Influence of Root System Characteristics on Black Spruce Seedling Responses to Limiting Conditions
Abstract
1. Introduction
2. Results
2.1. Experiment 1: Growth and Physiological Responses to Irrigation and Fertilization Treatments
2.2. Experiment 2: Root Cell Morphology
3. Discussion
4. Materials and Methods
4.1. Experiment 1
4.2. Experiment 2
4.3. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Viereck, L.A.; Johnston, W.F. Picea mariana (Mill.) BSP black spruce. In Silvics of North America, Volume 1: Conifers; Agriculture Handbook 654; U.S. Department of Agriculture, Forest Service: Washington, DC, USA, 1990; pp. 227–237. [Google Scholar]
- Lieffers, V.J.; Macdonald, S.E. Growth and foliar nutrient status of black spruce and tamarack in relation to depth of water table in some Alberta peatlands. Can. J. For. Res. 1990, 20, 805–809. [Google Scholar] [CrossRef]
- Islam, M.A.; Macdonald, S.E. Ecophysiological adaptations of black spruce (Picea mariana) and tamarack (Larix laricina) seedlings to flooding. Trees-Struct. Funct. 2004, 18, 35–42. [Google Scholar] [CrossRef]
- DesRochers, A.; Gagnon, R. Is ring count at ground level a good estimation of black spruce age? Can. J. For. Res. 1997, 27, 1263–1267. [Google Scholar] [CrossRef]
- Salmon, D. Ressources et industries forestières du Québec: Portrait statistique, Édition 2017; Gouvernement du Québec, Ministère des Forêts, de la Faune et des Parcs: Québec, QC, Canada, 2017.
- Grossnickle, S.C. Importance of root growth in overcoming planting stress. New For. 2005, 30, 273–294. [Google Scholar] [CrossRef]
- Wagner, R.G.; Robinson, A.P. Critical period of interspecific competition for four northern conifers: 10-year growth response and associated vegetation dynamics. Can. J. For. Res. 2006, 36, 2474–2485. [Google Scholar] [CrossRef]
- Johansson, K.; Nilsson, U.; Allen, H.L. Interactions between soil scarification and Norway spruce seedling types. New For. 2007, 33, 13–27. [Google Scholar] [CrossRef]
- Thiffault, N.; Hébert, F.; Jobidon, R. Planted Picea mariana growth and nutrition as influenced by silviculture × nursery interactions on an ericaceous-dominated site. Silva Fenn. 2012, 46, 667–682. [Google Scholar] [CrossRef]
- Burdett, A. Physiological processes in plantation establishment and the development of specifications for forest planting stock. Can. J. For. Res. 1990, 20, 415–427. [Google Scholar] [CrossRef]
- Margolis, H.A.; Brand, D.G. An ecophysiological basis for understanding plantation establishment. Can. J. For. Res. 1990, 20, 375–390. [Google Scholar] [CrossRef]
- de Montigny, L.M.; Weetman, G.F. The effects of ericaceous plants on forest productivity. In The Silvics and Ecology of Boreal Spruces; Titus, B.D., Lavigne, M.B., Newton, P.F., Meades, W.J., Eds.; Canadian Forest Service: St. John’s, NL, Canada; Forestry Canada Information Report N-X-271; 1990; pp. 83–90. [Google Scholar]
- Sutton, R. Planting stock quality, root growth capacity, and field performance of three boreal conifers. N. Z. J. For. Sci. 1980, 10, 54–71. [Google Scholar]
- Burdett, A.; Simpson, D.; Thompson, C. Root development and plantation establishment success. Plant Soil 1983, 71, 103–110. [Google Scholar] [CrossRef]
- Feret, P.P.; Kreh, R.E. Seedling Root Growth Potential as an Indicator of Loblolly Pine Field Performance. For. Sci. 1985, 31, 1005–1011. [Google Scholar] [CrossRef]
- Grossnickle, S.C.; Blake, T.J. Water relation patterns of bare-root and container jack pine and black spruce seedlings planted on boreal cut-over sites. New For. 1987, 1, 101–116. [Google Scholar] [CrossRef]
