Promising Potential of Lonchocarpus utilis against South American Myasis
Abstract
:1. Introduction
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Ethnobotanical Study
5.2. Bibliographic Review
5.3. In Silico Activity Test
- Rotenone;
- Rotenolone;
- Deguelin;
- Tephrosin;
- 3′metoxylupinifolin;
- 4 hydroxylonchocarpin;
- Lonchocarpene.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
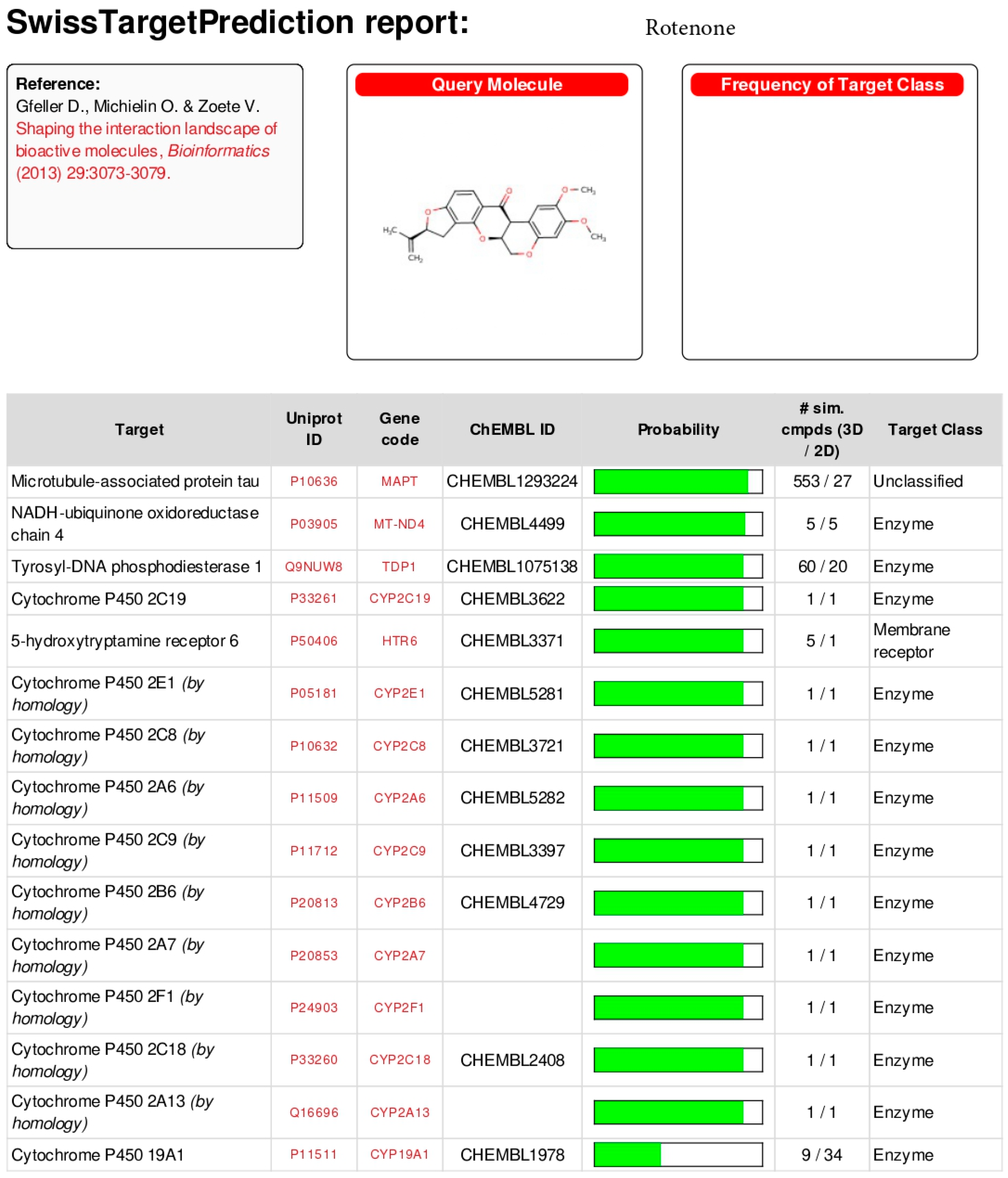

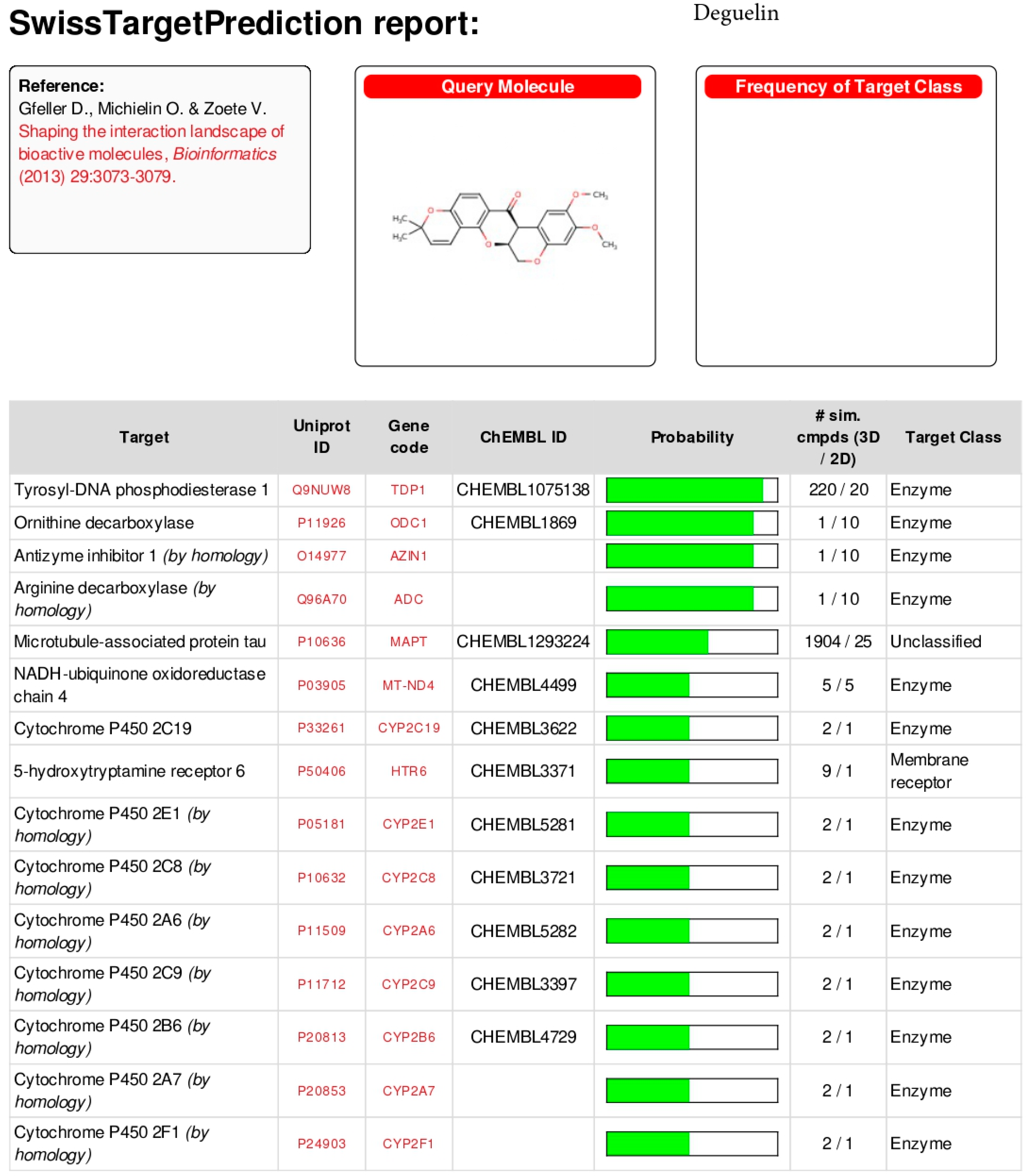




Appendix B
| Use Category | Part | Preparation | Traditional Knowledge | Native Community | Province of Ecuador |
|---|---|---|---|---|---|
| Medicinal | |||||
| Digestive system | R | Plaster | Stomach pain and diarrhea | Kichwa of Eastern | Napo |
| Unidentified ethnicity | Pastaza | ||||
| Skin and subcutaneous cellular tissue | R | Plaster | Chupos treatment: abscesses with pus | Kichwa of Eastern | Napo |
| Unidentified ethnicity | Pastaza | ||||
| Other infectious and parasitic diseases | R | Crushed | Mycosis treatment | Kichwa of Eastern | Orellana |
| Symptoms of undefined origin | L | Milled | Chronic pain caused by witchcraft | Kichwa of Eastern | Pastaza |
| Toxic uses | R, L, S | Crushed and spread in the river | Catch fish | Secoya | Sucumbíos |
| Poison, Insecticide Pesticide | Siona | Sucumbíos | |||
