Responses to Increased Salinity and Severe Drought in the Eastern Iberian Endemic Species Thalictrum maritimum (Ranunculaceae), Threatened by Climate Change
Abstract
1. Introduction
2. Results
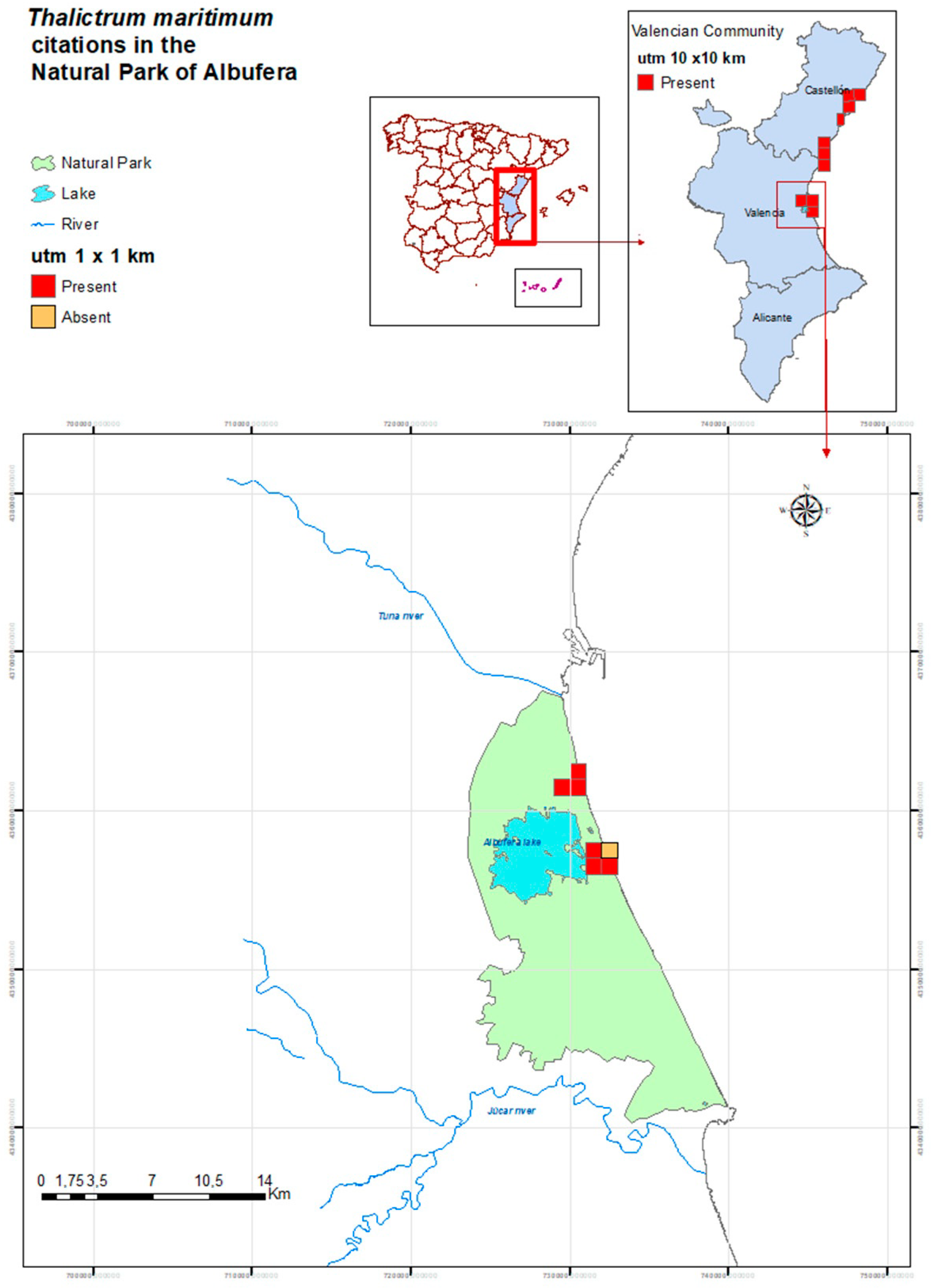
2.1. Habitat Characteristics of the Thalictrum maritimum Populations in the Natural Park of Albufera
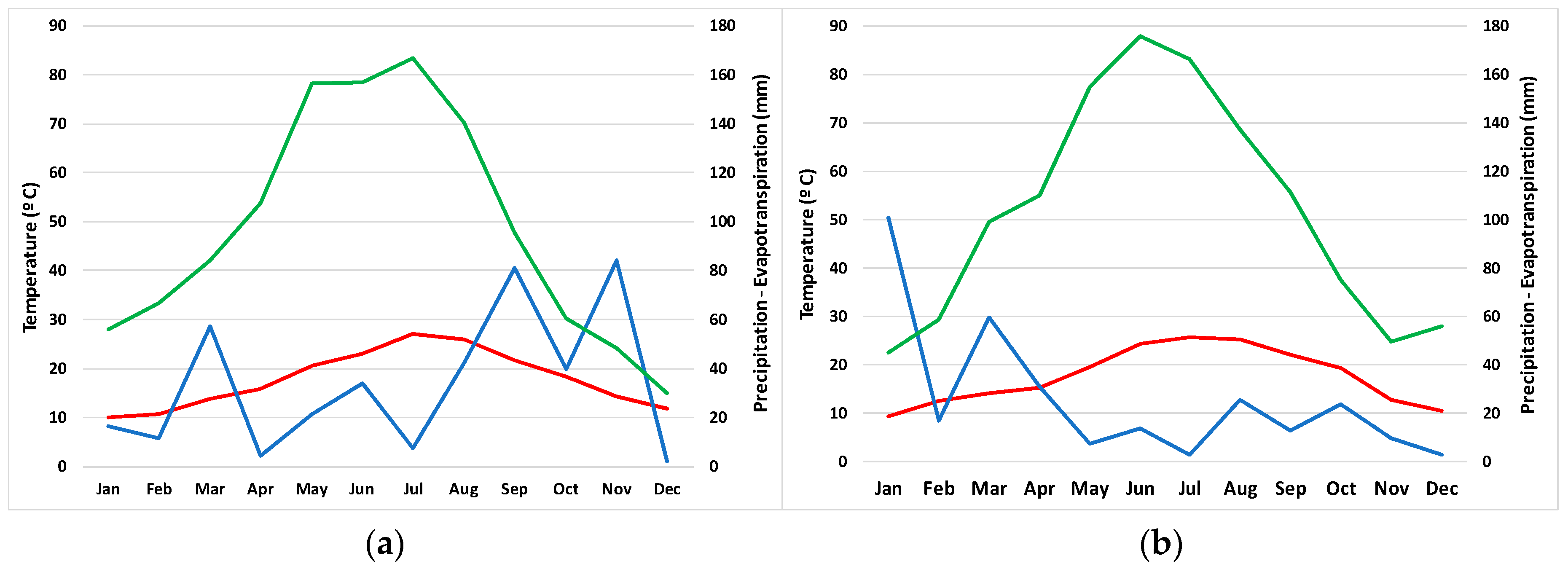
2.2. Climate Analysis
