Anthocyanin in the Vacuole of Red Onion Epidermal Cells Quenches Other Fluorescent Molecules
Abstract
:1. Introduction
2. Results
2.1. Measuring Anthocyanin in Onion Epidermal Cells
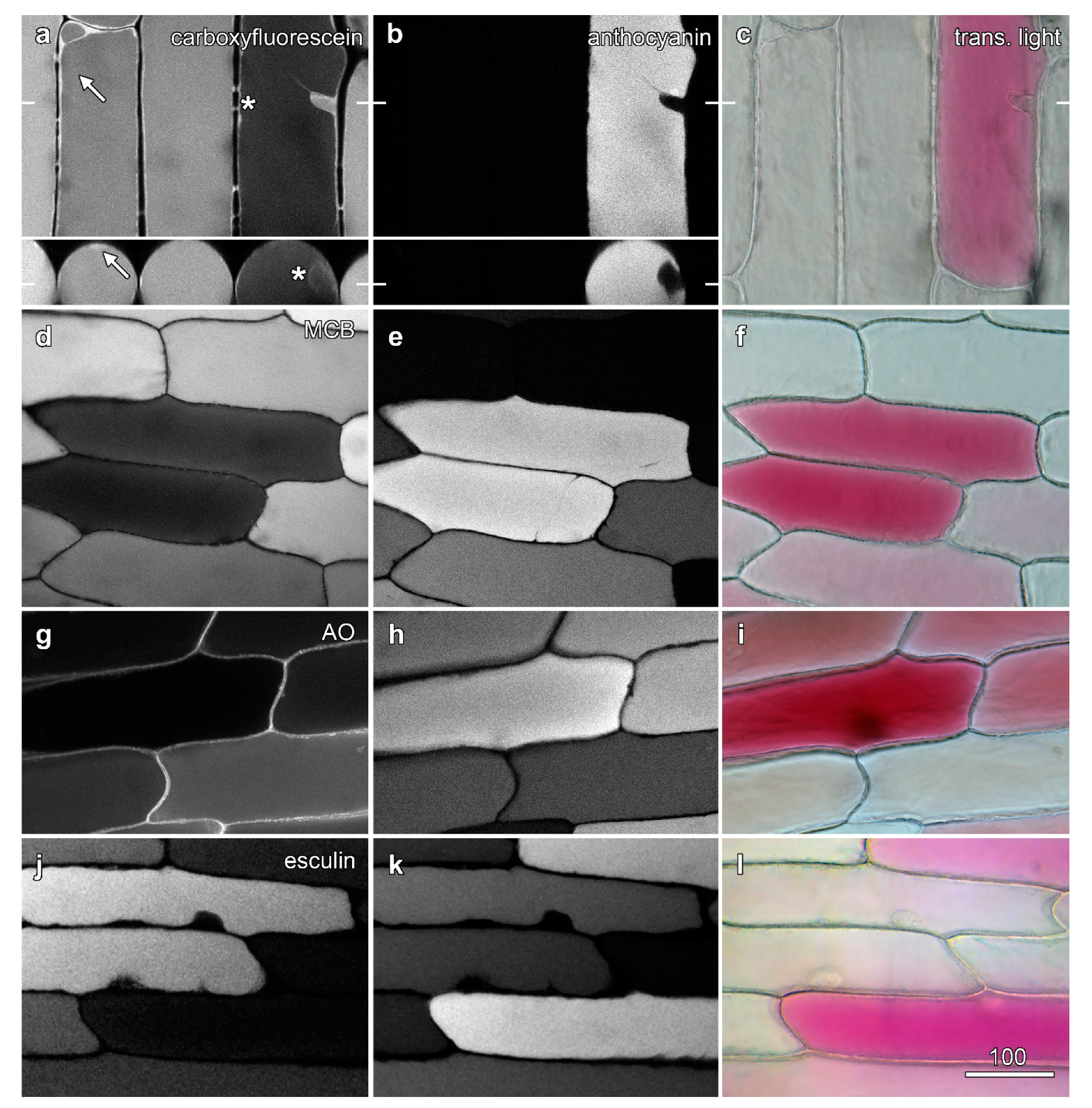
2.2. Anthocyanic Cells Show Reduced Fluorescence Vacuolar Dyes
2.3. Anthocyanin Reduces Carboxyfluorescein Fluorescence in the Vacuole of Other Species
2.4. Anthocyanic Cells Show Even Labelling of Tonoplast-Specific Dyes
2.5. Physiological Variations Do Not Account for Variations in Epidermal Cell Fluorescence
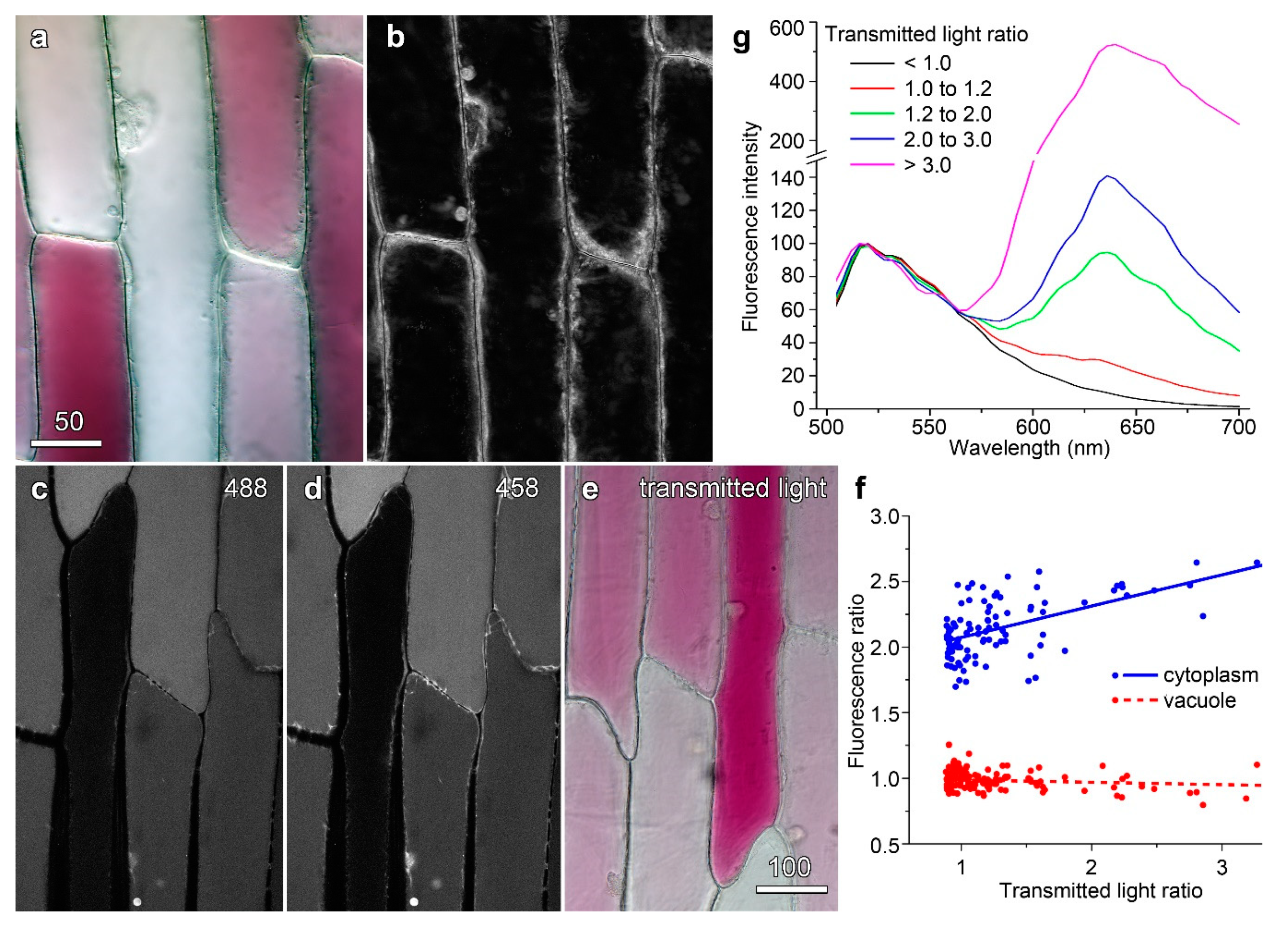
2.6. Quantifying Anthocyanin-Induced Quenching in the Vacuole
2.7. The Effect of Temperature on Quenching
3. Discussion
3.1. The Mechanism(s) Underlying Anthocyanin Quenching Phenomena
3.2. Future Directions
4. Materials and Methods
4.1. Plant Material
4.2. Fluorescent Dyes
4.3. Confocal Microscopy
4.4. Image Quantification
4.5. Image Processing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef] [PubMed]
- Sakuta, M. Diversity in plant red pigments: Anthocyanins and betacyanins. Plant Biotechnol. Rep. 2014, 8, 37–48. [Google Scholar] [CrossRef]
- Sasaki, N.; Nakayama, T. Achievements and perspectives in biochemistry concerning anthocyanin. Plant Cell Physiol. 2015, 56, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Dangles, O.; Fenger, J.-A. The chemical reactivity of anthocyanins and its consequences in food science and nutrition. Molecules 2018, 23, 1970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drabent, R.; Pliska, B.; Huszcza-Ciołkowska, G.; Smyk, B. Ultraviolet fluorescence of cyanidin an malvidin glycosides in aqueous environment. Spectrosc. Lett. 2007, 40, 165–182. [Google Scholar] [CrossRef]
- Poustka, F.; Irani, N.G.; Feller, A.; Lu, Y.; Pourcel, L.; Frame, K.; Grotewold, E. A trafficking pathway for anthocyanins overlaps with the endoplasmic reticulum-to-vacuole protein-sorting route in Arabidopsis and contributes to the formation of vacuolar inclusions. Plant Physiol. 2007, 145, 1323–1335. [Google Scholar] [CrossRef] [Green Version]
- Wiltshire, E.J.; Collings, D.A. New dynamics in an old friend: Dynamic tubular vacuoles radiate through the cortical cytoplasm of red onion epidermal cells. Plant Cell Physiol. 2009, 50, 1826–1839. [Google Scholar] [CrossRef] [Green Version]