| Unidentified ethnicity | Orellana | ||||
| Napo | |||||
| Zamora Chinchipe | |||||
| Tsa’chi | Pichincha | ||||
| Cofán | Sucumbíos | ||||
| Amazon | |||||
| Kichwa of Eastern | Sucumbíos | ||||
| Napo | |||||
| Orellana | |||||
| Pastaza | |||||
| Zamora Chinchipe | |||||
| Wao | Napo | ||||
| Orellana | |||||
| Shuar | Orellana | ||||
| Pastaza | |||||
| Morona Santiago | |||||
| Social, symbolic, and ritual uses | L | Leaves, alone or with ají leaves burned | Drives away evil spirits when you sleep in the forest | Unidentified ethnicity | Napo |
| Protection rituals | |||||
| Other handling | Collection and sale (rotenone content) | Cofán | Amazon | ||
| Commercialization | R |
| Use Categories | Part | Preparation | Method of Usage/Purpose of Use |
|---|---|---|---|
| Human Medicine | |||
| Used against myiasis: “to kill the tupe” (human bot fly) | R | Extraction of “milk” by pressure | The “milk” is deposited on a piece of paper and placed where tupe has stung |
| Hits and body aches | R | Crush roots | Crushed root is placed directly on the skin |
| Veterinary | |||
| External antiparasitic | R | Extraction of “milk” by pressure | The “milk” is deposited on a piece of paper and placed where tupe has stung |
| Toxic | |||
| Catch fish | R | Crushed roots to be used as soon as possible (in 1–2 days) | The “milk” obtained is spread in the water of rivers and ravines |
