under osmotic stresses. Targeted control of CK metabolism is a powerful tool to develop drought-tolerant plants [6,7,8,9,10]. This review assesses functions of enhanced CK contents in improving osmotic stress adaptability of plants without compromising yield.
2. Cytokinin Metabolism and Signal Transduction during Osmotic Stress
CKs primarily control cell growth and differentiation in plants. In response to osmotic stresses, concentration and transport of CKs in plants decrease drastically, whereas abscisic acid (ABA) levels increase [4,11,12,13,14,15]. Reduced CK levels inhibit cell division and cell growth. On the other hand, enhanced concentrations of ABA and ethylene promote the closure of stomatal apertures, thereby reducing water loss through transpiration, and activate the senescence-related Cys protease (phcp1) that promotes senescence of old leaves [16,17,18]. Characteristically, plants survive with minimum resources under osmotic stress. Two major enzymes concomitant with CK metabolism in plants are isopentenyl transferase (IPT), which is involved in the synthesis of CKs and cytokinin oxidase (CKX) that degrades CKs [19,20,21]. Genome surveys of different crops revealed the presence of more than one gene encoding each of these enzymes. In Arabidopsis, there are 9 IPT (AtIPT1 to AtIPT9) genes and 7 CKX (AtCKX1 to AtCKX7) genes [22,23] while in soybean, 14 CK biosynthetic (GmIPT) and 17 CK degradative (GmCKX) genes have been identified [19]. The high number of abiotic stress-inducible cis-elements on the promoter of CKX genes causes their upregulation under water deficit stress resulting in decreased CK levels [19,24,25,26]. Conversely, IPT transcript levels remain almost stationary in response to osmotic stress [19].
CK signal transduction culminates in the regulation of CK responsive genes and has been studied in several crop species, most extensively in Arabidopsis and rice [14,27,28,29,30,31]. CK signal perceptions and transduction pathways consist of three major components as histidine kinase (HK) receptors, authentic histidine phosphotransferases (AHPs) and response regulators (RRs) [32,33]. Signal transduction follows a multistep His-Asp phosphorelay, which involves four consecutive phosphorylation events that alternate between histidine and aspartate residues [34]. HK receptors are predominantly localized on endoplasmic reticulum (ER) membranes, while plasma membrane is reported to have relatively fewer HK receptors [35]. HK receptors have a conserved CK-binding CHASE (cyclases/histidine kinases-associated sensing extracellular) domain, two transmembrane domains, a histidine kinase domain, and two receiver domains [36]. During the process of CK perception and transduction, CK binding to the CHASE domain activates the cytosolic HK domain, which then undergoes an auto-phosphorylation of its conserved His residue. The phosphate is then transferred to a conserved Asp residue within the receiver domain [33,37]. The phosphate group is further transferred to downstream AHPs, which act as high-energy phosphodonors. AHPs have no catalytic activity and transfer the phosphate to an Asp residue within a type-B RR receiver domain [38]. Besides possessing a phosphate receiver domain, type-B RRs also have a large C-terminal extension that contains a Myb-like DNA-binding domain. DNA-binding motifs for type-B RRs have been identified upstream in many CK-regulated genes [39,40]. The process of CK signal transductions involves two checkpoints at the level of AHPs and type-B RRs for negative regulation. Pseudo-histidine phosphotransfer proteins (PHPs) mimic AHPs to accept phosphate from HK receiver domains but lack the histidine phosphorylation site to further transfer the phosphate group [37]. Plants also have type-A RRs that contain a phosphate receiver domain similar to type-B RRs; however, they lack a classic output domain to regulate transcription of CK genes [41]. Dephosphorylated type-A RR proteins are usually unstable and degraded by 26S proteasomes. Phosphorylation of type-A RRs leads to autoregulation of their transcription to ensure availability in response to CK signaling [42,43].
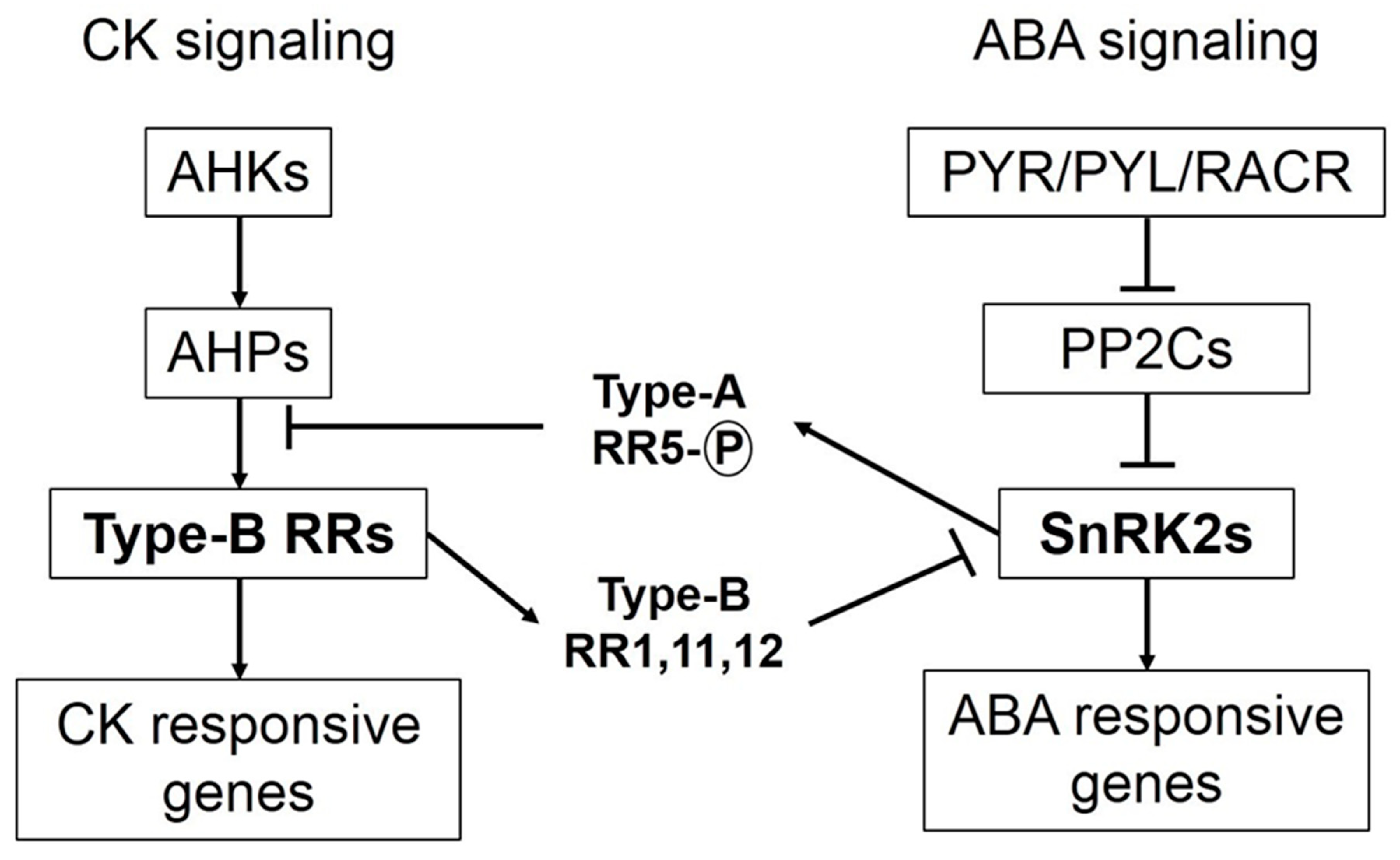
3. Cytokinins Antagonize ABA Signaling and ABA Arbitrated Adjustments during Osmotic Stress
CKs and ABA are unfriendly phytohormones, but both are equally vital for plant developmental processes. ABA promotes stress-induced signaling that enables plants to adjust under unfavorable environmental conditions [44,45]. ABA signaling stimulates CKX activity during osmotic stress and reduces CK concentration [19,26]. By contrast, elevated concentrations of CKs during osmotic stress conditions, either through exogenous application or IPT overexpression, counteract ABA arbitrated events [4,13]. Increased levels of CKs are known to affect both biosynthesis and sensitivity of ABA in plants under osmotic stress conditions. Key components of ABA signaling are SnRK2 (sucrose non-fermenting-1-related protein kinase 2) protein kinases, which phosphorylate downstream targets and trigger ABA-induced responses in plants. When ABA concentration is low, SnRK2 protein kinase activity is inhibited by PP2C (protein phosphatase type-2C) phosphatases. When ABA concentration increases during stress/exogenous application, ABA binds to its receptors, namely PYR/PYL/RCARs (pyrabactin resistance/ pyrabactin-like/ regulatory components of the ABA receptor), which in turn bind to PP2Cs and inactivate them. Consequently, PP2Cs are dissociated from SnRK2s resulting in activation of SnRK2s to initiate ABA-induced responses [46,47,48]. Crosstalk between signaling components of both ABA and CK reveals antagonism between the two phytohormones (Figure 1). Under osmotic stress conditions, when ABA concentration is high with prominent signaling in plants, SnRK2s phosphorylate and activate ARR5 (type-A response regulator 5). Phosphorylated AAR5 proteins auto-activate their transcription and competitively inhibit phosphate transfer to type-B RRs, thus hampering CK signaling. However, if CK concentration is high (in case of exogenous application/IPT overexpression) under osmotic stress, CK signaling components inhibit ABA signaling. Type-B RRs, which are positive regulators of CK signaling, inhibit SnRK2 activity [15].
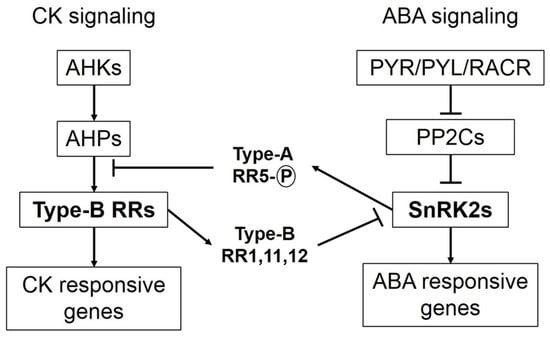
Figure 1.
