Ascorbate and Thiamin: Metabolic Modulators in Plant Acclimation Responses
Abstract
:1. Introduction
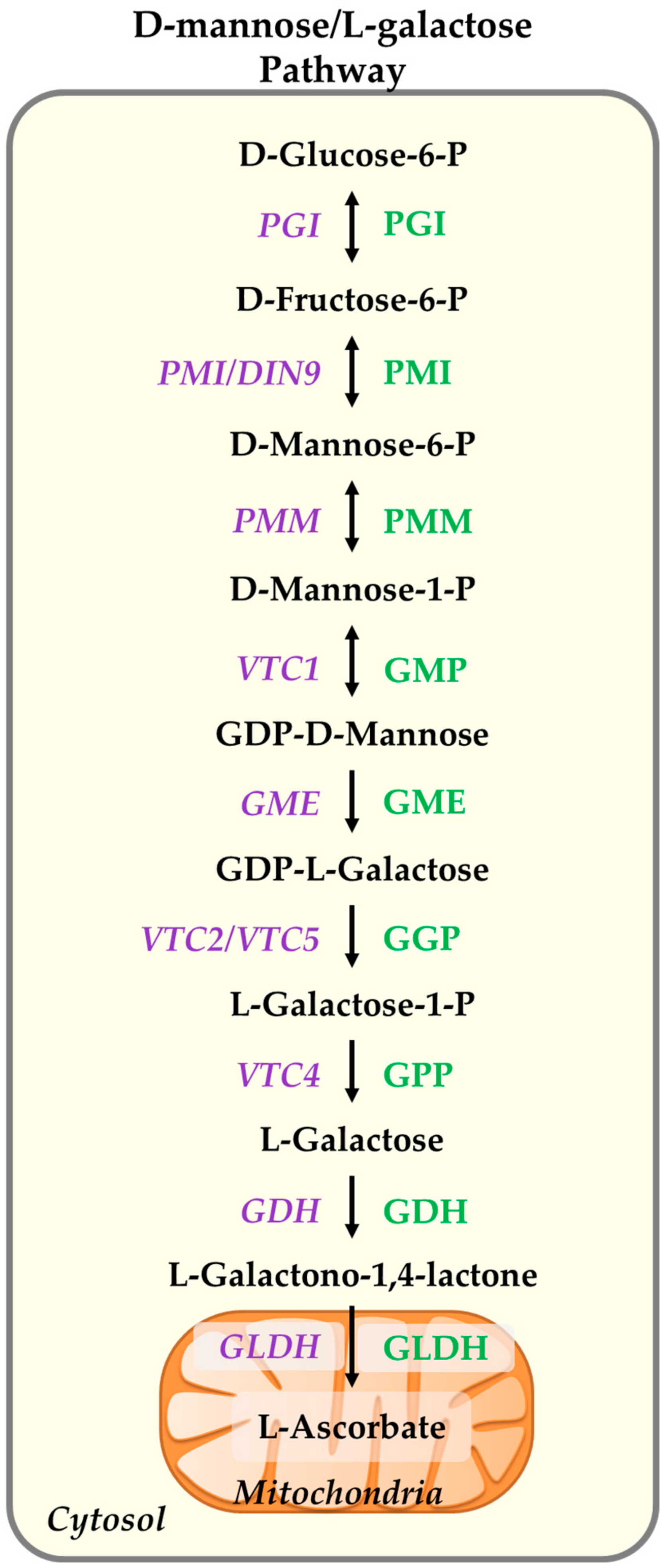
2. Ascorbate Biosynthesis and Subcellular Distribution
3. Role of Ascorbate in Light Acclimation
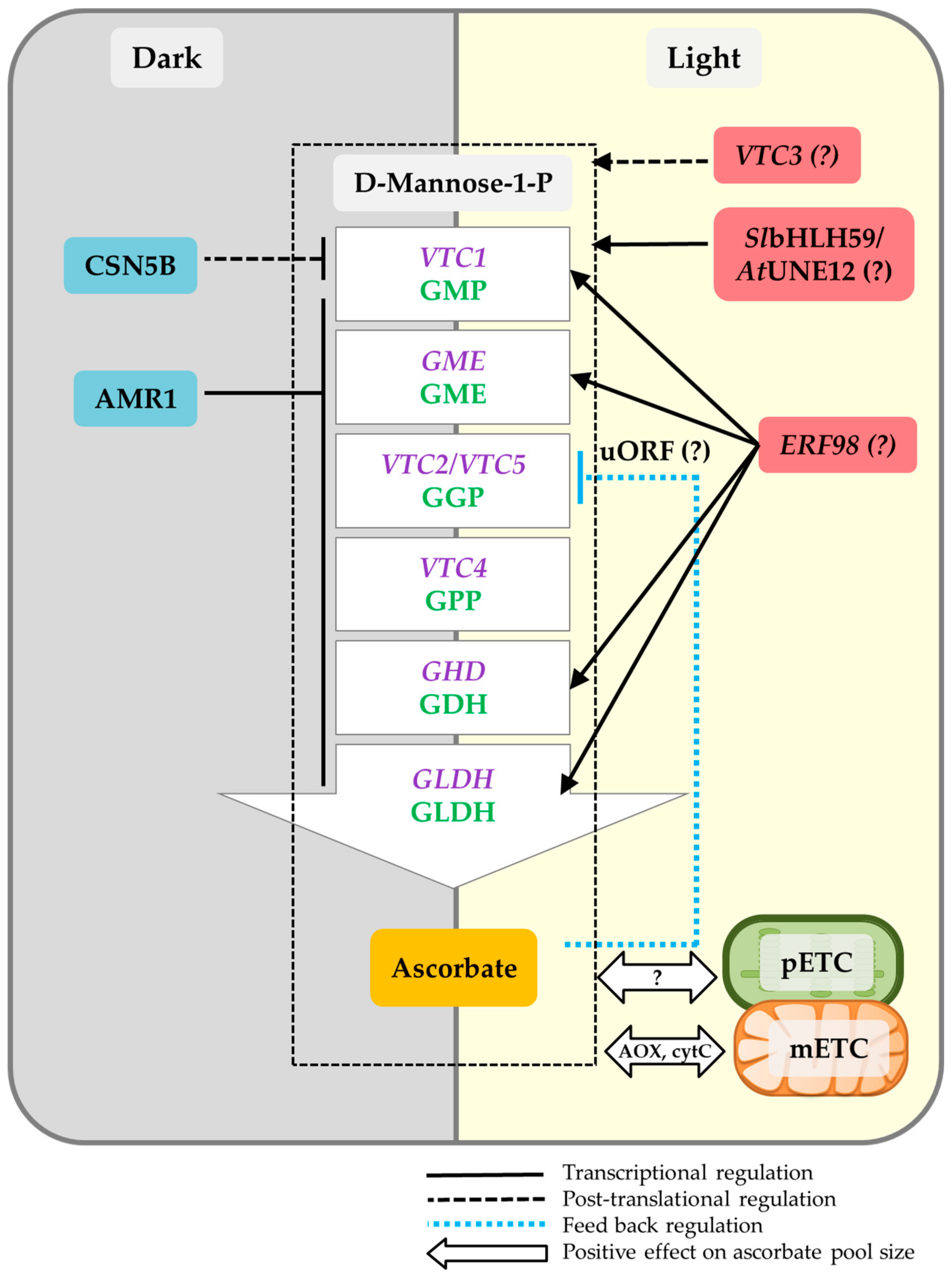
4. Light Regulation of Ascorbate
4.1. Transcriptional Regulation
4.2. Post-Translational Regulation
5. Role of Sugars, Photosynthesis, and Respiration in Light Regulation of Ascorbate
6. Role of Ascorbate in Photosynthesis Coordination of the Energy Systems of the Mitochondria and Chloroplast
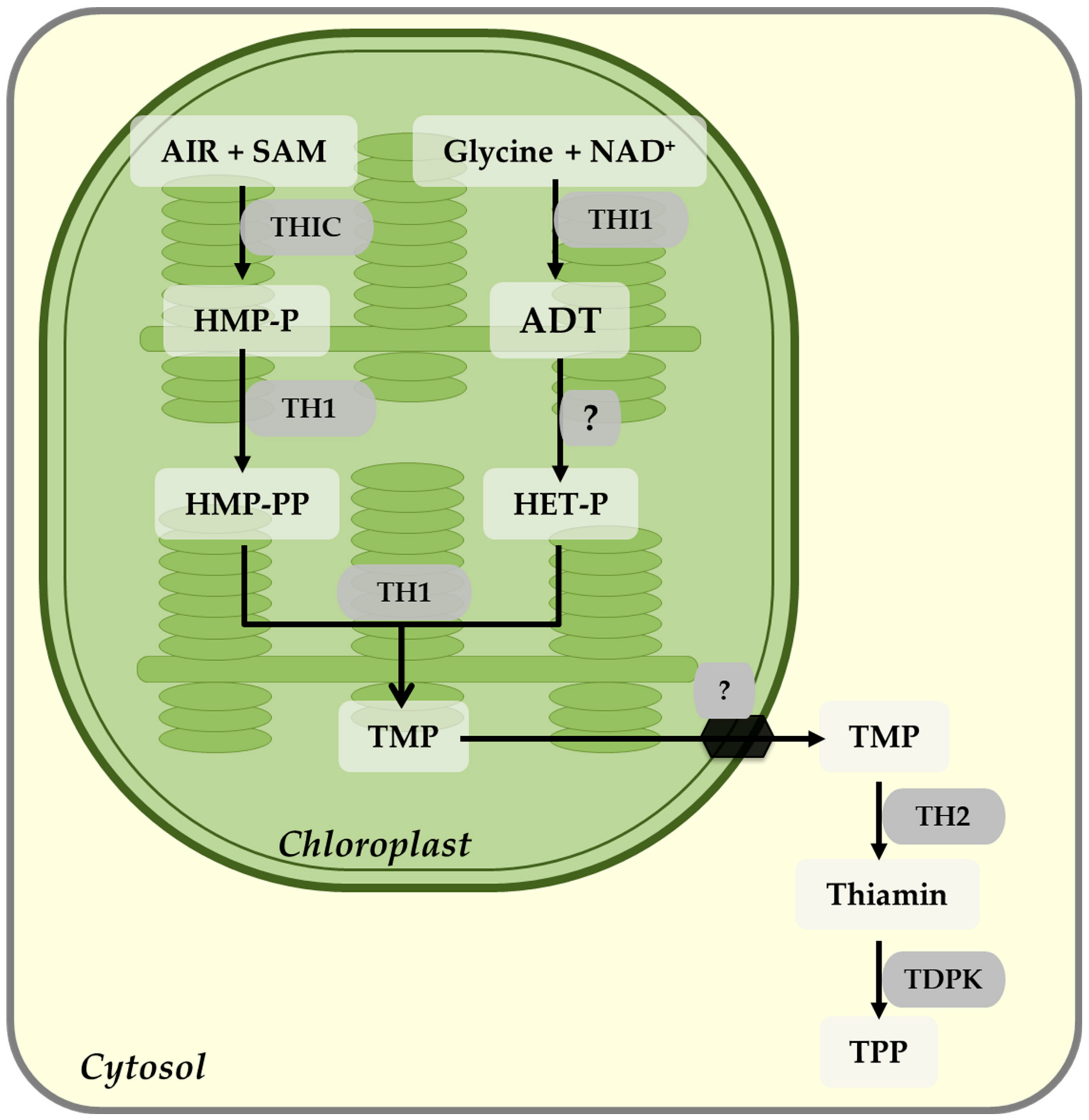
7. Thiamin Biosynthesis
Regulation of Thiamin Biosynthesis
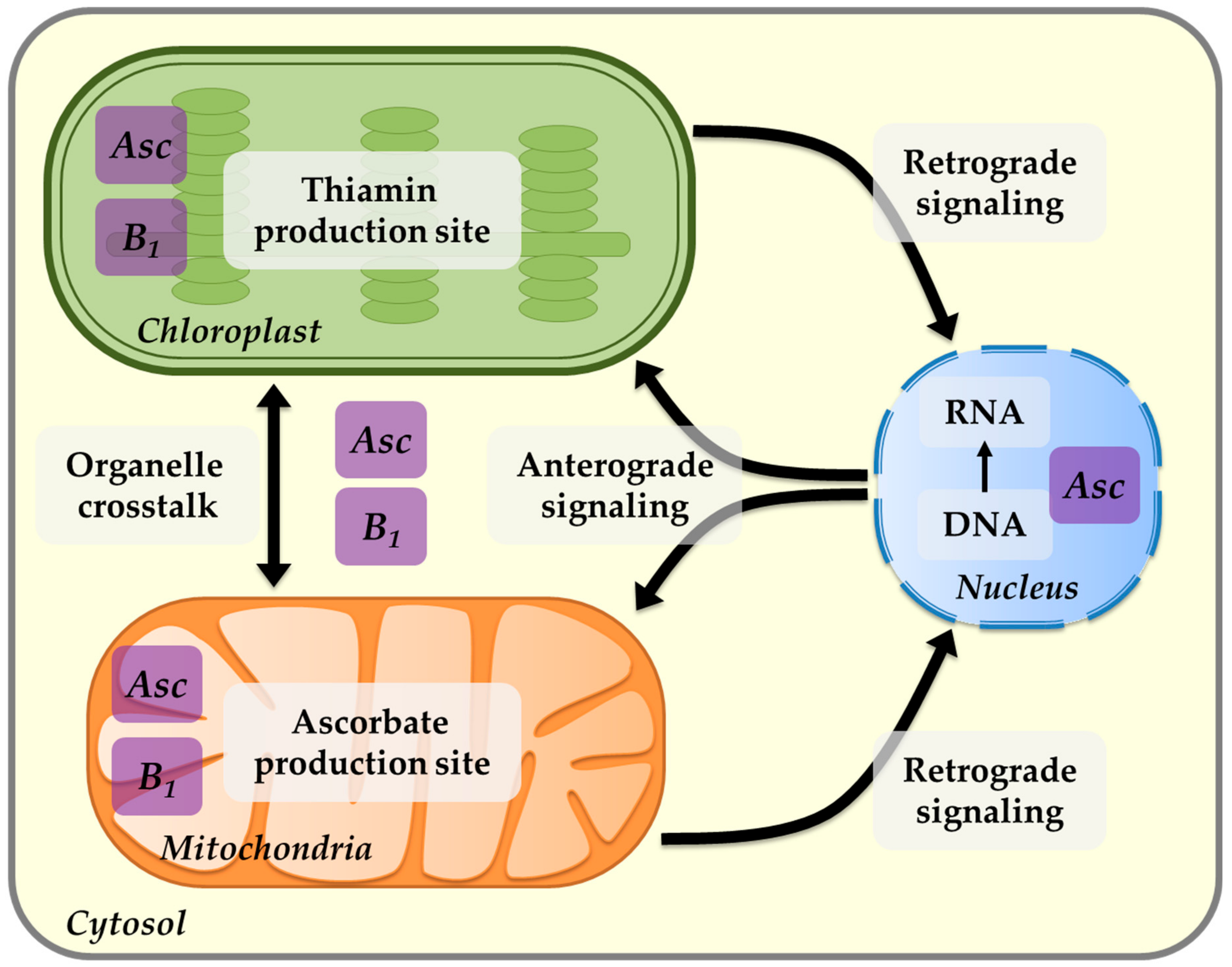
8. Thiamin and Chloroplast Interactions
9. Thiamin and Mitochondria Interactions
10. The Role of Thiamin in Mediating Plant Fitness and Acclimation
11. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Pesaresi, P.; Schneider, A.; Kleine, T.; Leister, D. Interorganellar communication. Curr. Opin. Plant Biol. 2007, 10, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Kleine, T.; Leister, D. Retrograde signaling: Organelles go networking. Biochim. Biophys. Acta 2016, 1857, 1313–1325. [Google Scholar] [CrossRef] [PubMed]
- Leister, D. Genomics-based dissection of the cross-talk of chloroplasts with the nucleus and mitochondria in arabidopsis. Gene 2005, 354, 110–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodson, J.D.; Chory, J. Coordination of gene expression between organellar and nuclear genomes. Nat. Rev. Genet. 2008, 9, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Bobik, K.; Burch-Smith, T.M. Chloroplast signaling within, between and beyond cells. Front. Plant Sci. 2015, 6, 781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koussevitzky, S.; Nott, A.; Mockler, T.C.; Hong, F.; Sachetto-Martins, G.; Surpin, M.; Lim, J.; Mittler, R.; Chory, J. Signals from chloroplasts converge to regulate nuclear gene expression. Science 2007, 316, 715–719. [Google Scholar] [CrossRef]
- Rhoads, D.M.; Subbaiah, C.C. Mitochondrial retrograde regulation in plants. Mitochondrion 2007, 7, 177–194. [Google Scholar] [CrossRef]
- Schwarzlander, M.; Finkemeier, I. Mitochondrial energy and redox signaling in plants. Antioxid. Redox Signal. 2013, 18, 2122–2144. [Google Scholar] [CrossRef]
- Dojcinovic, D.; Krosting, J.; Harris, A.J.; Wagner, D.J.; Rhoads, D.M. Identification of a region of the arabidopsis ataox1a promoter necessary for mitochondrial retrograde regulation of expression. Plant Mol. Biol. 2005, 58, 159–175. [Google Scholar] [CrossRef]
- Shapiguzov, A.; Nikkanen, L.; Fitzpatrick, D.; Vainonen, J.P.; Tiwari, A.; Gossens, R.; Alseekh, S.; Aarabi, F.; Blokhina, O.; Panzarová, K.; et al. Increased expression of mitochondrial dysfunction stimulon genes affects chloroplast redox status and photosynthetic electron transfer in arabidopsis. bioRxiv 2019. [Google Scholar] [CrossRef]
- Shapiguzov, A.; Vainonen, J.P.; Hunter, K.; Tossavainen, H.; Tiwari, A.; Jarvi, S.; Hellman, M.; Aarabi, F.; Alseekh, S.; Wybouw, B.; et al. Arabidopsis rcd1 coordinates chloroplast and mitochondrial functions through interaction with anac transcription factors. eLife 2019, 8. [Google Scholar] [CrossRef]
- Nunes-Nesi, A.; Araujo, W.L.; Fernie, A.R. Targeting mitochondrial metabolism and machinery as a means to enhance photosynthesis. Plant Physiol. 2011, 155, 101–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunes-Nesi, A.; Carrari, F.; Gibon, Y.; Sulpice, R.; Lytovchenko, A.; Fisahn, J.; Graham, J.; Ratcliffe, R.G.; Sweetlove, L.J.; Fernie, A.R. Deficiency of mitochondrial fumarase activity in tomato plants impairs photosynthesis via an effect on stomatal function. Plant J. 2007, 50, 1093–1106. [Google Scholar] [CrossRef] [PubMed]
- Nunes-Nesi, A.; Sulpice, R.; Gibon, Y.; Fernie, A.R. The enigmatic contribution of mitochondrial function in photosynthesis. J. Exp. Bot. 2008, 59, 1675–1684. [Google Scholar] [CrossRef] [Green Version]
- Chi, W.; Feng, P.; Ma, J.; Zhang, L. Metabolites and chloroplast retrograde signaling. Curr. Opin. Plant Biol. 2015, 25, 32–38. [Google Scholar] [CrossRef]
- Caldana, C.; Fernie, A.R.; Willmitzer, L.; Steinhauser, D. Unraveling retrograde signaling pathways: Finding candidate signaling molecules via metabolomics and systems biology driven approaches. Front. Plant Sci. 2012, 3, 267. [Google Scholar] [CrossRef] [Green Version]
- Asensi-Fabado, M.A.; Munne-Bosch, S. Vitamins in plants: Occurrence, biosynthesis and antioxidant function. Trends Plant Sci. 2010, 15, 582–592. [Google Scholar] [CrossRef]