References
- World Health Organization (WHO) Neglected Tropical Diseases. Available online: https://web.archive.org/web/20140227152033/; http://www.who.int/neglected_diseases/en/ (accessed on 13 December 2019).
- Katewa, S.S.; Chaudhary, B.L.; Jain, A. Folk herbal medicines from tribal area of Rajasthan, India. J. Ethnopharmacol. 2004, 92, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Carreño-Hidalgo, P.C. La etnobotánica y su importancia como herramienta para la articulación entre conocimientos ancestrales y científicos. In Trabajo de Grado para Optar a Título de Licenciado; Universidad Distrital Francisco José de Caldas: Bogotá, Colombia, 2016. [Google Scholar]
- Hotez, P.J.; Alvarado, M.; Basáñez, M.-G.; Bolliger, I.; Bourne, R.; Boussinesq, M.; Brooker, S.J.; Brown, A.S.; Buckle, G.; Budke, C.M. The global burden of disease study 2010: Interpretation and implications for the neglected tropical diseases. PLoS Negl. Trop. Dis. 2014, 8, e2865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemoga Soto, R. Globalizacion y transformacion de las formas juridicas: Apropiacion de material genetico. Pensam. Jurídico 1:138-148. ISSN 2357-6170. 2013. Available online: https://revistas.unal.edu.co/index.php/peju/article/view/38893 (accessed on 17 December 2019).
