The Development of Crassulacean Acid Metabolism (CAM) Photosynthesis in Cotyledons of the C4 Species, Portulaca grandiflora (Portulacaceae)
Abstract
:1. Introduction
2. Results
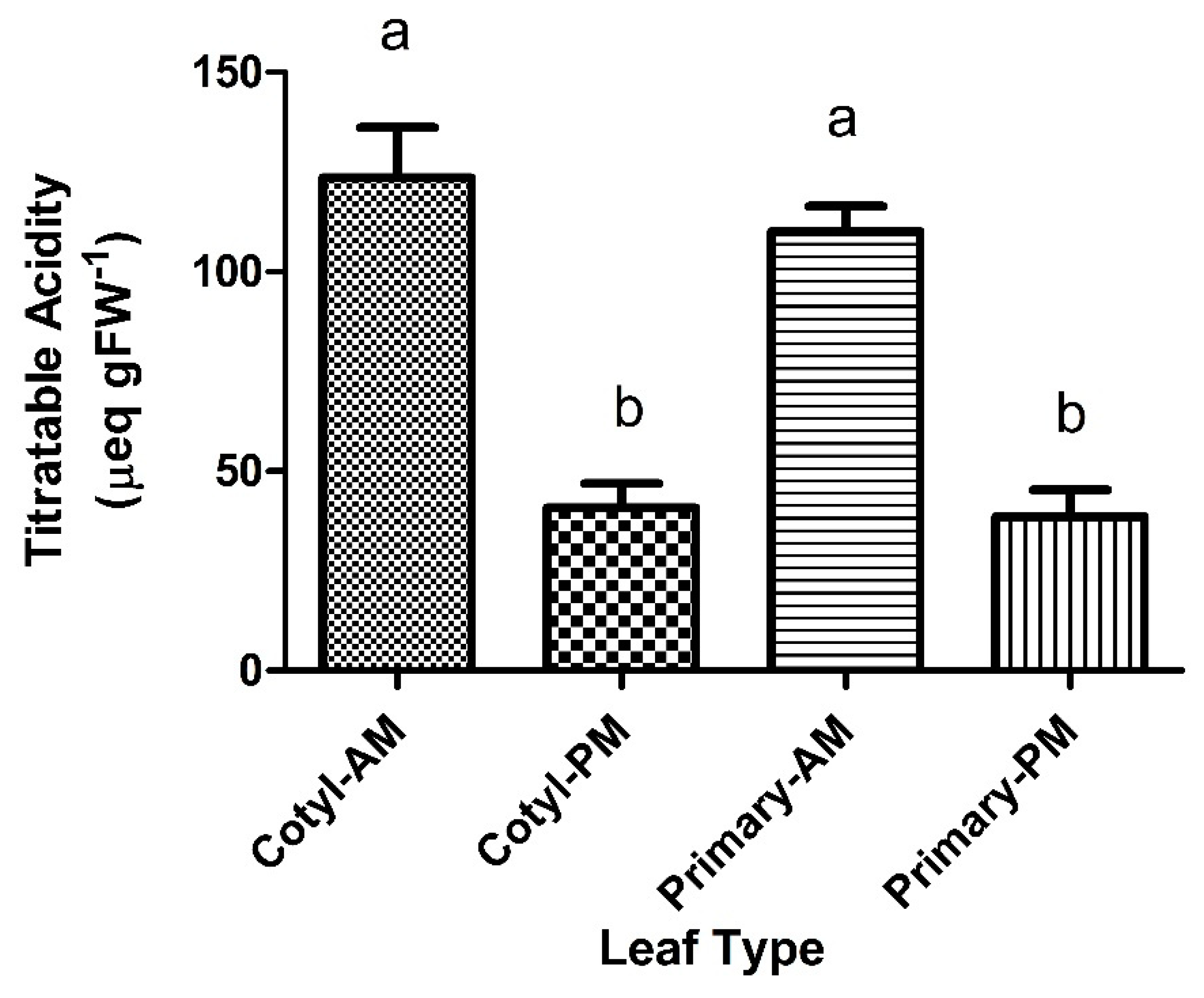
2.1. Titratable Acidity
2.2. Enzyme Activity: PEP Carboxylase and NADP-ME
2.3. Electron Transport Rate/JO2
2.4. Leaf Anatomy
3. Discussion
3.1. C4 Development
3.2. CAM Development
4. Materials and Methods
4.1. Plant Material
4.2. Titratable Acidity
4.3. Enzyme Activity
4.4. Leaf Anatomy
4.5. Electron Transport and JO2
4.6. Tissue Printing
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sage, R.F.; Monson, R.K. Preface. In C4 Plant Biology; Academic: Philip Drive Norwell, MA, USA, 1999; pp. xiii–xv. [Google Scholar]
- Voznesenskaya, E.V.; Koteyeva, N.K.; Edwards, G.E.; Ocampo, G. Revealing diversity in structural and biochemical forms of C4 photosynthesis and a C3-C4 intermediate in genus Portulaca L. (Portulacaceae). J. Exp. Bot. 2010, 61, 3647–3662. [Google Scholar] [CrossRef] [PubMed]
- Voznesenskaya, E.V.; Franceschi, V.R.; Kiirats, O.; Freitag, H.; Edwards, G.E. Kranz anatomy is not essential for terrestial C4 plant photosynthesis. Nature 2001, 414, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Voznesenskaya, E.V.; Franceschi, V.R.; Kiirats, O.; Artyusheva, E.G.; Freitag, H.; Edwards, G.E. Proof of C4 photosynthesis without Kranz anatomy in Bienertia cycloptera (Chenopodiaceae). Plant J. 2002, 31, 649–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osmond, C.B. Crassulacean acid metabolism: A curiosity in context. Ann. Rev. Plant Physiol. 1978, 29, 379–414. [Google Scholar] [CrossRef]
- Ting, I.P. Crassulacean acid metabolism. Ann. Rev. Plant Physiol. 1985, 36, 595–622. [Google Scholar] [CrossRef]
- Gibson, A. Anatomy of Succulence. In Crassulacean Acid Metabolism: Proceedings of the Fifth Symposium in Botany; Ting, I.P., Gibbs, M., Eds.; Waverly Press: Baltimore, MD, USA, 1982; pp. 1–17. [Google Scholar]
- Winter, K.; Smith, J.A.C. An Introduction to Crassulacean acid metabolism. In Crassulacean Acid Metabolism: Biochemical Principles and Ecological Diversity; Springer: Berlin/Heidelberg, Germany, 1996; pp. 1–13. [Google Scholar]
- Eggli, U.; Ford-Werntz, D. Portulacaceae. In Illustrated Handbook of Succulent Plants: Dicotyledons; Springer: Berlin/Heidelberg, Germany, 2002; p. 370. [Google Scholar]