- Smirnoff, N. Ascorbic acid metabolism and functions: A comparison of plants and mammals. Free Radic. Biol. Med. 2018, 122, 116–129. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, N.; Mittler, R. Reactive oxygen species and temperature stresses: A delicate balance between signalling and destruction. Physiol. Plant. 2006, 126, 41–51. [Google Scholar] [CrossRef]
- Schmitz, J.; Heinrichs, L.; Scossa, F.; Fernie, A.R.; Oelze, M.-L.; Dietz, K.-J.; Rothbart, M.; Grimm, B.; Flügge, U.-I.; Häusler, R.E. The essential role of sugar metabolism in the acclimation response of arabidopsis thaliana to high light intensities. J. Exp. Bot. 2014, 65, 1619–1636. [Google Scholar] [CrossRef] [PubMed]
- Streb, P.; Aubert, S.; Gout, E.; Bligny, R. Reversibility of cold- and light-stress tolerance and accompanying changes of metabolite and antioxidant levels in the two high mountain plant species soldanella alpina and ranunculus glacialis. J. Exp. Bot. 2003, 54, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Zechmann, B. Subcellular distribution of ascorbate in plants. Plant Signal. Behav. 2014, 6, 360–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bocobza, S.E.; Aharoni, A. Switching the light on plant riboswitches. Trends Plant Sci. 2008, 13, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Bocobza, S.E.; Malitsky, S.; Araújo, W.L.; Nunes-Nesi, A.; Meir, S.; Shapira, M.; Fernie, A.R.; Aharoni, A. Orchestration of thiamin biosynthesis and central metabolism by combined action of the thiamin pyrophosphate riboswitch and the circadian clock in arabidopsis. Plant Cell 2013, 25, 288–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khozaei, M.; Fisk, S.; Lawson, T.; Gibon, Y.; Sulpice, R.; Stitt, M.; Lefebvre, S.C.; Raines, C.A. Overexpression of plastid transketolase in tobacco results in a thiamine auxotrophic phenotype. Plant Cell 2015, 27, 432–447. [Google Scholar] [CrossRef] [Green Version]
- Tylicki, A.; Łotowski, Z.; Siemieniuk, M.; Ratkiewicz, A. Thiamine and selected thiamine antivitamins—Biological activity and methods of synthesis. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef] [Green Version]
- Sayed, S.A.; Gadallah, M.A.A. Effects of shoot and root application of thiamin on salt-stressed sunflower plants. Plant Growth Regul. 2002, 36, 71–80. [Google Scholar] [CrossRef]
- Rapala-Kozik, M.; Kowalska, E.; Ostrowska, K. Modulation of thiamine metabolism in zea mays seedlings under conditions of abiotic stress. J. Exp. Bot. 2008, 59, 4133–4143. [Google Scholar] [CrossRef] [Green Version]
- Rapala-Kozik, M.; Wolak, N.; Kujda, M.; Banas, A.K. The upregulation of thiamine (vitamin b1) biosynthesis in arabidopsis thaliana seedlings under salt and osmotic stress conditions is mediated by abscisic acid at the early stages of this stress response. BMC Plant Biol. 2012, 12, 2. [Google Scholar] [CrossRef] [Green Version]
- Tunc-Ozdemir, M.; Miller, G.; Song, L.; Kim, J.; Sodek, A.; Koussevitzky, S.; Misra, A.N.; Mittler, R.; Shintani, D. Thiamin confers enhanced tolerance to oxidative stress in arabidopsis. Plant Physiol. 2009, 151, 421–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosado-Souza, L.; Proost, S.; Moulin, M.; Bergmann, S.; Bocobza, S.E.; Aharoni, A.; Fitzpatrick, T.B.; Mutwil, M.; Fernie, A.R.; Obata, T. Appropriate thiamin pyrophosphate levels are required for acclimation to changes in photoperiod. Plant Physiol. 2019, 180, 185–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subki, A.; Abidin, A.; Balia Yusof, Z.N. The role of thiamine in plants and current perspectives in crop improvement. In B Group Vitamins—Current Uses and Perspectives; IntechOpen: London, UK, 2018; pp. 33–44. [Google Scholar]
- Lorence, A.; Chevone, B.I.; Mendes, P.; Nessler, C.L. Myo-inositol oxygenase offers a possible entry point into plant ascorbate biosynthesis. Plant Physiol. 2004, 134, 1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolucka, B.A.; Van Montagu, M. Gdp-mannose 3′,5′-epimerase forms gdp-l-gulose, a putative intermediate for the de novo biosynthesis of vitamin c in plants. J. Biol. Chem. 2003, 278, 47483–47490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agius, F.; Gonzalez-Lamothe, R.; Caballero, J.L.; Munoz-Blanco, J.; Botella, M.A.; Valpuesta, V. Engineering increased vitamin c levels in plants by overexpression of a d-galacturonic acid reductase. Nat. Biotechnol. 2003, 21, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Simkin, A.J. Genetic engineering for global food security: Photosynthesis and biofortification. Plants (Basel) 2019, 8, E586. [Google Scholar] [CrossRef] [Green Version]