- Torres Morocho, D.M.; Orea Igarza, U.; Brito Vallina, M.L.; Cordero Machado, E. Estudio de la extracción del follaje de Barbasco (Lonchocarpus nicou) como fuente biocida (en condiciones de la Amazonía en Ecuador). Rev. Cienc. Técnicas Agropecu. 2013, 22, 41–49. [Google Scholar]
- Luzuriaga-Quichimbo, C.X. Estudio Etnobotánico en Comunidades Kichwas Amazónicas de Pastaza, Ecuador. Ph.D. Thesis, Universidad de Extremadura, Badajoz, España, 2017. [Google Scholar]
- Borgtoft, H.; Skov, F.; Fjeldsa, J.; Øllgaard, B. People and Biodiversity. Two case studies from the Andean foothills of Ecuador. Centre for research on cultural and biological diversity of Andean rainforests. Diva Tech. Rep. 1998, 3, 1–190. [Google Scholar]
- Álvarez, C. Historias desde el Aula; Abya-Yala: Quito, Ecuador, 2006. [Google Scholar]
- GADP-Pastaza. Estudio del Impacto Ambiental del Proyecto de Construcción del Afirmado Camino Vecinal Latasas-Umupi, Parroquia Canelos, Provincia Pastaza; Gobierno Autónomo Descentralizado de Pastaza: Puyo, Ecuador, 2013.
- GADP-Pastaza. Plan de Desarrollo y Ordenamiento Territorial del Cantón Pastaza, 2015–2020; Gobierno autónomo Descentralizado de Pastaza: Puyo, Ecuador, 2015.
- Widdowson, M.-A.; Iuliano, A.D.; Dawood, F.S. Challenges to global pandemic mortality estimation. Lancet Infect. Dis. 2014. [Google Scholar] [CrossRef]
- Zúñiga Carrasco, I.R. Miasis: Un problema de salud poco estudiado en México. Rev. Enferm. Infecc. Pediatr. 2009, 22, 121–125. [Google Scholar]
- Tamir, J.; Haik, J.; Orenstein, A.; Schwartz, E. Dermatobia hominis myiasis among travelers returning from South America. J. Am. Acad. Derm. 2003, 48, 630–632. [Google Scholar] [CrossRef]
- Mathieu, M.E.; Wilson, B.B. Myiasis, 5th ed.; Co, C.L., Ed.; Churchill Livingstone Co: Philadelphia, PA, USA, 2000. [Google Scholar]
- Martinez-Estrada, V.; Aguilera, V.; Jurado, F. Miasis furunculoide. Comun. Caso. Dermatol. Rev. Mex 2002, 46, 280–284. [Google Scholar]
- Mengarelli, R.H.; Cevallos, M.V. Manejo de las miasis en heridas agudas y crónicas: Presentación de casos y revisión de la bibliografía. Rev. Argent. Dermatol. Ciudad Autónoma Buenos Aires 2012, 93, 1–8. [Google Scholar]
- Ginarte, M.; García Doval, I.; Peteiro, C.; Toribio, J. Miasis cutánea por Dermatobia hominis. Actas Dermosifiliogr. 1996, 87, 340–342. [Google Scholar]
- de Hollanda Ramírez, A.M.; Silva Rodríguez, A.R.; Zaracho, G. Invermectina in the treatment of Human Miasis. La Fac. Cienc. Médicas 2005, 38, 62–71. [Google Scholar]
- Manrique, A.; Manrique, D.; Catacora, J. Miasis cutánea: Reporte de un caso y revisión de la literatura. Folia Derm. Peru 2009, 20, 23–26. [Google Scholar]
- Rubio, C.; de Guevara, C.L.; Martín, M.A.; Campos, L.; Quesada, A.; Casado, M. Miasis cutáneas sobre lesiones tumorales: Presentación de tres casos. Actas Dermosifiliogr. 2006, 97, 39–42. [Google Scholar] [CrossRef]
- Izquierdo, M.J.; Pastor, M.A.; Carrasco, L.; Fariña, M.C.; Martín, L.; Requena, L.; Fernández, R.; Gadea, I. Miasis forunculoide: Descripción de dos casos con estudio histológico de las diferentes larvas. Actas Dermosifiliogr. 2001, 92, 456–460. [Google Scholar] [CrossRef]
- Chan, J.C.M.; Lee, J.S.W.; Dai, D.L.K.; Woo, J. Unusual cases of human myiasis due to Old World screwworm fly acquired indoors in Hong Kong. Trans. R. Soc. Trop. Med. Hyg. 2005, 99, 914–918. [Google Scholar] [CrossRef]