- Grossnickle, S.C.; El-Kassaby, Y.A. Bareroot versus container stocktypes: A performance comparison. New For. 2016, 47, 1–51. [Google Scholar] [CrossRef]
- Idris, M.; Salifu, K.; Timmer, V. Root plug effects on early growth and nutrition of container black spruce seedlings. For. Ecol. Manag. 2004, 195, 399–408. [Google Scholar] [CrossRef]
- Rose, R.; Haase, D.L. Root and shoot allometry of bareroot and container Douglas-fir seedlings. New For. 2005, 30, 215–233. [Google Scholar] [CrossRef]
- Jutras, S.; Thiffault, N.; Munson, A. Comparing large bareroot and container stock: Water stress as influenced by peat and soil water availability. Tree Plant. Notes 2007, 52, 15–18. [Google Scholar]
- Thiffault, N. Stock type in intensive silviculture: A (short) discussion about roots and size. Forest. Chron. 2004, 80, 463–468. [Google Scholar] [CrossRef]
- Krause, C.; Morin, H. Adventive-root development in mature black spruce and balsam fir in the boreal forests of Quebec, Canada. Can. J. For. Res. 2005, 35, 2642–2654. [Google Scholar] [CrossRef]
- Tarroux, E.; DesRochers, A.; Girard, J.-P. Growth and root development of black and white spruce planted after deep planting. For. Ecol. Manag. 2014, 318, 294–303. [Google Scholar] [CrossRef]
- Kozlowski, T. Responses of woody plants to flooding and salinity. Tree Physiol. Monogr. 1997, 1, 1–29. [Google Scholar] [CrossRef]
- Steffens, B.; Rasmussen, A. The physiology of adventitious roots. Plant Physiol. 2016, 170, 603–617. [Google Scholar] [CrossRef]
- Patterson, T.B.; Guy, R.D.; Dang, Q.L. Whole-plant nitrogen-and water-relations traits, and their associated trade-offs, in adjacent muskeg and upland boreal spruce species. Oecologia 1997, 110, 160–168. [Google Scholar] [CrossRef]
- Calvo-Polanco, M.; Señorans, J.; Zwiazek, J.J. Role of adventitious roots in water relations of tamarack (Larix laricina) seedlings exposed to flooding. BMC Plant Biol. 2012, 12, 99. [Google Scholar] [CrossRef]
- Drew, M.; Jackson, M.; Giffard, S. Ethylene-promoted adventitious rooting and development of cortical air spaces (aerenchyma) in roots may be adaptive responses to flooding in Zea mays L. Planta 1979, 147, 83–88. [Google Scholar] [CrossRef]
- Argus, R.; Colmer, T.; Grierson, P. Early physiological flood tolerance is followed by slow post-flooding root recovery in the dryland riparian tree Eucalyptus camaldulensis subsp. refulgens. Plant Cell Environ. 2015, 38, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Huber, H.; Beljaars, S.J.M.; Birnbaum, D.; de Best, S.; de Kroon, H.; Visser, E.J.W. Benefits of flooding-induced aquatic adventitious roots depend on the duration of submergence: Linking plant performance to root functioning. Ann. Bot. 2017, 120, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Wiengweera, A.; Greenway, H. Performance of seminal and nodal roots of wheat in stagnant solution: K+ and P uptake and effects of increasing O2 partial pressures around the shoot on nodal root elongation. J. Exp. Bot. 2004, 55, 2121–2129. [Google Scholar] [CrossRef] [PubMed]
- Pernot, C.; Thiffault, N.; DesRochers, A. Contribution of adventitious vs. initial roots to growth and physiology of black spruce seedlings. Physiol. Plant. 2019, 165, 29–38. [Google Scholar] [CrossRef]
- Pernot, C.; Thiffault, N.; DesRochers, A. Root system origin and structure influence planting shock of black spruce seedlings in boreal microsites. For. Ecol. Manag. 2019, 433, 594–605. [Google Scholar] [CrossRef]