- Gallie, D.R. The role of l-ascorbic acid recycling in responding to environmental stress and in promoting plant growth. J. Exp. Bot. 2013, 64, 433–443. [Google Scholar] [CrossRef] [Green Version]
- Szarka, A.; Bánhegyi, G.; Asard, H. The inter-relationship of ascorbate transport, metabolism and mitochondrial, plastidic respiration. Antioxid. Redox Signal. 2013, 19, 1036–1044. [Google Scholar] [CrossRef] [Green Version]
- Fernie, A.R.; Tóth, S.Z. Identification of the elusive chloroplast ascorbate transporter extends the substrate specificity of the pht family. Mol. Plant 2015, 8, 674–676. [Google Scholar] [CrossRef] [Green Version]
- Miyaji, T.; Kuromori, T.; Takeuchi, Y.; Yamaji, N.; Yokosho, K.; Shimazawa, A.; Sugimoto, E.; Omote, H.; Ma, J.F.; Shinozaki, K.; et al. Atpht4; 4 is a chloroplast-localized ascorbate transporter in arabidopsis. Nat. Commun. 2015, 6, 5928. [Google Scholar] [CrossRef] [Green Version]
- Dietz, K.-J. Efficient high light acclimation involves rapid processes at multiple mechanistic levels. J. Exp. Bot. 2015, 66, 2401–2414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsharafa, K.; Vogel, M.O.; Oelze, M.-L.; Moore, M.; Stingl, N.; König, K.; Friedman, H.; Mueller, M.J.; Dietz, K.-J. Kinetics of retrograde signalling initiation in the high light response of arabidopsis thaliana. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2014, 369, 20130424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mullineaux, P.M.; Exposito-Rodriguez, M.; Laissue, P.P.; Smirnoff, N. Ros-dependent signalling pathways in plants and algae exposed to high light: Comparisons with other eukaryotes. Free Radic. Biol. Med. 2018, 122, 52–64. [Google Scholar] [CrossRef]
- Müller, P.; Li, X.-P.; Niyogi, K.K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001, 125, 1558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller-Moulé, P.; Havaux, M.; Niyogi, K.K. Zeaxanthin deficiency enhances the high light sensitivity of an ascorbate-deficient mutant of arabidopsis. Plant Physiol. 2003, 133, 748–760. [Google Scholar] [CrossRef] [Green Version]
- Müller-Moulé, P.; Conklin, P.L.; Niyogi, K.K. Ascorbate deficiency can limit violaxanthin de-epoxidase activity in vivo. Plant Physiol. 2002, 128, 970–977. [Google Scholar] [CrossRef] [Green Version]
- Giacomelli, L.; Rudella, A.; van Wijk, K.J. High light response of the thylakoid proteome in arabidopsis wild type and the ascorbate-deficient mutant vtc2-2. A comparative proteomics study. Plant Physiol. 2006, 141, 685–701. [Google Scholar] [CrossRef] [Green Version]
- Berwal, M.K.; Ram, C. Superoxide dismutase: A stable biochemical marker for abiotic stress tolerance in higher plants. In Abiotic and Biotic Stress in Plants; IntechOpen: London, UK, 2018; p. 10. [Google Scholar]
- Schroda, M.; Vallon, O.; Wollman, F.A.; Beck, C.F. A chloroplast-targeted heat shock protein 70 (hsp70) contributes to the photoprotection and repair of photosystem ii during and after photoinhibition. Plant Cell 1999, 11, 1165–1178. [Google Scholar] [CrossRef] [Green Version]
- Li, X.P.; Bjorkman, O.; Shih, C.; Grossman, A.R.; Rosenquist, M.; Jansson, S.; Niyogi, K.K. A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 2000, 403, 391–395. [Google Scholar] [CrossRef]
- Nicol, L.; Nawrocki, W.J.; Croce, R. Disentangling the sites of non-photochemical quenching in vascular plants. Nat. Plants 2019, 5, 1177–1183. [Google Scholar] [CrossRef]
- Giacomelli, L.; Masi, A.; Ripoll, D.R.; Lee, M.J.; Van Wijk, K.J. Arabidopsis thaliana deficient in two chloroplast ascorbate peroxidases shows accelerated light-induced necrosis when levels of cellular ascorbate are low. Plant Mol. Biol. 2007, 65, 627–644. [Google Scholar] [CrossRef] [PubMed]
- Page, M.; Sultana, N.; Paszkiewicz, K.; Florance, H.; Smirnoff, N. The influence of ascorbate on anthocyanin accumulation during high light acclimation in arabidopsis thaliana: Further evidence for redox control of anthocyanin synthesis. Plant Cell Environ. 2012, 35, 388–404. [Google Scholar] [CrossRef] [PubMed]
- Vanderauwera, S.; Zimmermann, P.; Rombauts, S.; Vandenabeele, S.; Langebartels, C.; Gruissem, W.; Inzé, D.; Van Breusegem, F. Genome-wide analysis of hydrogen peroxide-regulated gene expression in arabidopsis reveals a high light-induced transcriptional cluster involved in anthocyanin biosynthesis. Plant Physiol. 2005, 139, 806–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dowdle, J.; Ishikawa, T.; Gatzek, S.; Rolinski, S.; Smirnoff, N. Two genes in arabidopsis thaliana encoding gdp-l-galactose phosphorylase are required for ascorbate biosynthesis and seedling viability. Plant J. 2007, 52, 673–689. [Google Scholar] [CrossRef]