Interaction between signaling components of cytokinin and abscisic acid. Osmotic stresses usually result in enhanced abscisic acid (ABA) biosynthesis and signaling. Binding of ABA receptors to PP2Cs results in activation of SnRK2s, which in turn phosphorylate and activate type-A response regulator 5s (RR5s). Type-A RR5s autoactivate their transcription and act as negative regulators of cytokinin (CK) signaling by hindering phosphate transfer from authentic histidine phosphotransferases (AHPs) to type-B RRs. Conversely, if plants have higher CK concentration (due to exogenous spray of synthetic cytokinins or isopentenyl transferase (IPT) overexpression) under osmotic stresses, CK signaling predominates. Type-B RRs (key components of CK signaling) activate some other type-B RRs (RR1, 11 and 12), which obstruct ABA signaling by inhibiting the activity of SnRK2s.
Increase in CK concentration in IPT overexpressing tobacco plants resulted in suppression of ABA biosynthetic genes [49]. Likewise, overexpression of IPT8 in Arabidopsis caused insensitivity in ABA treatments and prevented induction of ABI1 and ABI5 in seedlings [50]. Leaf fragments of Commelina and maize were assessed for the effects of CK and ABA treatments on stomatal behavior. When applied separately, ABA triggered stomatal closure in both Commelina and maize, while CKs (zeatin and kinetin at 1 to 100 m−3 mol/L) did not promote stomatal opening in either species. However, when applied simultaneously, CKs reversed ABA-induced closure of maize stomata but showed no effect on ABA-induced closure of Commelina stomata [51]. Under white light illumination, 10 μM ABA almost completely closed the stomata in isolated epidermal peels of Arabidopsis, and subsequent application of 10 μM BA (6-benzyladenine) reversed ABA-induced stomatal closure. Nevertheless, BA application had no effect on dark-induced stomatal closure, nor did it enhance stomatal opening under light conditions when applied separately. Results confirmed that CKs only revert stomatal closure induced by ABA and not under normal conditions [52]. Effects of exogenous application of benzyladenine (BA) and abscisic acid (ABA) were investigated separately as well as simultaneously on stomatal gas exchange in Phaseolus vulgaris L. leaves. When sprayed separately, 100 μM ABA decreased stomatal conductance, transpiration rate and net photosynthetic rate, while 10 μM BA had no significant effect on these parameters individually. By contrast, when applied simultaneously, application of 10 μM BA reversed the effects of 100 μM ABA on common bean leaves [53]. Furthermore, BA delayed the development of water deficit stress and increased photosynthetic rate in stressed leaves [53].
4. IPT Overexpression Influences Growth and Osmotic Stress Adaptability
Plants exhibit enhanced acclimation to osmotic stresses when endogenous CK content is high. The IPT gene catalyzes the rate-limiting step of cytokinin biosynthesis. Overexpressing IPT genes under control of senescence-induced pSAG12 or maturation and stress-induced pSARK promoters are the preferred approaches among researchers to manipulate CK levels [54,55]. The IPT gene from Agrobacterium tumefaciens has been frequently targeted for this purpose [56]. Increased CK contents through overexpression of the IPT gene, driven by senescence-activated promoter (pSAG12::IPT) in creeping bentgrass (Agrostis stolonifera), imparted enhanced tolerance to drought [8] and alleviated drought-induced damages to promote root growth [10]. Enhanced drought tolerance ability of IPT overexpression in A. stolonifera plants is attributed to accumulation of some essential metabolites, predominantly amino acids (proline, g-aminobutyric acid, alanine and glycine), carbohydrates (sucrose, fructose, maltose and ribose), and organic acids that are mainly involved in the citric acid cycle. These metabolites promote drought tolerance due to their well-known roles in osmotic adjustment, stress signaling and respiration for energy production [8]. In another experiment, IPT overexpression was investigated in Agrostis stolonifera plants under the control of SAG12 (pSAG12::IPT) and HSP18.2 (pHSP18.2::IPT) promoters. Transgenic lines showed higher CK contents with better drought adaptability and maintained a higher CK-to-ABA ratio. Furthermore, transgenic lines had better turf quality, photochemical efficiency, chlorophyll content, and leaf relative water content (RWC) under drought stress than NT (null transformant) plants [57].
Overexpression of the IPT gene, driven by SARK promoter (pSARK::IPT), augmented the synthesis of CK and contributed to enhanced osmotic stress tolerance in tobacco (Nicotiana tabacum) transgenic plants [58,59]. The transgenic plants displayed minimal reduction in biomass and seed yield (8–14%) under water deficit stress, compared to WT (wild type) plants, which showed a decline of 57% and 60% in biomass and seed yield, respectively. Improved yield of IPT overexpressing plants may be ascribed to a better photosynthetic rate and 2–3 times higher water use efficiency (WUE) compared to WT plants [58]. Synchronized expression of IPT (pSARK::IPT) significantly improved drought adaptability of peanut plants in both laboratory and field conditions. Transgenic peanut plants sustained higher photosynthetic rates, higher stomatal conductance, and higher transpiration than WT control plants under reduced irrigation conditions. Consequently, transgenic plants produced substantially higher yields than control plants in the field [7].
In rice plants, overexpression of IPT (pSARK::IPT) demonstrated greater tolerance to osmotic stress [60,61]. Enhanced stress adaptability of transgenic rice plants was facilitated by cytokinin-dependent harmonized regulation of carbon and nitrogen metabolism, which resulted in a healthier source to sink relationship under stress conditions [60,61]. Transgenic rice plants showed delayed response to stress, with significantly higher biomass and grain yield compared to WT plants [60]. A batch of pSARK::IPT transgenic cotton plants, grown in growth chamber condition, demonstrated enhanced tolerance to drought stress [9], which was accredited to delayed senescence of leaves and flowers. The IPT transgenic plants produced more root and shoot biomass, dropped fewer flowers, maintained higher chlorophyll contents, and higher photosynthetic rates under reduced irrigation conditions in comparison to the wild-type and segregated non-transgenic lines [9]. A gene expression study of IPT exposed a noteworthy shift in expression of hormone-related genes in transgenic plants. During water deficit stress, pSARK::IPT plants displayed increased transcription of brassinosteroid (BR)-related genes and repression of jasmonate (JA)-related genes [9,60]
Improved drought tolerance with delayed leaf senescence was displayed by pSAG12::IPT transgenic cassava plants. Detached leaves of the transgenic plants retained more chlorophyll compared to WT plants. Transgenic plants accumulated more trans-zeatin-type cytokinins with positive effects on photosynthesis, sugar allocation, and nitrogen partitioning [6]. Eggplants transformed with pSAG12::IPT also exhibited delayed senescence along with tolerance to drought and cold stresses. Compared to WT plants, transgenic eggplants displayed higher contents of chlorophyll, indole acetic acid (IAA), zeatin riboside, and gibberellic acid (GA) while ABA and malondialdehyde levels were low. Consequently, vegetative growth rates and yields of transgenic plants were relatively higher than those of WT plants [62]. In agreement with IPT gene insertions resulting in enhanced CK concentration and improved osmotic stress tolerance, CKX gene knockouts have also been tested for tolerance under salinity stress. Knockdown of OsCKX2 in rice using the RNAi-based approach resulted in a significant increase in cytokinins under salt stress condition. OsCKX2-knockdown plants displayed improved vegetative growth, relative water content, and photosynthetic efficiency compared to wild types under salinity stress [63].
Generally, elevated CK levels under water deficit stress condition are associated with enhanced osmotic stress tolerance and better growth. Nevertheless, some contrasting observations were also reported where decreased biosynthesis of CK in transgenic plants resulted in improved root growth and drought tolerance. Transgenic Arabidopsis and tobacco plants, with enhanced root-specific degradation of CK, exhibited larger root systems compared to WT plants with no effect on growth and development of shoots. Moreover, transgenic plants displayed higher survival rates after severe drought treatment [25]. In another investigation, barley (Hordeum vulgare) was transformed using the CKX1 gene from Arabidopsis thaliana (AtCKX1) under the control of mild root-specific b-glucosidase promoter. The transgenic plants were found to have low CK contents with better root systems and maintained high water contents under severe water deficit stress compared to WT plants [64].
5. Cytokinin Mediated Drought Acclimation is Primarily due to Delayed Senescence
Senescence is a natural physiological aging phenomenon in plants. The process involves the degradation of macromolecules, which mobilize nutrients from senescing tissues to sink tissues in order to sustain growth and development. Besides natural aging factors, senescence may also be triggered by various biotic and abiotic stresses [65,66]. Leaf senescence, as the final stage of development, follows a synchronized order of events involving loss of chlorophyll with subsequent reduction in photosynthesis, degradation of macromolecules, relocation of nutrients, dismantling of cellular components and, finally, cell death [67,68,69]. Senescence of plant leaves and flowers is achieved by the coordinated action of numerous senescence-associated genes (SAGs) with cysteine proteases as key components [70,71,72].
In the context of phytohormones, senescence is complemented by a decline in leaf CK content. An upsurge in the endogenous concentration or exogenous application of CKs results in nutrient mobilization and delays senescence [8,10,73]. Extracellular invertase and hexose transporters, responsible for apoplasmic phloem unloading, are co-induced by elevated levels of CKs, which instigate delay of senescence via an effect on source-sink relations [74]. Enhanced expression of the IPT gene from Agrobacterium tumefaciens, under the control of senescence-associated gene promoter (SAG12) is a widely used approach to delay senescence [6,8,75]. This IPT overexpression in plants with delayed senescence is useful for studying interactions of signaling mechanisms pertaining to CK based stress tolerance [6,62].
Environmental stresses, particularly drought, are responsible for premature leaf and flower senescence in plants by inducing synthesis of different types of cysteine proteases [76,77,78]. Delaying senescence through elevated levels of cytokinin during such environmental stresses has been proved beneficial for plants in terms of drought adaptation. Overexpression of the IPT gene under control of maturation and stress-induced promoter (pSARK::IPT) was reported to delay drought-induced senescence and thereby create drought tolerance in tobacco [58,59], peanut [7], rice [60,61] and cotton [9]. Likewise, IPT transformations under control of senescence-associated gene promoter (pSAG12::IPT) also proved advantageous in delaying senescence and imparting drought tolerance in cassava [6], Agrostis stolonifera [8,57] and brinjal [62].