- Aubin, N.K. Influence du contenu en eau du substrat et de la profondeur de plantation sur la formation de racines adventives caulinaires, la croissance et l’allocation glucidique de semis d’épinette noire (Picea mariana (Mill.) BSP). MSc. Thesis, Université du Québec à Chicoutimi, Chicoutimi, QC, Canada, 1996. [Google Scholar]
- Geiss, G.; Gutierrez, L.; Bellini, C. Adventitious root formation: New insights and perspectives. Annu. Plant Rev. 2009, 37, 127–156. [Google Scholar] [CrossRef]
- Bellini, C.; Pacurar, D.I.; Perrone, I. Adventitious roots and lateral roots: Similarities and differences. Annu. Rev. Plant Biol. 2014, 65, 639–666. [Google Scholar] [CrossRef] [PubMed]
- DesRochers, A.; Van den Driessche, R.; Thomas, B.R. NPK fertilization at planting of three hybrid poplar clones in the boreal region of Alberta. For. Ecol. Manag. 2006, 232, 216–225. [Google Scholar] [CrossRef]
- Hacke, U.G.; Plavcová, L.; Almeida-Rodriguez, A.; King-Jones, S.; Zhou, W.; Cooke, J.E. Influence of nitrogen fertilization on xylem traits and aquaporin expression in stems of hybrid poplar. Tree Physiol. 2010, 30, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Salifu, K.; Timmer, V. Nutrient retranslocation response of Picea mariana seedlings to nitrogen supply. Soil Sci. Soc. Am. J. 2001, 65, 905–913. [Google Scholar] [CrossRef]
- Houle, D.; Moore, J.-D. Soil solution, foliar concentrations and tree growth response to 3-year of ammonium-nitrate addition in two boreal forests of Québec, Canada. For. Ecol. Manag. 2008, 255, 2049–2060. [Google Scholar] [CrossRef]
- Brix, H. Effects of thinning and nitrogen fertilization on growth of Douglas-fir: Relative contribution of foliage quantity and efficiency. Can. J. For. Res. 1983, 13, 167–175. [Google Scholar] [CrossRef]
- Mooney, H.; Ferrar, P.J.; Slatyer, R. Photosynthetic capacity and carbon allocation patterns in diverse growth forms of Eucalyptus. Oecologia 1978, 36, 103–111. [Google Scholar] [CrossRef]
- Brix, H.; Ebell, L.F. Effects of nitrogen fertilization on growth, leaf area and photosynthesis rate in Douglas-fir. For. Sci. 1969, 15, 189–196. [Google Scholar] [CrossRef]
- Teskey, R.; Gholz, H.; Cropper Jr, W. Influence of climate and fertilization on net photosynthesis of mature slash pine. Tree Physiol. 1994, 14, 1215–1227. [Google Scholar] [CrossRef]
- Warren, C.R.; Adams, M.A. Phosphorus affects growth and partitioning of nitrogen to Rubisco in Pinus pinaster. Tree Physiol. 2002, 22, 11–19. [Google Scholar] [CrossRef]
- Warren, C.R.; McGrath, J.F.; Adams, M.A. Differential effects of N, P and K on photosynthesis and partitioning of N in Pinus pinaster needles. Ann. For. Sci. 2005, 62, 1–8. [Google Scholar] [CrossRef]
- Cannell, M.G.R. Dry matter partitioning in tree crops. In Attributes of Trees as Crop Plants; Cannell, M.G.R., Jackson, J.E., Eds.; Institute of Terrestrial Ecology: Abbotts Ripton, UK, 1985; pp. 160–193. [Google Scholar]
- Fan, S.; Blake, T.J.; Blumwald, E. The relative contribution of elastic and osmotic adjustments to turgor maintenance of woody species. Physiol. Plant. 1994, 90, 408–413. [Google Scholar] [CrossRef]
- Zine El Abidine, A.; Stewart, J.D.; Plamondon, A.P.; Bernier, P.Y. Diurnal and seasonal variations in gas exchange and water relations of lowland and upland black spruce ecotypes. Can. J. Bot. 1995, 73, 716–722. [Google Scholar] [CrossRef]
- Mexal, J.G.; South, D.B. Bareroot seedling culture. In Forest Regeneration Manual; Springer: Berlin/Heidelberg, Germany, 1991; pp. 89–115. [Google Scholar]