- Laing, W.; Norling, C.; Brewster, D.; Wright, M.; Bulley, S. Ascorbate concentration in arabidopsis thaliana and expression of ascorbate related genes using rnaseq in response to light and the diurnal cycle. bioRxiv 2017. [Google Scholar] [CrossRef] [Green Version]
- Müller-Moulé, P. An expression analysis of the ascorbate biosynthesis enzyme vtc2. Plant Mol. Biol. 2008, 68, 31–41. [Google Scholar] [CrossRef]
- Yabuta, Y.; Mieda, T.; Rapolu, M.; Nakamura, A.; Motoki, T.; Maruta, T.; Yoshimura, K.; Ishikawa, T.; Shigeoka, S. Light regulation of ascorbate biosynthesis is dependent on the photosynthetic electron transport chain but independent of sugars in arabidopsis. J. Exp. Bot. 2007, 58, 2661–2671. [Google Scholar] [CrossRef] [Green Version]
- Bulley, S.M.; Rassam, M.; Hoser, D.; Otto, W.; Schünemann, N.; Wright, M.; MacRae, E.; Gleave, A.; Laing, W. Gene expression studies in kiwifruit and gene over-expression in arabidopsis indicates that gdp-l-galactose guanyltransferase is a major control point of vitamin c biosynthesis. J. Exp. Bot. 2009, 60, 765–778. [Google Scholar] [CrossRef] [Green Version]
- Laing, W.A.; Wright, M.A.; Cooney, J.; Bulley, S.M. The missing step of the l-galactose pathway of ascorbate biosynthesis in plants, an l-galactose guanyltransferase, increases leaf ascorbate content. Proc. Natl. Acad. Sci. USA 2007, 104, 9534–9539. [Google Scholar] [CrossRef] [Green Version]
- Mellidou, I.; Chagné, D.; Laing, W.A.; Keulemans, J.; Davey, M.W. Allelic variation in paralogs of gdp-l-galactose phosphorylase is a major determinant of vitamin c concentrations in apple fruit. Plant Physiol. 2012, 160, 1613. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Liang, D.; Li, M.; Ma, F. Light and abiotic stresses regulate the expression of gdp-l-galactose phosphorylase and levels of ascorbic acid in two kiwifruit genotypes via light-responsive and stress-inducible cis-elements in their promoters. Planta 2013, 238, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Massot, C.; Stevens, R.; Génard, M.; Longuenesse, J.-J.; Gautier, H. Light affects ascorbate content and ascorbate-related gene expression in tomato leaves more than in fruits. Planta 2012, 235, 153–163. [Google Scholar] [CrossRef]
- Urzica, E.I.; Adler, L.N.; Page, M.D.; Linster, C.L.; Arbing, M.A.; Casero, D.; Pellegrini, M.; Merchant, S.S.; Clarke, S.G. Impact of oxidative stress on ascorbate biosynthesis in chlamydomonas via regulation of the vtc2 gene encoding a gdp-l-galactose phosphorylase. J. Biol. Chem. 2012, 287, 14234–14245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidal-Meireles, A.; Neupert, J.; Zsigmond, L.; Rosado-Souza, L.; Kovács, L.; Nagy, V.; Galambos, A.; Fernie, A.R.; Bock, R.; Tóth, S.Z. Regulation of ascorbate biosynthesis in green algae has evolved to enable rapid stress-induced response via the vtc2 gene encoding gdp-l-galactose phosphorylase. New Phytol. 2017, 214, 668–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenech, M.; Amaya, I.; Valpuesta, V.; Botella, M.A. Vitamin c content in fruits: Biosynthesis and regulation. Front. Plant Sci. 2018, 9, 2006. [Google Scholar] [CrossRef] [Green Version]
- Smirnoff, N.; Wheeler, G.L. Ascorbic acid in plants: Biosynthesis and function. Crit. Rev. Biochem. Mol. Biol. 2000, 35, 291–314. [Google Scholar] [CrossRef]
- Bartoli, C.G.; Yu, J.; Gomez, F.; Fernandez, L.; McIntosh, L.; Foyer, C.H. Inter-relationships between light and respiration in the control of ascorbic acid synthesis and accumulation in arabidopsis thaliana leaves. J. Exp. Bot. 2006, 57, 1621–1631. [Google Scholar] [CrossRef]
- Leferink, N.G.; van Duijn, E.; Barendregt, A.; Heck, A.J.; van Berkel, W.J. Galactonolactone dehydrogenase requires a redox-sensitive thiol for optimal production of vitamin c. Plant Physiol. 2009, 150, 596–605. [Google Scholar] [CrossRef] [Green Version]
- de Pinto, M.C.; Locato, V.; Paradiso, A.; De Gara, L. Role of redox homeostasis in thermo-tolerance under a climate change scenario. Ann. Bot. 2015, 116, 487–496. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.T.; Zhang, X.H.; Wang, Y.Z.; Cai, M.L.; Li, M.; Zhang, T.-J.; Peng, C. Identification of a gldh-overexpressing arabidopsis mutant and its responses to high-light stress. Photosynthetica 2018, 57. [Google Scholar] [CrossRef] [Green Version]