- Moya, J.; Spelta, G.; Gavazza, S.; Barbarulo, A.M.; Fontana, M.I.; Barerra, M.; Jurjo, L.L.; Azcune, R. Miasis cutánea Revisión sobre el tema y presentación de un caso de miasis forunculoide. Arch. Argent. Derm. 2007, 57, 217–222. [Google Scholar]
- Lima, T.C.; Santos, A.D.C.; Costa, D.T.M.; Souza, R.J.; Barison, A.; Steindel, M.; Biavatti, M.W. Chromenes from leaves of Calea pinnatifida and evaluation of their leishmanicidal activity. Rev. Bras. Farm. 2015, 25, 7–10. [Google Scholar] [CrossRef] [Green Version]
- Mishra, T.; Shukla, S.; Meena, S.; Singh, R.; Pal, M.; Upreti, D.K.; Datta, D. Isolation and identification of cytotoxic compounds from a fruticose lichen Roccella montagnei, and it’s in silico docking study against CDK-10. Rev. Bras. Farm. 2017, 27, 724–728. [Google Scholar] [CrossRef]
- dos Santos Passos, C.; Klein-Júnior, L.C.; de Mello Andrade, J.M.; Matté, C.; Henriques, A.T. The catechol-O-methyltransferase inhibitory potential of Z-vallesiachotamine by in silico and in vitro approaches. Rev. Bras. Farm. 2015, 25, 382–386. [Google Scholar] [CrossRef] [Green Version]
- Moraga-Nicolás, F.; Jara, C.; Godoy, R.; Iturriaga-Vásquez, P.; Venthur, H.; Quiroz, A.; Becerra, J.; Mutis, A.; Hormazábal, E. Rhodolirium andicola: A new renewable source of alkaloids with acetylcholinesterase inhibitory activity, a study from nature to molecular docking. Rev. Bras. Farm. 2018, 28, 34–43. [Google Scholar] [CrossRef]
- Moradi-Afrapoli, F.; Shokrzadeh, M.; Barzegar, F.; Gorji-Bahri, G.; Zadali, R.; Nejad Ebrahimi, S. Cytotoxic activity of abietane diterpenoids from roots of Salvia sahendica by HPLC-based activity profiling. Rev. Bras. Farm. 2018, 28, 27–33. [Google Scholar] [CrossRef]
- Mangul, S.; Martin, L.S.; Langmead, B.; Sanchez-Galan, J.E.; Toma, I.; Hormozdiari, F.; Pevzner, P.; Eskin, E. How bioinformatics and open data can boost basic science in countries and universities with limited resources. Nat. Biotechnol. 2019, 37, 324–326. [Google Scholar] [CrossRef] [PubMed]
- Fang, N.B.; Casida, J.E. Cube resin insecticide: Identification and biological activity of 29 rotenoid constituents. J. Agric. Food Chem. 1999, 47, 2130–2136. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, D.G.; de Almeida, C.M.C.; Silva, C.; Arruda, M.S.P.; Arruda, A.C.; Lopes, D.C.F.; Yamada, E.S.; da Costa, E.T.; da Silva, M.N. Flavonoids from the Leaves of Deguelia utilis (Leguminosae): Structural Elucidation and Neuroprotective Properties. J. Braz. Chem. Soc. 2012, 23, 1933–1939. [Google Scholar] [CrossRef] [Green Version]
- Lawson, M.A.; Kaouadji, M.; Allais, D.P.; Champavier, Y.; Chulia, A.J. Substituted tubaic acids, new oxidative rotenoid metabolites from Lonchocarpus nicou. Tetrahedron Lett. 2006, 47, 451–454. [Google Scholar] [CrossRef]
- Lawson, M.A.; Kaouadji, M.; Chulia, A.J. A single chalcone and additional rotenoids from Lonchocarpus nicou. Tetrahedron Lett. 2010, 51, 6116–6119. [Google Scholar] [CrossRef]
- Lawson, A.M.N.V. O-Benzoquinone and Ester-Linked Hydroxyfatatty Acid as Additional compounds from Lonchocarpus nicou. Open J. Plant Sci. 2016, 1, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Fang, N.B.; Casida, J.E. New bioactive flavonoids and stilbenes in cube resin insecticide. J. Nat. Prod. 2000, 63, 293. [Google Scholar] [CrossRef]