- Jinks, R.; Mason, B. Effects of seedling density on the growth of Corsican pine (Pinus nigra var. maritima Melv.), Scots pine (Pinus sylvestris L.) and Douglas-fir (Pseudotsuga menziesii Franco) in containers. Ann. For. Sci. 1998, 55, 407–423. [Google Scholar] [CrossRef]
- South, D.B.; Harris, S.W.; Barnett, J.P.; Hainds, M.J.; Gjerstad, D.H. Effect of container type and seedling size on survival and early height growth of Pinus palustris seedlings in Alabama, USA. For. Ecol. Manag. 2005, 204, 385–398. [Google Scholar] [CrossRef]
- Young, E.; Hanover, J.W. Development of the shoot apex of blue spruce (Picea pungens). Can. J. For. Res. 1977, 7, 614–620. [Google Scholar] [CrossRef]
- Grossnickle, S.C. Ecophysiology of Northern Spruce Species: The Performance of Planted Seedlings; NRC Research Press: Ottawa, ON, Canada, 2000; 409p. [Google Scholar]
- Blake, T.J.; Sutton, R.F. Variation in water relations of black spruce stock types planted in Ontario. Tree Physiol. 1987, 3, 331–344. [Google Scholar] [CrossRef]
- Barnett, J.P.; Brissette, J.C. Stock type affects performance of shortleaf pine planted in the Ouachita Mountains through 10 years. In Proceedings of the 12th Biennial Southern Silvicultural Research Conference; Gen. Tech. Rep. SRS-71; US Department of Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 2004; pp. 420–422. [Google Scholar]
- Mena-Petite, A.; Ortega-Lasuen, U.; González-Moro, M.; Lacuesta, M.; Muñoz-Rueda, A. Storage duration and temperature effect on the functional integrity of container and bare-root Pinus radiata D. Don stock-types. Trees 2001, 15, 289–296. [Google Scholar] [CrossRef]
- Helenius, P.; Luoranen, J.; Rikala, R.; Leinonen, K. Effect of drought on growth and mortality of actively growing Norway spruce container seedlings planted in summer. Scand. J. For. Res. 2002, 17, 218–224. [Google Scholar] [CrossRef]
- Helenius, P. Extension of the planting period of Norway spruce container seedlings: Risks related to the drought-growth stage dynamics and handling practices. Ph.D. Thesis, Finnish Forest Research Institute, Helsinki, Finland, 2005. [Google Scholar]
- Van Cleve, K.; Oechel, W.C.; Hom, J.L. Response of black spruce (Picea mariana) ecosystems to soil temperature modification in interior Alaska. Can. J. For. Res. 1990, 20, 1530–1535. [Google Scholar] [CrossRef]
- Lamhamedi, M.; Bernier, P. Ecophysiology and field performance of black spruce (Picea mariana): A review. Ann. For. Sci. 1994, 51, 529–551. [Google Scholar] [CrossRef]
- Renou-Wilson, F.; Keane, M.; Farrell, E. Effect of planting stocktype and cultivation treatment on the establishment of Norway spruce on cutaway peatlands. New For. 2008, 36, 307–330. [Google Scholar] [CrossRef]
- DesRochers, A.; Tremblay, F. The effect of root and shoot pruning on early growth of hybrid poplars. For. Ecol. Manag. 2009, 258, 2062–2067. [Google Scholar] [CrossRef]
- Timmis, R.; Tanaka, Y. Effects of container density and plant water stress on growth and cold hardiness of Douglas-fir seedlings. For. Sci. 1976, 22, 167–172. [Google Scholar] [CrossRef]
- Simpson, D.G. Growing density and container volume affect nursery and field growth of interior spruce seedlings. North J. Appl. For. 1991, 8, 160–165. [Google Scholar] [CrossRef]
- Brand, D.G.; Janas, P.S. Growth and acclimation of planted white pine and white spruce seedlings in response to environmental conditions. Can. J. For. Res. 1988, 18, 320–329. [Google Scholar] [CrossRef]