Effects of high CK content on ethylene synthesis and sensitivity and ABA accumulation were examined in petunia plants transformed with IPT under control of pSAG12 promoter (pSAG12::IPT). Floral senescence in transgenic lines was delayed by 6 to 10 days compared to WT flowers. Endogenous ethylene biosynthesis was induced by pollination in both transgenic and WT flowers but biosynthesis was postponed in IPT transgenic flowers. Moreover, flowers from IPT transgenic plants were relatively less sensitive to exogenous ethylene and required longer treatment times to induce endogenous ethylene production, corolla senescence and upregulation of the senescence-related Cys protease phcp1. ABA accumulation was relatively less in flowers of IPT transgenic plants [79]. Cassava plants transformed with pSAG12::IPT exhibited delayed senescence under drought stress and retained relatively high levels of chlorophyll compared to WT plants. Induced expression of IPT also had positive effects on photosynthesis, sugar allocation and nitrogen partitioning. Furthermore, the transgenic lines showed significant drought tolerance as indicated by stay-green capacity after drought stress treatments [6].
6. Cytokinins Uphold Plant Growth during Abiotic Stresses
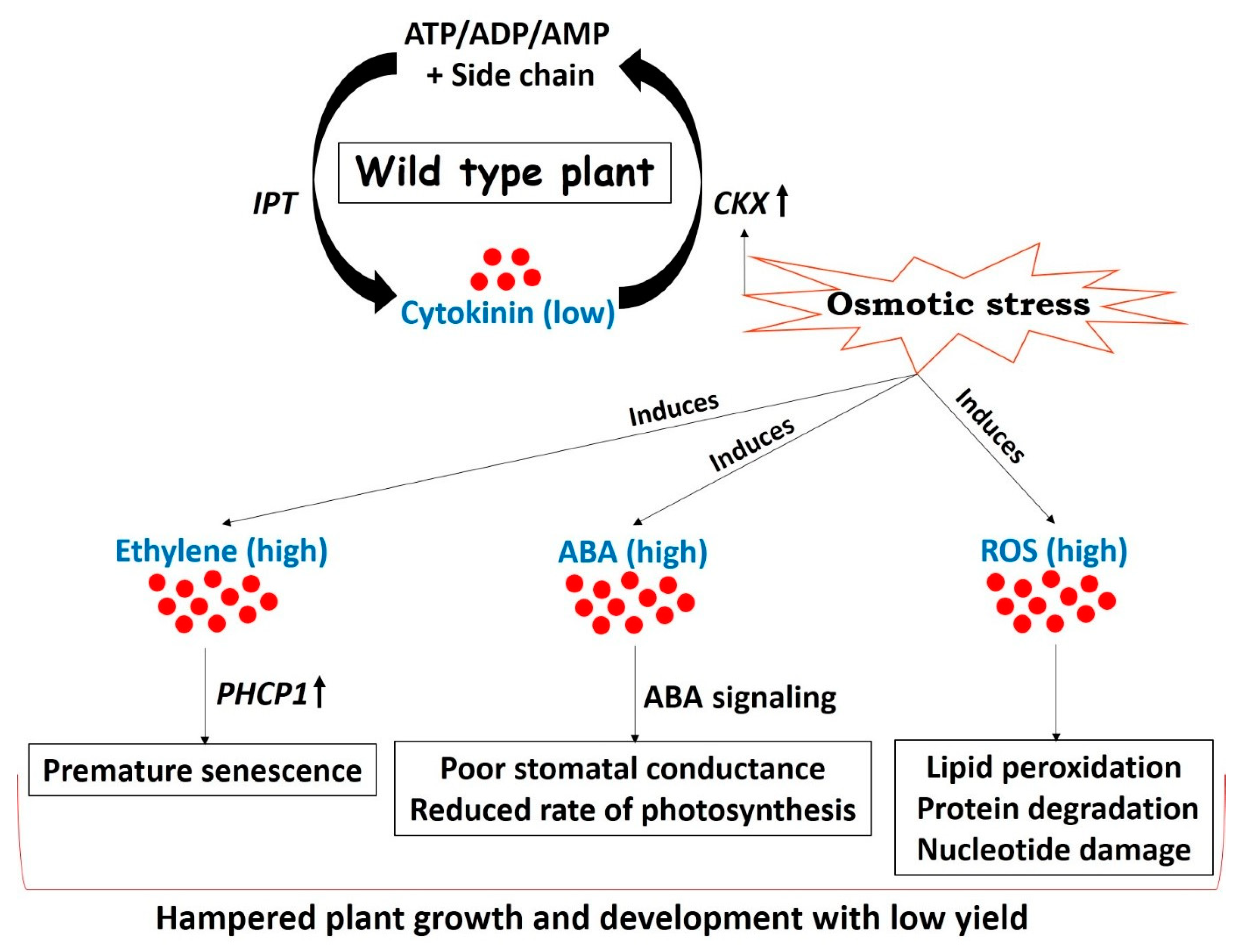
One of the prime objectives of plant biologists is to improve plant performances under less favorable environmental conditions. Approaches frequently used to counter drought stress are largely based on the overexpression of either regulatory or functional genes that are upregulated during stress conditions [80,81,82,83]. Transgenic plants created by altering the expression of such drought responsive genes are capable of withstanding drought up to a certain extent but exhibit a considerable drop in yield [84,85]. This loss of yield under drought stress is attributed to reduced CK biosynthesis followed by stress adaptive plant mechanisms including remobilization of nutrients triggering senescence, closure of stomata leading to reduced transpiration and gaseous exchange, and reduced photosynthetic rate [26] (Figure 2). Ultimately, plants follow the strategy of “survive with minimum” during stress conditions.
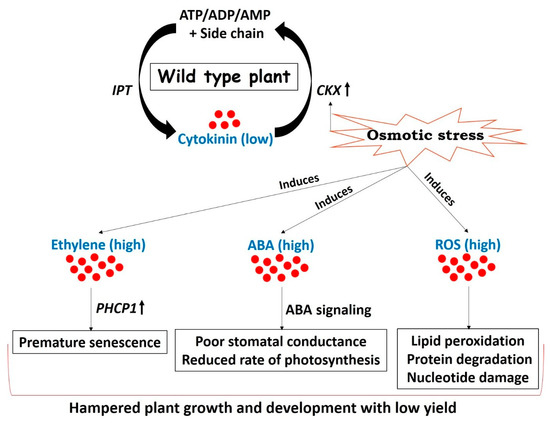
Figure 2.
Drought arbitrated adjustments in wild type plants resulting in mild tolerance and low yield. Cytokinin concentration in plants is maintained by two key genes involved in biosynthesis (IPT) and degradation (cytokinin oxidase (CKX)) of cytokinin. CKX is induced under drought stress due to the presence of abiotic stress-inducible cis-elements on its promoter [19,24,25,26], thereby decreasing the concentration of cytokinin. The onset of drought stress enhances the concentrations of stress responsive hormones such as ABA [86] and ethylene [87]. Reactive oxygen species (ROS) are also generated in chloroplasts and mitochondrial electron transport chains in response to stress [88]. Ethylene promotes premature senescence of leaves through a series of coordinated events and enhances the activity of senescence-related Cys protease (PHCP1) [17,18]. Increased biosynthesis and signaling of ABA leads to reduced stomatal conductance and decreases the photosynthetic rate [26,89]. Stress-induced elevated levels of ROS are unfavorable for plant growth and development and are responsible for lipid peroxidation, protein degradation, and nucleotide damage in the worst cases [90,91]. All these stress adaptive events slow down the normal growth and development of a plant resulting in poor yield. (IPT = isopentenyl transferase, CKX = cytokinin oxidase, ABA = abscisic acid, PHCP1 = senescence - related Cys protease, ROS = reactive oxygen species).
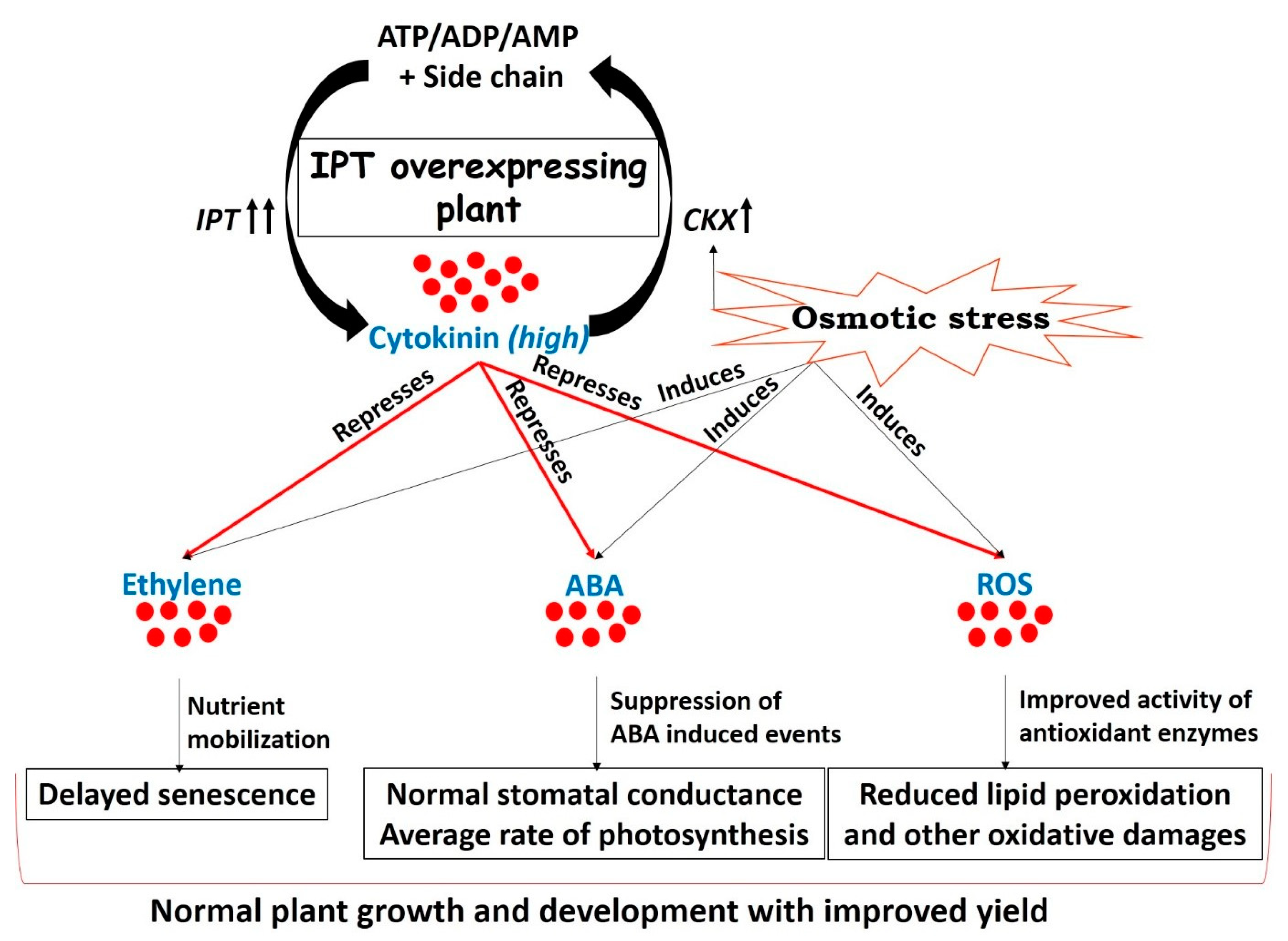
The novelty of CK mediated drought acclimation during the past decade has revolutionized the research pertaining to stress tolerance. Transgenic plants generated by overexpression of the IPT gene delivered better yield and tolerated drought for an extended length of time. Improved yield of IPT transgenic plants under limited water conditions may be ascribed to higher CK levels that counteract leaf senescence by mobilizing the remobilized nutrients [6,10,62], improving photosynthetic efficiency [7,58,92], interrupting drought-induced ABA signaling [15,50,93], and eventually stopping all those events that guide the plant to “survive with minimum” (Figure 3).