- Lawson, M.A.; Kaouadji, M.; Chulia, A.J. Nor-dehydrodeguelin and nor-dehydrorotenone, C(22) coumaronochromones from Lonchocarpus nicou. Tetrahedron Lett. 2008, 49, 2407–2409. [Google Scholar] [CrossRef]
- Kaouadji, M.; Agban, A.; Mariotte, A.M. Lonchocarpene, a stilbene, and lonchocarpusone, an isoflavone—2 new pyronopolyphenols from Lonchocarpus nicou roots. J. Nat. Prod. 1986, 49, 281–285. [Google Scholar] [CrossRef]
- Fang, N.B.; Casida, J.E. Anticancer action of cube insecticide: Correlation for rotenoid constituents between inhibition of NADH: Ubiquinone oxidoreductase and induced ornithine decarboxylase activities. Proc. Natl. Acad. Sci. USA 1998, 95, 3380–3384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhaisen, H.M.H. Introduction and Interpretation of Flavonoids. Adv. Sci. Eng. Med. 2014, 6, 1–16. [Google Scholar] [CrossRef]
- Caboni, P.; Sherer, T.B.; Zhang, N.J.; Taylor, G.; Na, H.M.; Greenamyre, J.T.; Casida, J.E. Rotenone, deguelin, their metabolites, and the rat model of Parkinson’s disease. Chem. Res. Toxicol. 2004, 17, 1540–1548. [Google Scholar] [CrossRef]
- Fuchino, H.; Kiuchi, F.; Yamanaka, A.; Obu, A.; Wada, H.; Mori-Yasumoto, K.; Kawahara, N.; Flores, D.; Palacios, O.; Sekita, S.; et al. New Leishmanicidal Stilbenes from a Peruvian Folk Medicine, Lonchocarpus nicou. Chem. Pharm. Bull. (Tokyo) 2013, 61, 979–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, B.; M’Batchi, B.; Mounzeo, H.; Bourobou, H.P.B.; Posso, P. Effect of Tephrosia vogelii and Justicia extensa on Tilapia nilotica in vivo. J. Ethnopharmacol. 2000, 69, 99–104. [Google Scholar] [CrossRef]
- Sarwar, M. The killer chemicals for control of agriculture insect pests: The botanical insecticides. Int. J. Chem. Biomol. Sci. 2015, 1, 123–128. [Google Scholar]
- Fuchino, H.; Sekita, S.; Mori, K.; Kawahara, N.; Satake, M.; Kiuchi, F. A New Leishmanicidal Saponin from Brunfelsia grandiflora. Chem. Pharm. Bull. (Tokyo) 2008, 56, 93–96. [Google Scholar] [CrossRef] [Green Version]
- Preston, S.; Korhonen, P.K.; Mouchiroud, L.; Cornaglia, M.; McGee, S.L.; Young, N.D.; Davis, R.A.; Crawford, S.; Nowell, C.; Ansell, B.R.E.; et al. Deguelin exerts potent nematocidal activity via the mitochondrial respiratory chain. FASEB J. 2017, 31, 4515–4532. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.Y.; Chang, D.J.; Hennessy, B.; Seo, S.Y. A Novel Derivative of the Natural Agent Deguelin for Cancer Chemoprevention and Therapy. Cancer Prev. Res. 2009, 2, 186. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-G.; Kim, M.-M. Anti-inflammatory Effect of Scopoletin in RAW264.7 Macrophages. J. Life Sci. 2015, 25, 1377–1383. [Google Scholar] [CrossRef] [Green Version]
- Murillo, G.; Salti, G.I.; Kosmeder, J.W.; Pezzuto, J.M.; Mehta, R.G. Deguelin inhibits the growth of colon cancer cells through the induction of apoptosis and cell cycle arrest. Eur. J. Cancer 2002, 38, 2446–2454. [Google Scholar] [CrossRef]
- Lee, H.Y.; Oh, S.H.; Woo, J.K.; Kim, W.Y.; Van Pelt, C.S.; Price, R.E.; Cody, D.; Tran, H.; Pezzuto, J.M.; Moriarty, R.M.; et al. Chemopreventive effects of deguelin, a novel Akt inhibitor, on tobacco-induced lung tumorigenesis. J. Natl. Cancer Inst. 2005, 97, 1695–1699. [Google Scholar] [CrossRef] [PubMed]