- Groot, A. Effects of shelter and competition on the early growth of planted white spruce (Picea glauca). Can. J. For. Res. 1999, 29, 1002–1014. [Google Scholar] [CrossRef]
- Lanner, R.M. On the insensitivity of height growth to spacing. For. Ecol. Manag. 1985, 13, 143–148. [Google Scholar] [CrossRef]
- Wonn, H.T.; O’Hara, K.L. Height:Diameter Ratios and Stability Relationships for Four Northern Rocky Mountain Tree Species. West. J. Appl. For. 2001, 16, 87–94. [Google Scholar] [CrossRef]
- Zedaker, S.; Burkhart, H.; Stage, A. General principles and patterns of conifer growth and yield. In Forest Vegetation Management for Conifer Production; Walstad, J.D., Kuch, P.J., Eds.; John Wiley & Sons, Inc.: New York, NY, USA, 1987; pp. 203–241. [Google Scholar]
- Lupi, C.; Morin, H.; Deslauriers, A.; Rossi, S.; Houle, D. Role of Soil Nitrogen for the Conifers of the Boreal Forest: A Critical Review. Int. J. Plant Soil Sci. 2013, 2, 155–189. [Google Scholar] [CrossRef] [PubMed]
- Jupp, A.; Newman, E. Morphological and anatomical effects of severe drought on the roots of Lolium perenne L. New Phytol. 1987, 105, 393–402. [Google Scholar] [CrossRef]
- Spaeth, S.C.; Cortes, P.M. Root cortex death and subsequent initiation and growth of lateral roots from bare steles of chickpeas. Can. J. Bot. 1995, 73, 253–261. [Google Scholar] [CrossRef]
- Cuneo, I.; Knipfer, T.; Brodersen, C.; McElrone, A.J. Mechanical failure of fine root cortical cells initiates plant hydraulic decline during drought. Plant Physiol. 2016, 72, 1669–1678. [Google Scholar] [CrossRef]
- Clarkson, D.T.; Sanderson, J.; Russell, R.S. Ion uptake and root age. Nature 1968, 220, 805–806. [Google Scholar] [CrossRef]
- Enstone, D.E.; Peterson, C.A.; Ma, F. Root endodermis and exodermis: Structure, function, and responses to the environment. J. Plant Growth Regul. 2002, 21, 335–351. [Google Scholar] [CrossRef]
- Stasovski, E.; Peterson, C.A. The effects of drought and subsequent rehydration on the structure and vitality of Zea mays seedling roots. Can. J. Bot. 1991, 69, 1170–1178. [Google Scholar] [CrossRef]
- McDonald, M.; Galwey, N.; Colmer, T. Similarity and diversity in adventitious root anatomy as related to root aeration among a range of wetland and dryland grass species. Plant Cell Environ. 2002, 25, 441–451. [Google Scholar] [CrossRef]
- Takahashi, H.; Yamauchi, T.; Colmer, T.D.; Nakazono, M. Aerenchyma formation in plants. In Low-Oxygen Stress in Plants; Springer: Berlin/Heidelberg, Germany, 2014; pp. 247–265. [Google Scholar]
- Angeles, G.; Evert, R.; Kozlowski, T. Development of lenticels and adventitious roots in flooded Ulmus americana seedlings. Can. J. For. Res. 1986, 16, 585–590. [Google Scholar] [CrossRef]
- Pezeshki, S. Root responses of flood-tolerant and flood-sensitive tree species to soil redox conditions. Trees 1991, 5, 180–186. [Google Scholar] [CrossRef]
- Boggie, R. Water-table depth and oxygen content of deep peat in relation to root growth of Pinus contorta. Plant Soil 1977, 48, 447–454. [Google Scholar] [CrossRef]
- Brewer, P.B.; Heisler, M.G.; Hejátko, J.; Friml, J.; Benková, E. In situ hybridization for mRNA detection in Arabidopsis tissue sections. Nat. Protoc. 2006, 1, 1462. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Fondation for Statical Computing: Vienna, Austria, 2015. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; Team, R.C. nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1–120. 2015. Available online: https://CRAN.R-project.org/package=nlme (accessed on 18 July 2018).