- Guralnick, L.J.; Jackson, M.D. The occurrence and phylogenetics of Crassulacean acid metabolism activity in the Portulacaceae. Int. J. Plant Sci. 2001, 162, 257–262. [Google Scholar] [CrossRef]
- Guralnick, L.J.; Cline, A.; Smith, M.; Sage, R. Evolutionary Physiology: The extent of C4 and CAM photosynthesis in the Genera Anacampseros and Grahamia of the Portulacaceae. J. Exp. Bot. 2008, 59, 1735–1742. [Google Scholar] [CrossRef] [Green Version]
- Hershokivitz, M.A.; Zimmer, E.A. On the evolutionary origins of the cacti. Taxon 1997, 46, 217–232. [Google Scholar] [CrossRef]
- Lara, M.V.; Andreo, C.S. Photosynthesis in nontypical C4 species. In Handbook of Photosyntheis, 2nd ed.; Pessarakli, M., Ed.; Taylor & Francis: London, UK, 2005; pp. 392–421. [Google Scholar]
- Koch, K.E.; Kennedy, R.A. Characteristics of Crassulacean acid metabolism in the succulent C4 dicot, Portulaca oleracea L. Plant Physiol. 1980, 65, 193–197. [Google Scholar] [CrossRef] [Green Version]
- Koch, K.E.; Kennedy, R.A. Crassulacean acid metabolism in the succulent C4 dicot, Portulaca oleracea L. under natural environmental conditions. Plant Physiol. 1982, 69, 757–761. [Google Scholar] [CrossRef] [Green Version]
- Kraybill, A.A.; Martin, C.E. Crassulacean acid metabolism in three of the C4 genus Portulaca. Int. J. Plant Sci. 1996, 57, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Mazen, A.M.A. Changes in the levels of phosphoenolpyruvate carboxylase with induction of Crassulacean acid metabolism (CAM)-like behaviour in the C4 plant Portulaca oleracea. Physiol. Plant. 1996, 98, 111–116. [Google Scholar] [CrossRef]
- Winter, K.; Sage, R.F.; Edwards, E.J.; Virgo, A.; Holtum, J.A.M. Facultative Crassulacean acid metabolism in a C3-C4 intermediate. J. Exp. Bot. 2019. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.B.; Shieh, Y.J.; Reger, B.J.; Black, C.C. Photosynthetic characteristics of Portulaca grandiflora, a succulent C4 dicot. Plant Physiol. 1981, 68, 1073–1080. [Google Scholar] [CrossRef] [Green Version]
- Guralnick, L.J.; Edwards, G.E.; Ku, M.S.B.; Hockema, B.; Franceschi, V.R. Photosynthetic and anatomical characteristics in the C4-crassulacean acid metabolism-cycling plant, Portulaca grandiflora. Funct. Plant Biol. 2002, 29, 763–773. [Google Scholar] [CrossRef]
- Sage, R.F. Are CAM and C4 photosynthesis incompatible? Funct. Plant Biol. 2002, 29, 775–785. [Google Scholar] [CrossRef]