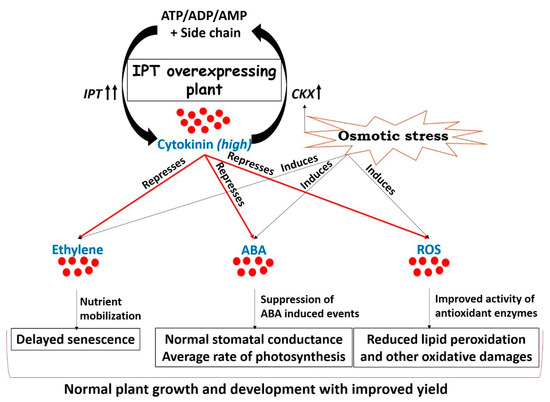
Figure 3.
Drought arbitrated adjustments in IPT transgenic plants, resulting in tolerance to water deficit stress without compromising yield. Cytokinin concentration in plants is maintained by two key genes involved in biosynthesis (IPT) and degradation (CKX) of cytokinin. Though CKX is induced under drought stress [19,25,26] but overexpression of the bacterial IPT gene in transgenic plants helps to maintain cytokinin concentration at higher levels [57,58,59,62] Elevated concentration of cytokinin during drought stress limits the biosynthesis and sensitivity of ethylene [79], helps in nutrient mobilization [8,10], and improves source-sink relations [74]. All these events eventually lead to a delay in the process of senescence in IPT overexpressing plants [6,7,58,60,61] or plants sprayed with cytokinin exogenously [53,94]. Increased levels of cytokinin during drought stress also affect biosynthesis [49] and sensitivity [50] of ABA, ultimately reverting the ABA-induced events [4,13]. Hence, IPT transgenic plants have better stomatal conductance and improved photosynthetic efficiency [7,58,92] compared to wild types. Elevated cytokinin levels in IPT overexpressing plants positively modulate the activities of antioxidant enzymes during drought stress and mitigate ROS driven damages [8,95,96]. Overall, IPT transgenic plants sustain normal growth and development under drought stress and offer an improved yield compared to wild types. (IPT = isopentenyl transferase, CKX = cytokinin oxidase, ABA = abscisic acid, ROS = reactive oxygen species).
Leaf discs of pSAG12::IPT modified gerbera plants, incubated in 40% (w/v) polyethylene glycol (PEG) for 20 h under continuous light [130 μmol/(m2·s)], retained relatively higher contents of chlorophyll, carotenoids, and soluble proteins compared to control plants [96]. Tomato roots, transiently expressing the IPT gene (pHSP70::IPT), exhibited a 2–3-fold increase in root CK concentration and improved plant growth and yield under salinity stress (100 mM NaCl for 22 days). Enhanced CK concentrations in transgenic tomato plants delayed stomatal closure and leaf senescence and virtually doubled shoot growth compared to WT plants. Furthermore, ABA and toxic Na+ ion concentrations decreased by 20–40% and 30% respectively with concomitant increases in the essential K+ ion by 20% in mature leaves [93]. In another experiment, WT shoots were grafted onto a constitutive IPT expressing rootstock (p35S::IPT); plant yield was enhanced by 30% compared to WT under salinity stress [93]. High yield and superior growth rate of IPT overexpressing plants may also be substantiated by improved N-use efficiency. Transgenic tobacco plants (pSARK::IPT) with increased CK biosynthesis, maintained adequate biomass and growth rates under limited N conditions compared to WT plants [97]. Higher CK biosynthesis in pSAG12::IPT modified rice plants resulted in vigorous growth even after a long drought period that killed the control plants. The transgenic plants displayed improved photosynthetic activity and maintained high water contents during drought [58]. Similar modifications (pSAG12::IPT) in transgenic peanut plants also resulted in higher photosynthetic rates with improved stomatal conductance and transpiration, compared to WT control plants under limited irrigation conditions. All these CK mediated changes in transgenic peanut plants resulted in significantly higher yields than wild-type control plants in the field [7]. Experiments involving CK-deficient mutants also revealed reduced growth and yield. Loss-of-function mutants of CK receptors AHK2, AHK3 and CRE1/AHK4 in Arabidopsis displayed rapid seed germination but the leaves of mutants formed fewer cells and had reduced chlorophyll content [98]. Arabidopsis CK-deficient ipt1, 3, 5 and 7 mutants showed better salt and drought tolerance but had reduced yield compared to WT [26].
Elevated concentrations of CK under drought stress counteract drought-induced signaling and facilitate plants to act ordinarily [4,99]. Hence, plants with high CK concentration are able to maintain normal levels of leaf water content, photosynthetic rate and stomatal conductance even under stress conditions. All these factors cooperatively lead to better growth and development of plants under stress. Besides IPT modulations, exogenous applications of synthetic cytokinins have also proved equally beneficial in improving plant growth under stress environments. Foliar spray of CPPU enhanced salt tolerance in rice by maintaining rates of photosynthesis, soluble sugars and free proline concentration under salinity stress [92]. Exogenous application of 100 μM CK onto creeping bentgrass improved turf quality and delayed leaf wilting under drought stress and elevated N conditions [94]. In a recent study on wheat, exogenous application of the optimized dose of 10 mg L−1 BAP significantly increased membrane stability index (MSI), photosynthetic pigment contents, chlorophyll stability index, and other growth parameters under drought and high temperature stress conditions [100].
7. Cytokinins Moderate ROS Levels during Osmotic Stresses
Reactive oxygen species (ROS) are generated in chloroplasts and mitochondrial electron transport chains in response to both abiotic and biotic stresses [88]. Drought-induced oxidative damage can lead to lipid peroxidation, protein degradation and nucleotide damage, further inhibiting a wide range of plant cellular processes [90,91,101]. Major ROS scavenging enzymatic systems in plants are dehydroascorbate reductase (DHAR), ascorbate peroxidase (APX), guaiacol peroxidase (GPX), glutathione reductase (GR), superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD) [102,103]. Increasing a plant’s CK contents either through exogenous application [104,105] or by overexpression of IPT [8,57,93,106,107] can mitigate oxidative stress and improve drought tolerance. CKs do this by positively modulating antioxidant enzymatic activities (i.e., POD, SOD and CAT) of plants, thereby aiding plant defenses to abiotic stresses [95,104,106,108,109,110].
Tobacco plants overexpressing the IPT gene under control of the promoter of a small subunit of rubisco (pSSU::IPT) exhibited delayed senescence and predominance of zeatin and zeatin riboside type CKs [95]. The transgenic plants demonstrated increased activities of antioxidant enzymes (CAT, GR, APX) compared to control plants [95,111]. Furthermore, electron microscopic investigation revealed relatively higher numbers of crystal-like pores in peroxisomes and abnormal interactions among organelles in transgenic tobacco plants [95]. In another report, pSARK::IPT transgenic tobacco plants grown under limited nitrogen (N) conditions demonstrated reduced oxidative damage and higher biomass compared to WT plants [97]. Gerbera plants, modified by the pSAG12::IPT chimeric gene and induced by 40% (w/v) polyethylene glycol (PEG) mediated osmotic stress for 20 h, revealed higher activities of SOD, CAT, APX, GPX and DHAR compared to control plants [96]. Additionally, transgenic gerbera plants showed reduced lipid peroxidation rate (measured by thiobarbituric acid reactive substance (TBARS)) under PEG-induced osmotic stress [96].
pSAG12::IPT modification in creeping bentgrass (Agrostis stolonifera L.) led to greater antioxidant enzyme activities of SOD, POD and CAT, with relatively lower lipid peroxidation in leaves under osmotic stress than NT plants [8]. In another experiment, creeping bentgrass was modified using the pSAG12::IPT gene cassette. Root physiological analysis of transgenic plants showed significantly lower contents of ROS (hydrogen peroxide and superoxide) and less lipid peroxidation compared to WT roots under drought stress. Enzymatic assays and transcript abundance analysis further revealed predominantly higher activities of SOD, POD, CAT and DHAR in roots of transgenic bentgrass under drought stress [10]. Antioxidant metabolism of drought-stressed creeping bentgrass was also studied under simultaneous effects of CK (0, 10 and 100 μM) and N (low = 2.5 and high = 7.5 kg N/ha every 15 days) applied exogenously. Plants with CK10 and CK100 treatments had lower O2− and H2O2 concentration than control CK0 plants. The CK100 treatment boosted activities of SOD, APX, CAT and POD by 25%, 22%, 17%, and 24%, respectively compared to CK0 [94]. However, the activity of these antioxidant enzymes enhanced more significantly under high N condition relative to low N condition, in contrast to findings of Rubio-Wilhelmi [97], where pSARK::IPT transgenic tobacco plants showed less oxidative damage under limited N conditions. Nevertheless, enhancing the concentration of CK, either through exogenous application or endogenously via IPT modifications, always proved advantageous for the plant in terms of reducing oxidative damage.