- Lenth, R.V. Least-squares means: The R package lsmeans. J. Stat. Softw. 2016, 69, 1–33. [Google Scholar] [CrossRef]


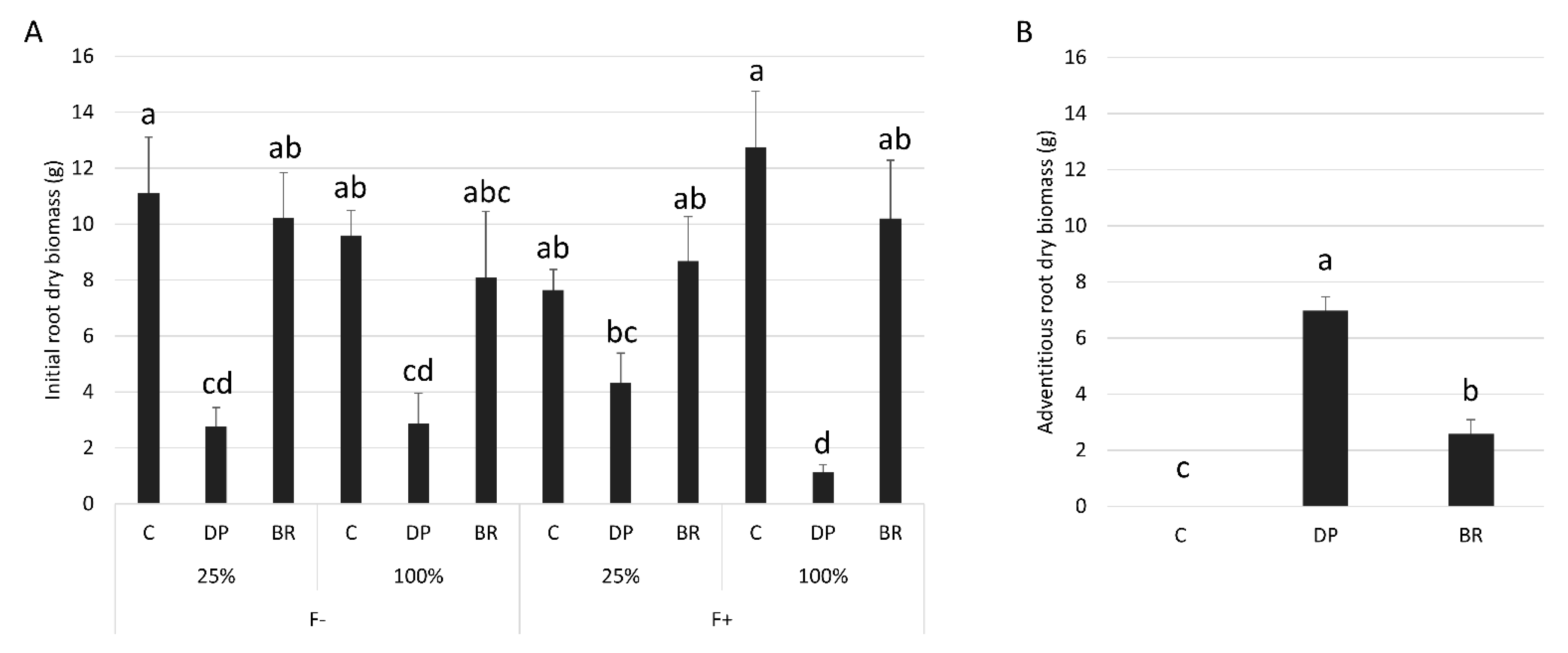

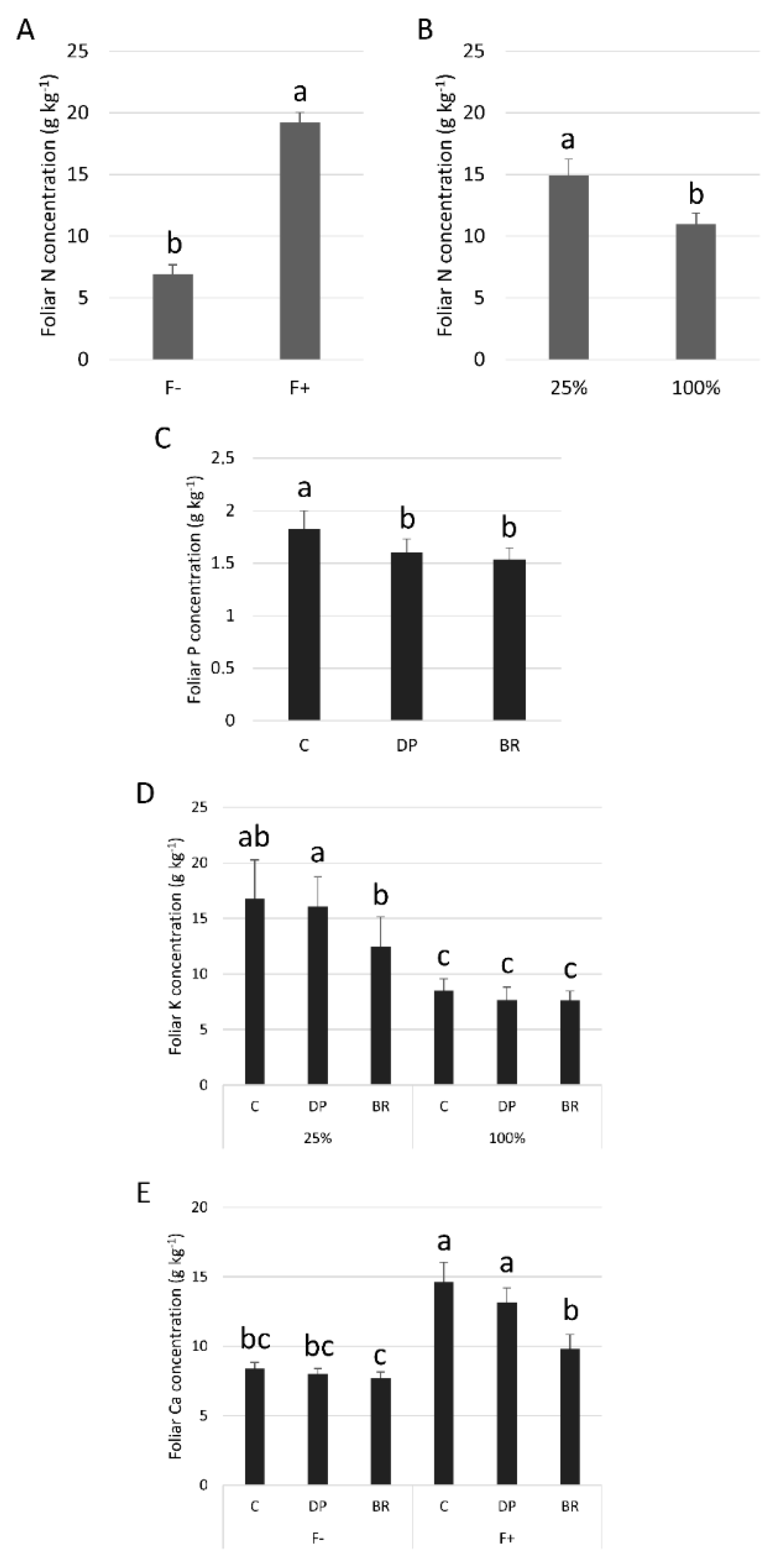

| Variables | Fertilization | Irrigation | Stock Type | Fertilization × Irrigation | Fertilization × Stock Type | Irrigation × Stock Type | Fertilization × Irrigation × Stock Type | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F-Value | P-Value | F-Value | P-Value | F-Value | P-Value | F-value | P-Value | F-Value | P-Value | F-Value | P-Value | F-Value | P-Value | |
| Growth | ||||||||||||||
| RGRheight (log) | 43.88 | <0.001 | 3.68 | 0.060 | 25.89 | <0.001 | 8.64 | 0.005 | 0.94 | 0.396 | 1.36 | 0.266 | 0.52 | 0.595 |
| RGRdiameter | 37.52 | <0.001 | 6.30 | 0.015 | 2.14 | 0.127 | 13.85 | 0.001 | 4.88 | 0.011 | 1.34 | 0.271 | 0.09 | 0.912 |
| Total dry biomass | 21.54 | <0.001 | 0.49 | 0.486 | 5.21 | 0.009 | 0.79 | 0.379 | 0.59 | 0.560 | 2.07 | 0.137 | 1.67 | 0.198 |
| Shoot dry biomass | 45.52 | <0.001 | 0.58 | 0.449 | 5.92 | 0.005 | 0.18 | 0.669 | 1.37 | 0.263 | 1.62 | 0.207 | 0.71 | 0.495 |
| Total root dry biomass (log) | 0.04 | 0.852 | 0.20 | 0.658 | 2.26 | 0.115 | 1.46 | 0.232 | 0.32 | 0.727 | 2.31 | 0.109 | 2.96 | 0.060 |
| Adventitious root dry biomass (logx + 1) | 0.01 | 0.993 | 0.80 | 0.376 | 103.28 | <0.001 | 0.01 | 0.907 | 0.68 | 0.509 | 1.03 | 0.363 | 0.03 | 0.971 |