- Chrisitin, P.A.; Arakaki, M.; Osborne, C.P.; Brautigam, A.; Sage, R.F.; Hibberd, J.M.; Kelly, S.; Covshoff, S.; Wong, G.K.S.; Hancock, L.; et al. Shared origins of key enzyme during the evolution of C4 photosynthesis and CAM metabolism. J. Exp. Bot. 2014, 65, 3609–3621. [Google Scholar] [CrossRef] [Green Version]
- Voznesenskaya, E.V.; Koteyeva, N.K.; Edwards, G.E.; Ocampo, G. Unique photsynthetic phenotypes in Portulaca (Portulacceae): C3-C4 intermediates and NAD-ME C4 species with Pilosoid-type Kranz anatomy. J. Exp. Bot. 2017, 68, 225–239. [Google Scholar] [CrossRef] [Green Version]
- Dengler, N.G.; Dengler, R.E.; Donnelly, P.M.; Filosa, M.F. Expression of the C4 pattern of photosynthetic enzyme accumulation during leaf development in Atriplex rosea (Chenopodiaceae). Am. J. Bot. 1995, 82, 319–327. [Google Scholar] [CrossRef]
- Von Willert, D.J.; EUer, B.M.; Werger, M.J.A.; Brinckmann, E.; Ihlenfeldt, H.-D. Life Strategies of Succulents in Deserts. With Special Reference to the Namib Desert. Cambridge Studies in Ecology; von Willert, D.J., Matyssek, R., Herppich, W.B., Eds.; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- Guralnick, L.J.; Marsh, C.; Asp, R.; Karjala, A. Physiological and anatomical aspects of CAM-cycling in Lewisia cotyledon var cotyledon (Portulacaceae). Madrono 2001, 48, 131–137. [Google Scholar]
- Hernandez-Gonzales, O.; Villarreal, O.B. Crassulacean acid metabolism photosynthesis in columnar cactus seedling during ontogeny: The effect of light on nocturnal acidity accumulation and chlorophyll fluorescence. Am. J. Bot. 2007, 94, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Winter, K.; Garcia, M.; Holtum, J.A.M. Drought-stress-induced up-regulation of CAM in seedlings of a tropical cactus, Opuntia elatior, operating predominantly in the C3 mode. J. Exp. Bot. 2011, 62, 4037–4042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayala-Cordero, G.; Terrazas, T.; Lopez-Mata, L.; Trejo, C. Morpho-anatomical changes and photosynthetic metabolism of Stenocereus beneckei seedlings under soil water deficit. J. Exp. Bot. 2006, 57, 3165–3174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Secorum, A.C.; de Souza, L.A. Morphology and anatomy of Rhipsalis cereuscula, Rhipsalis floccosa subsp. Hohenauensis and Lepismium cruciforme (Cactaceae) seedlings. Rev. Mex. Biodivers. 2011, 82, 131–143. [Google Scholar]