- Ntagkas, N.; Woltering, E.J.; Marcelis, L.F.M. Light regulates ascorbate in plants: An integrated view on physiology and biochemistry. Environ. Exp. Bot. 2018, 147, 271–280. [Google Scholar] [CrossRef]
- Fukunaga, K.; Fujikawa, Y.; Esaka, M. Light regulation of ascorbic acid biosynthesis in rice via light responsive cis-elements in genes encoding ascorbic acid biosynthetic enzymes. Biosci. Biotechnol. Biochem. 2010, 74, 888–891. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Badejo, A.A.; Shibata, H.; Sawa, Y.; Maruta, T.; Shigeoka, S.; Page, M.; Smirnoff, N.; Ishikawa, T. Expression analysis of the vtc2 and vtc5 genes encoding gdp-l-galactose phosphorylase, an enzyme involved in ascorbate biosynthesis, in arabidopsis thaliana. Biosci. Biotech. Biochem. 2011, 75, 1783–1788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Lorence, A.; Gruszewski, H.A.; Chevone, B.I.; Nessler, C.L. Amr1, an arabidopsis gene that coordinately and negatively regulates the mannose/l-galactose ascorbic acid biosynthetic pathway. Plant Physiol. 2009, 150, 942–950. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Wang, J.; Zhang, R.; Huang, R. The ethylene response factor aterf98 enhances tolerance to salt through the transcriptional activation of ascorbic acid synthesis in arabidopsis. Plant J. 2012, 71, 273–287. [Google Scholar] [CrossRef]
- Ye, J.; Li, W.; Ai, G.; Li, C.; Liu, G.; Chen, W.; Wang, B.; Wang, W.; Lu, Y.; Zhang, J.; et al. Genome-wide association analysis identifies a natural variation in basic helix-loop-helix transcription factor regulating ascorbate biosynthesis via d-mannose/l-galactose pathway in tomato. PLoS Genet. 2019, 15, e1008149. [Google Scholar] [CrossRef]
- Bulley, S.; Laing, W. The regulation of ascorbate biosynthesis. Curr. Opin. Plant Biol. 2016, 33, 15–22. [Google Scholar] [CrossRef]
- Mellidou, I.; Kanellis, A.K. Genetic control of ascorbic acid biosynthesis and recycling in horticultural crops. Front. Chem. 2017, 5, 50. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Yu, Y.; Zhang, Z.; Quan, R.; Zhang, H.; Ma, L.; Deng, X.W.; Huang, R. Arabidopsis csn5b interacts with vtc1 and modulates ascorbic acid synthesis. Plant Cell 2013, 25, 625–636. [Google Scholar] [CrossRef] [Green Version]
- Laing, W.A.; Martinez-Sanchez, M.; Wright, M.A.; Bulley, S.M.; Brewster, D.; Dare, A.P.; Rassam, M.; Wang, D.; Storey, R.; Macknight, R.C.; et al. An upstream open reading frame is essential for feedback regulation of ascorbate biosynthesis in arabidopsis. Plant Cell 2015, 27, 772–786. [Google Scholar] [CrossRef] [Green Version]
- Conklin, P.L.; De Paolo, D.; Wintle, B.; Schatz, C.; Buckenmeyer, G. Identification of arabidopsis vtc3 as a putative and unique dual function protein kinase: Protein phosphatase involved in the regulation of the ascorbic acid pool in plants. J. Exp. Bot. 2013, 64, 2793–2804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ntagkas, N.; Woltering, E.; Bouras, S.; de Vos, R.C.H.; Dieleman, J.A.; Nicole, C.C.S.; Labrie, C.; Marcelis, L.F.M. Light-induced vitamin c accumulation in tomato fruits is independent of carbohydrate availability. Plants (Basel) 2019, 8, E86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massot, C.; Génard, M.; Stevens, R.; Gautier, H. Fluctuations in sugar content are not determinant in explaining variations in vitamin c in tomato fruit. Plant Physiol. Biochem. 2010, 48, 751–757. [Google Scholar] [CrossRef] [Green Version]
- Ntagkas, N.; Woltering, E.; Nicole, C.; Labrie, C.; Marcelis, L.F.M. Light regulation of vitamin c in tomato fruit is mediated through photosynthesis. Environ. Exp. Bot. 2019, 158, 180–188. [Google Scholar] [CrossRef]
- Bartoli, C.G.; Pastori, G.M.; Foyer, C.H. Ascorbate biosynthesis in mitochondria is linked to the electron transport chain between complexes iii and iv. Plant Physiol. 2000, 123, 335–343. [Google Scholar] [CrossRef] [Green Version]
- Schimmeyer, J.; Bock, R.; Meyer, E.H. L-galactono-1,4-lactone dehydrogenase is an assembly factor of the membrane arm of mitochondrial complex i in arabidopsis. Plant Mol. Biol. 2016, 90, 117–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del-Saz, N.F.; Ribas-Carbo, M.; McDonald, A.E.; Lambers, H.; Fernie, A.R.; Florez-Sarasa, I. An In Vivo Perspective of the Role(s) of the Alternative Oxidase Pathway; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; Volume 23, pp. 206–219. [Google Scholar]