| Initial root dry biomass (log) | 0.31 | 0.581 | 2.39 | 0.128 | 47.84 | <0.001 | 0.04 | 0.845 | 0.37 | 0.691 | 3.49 | 0.038 | 4.32 | 0.018 |
| Root/Shoot ratio | 35.80 | <0.001 | 0.01 | 0.937 | 0.57 | 0.567 | 0.82 | 0.369 | 1.24 | 0.298 | 0.20 | 0.815 | 2.33 | 0.107 |
| Physiology | ||||||||||||||
| Shoot water potential | 22.18 | <0.001 | 174.92 | <0.001 | 1.20 | 0.308 | 20.61 | <0.001 | 1.35 | 0.269 | 0.78 | 0.464 | 3.23 | 0.047 |
| Net photosynthesis | 0.99 | 0.324 | 0.47 | 0.497 | 15.96 | <0.001 | 1.65 | 0.203 | 4.28 | 0.043 | 3.62 | 0.034 | 2.62 | 0.082 |
| Stomatal conductance (log) | 0.05 | 0.833 | 4.75 | 0.034 | 7.25 | 0.002 | 0.01 | 0.944 | 2.96 | 0.060 | 9.87 | 0.000 | 3.89 | 0.027 |
| Nutrient concentration | ||||||||||||||
| N (log) | 1092.67 | <0.001 | 101.54 | <0.001 | 0.28 | 0.755 | 0.38 | 0.542 | 2.60 | 0.064 | 1.32 | 0.279 | 0.41 | 0.668 |
| P (log) | 313.57 | <0.001 | 3.40 | 0.071 | 3.15 | 0.050 | 4.02 | 0.048 | 1.73 | 0.187 | 1.29 | 0.285 | 2.82 | 0.069 |
| K (log) | 353.91 | <0.001 | 77.33 | <0.001 | 2.18 | 0.123 | 36.82 | <0.001 | 2.37 | 0.104 | 3.91 | 0.044 | 1.29 | 0.285 |
| Ca (log) | 85.11 | <0.001 | 29.90 | <0.001 | 9.80 | 0.000 | 11.40 | 0.001 | 3.69 | 0.032 | 1.19 | 0.312 | 1.70 | 0.194 |
| Variables | Fertilization | Stock Type | |||
|---|---|---|---|---|---|