Plenty of evidences suggest that drought induces senescence of leaves with concurrent enhancement in ROS production. Both senescence and ROS production are moderated by higher CK levels inside the plant. However, when plants are not under stress, contrasting results deny the correlation of senescence progression with ROS-triggered lipid peroxidation. Recently, leaves of pSAG12::IPT overexpressing tobacco plants were allowed to undergo natural senescence and lipid peroxidation rate was determined by GS-MS using end product malondialdehyde (MDA). Though leaves of pSAG12::IPT remained green due to delayed senescence, lipid peroxidation was much higher compared to WT leaves of the same age [112]. Results indicated that lipid peroxidation cannot be correlated with leaf senescence. Further, ROS generation is not always moderated by higher CK levels in plants; however, stress-induced enhancement of ROS is controlled by elevated CK levels as evidenced by earlier reports.
8. Cytokinin-Induced Transcriptomic and Proteomic Changes during Osmotic Stresse
A transcriptome study of pSARK::IPT modified tobacco plants, performed under prolonged water deficit conditions, revealed repression of carotenoid pathway genes which are implicated in ABA biosynthesis [49]. By contrast, higher transcript abundance of genes involved in brassinosteroid biosynthetic pathways was witnessed in transgenic plants. Furthermore, transgenic plants displayed significantly higher transcript levels of genes associated with PSI, PSII, cytochrome b6/f complex, NADH oxidoreductase and the adenosine triphosphate (ATP) complex. Differential transcript levels in pSARK::IPT tobacco plants were further complemented by assessing expression of corresponding proteins using Western Blot [49]. CK arbitrated transcriptome changes, as observed by overexpression of IPT (pSAG12::IPT) in creeping bentgrass under drought stress, exposed differential expression of 252 genes related to energy production, metabolism, stress defense, signaling, protein synthesis and transport and membrane transport. Substantially higher transcript levels were observed for genes encoding proteins like Mg-protoporphyrin IX, chloroplast localized ToxA binding protein (Pr ToxA), CAT, aquaporin Pip1–2, Leu-rich repeat (LRR) receptor kinases, universal stress protein (USP), and isoflavone reductase-like protein 5. Conversely, transgenic plants showed reduced transcript levels of genes encoding malate dehydrogenase, glycogen synthase kinase (GSK), DELLA proteins, and ATP binding cassette (ABC) transporters [113]. To identify transcription factors (TFs) and their downstream genes associated with high CK mediated drought acclimation, transcriptomic profiling of IPT-transgenic creeping bentgrass was performed under drought stress. Among 127 differentially expressing TFs, 65 exhibited upregulation and 62 were downregulated in IPT-transgenic plants, compared to WT. The downstream genes of 15 TFs also expressed differentially in IPT-transgenic plants. Significant transcriptional upregulation was detected in central hubs of bHLH148, MYB4/4-like and WRKY28/53/71. These TFs are ascribed to trigger the genes involved in jasmonic acid (JA) signaling and suppress the genes associated with ABA signaling [114].
Differential proteomic analyses of leaves and roots of Agrostis stolonifera, expressing IPT under control of two different inducible promoters (SAG12 and HSP18), were performed under heat stress (35 °C). Significant changes were detected in proteins related to energy metabolism, localization and storage, and stress defense. Transgenic plants displayed predominantly higher abundance of enolase, oxygen-evolving enhancer protein 2, putative oxygen-evolving complex, rubisco small subunit, Hsp90, and glycolate oxidase in leaves under heat stress, compared to NT plants. Similarly, root proteome revealed relatively higher abundance of Fd-GOGAT, nucleotide-sugar dehydratase, NAD-dependent isocitrate dehydrogenase, ferredoxin-NADP reductase precursor, putative heterogeneous nuclear ribonucleoprotein A2, ascorbate peroxidase, and dDTP-glucose 4–6-dehydratases-like protein [115]. In another investigation, leaf and root proteome of similarly modified (pSAG12::IPT) transgenic creeping bentgrass under drought stress revealed higher abundance of proteins involved in energy production during the processes of photosynthesis and respiration (ribulose 1,5-bisphosphate carboxylase (RuBisCO) and glyceraldehyde phosphate dehydrogenase (GAPDH)), compared to WT plants. Furthermore, transgenic plants showed higher abundance of proteins involved in methionine and glutamine synthesis, chloroplastic elongation factor (EF-Tu), protein disulphide isomerases (PDIs), and antioxidant enzymes (catalase and peroxidase), than WT plants [8]. To elucidate the effects of altered endogenous CK content on the proteome of the chloroplast and its subfractions (stroma and thylakoids), transgenic tobacco plants with high (pSSU::IPT) and low (p35S:CKX1) endogenous CK were analyzed. Results revealed substantial quantitative differences in stroma proteins of both the transgenic plants but with no qualitative change in chloroplast proteome [116].
9. Conclusions
Contrasting observations revealed that CK-deficient plants display a strong stress-tolerant phenotype with increased cell membrane integrity and abscisic acid (ABA) hypersensitivity [26] but at the cost of growth and yield [97]. Alternatively, high CKs in plants facilitate acclimation to osmotic stresses with satisfactory growth and yield by relapsing the conventional transcriptional program activated under abiotic stress [7,58,94,99]. Evaluation of two contrasting Arabidopsis transformants with overexpression of CKX and IPT genes confirmed the constructive role of cytokinins in drought acclimation [55]. CK allows sustainable plant growth and development under stress conditions by stimulating the expression of genes related to growth processes and inhibiting the expression of genes associated with premature senescence. A reciprocal regulation mechanism exists between CK and ABA metabolisms that fine-tunes different processes related stress adaptations as well as plant growth and development. Metabolic resentment between CK and ABA was discovered a long time ago when elevated levels of CK suppressed the activity of xanthine dehydrogenase, one of the key enzymes involved in ABA biosynthesis [117]. Higher concentrations of CK during drought stress act as antagonists to ethylene-induced senescence [118] and biosynthesis, sensitivity, and signaling of ABA (reviewed above). Application of ethylene-based chemical defoliants (thidiazuron and ethephon) upregulated the transcription of CKX and ethylene-related genes in cotton [119], proposing CKX as the common target for CK crosstalk with both ABA and ethylene. Apart from ABA and ethylene, CK crosstalk has also been reported with other phytohormones. IPT overexpression caused the upregulation of BR (brassinosteroid)-biosynthesis (DWF5 and HYD1) and BR-signaling (BRL3, BRI1, BRH1, BIM1, SERK1) genes under water deficit stress, suggesting a positive correlation between CK and BR [9,60]. Conversely, elevated CK concentration repressed JA (jasmonic acid)-related genes (JAZ12, JAZ1, OPR2 and MES3) [9,60], while high JA concentration in plants attenuated cytokinin signaling by repressing the cytokinin receptor AHK4 and stimulating expression of AHP6, a negative regulator of cytokinin signaling [120]. Contrasting observations were reported while investigating the correlation of IAA with elevated levels of CK. Expression of auxin transport genes, OsPIN6 and OsPIN3a, was downregulated in IPT overexpressing rice plants [60] but pSAG12::IPT transgenic eggplants displayed relatively higher contents of IAA and GA under drought stress [62]. Nonetheless, more knowledge is required to comprehend interactions among phytohormone signaling pathways. CK mediated drought acclimation has been investigated at length during the past decade but the precise molecular mechanism remains unclear. With the advent of new biotechnological approaches like CRISPR/Cas and the availability of whole genome sequences, future genetic manipulation to enhance abiotic stress tolerance will continuously improve. Future research should focus on excavating novel drought responsive genes/proteins and the molecular mechanisms involved in cytokinin responsive drought tolerance.
Author Contributions
K.S. and R.S.G. perceived the idea of a manuscript on this topic. R.S.G. conducted the literature search while K.S. critically edited and evaluated the written text. All authors approved the final document.
Funding
This research received no external funding.
Acknowledgments
We thank all researchers at the Faculty of Science, Mahidol University, for providing useful suggestions and support. Extensive previous studies on this topic provided the literature for this review. The authors apologize to those whose contributions were not cited.
Conflicts of Interest
The authors declare that this manuscript was researched and written independently with no financial conflicts of interest.