- Boreddy, S.R.; Srivastava, S.K. Deguelin suppresses pancreatic tumor growth and metastasis by inhibiting epithelial-to-mesenchymal transition in an orthotopic model. Oncogene 2013, 32, 3980–3991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thamilselvan, V.; Menon, M.; Thamilselvan, S. Anticancer efficacy of deguelin in human prostate cancer cells targeting glycogen synthase kinase-3 beta/beta-catenin pathway. Int. J. Cancer 2011, 129, 2916–2927. [Google Scholar] [CrossRef]
- Wang, A.M.; Wang, W.N.; Chen, Y.Q.; Ma, F.Q.; Wei, X.M.; Bi, Y.Y. Deguelin induces PUMA-mediated apoptosis and promotes sensitivity of lung cancer cells (LCCs) to doxorubicin (Dox). Mol. Cell. Biochem. 2018, 442, 177–186. [Google Scholar] [CrossRef]
- Bortul, R.; Tazzari, P.L.; Billi, A.M.; Tabellini, G.; Mantovani, I.; Cappellini, A.; Grafone, T.; Martinelli, G.; Conte, R.; Martelli, A.M.; et al. PI3K/AKT inhibitor, enhances chemosensitivity of leukaemia cells with an active PI3K/AKT pathway. Br. J. Haematol. 2005, 129, 677–686. [Google Scholar] [CrossRef]
- Ackerman, J.L.; Bellwood, D.R. Reef fish assemblages: A re-evaluation using enclosed rotenone stations. Mar. Ecol. Prog. Ser. 2000, 206, 227–237. [Google Scholar] [CrossRef]
- Aladdin, N.-A.; Jamal, J.A.; Talip, N.; Hamsani, N.A.M.; Rahman, M.R.A.; Sabandar, C.W.; Muhammad, K.; Husain, K.; Jalil, J.; Lima, N.M.; et al. Antifungal activity of extracts and phenolic compounds from Deguelia duckeana. Rev. Bras. Farm. 2018, 28, 697–702. [Google Scholar] [CrossRef] [Green Version]
- Lobo, L.T.; da Silva, G.A.; de Freitas, M.C.C.; Souza, A.P.S.; da Silva, M.N.; Arruda, A.C.; Guilhon, G.; Santos, L.S.; Santos, A.S.; Arruda, M.S.P. Stilbenes from Deguelia rufescens var. urucu (Ducke) A. M. G. Azevedo Leaves: Effects on Seed Germination and Plant Growth. J. Braz. Chem. Soc. 2010, 21, 1838–1844. [Google Scholar] [CrossRef] [Green Version]
- Omura, S. Ivermectin: 25 years and still going strong. Int. J. Antimicrob. Agents 2008, 31, 91–98. [Google Scholar] [CrossRef]
- Õmura, S.; Crump, A. The life and times of ivermectin—a success story. Nat. Rev. Microbiol. 2004, 2, 984. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.; Lagunes, A.; Rodriguez, J.C.; Rodriguez, D. Insecticidas vegetales; una vieja y nueva alternativa en el manejo de insectos. Rev. Manejo Integr. Plagas Agroecol. 2002, 66, 4–12. [Google Scholar]
- Gupta, R.C. Biomarkers in Toxicology; Academic Press: Cambridge, MT, USA, 2014; ISBN 9780124046306. [Google Scholar]
- McGarry, J.W. Tropical myiases: Neglected and well travelled. Lancet Infect. Dis. 2014, 14, 672–674. [Google Scholar] [CrossRef]
- World Health Organization (WHO), International Programme on Chemical Safety. The WHO Recommended Classification of Pesticides by Hazard. Guidelines to Classification; World Health Organization: Geneve, Switzerland, 2009; ISBN 9789241547963. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gfeller, D.; Michielin, O.; Zoete, V. Shaping the interaction landscape of bioactive molecules. Bioinformatics 2013, 29, 3073–3079. [Google Scholar] [CrossRef]
- Gfeller, D.; Grosdidier, A.; Wirth, M.; Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: A web server for target prediction of bioactive small molecules. Nucleic Acids Res. 2014, 42, 32–38. [Google Scholar] [CrossRef]