| F− | F+ | C | DP | BR | |
| Shoot dry biomass (g) | 18.4 b ± 0.8 | 27.1 a ± 1.1 | 20.6 b ± 1.1 | 21.9 b ± 1.4 | 25.6 a ± 1.8 |
| Root dry biomass (g) | 10.6 a ± 0.6 | 10.7 a ± 0.6 | 10.3 a ± 0.8 | 9.8 a ± 0.5 | 11.9 a ± 0.8 |
| Total dry biomass (g) | 29.0 b ± 1.3 | 37.8 a ± 1.5 | 30.8 b ± 1.7 | 31.7 b ± 1.7 | 37.5 a ± 2.3 |
| Root/Shoot ratio (g g−1) | 0.58 a ± 0.03 | 0.40 b ± 0.02 | 0.51 a ± 0.03 | 0.47 a ± 0.02 | 0.50 a ± 0.04 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pernot, C.; Thiffault, N.; DesRochers, A. Influence of Root System Characteristics on Black Spruce Seedling Responses to Limiting Conditions. Plants 2019, 8, 70. https://doi.org/10.3390/plants8030070
Pernot C, Thiffault N, DesRochers A. Influence of Root System Characteristics on Black Spruce Seedling Responses to Limiting Conditions. Plants. 2019; 8(3):70. https://doi.org/10.3390/plants8030070
Chicago/Turabian StylePernot, Clémentine, Nelson Thiffault, and Annie DesRochers. 2019. "Influence of Root System Characteristics on Black Spruce Seedling Responses to Limiting Conditions" Plants 8, no. 3: 70. https://doi.org/10.3390/plants8030070
APA StylePernot, C., Thiffault, N., & DesRochers, A. (2019). Influence of Root System Characteristics on Black Spruce Seedling Responses to Limiting Conditions. Plants, 8(3), 70. https://doi.org/10.3390/plants8030070