- Chew, O.; Whelan, J.; Millar, A.H. Molecular definition of the ascorbate-glutathione cycle in arabidopsis mitochondria reveals dual targeting of antioxidant defenses in plants. J. Biol. Chem. 2003, 278, 46869–46877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karpinska, B.; Zhang, K.; Rasool, B.; Pastok, D.; Morris, J.; Verrall, S.R.; Hedley, P.E.; Hancock, R.D.; Foyer, C.H. The redox state of the apoplast influences the acclimation of photosynthesis and leaf metabolism to changing irradiance. Plant Cell Environ. 2018, 41, 1083–1097. [Google Scholar] [CrossRef]
- Müller-Moulé, P.; Golan, T.; Niyogi, K.K. Ascorbate-deficient mutants of arabidopsis grow in high light despite chronic photooxidative stress. Plant Physiol. 2004, 134, 1163. [Google Scholar] [CrossRef] [Green Version]
- Asada, K. The water-water cycle as alternative photon and electron sinks. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2000, 355, 1419–1431. [Google Scholar] [CrossRef] [Green Version]
- Foyer, C.H. The role of ascorbate in plants, interactions with photosynthesis, and regulatory significance. Curr. Top. Plant Physiol. 1991, 6, 131–144. [Google Scholar]
- Heber, U. Irrungen, wirrungen? The mehler reaction in relation to cyclic electron transport in c3 plants. In Discoveries in Photosynthesis; Govindjee, G., Beatty, J.T., Gest, H., Allen, J.F., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 551–559. [Google Scholar]
- Mano, J.I.; Hideg, E.; Asada, K. Ascorbate in thylakoid lumen functions as an alternative electron donor to photosystem ii and photosystem i. Arch. Biochem. Biophys. 2004, 429, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Tóth, S.Z.; Puthur, J.T.; Nagy, V.; Garab, G. Experimental evidence for ascorbate-dependent electron transport in leaves with inactive oxygen-evolving complexes. Plant Physiol. 2009, 149, 1568. [Google Scholar] [CrossRef] [PubMed]
- Kiddle, G.; Pastori, G.M.; Bernard, S.; Pignocchi, C.; Antoniw, J.; Verrier, P.J.; Foyer, C.H. Effects of leaf ascorbate content on defense and photosynthesis gene expression in arabidopsis thaliana. Antioxid. Redox Signal. 2003, 5, 23–32. [Google Scholar] [CrossRef]
- Plumb, W.; Townsend, A.J.; Rasool, B.; Alomrani, S.; Razak, N.; Karpinska, B.; Ruban, A.V.; Foyer, C.H. Ascorbate-mediated regulation of growth, photoprotection, and photoinhibition in arabidopsis thaliana. J. Exp. Bot. 2018, 69, 2823–2835. [Google Scholar] [CrossRef]
- Nunes-Nesi, A.; Carrari, F.; Lytovchenko, A.; Smith, A.M.; Loureiro, M.E.; Ratcliffe, R.G.; Sweetlove, L.J.; Fernie, A.R. Enhanced photosynthetic performance and growth as a consequence of decreasing mitochondrial malate dehydrogenase activity in transgenic tomato plants. Plant Physiol. 2005, 137, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Alhagdow, M.; Mounet, F.; Gilbert, L.; Nunes-Nesi, A.; Garcia, V.; Just, D.; Petit, J.; Beauvoit, B.; Fernie, A.R.; Rothan, C.; et al. Silencing of the mitochondrial ascorbate synthesizing enzyme l-galactono-1,4-lactone dehydrogenase affects plant and fruit development in tomato. Plant Physiol. 2007, 145, 1408–1422. [Google Scholar] [CrossRef] [Green Version]
- Tomaz, T.; Bagard, M.; Pracharoenwattana, I.; Linden, P.; Lee, C.P.; Carroll, A.J.; Stroher, E.; Smith, S.M.; Gardestrom, P.; Millar, A.H. Mitochondrial malate dehydrogenase lowers leaf respiration and alters photorespiration and plant growth in arabidopsis. Plant Physiol. 2010, 154, 1143–1157. [Google Scholar] [CrossRef] [Green Version]
- Bocobza, S.; Adato, A.; Mandel, T.; Shapira, M.; Nudler, E.; Aharoni, A. Riboswitch-dependent gene regulation and its evolution in the plant kingdom. Genes Dev. 2007, 21, 2874–2879. [Google Scholar] [CrossRef] [Green Version]
- Raschke, M.; Burkle, L.; Muller, N.; Nunes-Nesi, A.; Fernie, A.R.; Arigoni, D.; Amrhein, N.; Fitzpatrick, T.B. Vitamin b1 biosynthesis in plants requires the essential iron sulfur cluster protein, thic. Proc. Natl. Acad. Sci. USA 2007, 104, 19637–19642. [Google Scholar] [CrossRef] [Green Version]