References
- Burke, E.J.; Brown, S.J.; Christidis, N. Modeling the recent evolution of global drought and projections for the twenty-first century with the Hadley Centre climate model. J. Hydrometeorol. 2006, 7, 1113–1125. [Google Scholar] [CrossRef]
- Rengasamy, P. World salinization with emphasis on Australia. J. Exp. Bot. 2006, 57, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasulu, N.; Harshavardhan, V.T.; Govind, G.; Seiler, C.; Kohli, A. Contrapuntal role of ABA: Does it mediate stress tolerance or plant growth retardation under long-term drought stress? Gene 2012, 506, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Herrera-Estrella, L.; Tran, L.S.P. The Yin–Yang of cytokinin homeostasis and drought acclimation/adaptation. Trends Plant Sci. 2016, 21, 548–550. [Google Scholar] [CrossRef]
- Worakan, P.; Karaket, N.; Maneejantra, N.; Supaibulwatana, K. A Phenylurea Cytokinin, CPPU, Elevated Reducing Sugar and Correlated to Andrographolide Contents in Leaves of Andrographis paniculata (Burm. F.) Wall. Ex Nees. Appl. Biochem. Biotechnol. 2017, 181, 638–649. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, W.Q.; Zhang, G.L.; Kaminek, M.; Dobrev, P.; Xu, J.; Gruissem, W. Senescence-inducible expression of isopentenyl transferase extends leaf life, increases drought stress resistance and alters cytokinin metabolism in cassava. J. Integr. Plant Biol. 2010, 52, 653–669. [Google Scholar] [CrossRef]
- Qin, H.; Gu, Q.; Zhang, J.; Sun, L.; Kuppu, S.; Zhang, Y.; Zhang, H. Regulated expression of an isopentenyltransferase gene (IPT) in peanut significantly improves drought tolerance and increases yield under field conditions. Plant Cell Physiol. 2011, 52, 1904–1914. [Google Scholar] [CrossRef]
- Merewitz, E.B.; Gianfagna, T.; Huang, B. Protein accumulation in leaves and roots associated with improved drought tolerance in creeping bentgrass expressing an ipt gene for cytokinin synthesis. J. Exp. Bot. 2011, 62, 5311–5333. [Google Scholar] [CrossRef]
- Kuppu, S.; Mishra, N.; Hu, R.; Sun, L.; Zhu, X.; Shen, G.; Zhang, H. Water-deficit inducible expression of a cytokinin biosynthetic gene IPT improves drought tolerance in cotton. PLoS ONE 2013, 8, e64190. [Google Scholar] [CrossRef]
- Xu, Y.; Burgess, P.; Zhang, X.; Huang, B. Enhancing cytokinin synthesis by overexpressing ipt alleviated drought inhibition of root growth through activating ROS-scavenging systems in Agrostis stolonifera. J. Exp. Bot. 2016, 67, 1979–1992. [Google Scholar] [CrossRef]
- Davies, W.J.; Kudoyarova, G.; Hartung, W. Long-distance ABA signaling and its relation to other signaling pathways in the detection of soil drying and the mediation of the plant’s response to drought. J. Plant Growth Regul. 2005, 24, 285. [Google Scholar] [CrossRef]
- Kudoyarova, G.R.; Vysotskaya, L.B.; Cherkozyanova, A.; Dodd, I.C. Effect of partial rootzone drying on the concentration of zeatin-type cytokinins in tomato (Solanum lycopersicum L.) xylem sap and leaves. J. Exp. Bot. 2006, 58, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Argueso, C.T.; Ferreira, F.J.; Kieber, J.J. Environmental perception avenues: The interaction of cytokinin and environmental response pathways. Plant Cell Environ. 2009, 32, 1147–1160. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.A.; Benková, E. Cytokinin cross-talking during biotic and abiotic stress responses. Front. Plant Sci. 2013, 4, 451. [Google Scholar] [CrossRef]
- Huang, X.; Hou, L.; Meng, J.; You, H.; Li, Z.; Gong, Z.; Shi, Y. The antagonistic action of abscisic acid and cytokinin signaling mediates drought stress response in Arabidopsis. Mol. Plant 2018, 11, 970–982. [Google Scholar] [CrossRef]
- Jones, M.L.; Chaffin, G.S.; Eason, J.R.; Clark, D.G. Ethylene-sensitivity regulates proteolytic activity and cysteine protease gene expression in petunia corollas. J. Exp. Bot. 2005, 56, 2733–2744. [Google Scholar] [CrossRef]
- Schaller, G.E.; Street, I.H.; Kieber, J.J. Cytokinin and the cell cycle. Curr. Opin. Plant Biol. 2014, 21, 7–15. [Google Scholar] [CrossRef]
- Albert, R.; Acharya, B.R.; Jeon, B.W.; Zañudo, J.G.; Zhu, M.; Osman, K.; Assmann, S.M. A new discrete dynamic model of ABA-induced stomatal closure predicts key feedback loops. PLoS Boil. 2017, 15, e2003451. [Google Scholar] [CrossRef]
- Le, D.T.; Nishiyama, R.; Watanabe, Y.; Vankova, R.; Tanaka, M.; Seki, M.; Tran, L.S.P. Identification and expression analysis of cytokinin metabolic genes in soybean under normal and drought conditions in relation to cytokinin levels. PLoS ONE 2012, 7, e42411. [Google Scholar] [CrossRef]
- Yamburenko, M.V.; Kieber, J.J.; Schaller, G.E. Dynamic patterns of expression for genes regulating cytokinin metabolism and signaling during rice inflorescence development. PLoS ONE 2017, 12, e0176060. [Google Scholar] [CrossRef]
- Bielach, A.; Hrtyan, M.; Tognetti, V. Plants under stress: Involvement of auxin and cytokinin. Int. J. Mol. Sci. 2017, 18, 1427. [Google Scholar] [CrossRef] [PubMed]
- Werner, T.; Köllmer, I.; Bartrina, I.; Holst, K.; Schmülling, T. New insights into the biology of cytokinin degradation. Plant Biol. 2006, 8, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.; Vankova, R.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.S.P. Cytokinins: Metabolism and function in plant adaptation to environmental stresses. Trends Plant Sci. 2012, 17, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Brugière, N.; Jiao, S.; Hantke, S.; Zinselmeier, C.; Roessler, J.A.; Niu, X.; Habben, J.E. Cytokinin oxidase gene expression in maize is localized to the vasculature, and is induced by cytokinins, abscisic acid, and abiotic stress. Plant Physiol. 2003, 132, 1228–1240. [Google Scholar] [CrossRef] [PubMed]
- Werner, T.; Nehnevajova, E.; Köllmer, I.; Novák, O.; Strnad, M.; Krämer, U.; Schmülling, T. Root-specific reduction of cytokinin causes enhanced root growth, drought tolerance, and leaf mineral enrichment in Arabidopsis and tobacco. Plant Cell 2010, 22, 3905–3920. [Google Scholar] [CrossRef]
- Nishiyama, R.; Watanabe, Y.; Fujita, Y.; Le, D.T.; Kojima, M.; Werner, T.; Sakakibara, H. Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. Plant Cell 2011, 23, 2169–2183. [Google Scholar] [CrossRef]
- Pareek, A.; Singh, A.; Kumar, M.; Kushwaha, H.R.; Lynn, A.M.; Singla-Pareek, S.L. Whole-genome analysis of Oryza sativa reveals similar architecture of two-component signaling machinery with Arabidopsis. Plant Physiol. 2006, 142, 380–397. [Google Scholar] [CrossRef]
- Du, L.; Jiao, F.; Chu, J.; Jin, G.; Chen, M.; Wu, P. The two-component signal system in rice (Oryza sativa L.): A genome-wide study of cytokinin signal perception and transduction. Genomics 2007, 89, 697–707. [Google Scholar] [CrossRef]
- Tran, L.S.P.; Urao, T.; Qin, F.; Maruyama, K.; Kakimoto, T.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional analysis of AHK1/ATHK1 and cytokinin receptor histidine kinases in response to abscisic acid, drought, and salt stress in Arabidopsis. Proc. Natl. Acad. Sci. USA 2007, 104, 20623–20628. [Google Scholar] [CrossRef]
- Schaller, G.E.; Shiu, S.H.; Armitage, J.P. Two-component systems and their co-option for eukaryotic signal transduction. Curr. Biol. 2011, 21, R320–R330. [Google Scholar] [CrossRef]
- Zwack, P.J.; Rashotte, A.M. Interactions between cytokinin signalling and abiotic stress responses. J. Exp. Bot. 2015, 66, 4863–4871. [Google Scholar] [CrossRef] [PubMed]
- Thu, N.B.A.; Hoang, X.L.T.; Truc, M.T.; Sulieman, S.; Thao, N.P.; Tran, L.S.P. Cytokinin signaling in plant response to abiotic stresses. Mech. Plant Horm. Signal. Under Stress 2017, 1, 71–100. [Google Scholar]
- Kieber, J.J.; Schaller, G.E. Cytokinin signaling in plant development. Development 2018, 145, dev149344. [Google Scholar] [CrossRef] [PubMed]
- Pils, B.; Heyl, A. Unraveling the evolution of cytokinin signaling. Plant Physiol. 2009, 151, 782–791. [Google Scholar] [CrossRef]
- Zürcher, E.; Müller, B. Cytokinin synthesis, signaling, and function—advances and new insights. In International Review of Cell and Molecular Biology; Academic Press: Cambridge, MA, USA, 2016; Volume 324, pp. 1–38. [Google Scholar]
- Higuchi, M.; Pischke, M.S.; Mähönen, A.P.; Miyawaki, K.; Hashimoto, Y.; Seki, M.; Helariutta, Y. In planta functions of the Arabidopsis cytokinin receptor family. Proc. Natl. Acad. Sci. USA. 2004, 101, 8821–8826. [Google Scholar] [CrossRef]
- Suzuki, T.; Zakurai, K.; Imamura, A.; Nakamura, A.; Ueguchi CMizuno, T. Compilation and characterization of histidine-containing phosphotransmitters implicated in His-to-Asp phosphorelay in plants: AHP signal transducers of Arabidopsis thaliana. Biosci. Biotechnol. Biochem. 2000, 64, 2482–2485. [Google Scholar] [CrossRef]
- Hutchison, C.E.; Li, J.; Argueso, C.; Gonzalez, M.; Lee, E.; Lewis, M.W.; Ecker, J.R. The Arabidopsis histidine phosphotransfer proteins are redundant positive regulators of cytokinin signaling. Plant Cell 2006, 18, 3073–3087. [Google Scholar] [CrossRef]
- Raines, T.; Blakley, I.C.; Tsai, Y.C.; Worthen, J.M.; Franco-Zorrilla, J.M.; Solano, R.; Kieber, J.J. Characterization of the cytokinin-responsive transcriptome in rice. BMC Plant Biol. 2016, 16, 260. [Google Scholar] [CrossRef]