- Guralnick, L.J.; Ting, I.P. Seasonal response to drought and rewatering in Portulacaria afra (L.) Jacq. Oecologia 1987, 70, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Pyankov, V.I.; Voznesenskaya, E.V.; Kuz’min, A.N.; Ku, M.S.B.; Ganko, E.; Franceschi, V.R.; Black, C.C.; Edwards, G.E. Occurrence of C3 and C4 photosynthesis in coltyledons of leaves of Salsola species (Chenopodiaceae). Photosynth. Res. 2000, 63, 69–84. [Google Scholar] [CrossRef]
- Artyushera, E.G.; Edwards, G.E.; P’yankov, V.I. Photosynthesizing tissue development in C4 cotyledons of two Salsola species (Chenopodiaceae). Russ. J. Plant Physiol. 2003, 50, 4–18. [Google Scholar] [CrossRef]
- Keerberg, O.; Parnik, T.; Ivanova, H.; Bassuner, B.; Bauwe, H. C2 photosynthesis generates about 3-fold elevated leaf CO2 levels in the C3–C4 intermediate species Flaveria pubescens. J. Exp. Bot. 2014, 65, 3649–3656. [Google Scholar] [CrossRef] [Green Version]
- Lal, A.; Edwards, G.E. Analysis of inhibition of photosynthesis wunder waer stress in the C4 species Amaranthus cruentus and Zea mays: Electron transport, CO2 fixation, and carboxylation capacity. Australian J. Exp. Bot. 1996, 23, 403–412. [Google Scholar]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guralnick, L.J.; Gilbert, K.E.; Denio, D.; Antico, N. The Development of Crassulacean Acid Metabolism (CAM) Photosynthesis in Cotyledons of the C4 Species, Portulaca grandiflora (Portulacaceae). Plants 2020, 9, 55. https://doi.org/10.3390/plants9010055
Guralnick LJ, Gilbert KE, Denio D, Antico N. The Development of Crassulacean Acid Metabolism (CAM) Photosynthesis in Cotyledons of the C4 Species, Portulaca grandiflora (Portulacaceae). Plants. 2020; 9(1):55. https://doi.org/10.3390/plants9010055
Chicago/Turabian StyleGuralnick, Lonnie J., Kate E. Gilbert, Diana Denio, and Nicholas Antico. 2020. "The Development of Crassulacean Acid Metabolism (CAM) Photosynthesis in Cotyledons of the C4 Species, Portulaca grandiflora (Portulacaceae)" Plants 9, no. 1: 55. https://doi.org/10.3390/plants9010055
APA StyleGuralnick, L. J., Gilbert, K. E., Denio, D., & Antico, N. (2020). The Development of Crassulacean Acid Metabolism (CAM) Photosynthesis in Cotyledons of the C4 Species, Portulaca grandiflora (Portulacaceae). Plants, 9(1), 55. https://doi.org/10.3390/plants9010055