- Kong, D.; Zhu, Y.; Wu, H.; Cheng, X.; Liang, H.; Ling, H.Q. Atthic, a gene involved in thiamine biosynthesis in arabidopsis thaliana. Cell Res. 2008, 18, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Machado, C.R.; de Oliveira, R.L.; Boiteux, S.; Praekelt, U.M.; Meacock, P.A.; Menck, C.F. Thi1, a thiamine biosynthetic gene in arabidopsis thaliana, complements bacterial defects in DNA repair. Plant Mol. Biol. 1996, 31, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Abeydeera, N.D.; Bale, S.; Pai, P.J.; Dorrestein, P.C.; Russell, D.H.; Ealick, S.E.; Begley, T.P. Saccharomyces cerevisiae thi4p is a suicide thiamine thiazole synthase. Nature 2011, 478, 542–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, A.; Jurgenson, C.T.; Schroeder, F.C.; Ealick, S.E.; Begley, T.P. Biosynthesis of thiamin thiazole in eukaryotes: Conversion of nad to an advanced intermediate. J. Am. Chem. Soc. 2007, 129, 2914–2922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, A.; Schroeder, F.C.; Jurgenson, C.T.; Ealick, S.E.; Begley, T.P. Biosynthesis of the thiamin-thiazole in eukaryotes: Identification of a thiazole tautomer intermediate. J. Am. Chem. Soc. 2008, 130, 11394–11398. [Google Scholar] [CrossRef]
- Ajjawi, I.; Tsegaye, Y.; Shintani, D. Determination of the genetic, molecular, and biochemical basis of the arabidopsis thaliana thiamin auxotroph th1. Arch. Biochem. Biophys. 2007, 459, 107–114. [Google Scholar] [CrossRef]
- Hsieh, W.-Y.; Liao, J.-C.; Wang, H.-T.; Hung, T.-H.; Tseng, C.-C.; Chung, T.-Y.; Hsieh, M.-H. The arabidopsis thiamin-deficient mutant pale green1 lacks thiamin monophosphate phosphatase of the vitamin b1 biosynthesis pathway. Plant J. 2017, 91, 145–157. [Google Scholar] [CrossRef] [Green Version]
- Dong, W.; Stockwell, V.O.; Goyer, A. Enhancement of thiamin content in arabidopsis thaliana by metabolic engineering. Plant Cell Physiol. 2015, 56, 2285–2296. [Google Scholar] [CrossRef] [Green Version]
- Colinas, M.; Fitzpatrick, T.B. Natures balancing act: Examining biosynthesis de novo, recycling and processing damaged vitamin b metabolites. Curr. Opin. Plant Biol. 2015, 25, 98–106. [Google Scholar] [CrossRef]
- Goyer, A. Thiamin biofortification of crops. Curr. Opin. Biotechnol. 2016, 44, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Colinas, M.; Eisenhut, M.; Tohge, T.; Pesquera, M.; Fernie, A.R.; Weber, A.P.M.; Fitzpatrick, T.B. Balancing of b6 vitamers is essential for plant development and metabolism in arabidopsis. Plant Cell 2016, 28, 439–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goyer, A. Thiamine in plants: Aspects of its metabolism and functions. Phytochemistry 2010, 71, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Wachter, A.; Tunc-Ozdemir, M.; Grove, B.C.; Green, P.J.; Shintani, D.K.; Breaker, R.R. Riboswitch control of gene expression in plants by splicing and alternative 3’ end processing of mrnas. Plant Cell 2007, 19, 3437–3450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenwick, M.K.; Mehta, A.P.; Zhang, Y.; Abdelwahed, S.H.; Begley, T.P.; Ealick, S.E. Non-canonical active site architecture of the radical sam thiamin pyrimidine synthase. Nat. Commun. 2015, 6, 6480. [Google Scholar] [CrossRef] [Green Version]
- Rapala-Kozik, M.; Olczak, M.; Ostrowska, K.; Starosta, A.; Kozik, A. Molecular characterization of the thi3 gene involved in thiamine biosynthesis in zea mays: Cdna sequence and enzymatic and structural properties of the recombinant bifunctional protein with 4-amino-5-hydroxymethyl-2-methylpyrimidine (phosphate) kinase and thiamine monophosphate synthase activities. Biochem. J. 2007, 408, 149–159. [Google Scholar]
- Li, C.-L.; Wang, M.; Wu, X.-M.; Chen, D.-H.; Lv, H.-J.; Shen, J.-L.; Qiao, Z.; Zhang, W. Thi1, a thiamine thiazole synthase, interacts with ca(2+)-dependent protein kinase cpk33 and modulates the s-type anion channels and stomatal closure in arabidopsis. Plant Physiol. 2016, 170, 1090–1104. [Google Scholar] [CrossRef] [Green Version]
- Fitzpatrick, T.B.; Thore, S. Complex behavior: From cannibalism to suicide in the vitamin b1 biosynthesis world. Curr. Opin. Struct. Biol. 2014, 29, 34–43. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Chapter 8—Photosynthesis: Carbon reactions. In Plant Physiol, 4th ed.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2006; pp. 145–170. [Google Scholar]
- Julliard, J.H.; Douce, R. Biosynthesis of the thiazole moiety of thiamin (vitamin b1) in higher plant chloroplasts. Proc. Natl. Acad. Sci. USA 1991, 88, 2042–2045. [Google Scholar] [CrossRef] [Green Version]
- Binder, S. Branched-chain amino acid metabolism in arabidopsis thaliana. Arab Book 2010, 8, e0137. [Google Scholar] [CrossRef] [Green Version]
- Du, Q.; Wang, H.; Xie, J. Thiamin (vitamin b1) biosynthesis and regulation: A rich source of antimicrobial drug targets? Int. J. Biol. Sci. 2011, 7, 41–52. [Google Scholar] [CrossRef] [Green Version]