- Wiltshire, E.J.; Eady, C.C.; Collings, D.A. Induction and inhibition of anthocyanin in the inner epidermis of red onion leaves by environmental stimuli, and through transient expression of transcription factors. Plant Cell Rep. 2017, 36, 987–1000. [Google Scholar] [CrossRef]
- Andersen, Ø.M.; Jordheim, M. The anthocyanins. In Flavonoids: Chemistry, Biochemistry and Applications; Andersen, Ø.M., Markham, K.R., Eds.; CRC Press: Boca Raton, FL, USA, 2005; pp. 471–552. [Google Scholar]
- Zhang, H.; Wang, L.; Deroles, S.; Bennett, R.; Davies, K. New insight into the structure and formation of anthocyanic vacuolar inclusions in flower petals. BMC Plant Biol. 2006, 6, 29. [Google Scholar] [CrossRef] [Green Version]
- Alfenito, M.R.; Souer, E.; Goodman, C.D.; Buell, R.; Mol, J.; Koes, R.; Walbot, V. Functional complementation of anthocyanin sequestration in the vacuole by widely divergent glutathione S-transferases. Plant Cell 1998, 10, 1135–1149. [Google Scholar] [CrossRef] [Green Version]
- Koes, R.; Verweij, W.; Quattrocchio, F. Flavonoids: A colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci. 2005, 10, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Chalker-Scott, L. Environmental significance of anthocyanins in plant stress responses. Photochem. Photobiol. 1999, 70, 1–9. [Google Scholar] [CrossRef]
- Gould, K.S.; Lister, C. Flavonoid functions in plants. In Flavonoids: Chemistry, Biochemistry and Applications; Anderson, Ø.M., Markham, K.R., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 397–441. [Google Scholar]
- De Rosso, V.V.; Morán Vierya, F.E.; Mercadante, A.Z.; Borsarelli, C.D. Singlet oxygen quenching by anthocyanin’s flavylium cations. Free Radic. Res. 2008, 42, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Hernández, I.; Alegre, L.; van Breusegem, F.; Munné-Bosch, S. How relevant are flavonoids as antioxiants in plants? Trends Plant Sci. 2009, 14, 125–132. [Google Scholar] [CrossRef]
- Agati, G.; Matteini, P.; Goti, A.; Tattini, M. Chloroplast-located flavonoids can scavenge singlet oxygen. New Phytol. 2007, 174, 77–89. [Google Scholar] [CrossRef]