- De la Torre, L.; Navarrete, H.; Muriel, P.; Maciá, J.; Balslev, H. Enciclopedia de las plantas Útiles en Ecuador; Escuela de Ciencias Biológicas de la Pontífica Universidad Católica del Ecuador & Herbario AAU del Departamento de Ciencias Biológicas de la Universidad de Aarhus: Quito, Ecuador; Aahrus, Dinamarca, 2008; ISBN 978-9978-77-135-8. [Google Scholar]

| Molecule | Tested in | Activity | References |
|---|---|---|---|
| Rotenone | Rat | Inhibition of mitochondrial activity (diminished NADH: ubiquinone oxidoreductase activity) | [41] |
| Cell | Inhibition of growth | [31] | |
| Lehismania | Antilehismaniasic | [42] | |
| Cell | Antiproliferative | [39] | |
| Fish | Toxic for fish | [41,43] | |
| Insect | Insecticide and pesticide | [44] | |
| Rotenolone | Rat | Inhibition of mitochondrial activity (diminished NADH: ubiquinone oxidoreductase activity) (25% less active than rotenone). | [41] |
| Cell | Inhibition of growth | [31] | |
| Deguelin | Inhibition of mitochondrial activity (diminished NADH: ubiquinone oxidoreductase activity) (50% less active than rotenone). | [41] | |
| Cell | Inhibition of growth | [31,45] | |
| Cell | Antiproliferative | [39] | |
| Nematode | Nematocide | [46] | |
| Anti-inflammatory in transplants | [46,47] | ||
| Cell | Potent apoptotic and antiangiogenic | [48,49] | |
| Cell | Inhibition of progression of tumors such as lung, stomach, prostate, colon, ovary, and pancreas. | [49,50,51,52,53] | |
| Cell | Inhibition of tumor cell growth and metastasis. | [51,52] | |
| Cell | Chemical adjuvant against leukemia | [54] | |
| Tephrosin | Rat | Inhibition of mitochondrial activity (diminished NADH: ubiquinone oxidoreductase activity) | [41] |
| Cell | Inhibition of growth | [43] | |
| Prenyl-urucuol A | Cell | Cytoprotective activity of neurons in rats (Complete fraction) | [55] |
| Prenyl-isotirumalin | |||
| Prenylutilinol | |||
| 3′-methoxylupinifolin | |||
| Prenylutiline | |||
| (2S)-6-(γ,γ-dimethylallyl)-5,4′-dihydroxy-3′-methoxy-6″,6″-dimethylpyran [2 ″,3″:7,8] flavanone | Cell | Inhibition of growth | [36] |
| 4-hydroxylonchocarpin | Antifungal | [56] | |
| Lonchocarpene | Seedling | Inhibition of growth/development | [57] |
| 4-methoxylonchocarpene | Seedling | ||
| 3,5-dimethoxy-4-hydroxy-3-prenyl-trans-stilbene | Seedling |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luzuriaga-Quichimbo, C.X.; Blanco-Salas, J.; Cerón-Martínez, C.E.; Alías-Gallego, J.C.; Ruiz-Téllez, T. Promising Potential of Lonchocarpus utilis against South American Myasis. Plants 2020, 9, 33. https://doi.org/10.3390/plants9010033
Luzuriaga-Quichimbo CX, Blanco-Salas J, Cerón-Martínez CE, Alías-Gallego JC, Ruiz-Téllez T. Promising Potential of Lonchocarpus utilis against South American Myasis. Plants. 2020; 9(1):33. https://doi.org/10.3390/plants9010033
Chicago/Turabian StyleLuzuriaga-Quichimbo, Carmen X., José Blanco-Salas, Carlos E. Cerón-Martínez, Juan Carlos Alías-Gallego, and Trinidad Ruiz-Téllez. 2020. "Promising Potential of Lonchocarpus utilis against South American Myasis" Plants 9, no. 1: 33. https://doi.org/10.3390/plants9010033
APA StyleLuzuriaga-Quichimbo, C. X., Blanco-Salas, J., Cerón-Martínez, C. E., Alías-Gallego, J. C., & Ruiz-Téllez, T. (2020). Promising Potential of Lonchocarpus utilis against South American Myasis. Plants, 9(1), 33. https://doi.org/10.3390/plants9010033