- Bunik, V.I.; Fernie, A.R. Metabolic control exerted by the 2-oxoglutarate dehydrogenase reaction: A cross-kingdom comparison of the crossroad between energy production and nitrogen assimilation. Biochem. J. 2009, 422, 405–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araújo, W.L.; Martins, A.O.; Fernie, A.R.; Tohge, T. 2-oxoglutarate: Linking tca cycle function with amino acid, glucosinolate, flavonoid, alkaloid, and gibberellin biosynthesis. Front. Plant Sci. 2014, 5, 552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araújo, W.L.; Nunes-Nesi, A.; Nikoloski, Z.; Sweetlove, L.J.; Fernie, A.R. Metabolic control and regulation of the tricarboxylic acid cycle in photosynthetic and heterotrophic plant tissues. Plant Cell Environ. 2012, 35, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bunik, V. Vitamin-Dependent Multienzyme Complexes of 2-Oxo Acid Dehydrogenases: Structure, Function, Regulation and Medical Implications; Nova Science Publisher: Hauppauge, NY, USA, 2017. [Google Scholar]
- Peng, C.; Uygun, S.; Shiu, S.H.; Last, R.L. The impact of the branched-chain ketoacid dehydrogenase complex on amino acid homeostasis in arabidopsis. Plant Physiol. 2015, 169, 1807–1820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gass, N.; Glagotskaia, T.; Mellema, S.; Stuurman, J.; Barone, M.; Mandel, T.; Roessner-Tunali, U.; Kuhlemeier, C. Pyruvate decarboxylase provides growing pollen tubes with a competitive advantage in petunia. Plant Cell 2005, 17, 2355–2368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bunik, V.I.; Tylicki, A.; Lukashev, N.V. Thiamin diphosphate-dependent enzymes: From enzymology to metabolic regulation, drug design and disease models. FEBS J. 2013, 280, 6412–6442. [Google Scholar] [CrossRef] [PubMed]
- Martinis, J.; Gas-Pascual, E.; Szydlowski, N.; Crevecoeur, M.; Gisler, A.; Burkle, L.; Fitzpatrick, T.B. Long-distance transport of thiamine (vitamin b1) is concomitant with that of polyamines. Plant Physiol. 2016, 171, 542–553. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Li, B.; Yang, Y.; Mu, P.; Qian, W.; Dong, L.; Zhang, K.; Liu, X.; Qin, H.; Ling, H.; et al. A novel allele of l-galactono-1,4-lactone dehydrogenase is associated with enhanced drought tolerance through affecting stomatal aperture in common wheat. Sci. Rep. 2016, 6, 30177. [Google Scholar] [CrossRef] [Green Version]
- Ahn, I.P.; Kim, S.; Lee, Y.H. Vitamin b1 functions as an activator of plant disease resistance. Plant Physiol. 2005, 138, 1505–1515. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, D.T.; Farias, L.P.; de Almeida, J.D.; Kashiwabara, P.M.; Ribeiro, A.F.; Silva-Filho, M.C.; Menck, C.F.; Van Sluys, M.A. Functional characterization of the thi1 promoter region from arabidopsis thaliana. J. Exp. Bot. 2005, 56, 1797–1804. [Google Scholar] [CrossRef]
- Ferreira, S.; Hjerno, K.; Larsen, M.; Wingsle, G.; Larsen, P.; Fey, S.; Roepstorff, P.; Salome Pais, M. Proteome profiling of populus euphratica oliv. Upon heat stress. Ann. Bot. 2006, 98, 361–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, C.E.; Li, Y.; Labbe, A.; Guevara, D.; Nuin, P.; Whitty, B.; Diaz, C.; Golding, G.B.; Gray, G.R.; Weretilnyk, E.A.; et al. Transcriptional profiling implicates novel interactions between abiotic stress and hormonal responses in thellungiella, a close relative of arabidopsis. Plant Physiol. 2006, 140, 1437–1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, I.P.; Kim, S.; Lee, Y.H.; Suh, S.C. Vitamin b1-induced priming is dependent on hydrogen peroxide and the npr1 gene in arabidopsis. Plant Physiol. 2007, 143, 838–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, W.; Sun, X.; Zhang, L. Intracellular signaling from plastid to nucleus. Annu. Rev. Plant Biol. 2013, 64, 559–582. [Google Scholar] [CrossRef] [Green Version]
- Pogson, B.J.; Woo, N.S.; Forster, B.; Small, I.D. Plastid signalling to the nucleus and beyond. Trends Plant Sci. 2008, 13, 602–609. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosado-Souza, L.; Fernie, A.R.; Aarabi, F. Ascorbate and Thiamin: Metabolic Modulators in Plant Acclimation Responses. Plants 2020, 9, 101. https://doi.org/10.3390/plants9010101
Rosado-Souza L, Fernie AR, Aarabi F. Ascorbate and Thiamin: Metabolic Modulators in Plant Acclimation Responses. Plants. 2020; 9(1):101. https://doi.org/10.3390/plants9010101
Chicago/Turabian StyleRosado-Souza, Laise, Alisdair R. Fernie, and Fayezeh Aarabi. 2020. "Ascorbate and Thiamin: Metabolic Modulators in Plant Acclimation Responses" Plants 9, no. 1: 101. https://doi.org/10.3390/plants9010101
APA StyleRosado-Souza, L., Fernie, A. R., & Aarabi, F. (2020). Ascorbate and Thiamin: Metabolic Modulators in Plant Acclimation Responses. Plants, 9(1), 101. https://doi.org/10.3390/plants9010101