- Neill, S.O.; Gould, K.S. Anthocyanins in leaves: Light attenuators or antioxidants? Funct. Plant Biol. 2003, 30, 865–873. [Google Scholar] [CrossRef] [Green Version]
- Gould, K.S.; McKelvie, J.; Markham, K.R. Do anthocyanins function as antioxidants in leaves? Imaging of H2O2 in red and green leaves after mechanical injury. Plant Cell Environ. 2002, 25, 1261–1269. [Google Scholar] [CrossRef]
- Donner, H.; Gao, L.; Mazza, G. Separation and characterization of simple and malonylated anthocyanins in red onions, Allium cepa L. Food Res. Int 1997, 30, 637–643. [Google Scholar] [CrossRef]
- Slimestad, R.; Fossen, T.; Vågen, I.M. Onions: A source of unique dietary flavonoids. J. Agric. Food Chem. 2007, 55, 10067–10080. [Google Scholar] [CrossRef]
- Cordes, T.; Maiser, A.; Steinhauer, C.; Schermelleh, L.; Tinnefeld, P. Mechanisms and advancement of antifading agents for fluorescence microscopy and single-molecule spectroscopy. Phys. Chem. Chem. Phys. 2011, 13, 6699–6709. [Google Scholar] [CrossRef] [Green Version]
- Cole, L.; Coleman, J.; Evans, D.; Hawes, C. Internalisation of fluorescein isothiocyanate and fluorescein isothiocyanate-dextran by suspension-cultured plant cells. J. Cell Sci. 1990, 96, 721–730. [Google Scholar]
- Oparka, K.J. Uptake and compartmentation of fluorescent probes by plant cells. J. Exp. Bot. 1991, 42, 565–579. [Google Scholar] [CrossRef]
- Coleman, J.O.D.; Randall, R.; Blake-Kalff, M.M.A. Detoxification of xenobiotics in plant cells by glutathione conjugation and vacuolar compartmentalization: A fluorescent assay using monochlorobimane. Plant Cell Environ. 1997, 20, 449–460. [Google Scholar] [CrossRef] [Green Version]
- Swanson, S.J.; Bethke, P.C.; Jones, R.L. Barley aleurone cells contain two types of vacuoles: Characterization of lytic organelles by use of fluorescent probes. Plant Cell 1998, 10, 685–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haugland, R.P. The Handbook: A Guide to Fluorescent Probes and Labeling Technologies; Molecular Probes: Eugene, OR, USA, 2005. [Google Scholar]