- Zubo, Y.; Blakley, I.C.; Yamburenko, M.; Worthen, J.M.; Street, I.; Franco Zorrilla, J.M.; Zhang, W.; Hill, K.; Raines, T.; Solano, R. Cytokinin induces genome-wide binding of the type-B response regulator ARR10 to regulate growth and development in Arabidopsis. Proc. Natl. Acad. Sci. USA 2017, 114, E5995–E6004. [Google Scholar] [CrossRef]
- D’Agostino, I.B.; Deruere, J.; Kieber, J.J. Characterization of the response of the Arabidopsis response regulator gene family to cytokinin. Plant Physiol. 2000, 124, 1706–1717. [Google Scholar] [CrossRef]
- Taniguchi, M.; Sasaki, N.; Tsuge, T.; Aoyama, T.; Oka, A. ARR1 directly activates cytokinin response genes that encode proteins with diverse regulatory functions. Plant Cell Physiol. 2007, 48, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Liang, Y.; Deng, Y.; Chen, Q.; Zhang, J.; Yang, X.; Zuo, J. Genome-wide comparative analysis of type-A Arabidopsis response regulator genes by overexpression studies reveals their diverse roles and regulatory mechanisms in cytokinin signaling. Cell Res. 2009, 19, 1178. [Google Scholar] [CrossRef] [PubMed]
- Gujjar, R.S.; Akhtar, M.; Singh, M. Transcription factors in abiotic stress tolerance. Indian J. Plant Physiol. 2014, 19, 306–316. [Google Scholar] [CrossRef]
- Yoshida, T.; Mogami, J.; Yamaguchi-Shinozaki, K. ABA-dependent and ABA-independent signaling in response to osmotic stress in plants. Curr. Opin. Plant Biol. 2014, 21, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, A.S.; Gonugunta, V.K.; Christmann, A.; Grill, E. ABA perception and signalling. Trends Plant Sci. 2010, 15, 395–401. [Google Scholar] [CrossRef]
- Danquah, A.; de Zelicourt, A.; Colcombet, J.; Hirt, H. The role of ABA and MAPK signaling pathways in plant abiotic stress responses. Biotechnol. Adv. 2014, 32, 40–52. [Google Scholar] [CrossRef]
- Ullah, A.; Manghwar, H.; Shaban, M.; Khan, A.H.; Akbar, A.; Ali, U.; Fahad, S. Phytohormones enhanced drought tolerance in plants: A coping strategy. Environ. Sci. Pollut. Res. 2018, 25, 33103–33118. [Google Scholar] [CrossRef]
- Rivero, R.M.; Gimeno, J.; Van Deynze, A.; Walia, H.; Blumwald, E. Enhanced cytokinin synthesis in tobacco plants expressing PSARK: IPT prevents the degradation of photosynthetic protein complexes during drought. Plant Cell Physiol. 2010, 51, 1929–1941. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Ye, T.; Zhao, S.; Liu, Z.; Feng, Y.Q.; Wu, Y. Cytokinin antagonizes ABA suppression to seed germination of Arabidopsis by downregulating ABI5 expression. Plant J. 2011, 68, 249–261. [Google Scholar] [CrossRef]
- Blackman, P.G.; Davies, W.J. The effects of cytokinins and ABA on stomatal behaviour of maize and Commelina. J. Exp. Bot. 1983, 34, 1619–1626. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sano, T.; Tamaoki, M.; Nakajima, N.; Kondo, N.; Hasezawa, S. Cytokinin and auxin inhibit abscisic acid-induced stomatal closure by enhancing ethylene production in Arabidopsis. J. Exp. Bot. 2006, 57, 2259–2266. [Google Scholar] [CrossRef]
- Pospíšilová, J. Interaction of cytokinins and abscisic acid during regulation of stomatal opening in bean leaves. Photosynthetica 2003, 41, 49–56. [Google Scholar] [CrossRef]
- Hajouj, T.; Michelis, R.; Gepstein, S. Cloning and characterization of a receptor-like protein kinase gene associated with senescence. Plant Physiol. 2000, 124, 1305–1314. [Google Scholar] [CrossRef]
- Prerostova, S.; Dobrev, P.; Gaudinova, A.; Knirsch, V.; Körber, N.; Pieruschka, R.; Humplik, J. Cytokinins: Their impact on molecular and growth responses to drought stress and recovery in Arabidopsis. Front. Plant Sci. 2018, 9, 655. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.H. Genetic engineering of cytokinins and their application to agriculture. Crit. Rev. Biotechnol. 2008, 28, 213–232. [Google Scholar] [CrossRef]
- Merewitz, E.B.; Gianfagna, T.; Huang, B. Effects of SAG12-ipt and HSP18. 2-ipt expression on cytokinin production root growth and leaf senescence in creeping bentgrass exposed to drought stress. J. Am. Soc. Hortic. Sci. 2010, 135, 230–239. [Google Scholar] [CrossRef]
- Rivero, R.M.; Kojima, M.; Gepstein, A.; Sakakibara, H.; Mittler, R.; Gepstein, S.; Blumwald, E. Delayed leaf senescence induces extreme drought tolerance in a flowering plant. Proc. Natl. Acad. Sci. USA 2007, 104, 19631–19636. [Google Scholar] [CrossRef]
- Delatorre, C.A.; Cohen, Y.; Liu, L.; Peleg, Z.; Blumwald, E. The regulation of the SARK promoter activity by hormones and environmental signals. Plant Sci. 2012, 193, 39–47. [Google Scholar] [CrossRef]
- Peleg, Z.; Reguera, M.; Tumimbang, E.; Walia, H.; Blumwald, E. Cytokinin-mediated source/sink modifications improve drought tolerance and increase grain yield in rice under water-stress. Plant Biotechnol. J. 2011, 9, 747–758. [Google Scholar] [CrossRef]
- Reguera, M.; Peleg, Z.; Abdel-Tawab, Y.M.; Tumimbang, E.B.; Delatorre, C.A.; Blumwald, E. Stress-induced cytokinin synthesis increases drought tolerance through the coordinated regulation of carbon and nitrogen assimilation in rice. Plant Physiol. 2013, 163, 1609–1622. [Google Scholar] [CrossRef]
- Xiao, X.O.; Zeng, Y.M.; Cao, B.H.; Lei, J.J.; Chen, Q.H.; Meng, C.M.; Cheng, Y.J. PSAG12-IPT overexpression in eggplant delays leaf senescence and induces abiotic stress tolerance. J. Hortic. Sci. Biotechnol. 2017, 92, 349–357. [Google Scholar] [CrossRef]
- Joshi, R.; Sahoo, K.K.; Tripathi, A.K.; Kumar, R.; Gupta, B.K.; Pareek, A.; Singla-Pareek, S.L. Knockdown of an inflorescence meristem-specific cytokinin oxidase–OsCKX2 in rice reduces yield penalty under salinity stress condition. Plant Cell Environ. 2018, 41, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Pospíšilová, H.; Jiskrova, E.; Vojta, P.; Mrizova, K.; Kokáš, F.; Čudejková, M.M.; Dzurova, L. Transgenic barley overexpressing a cytokinin dehydrogenase gene shows greater tolerance to drought stress. New Biotechnol. 2016, 33, 692–705. [Google Scholar]
- Pogány, M.; Koehl, J.; Heiser, I.; Elstner, E.F.; Barna, B. Juvenility of tobacco induced by cytokinin gene introduction decreases susceptibility to Tobacco necrosis virus and confers tolerance to oxidative stress. Physiol. Mol. Plant Pathol. 2004, 65, 39–47. [Google Scholar] [CrossRef]
- Sade, N.; del Mar Rubio-Wilhelmi, M.; Umnajkitikorn, K.; Blumwald, E. Stress-induced senescence and plant tolerance to abiotic stress. J. Exp. Bot. 2017, 69, 845–853. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Alegre, L. Die and let live: Leaf senescence contributes to plant survival under drought stress. Funct. Plant Biol. 2004, 31, 203–216. [Google Scholar]
- Jing, H.C.; Nam, H.G. Leaf senescence in plants: From model plants to crops, still so many unknowns. J. Integr. Plant Biol. 2012, 54, 514–515. [Google Scholar] [CrossRef]
- Krupinska, K.; Mulisch, M.; Hollmann, J.; Tokarz, K.; Zschiesche, W.; Kage, H.; Bilger, W. An alternative strategy of dismantling of the chloroplasts during leaf senescence observed in a high-yield variety of barley. Physiol. Plant. 2012, 144, 189–200. [Google Scholar] [CrossRef]
- Drake, R.; John, I.; Farrell, A.; Cooper, W.; Schuch, W.; Grierson, D. Isolation and analysis of cDNAs encoding tomato cysteine proteases expressed during leaf senescence. Plant Mol. Biol. 1996, 30, 755–767. [Google Scholar] [CrossRef]
- Battelli, R.; Lombardi, L.; Picciarelli, P.; Lorenzi, R.; Frigerio, L.; Rogers, H.J. Expression and localisation of a senescence-associated KDEL-cysteine protease from Lilium longiflorum tepals. Plant Sci. 2014, 214, 38–46. [Google Scholar] [CrossRef]
- Díaz-Mendoza, M.; Velasco-Arroyo, B.; González-Melendi, P.; Martínez, M.; Díaz, I. C1A cysteine protease–cystatin interactions in leaf senescence. J. Exp. Bot. 2014, 65, 3825–3833. [Google Scholar] [CrossRef] [PubMed]
- Gan, S.; Amasino, R.M. Inhibition of leaf senescence by autoregulated production of cytokinin. Science 1995, 2705244, 1986–1988. [Google Scholar] [CrossRef] [PubMed]
- Lara, M.E.B.; Garcia, M.C.G.; Fatima, T.; Ehneß, R.; Lee, T.K.; Proels, R.; Roitsch, T. Extracellular invertase is an essential component of cytokinin-mediated delay of senescence. Plant Cell 2004, 16, 1276–1287. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.G.; Dervinis, C.; Barrett, J.E.; Klee, H.; Jones, M. Drought-induced leaf senescence and horticultural performance of transgenic PSAG12-ipt petunias. J. Am. Soc. Hortic. Sci. 2004, 129, 93–99. [Google Scholar] [CrossRef]
- Khanna-Chopra, R.; Srivalli, B.; Ahlawat, Y.S. Drought induces many forms of cysteine proteases not observed during natural senescence. Biochem. Biophys. Res. Commun. 1999, 255, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Coupe, S.A.; Sinclair, B.K.; Watson, L.M.; Heyes, J.A.; Eason, J.R. Identification of dehydration-responsive cysteine proteases during post-harvest senescence of broccoli florets. J. Exp. Bot. 2003, 54, 1045–1056. [Google Scholar] [CrossRef]
- Botha, A.M.; Kunert, K.J.; Cullis, C.A. Cysteine proteases and wheat (Triticum aestivum L) under drought: A still greatly unexplored association. Plant Cell Environ. 2017, 40, 1679–1690. [Google Scholar] [CrossRef]
- Chang, H.; Jones, M.L.; Banowetz, G.M.; Clark, D.G. Overproduction of cytokinins in petunia flowers transformed with PSAG12-IPT delays corolla senescence and decreases sensitivity to ethylene. Plant Physiol. 2003, 132, 2174–2183. [Google Scholar] [CrossRef]
- Bao, G.; Zhuo, C.; Qian, C.; Xiao, T.; Guo, Z.; Lu, S. Co-expression of NCED and ALO improves vitamin C level and tolerance to drought and chilling in transgenic tobacco and stylo plants. Plant Biotechnol. J. 2016, 14, 206–214. [Google Scholar] [CrossRef]
- Kudo, M.; Kidokoro, S.; Yoshida, T.; Mizoi, J.; Todaka, D.; Fernie, A.R.; Yamaguchi-Shinozaki, K. Double overexpression of DREB and PIF transcription factors improves drought stress tolerance and cell elongation in transgenic plants. Plant Biotechnol. J. 2017, 15, 458–471. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, L.; Wei, N.; Liu, Z.H.; Hu, S.; Li, X.B. Overexpression of cotton PYL genes in Arabidopsis enhances the transgenic plant tolerance to drought stress. Plant Physiol. Biochem. 2017, 115, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Thirumalaikumar, V.P.; Devkar, V.; Mehterov, N.; Ali, S.; Ozgur, R.; Turkan, I.; Balazadeh, S. NAC transcription factor JUNGBRUNNEN 1 enhances drought tolerance in tomato. Plant Biotechnol. J. 2018, 16, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Ann. Rev. Plant Biol. 2006, 57, 781–803. [Google Scholar] [CrossRef] [PubMed]
- Claeys, H.; Inzé, D. The agony of choice: How plants balance growth and survival under water-limiting conditions. Plant Physiol. 2013, 162, 1768–1779. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. Organization of cis-acting regulatory elements in osmotic-and cold-stress-responsive promoters. Trends Plant Sci. 2005, 10, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Apelbaum, A.; Yang, S.F. Biosynthesis of stress ethylene induced by water deficit. Plant Physiol. 1981, 68, 594–596. [Google Scholar] [CrossRef]
- Nath, M.; Bhatt, D.; Prasad, R.; Tuteja, N. Reactive oxygen species (ROS) metabolism and signaling in plant-mycorrhizal association under biotic and abiotic stress conditions. In Mycorrhiza-Eco-Physiology Secondary Metabolites, Nanomaterials; Springer: Cham, Switzerland, 2017; pp. 223–232. [Google Scholar]
- Liu, F.; Song, R.; Zhang, X.; Shahnazari, A.; Andersen, M.N.; Plauborg, F.; Jensen, C.R. Measurement and modelling of ABA signalling in potato (Solanum tuberosum L.) during partial root-zone drying. Environ. Exp. Bot. 2008, 63, 385–391. [Google Scholar] [CrossRef]
- Demidchik, V. Mechanisms of oxidative stress in plants: From classical chemistry to cell biology. Environ. Exp. Bot. 2015, 109, 212–228. [Google Scholar] [CrossRef]
- Tognetti, V.B.; Bielach, A.; Hrtyan, M. Redox regulation at the site of primary growth: Auxin, cytokinin and ROS crosstalk. Plant Cell Environ. 2017, 40, 2586–2605. [Google Scholar] [CrossRef]
- Gashaw, A.; Theerawitaya, C.; Samphumphuang, T.; Cha-um, S.; Supaibulwatana, K. CPPU elevates photosynthetic abilities, growth performances and yield traits in salt stressed rice (Oryza sativa L. spp. indica) via free proline and sugar accumulation. Pestic. Biochem. Physiol. 2014, 108, 27–33. [Google Scholar] [CrossRef]
- Ghanem, M.E.; Albacete, A.; Smigocki, A.C.; Frébort, I.; Pospíšilová, H.; Martínez-Andújar, C.; Pérez-Alfocea, F. Root-synthesized cytokinins improve shoot growth and fruit yield in salinized tomato (Solanum lycopersicum L.) plants. J. Exp. Bot. 2010, 62, 125–140. [Google Scholar] [CrossRef]
- Chang, Z.; Liu, Y.; Dong, H.; Teng, K.; Han, L.; Zhang, X. Effects of cytokinin and nitrogen on drought tolerance of creeping bentgrass. PLoS ONE 2016, 11, e0154005. [Google Scholar] [CrossRef]
- Synkova, H.; Semoradova, S.; Schnablová, R.; Witters, E.; Husak, M.; Valcke, R. Cytokinin-induced activity of antioxidant enzymes in transgenic Pssu-ipt tobacco during plant ontogeny. Biol. Plant. 2006, 50, 31–41. [Google Scholar] [CrossRef]
- Lai, Q.X.; Bao, Z.Y.; Zhu, Z.J.; Qian, Q.Q.; Mao, B.Z. Effects of osmotic stress on antioxidant enzymes activities in leaf discs of P SAG12-IPT modified gerbera. J. Zhejiang Univ. Sci. B 2007, 8, 458–464. [Google Scholar] [CrossRef]
- Rubio-Wilhelmi, M.M.; Sanchez-Rodriguez, E.; Rosales, M.A.; Begona, B.; Rios, J.J.; Romero, L.; Blumwald, E.; Ruiz, J.M. Effect of cytokinins on oxidative stress in tobacco plants under nitrogen deficiency. Environ. Exp. Bot. 2011, 72, 167–173. [Google Scholar] [CrossRef]
- Riefler, M.; Novak, O.; Strnad, M.; Schmülling, T. Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. Plant Cell 2006, 18, 40–54. [Google Scholar] [CrossRef]
- Golan, Y.; Shirron, N.; Avni, A.; Shmoish, M.; Gepstein, S. Cytokinins induce transcriptional reprograming and improve arabidopsis plant performance under drought and salt stress conditions. Front. Environ. Sci. 2016, 4, 63. [Google Scholar] [CrossRef]
- Kumari, S.; Kumar, S.; Prakash, P. Exogenous application of cytokinin (6-BAP) ameliorates the adverse effect of combined drought and high temperature stress in wheat seedling. J. Pharmacogn. Phytochem. 2018, 7, 1176–1180. [Google Scholar]
- Munne-Bosch, S.; Pinto-Marijuan, M. Free radicals, oxidative stress and antioxidants. Encycl. Appl. Plant Sci 2016, 2, 16–19. [Google Scholar]
- Xu, J.; Duan, X.; Yang, J.; Beeching, J.R.; Zhang, P. Enhanced reactive oxygen species scavenging by overproduction of superoxide dismutase and catalase delays postharvest physiological deterioration of cassava storage roots. Plant Physiol. 2013, 161, 1517–1528. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Zavaleta-Mancera, H.A.; López-Delgado, H.; Loza-Tavera, H.; Mora-Herrera, M.; Trevilla-García, C.; Vargas-Suárez, M.; Ougham, H. Cytokinin promotes catalase and ascorbate peroxidase activities and preserves the chloroplast integrity during dark-senescence. J. Plant Physiol. 2007, 164, 1572–1582. [Google Scholar] [CrossRef]
- Baque, M.A.; Hahn, E.J.; Paek, K.Y. Growth, secondary metabolite production and antioxidant enzyme response of Morinda citrifolia adventitious root as affected by auxin and cytokinin. Plant Biotechnol. Rep. 2010, 4, 109–116. [Google Scholar] [CrossRef]
- Zhang, X.; Ervin, E.H. Impact of seaweed extract-based cytokinins and zeatin riboside on creeping bentgrass heat tolerance. Crop Sci. 2008, 48, 364–370. [Google Scholar] [CrossRef]
- Rivero, R.M.; Shulaev, V.; Blumwald, E. Cytokinin-dependent photorespiration and the protection of photosynthesis during water deficit. Plant Physiol. 2009, 150, 1530–1540. [Google Scholar] [CrossRef]
- Petit-Paly, G.; Franck, T.; Brisson, L.; Kevers, C.; Chénieux, J.C.; Rideau, M. Cytokinin modulates catalase activity and cournarin accumulation in in vitro cultures of tobacco. J. Plant Physiol. 1999, 155, 9–15. [Google Scholar] [CrossRef]
- Zimmermann, P.E.; Zentgraf, U.L. The correlation between oxidative stress and leaf senescence during plant development. Cell. Mol. Biol. Lett. 2005, 10, 515. [Google Scholar]
- Hönig, M.; Plíhalová, L.; Husičková, A.; Nisler, J.; Doležal, K. Role of cytokinins in senescence, antioxidant defence and photosynthesis. Int. J. Mol. Sci. 2018, 19, 4045. [Google Scholar]
- Synkova, H.; Semorádová, Š.; Burketova, L. High content of endogenous cytokinins stimulates activity of enzymes and proteins involved in stress response in Nicotiana tabacum. Plant Cell Tissue Organ Cult. 2004, 79, 169–179. [Google Scholar] [CrossRef]
- Pilarska, M.; Skowron, E.; Pietraś, R.; Krupinska, K.; Niewiadomska, E. Changes in lipid peroxidation in stay-green leaves of tobacco with senescence-induced synthesis of cytokinins. Plant Physiol. Biochem. 2017, 118, 161–167. [Google Scholar] [CrossRef]
- Merewitz, E.; Xu, Y.; Huang, B. Differentially expressed genes associated with improved drought tolerance in creeping bentgrass overexpressing a gene for cytokinin biosynthesis. PLoS ONE 2016, 11, e0166676. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, B. Transcriptional factors for stress signaling, oxidative protection, and protein modification in ipt-transgenic creeping bentgrass exposed to drought stress. Environ. Exp. Bot. 2017, 144, 49–60. [Google Scholar] [CrossRef]
- Xu, Y.; Gianfagna, T.; Huang, B. Proteomic changes associated with expression of a gene (ipt) controlling cytokinin synthesis for improving heat tolerance in a perennial grass species. J. Exp. Bot. 2010, 61, 3273–3289. [Google Scholar] [CrossRef]
- Cortleven, A.; Noben, J.P.; Valcke, R. Analysis of the photosynthetic apparatus in transgenic tobacco plants with altered endogenous cytokinin content: A proteomic study. Proteome Sci. 2011, 9, 1. [Google Scholar] [CrossRef]
- Cowan, A.K.; Cairns, A.L.; Bartels-Rahm, B. Regulation of abscisic acid metabolism: Towards a metabolic basis for abscisic acid-cytokinin antagonism. J. Exp. Bot. 1999, 50, 595–603. [Google Scholar] [CrossRef]
- Liu, J.; Moore, S.; Chen, C.; Lindsey, K. Crosstalk complexities between auxin, cytokinin, and ethylene in Arabidopsis root development: From experiments to systems modeling, and back again. Mol. Plant 2017, 10, 1480–1496. [Google Scholar] [CrossRef]
- Xu, J.; Chen, L.; Sun, H.; Wusiman, N.; Sun, W.; Li, B.; Xu, H. Crosstalk between cytokinin and ethylene signaling pathways regulates leaf abscission in cotton in response to chemical defoliants. J. Exp. Bot. 2019, 70, 1525–1538. [Google Scholar] [CrossRef]
- Pavlů, J.; Novak, J.; Koukalová, V.; Luklova, M.; Brzobohatý, B.; Černý, M. Cytokinin at the crossroads of abiotic stress signalling pathways. Int. J. Mol. Sci. 2018, 19, 2450. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).