- Rottmann, T.M.; Fritz, C.; Lauter, A.; Schneider, S.; Ficher, C.; Danzberger, N.; Diterich, P.; Sauer, N.; Stadler, R. Protoplast-esculin assay as a novel method to assay plant sucrose transporters: Characterization of AtSUC6 and ATSUC7 in Arabidopsis Col-0 ecotype. Front. Plant Sci. 2018, 9, 430. [Google Scholar] [CrossRef] [Green Version]
- Collings, D.A. Subcellular localization of transiently expressed fluorescent fusion proteins. Methods Mol. Biol. 2013, 1069, 227–258. [Google Scholar]
- Collings, D.A. Optimisation approaches for concurrent transmitted light imaging during confocal microscopy. Plant Methods 2015, 11, 40. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, K.; Toyama-Kato, Y.; Kameda, K.; Kondo, T. Sepal color variation of Hydrangea macrophylla and vacuolar pH measured with a proton-selective microelectrode. Plant Cell Physiol. 2003, 44, 262–268. [Google Scholar] [CrossRef] [Green Version]
- Brauer, D.; Uknalis, J.; Triana, R.; Tu, S.-I. Effects of external pH and ammonium on vacuolar pH in maize roothair cells. Plant Physiol. Biochem. 1997, 35, 31–39. [Google Scholar]
- Roos, W.; Evers, S.; Heike, M.; Tschöpe, M.; Schumann, B. Shifts in intracellular pH distribution as a part of the signal mechanism leading to the elicitation of benzophenanthridine alkaloids. Plant Physiol. 1998, 118, 349–364. [Google Scholar] [CrossRef] [Green Version]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Hatier, J.H.B.; Gould, K.S. Anthocyanin function in vegetative organs. In Anthocyanins: Biosynthesis, Functions, and Applications; Gould, K.S., Davies, K., Winefield, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–20. [Google Scholar]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Sivitz, A.B.; Reinders, A.; Ward, J.M. Arabidopsis sucrose transporter AtSUC1 is important for pollen germination and sucrose-induced anthocyanin accumulation. Plant Physiol. 2008, 147, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Oancea, A.M.; Aprodu, I.; Râpeanu, G.; Bahrim, G.; Stanciuc, N. The binding mechanism of anthocyanins from sour cherries (Prunus cerasus L.) skins to bovine b-lactoglobulin: A fluorescence and in silico-based approach. Int. J. Food Prop. 2017, 20, S3096–S3111. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Xu, D.; Zhang, X.; Yang, J.; Wang, Q. Evaluation of anthocyanins in Aronia melanocarpa/BSA binding by spectroscopic studies. Amb Express 2018, 8, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Gidley, M.J.; Waren, F.J. The mechanism of interactions between tea polyphenols and porcine pancreatic alpha-amylase: Analysis by inhibition kinetics, fluorescence quenching, differential scanning calorimetry and isothermal titration calorimetry. Mol. Nutr. Food Res. 2017, 61, 1700324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Deng, P.; Xu, Y.; Lu, S.; Wang, J. Quantification and analysis of anthocyanin and flavonoid composition, and antioxidant activities in onions with three different colors. J. Integr. Agric. 2016, 15, 2175–2181. [Google Scholar] [CrossRef] [Green Version]
- Fu, Q.; Tang, J.; Cui, M.; Xing, J.; Liu, Z.; Liu, S. Application of porous metal enrichment probe sampling in single cells analysis using matrix-assisted laser desorption ionization time of flight mass spectrometry (MALDI-TOF-MS). J. Mass Spectrom. 2015, 51, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Saha-Shah, A.; Green, C.M.; Abraham, D.H.; Baker, L.A. Segmented flow sampling with push-pull theta pipettes. Analyst 2016, 141, 1958. [Google Scholar] [CrossRef]
- Bücherl, C.A.; Bader, A.; Westphal, A.H.; Laptenok, S.P.; Borst, J.W. FRET-FLIM applications in plant systems. Protoplasma 2014, 251, 383–394. [Google Scholar] [CrossRef]
- Chanoca, A.; Burkel, B.; Kovinich, N.; Grotewold, E.; Eliceiri, K.W.; Otegui, M.S. Using fluorescence lifetime microscopy to study the subcellular localization of anthocyanins. Plant J. 2016, 88, 895–903. [Google Scholar] [CrossRef]
- Eady, C.C.; Weld, R.J.; Lister, C.E. Agrobacterium tumefaciens-mediated transformation and transgenic-plant regeneration of onion (Allium cepa L.). Plant Cell Rep. 2000, 19, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Kenel, F.; Eady, C.; Brinch, S. Efficient Agrobacterium tumefaciens-mediated transformation and regeneration of gralic (Allium sativum L.) immature leaf tissue. Plant Cell Rep. 2010, 29, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Teng, S.; Keurentjes, J.; Bentsink, L.; Koornneef, M.; Smeekens, S. Sucrose-specific induction of anthocyanin biosynthesis in Arabidopsis requires the MYB75/PAP1 gene. Plant Physiol. 2005, 139, 1840–1852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovinich, N.; Kayanja, G.; Chanoca, A.; Riedl, K.; Otegui, M.S.; Grotewold, E. Not all anthocyanins are born equal: Distinct patterns induced by stress in Arabidopsis. Planta 2014, 240, 931–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Fluorophore | Source | Stock 1 (mM) | Used (µM) | Loading (Hours) | Excitation 2 (nm) | Emission (nm) |
|---|---|---|---|---|---|---|
| esterase released dyes | ||||||
| fluorescein diacetate | Invitrogen | 10 | 10 | 6 | 488 | 500–530 |
| carboxyfluorescein diacetate | Invitrogen | 100 | 10 | 6 | 458, 488 3 | 500–530 |
| BCECF-AM ester | Invitrogen | 10 | 10 | 6 | 488 | 500–530 |
| Cell Tracker green (5-chloro-methylfluorescein diacetate) | Invitrogen | 10 | 10 | 6 | 488 | 500–530 |
| other enzyme-released dyes | ||||||
| monochlorobimane | Fluka | 100 | 1000 | 6 | 405 | 420–470 |
| Cell Tracker blue (CMAC) | Invitrogen | 10 | 100 | 6 | 405 | 420–470 |
| weak acid dyes | ||||||
| acridine orange | Sigma | 50 | 20 | 6 | 488 | 500–530 |
| sugar analogues | ||||||
| esculin | Sigma | 30 | -- | 4 h | 405 | 420–460 |
| tonoplast membrane probes | ||||||
| MDY-64 | Invitrogen | 10 | 10 | 0.1 | 473 | 480–530 |
| Lysotracker Red DND-99 | Invitrogen | 0.2 | 0.2 | 0.1 | 559 | 570–600 |
| Plant-generated molecules | ||||||
| anthocyanin | -- | -- | -- | -- | 561 | 570–650 4 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collings, D.A. Anthocyanin in the Vacuole of Red Onion Epidermal Cells Quenches Other Fluorescent Molecules. Plants 2019, 8, 596. https://doi.org/10.3390/plants8120596
Collings DA. Anthocyanin in the Vacuole of Red Onion Epidermal Cells Quenches Other Fluorescent Molecules. Plants. 2019; 8(12):596. https://doi.org/10.3390/plants8120596
Chicago/Turabian StyleCollings, David A. 2019. "Anthocyanin in the Vacuole of Red Onion Epidermal Cells Quenches Other Fluorescent Molecules" Plants 8, no. 12: 596. https://doi.org/10.3390/plants8120596
APA StyleCollings, D. A. (2019). Anthocyanin in the Vacuole of Red Onion Epidermal Cells Quenches Other Fluorescent Molecules. Plants, 8(12), 596. https://doi.org/10.3390/plants8120596