2.3. Soil Characteristics
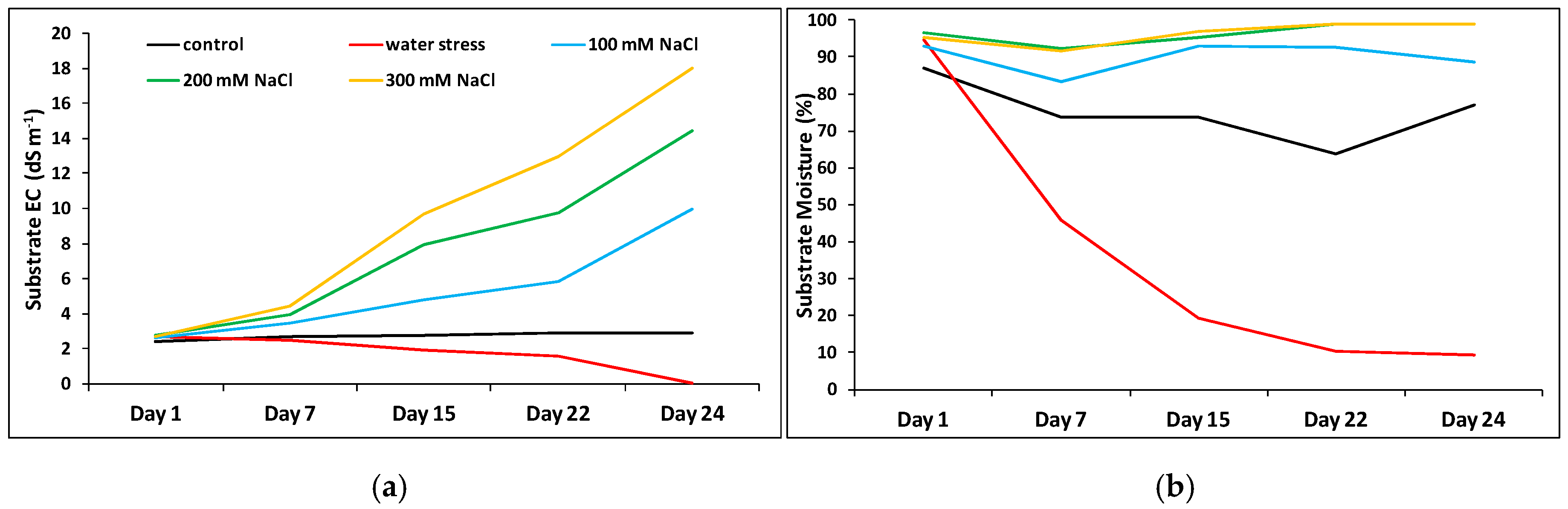
2.4. Substrate Electric Conductivity (EC) and Moisture
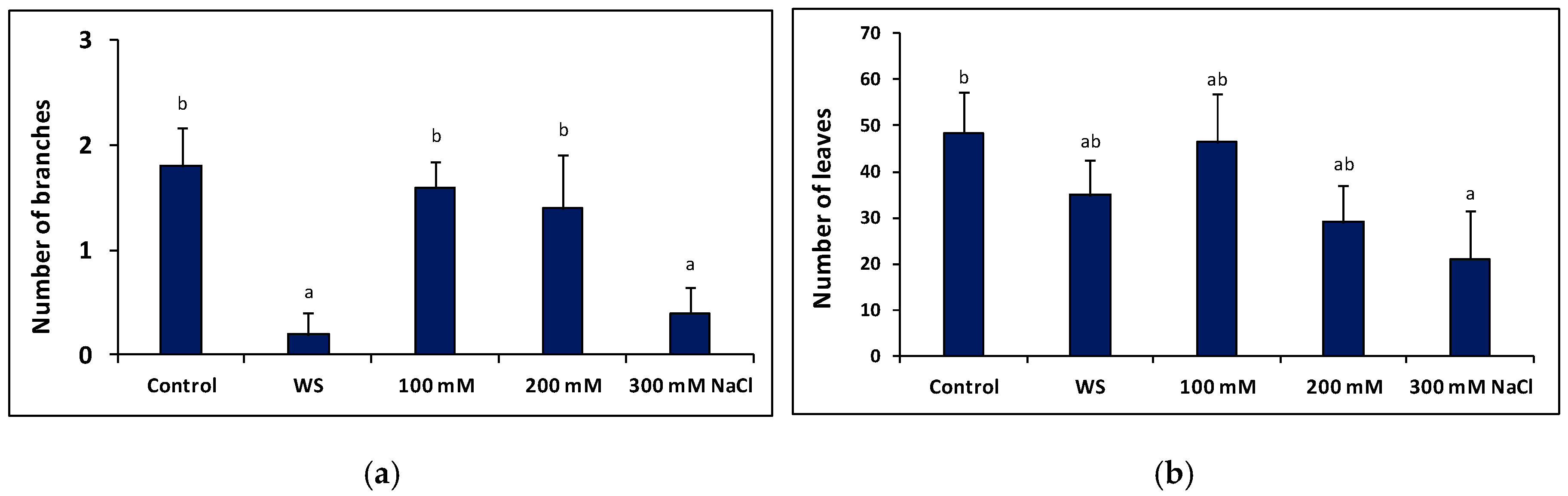
2.5. Plant Growth under Stress in the Greenhouse
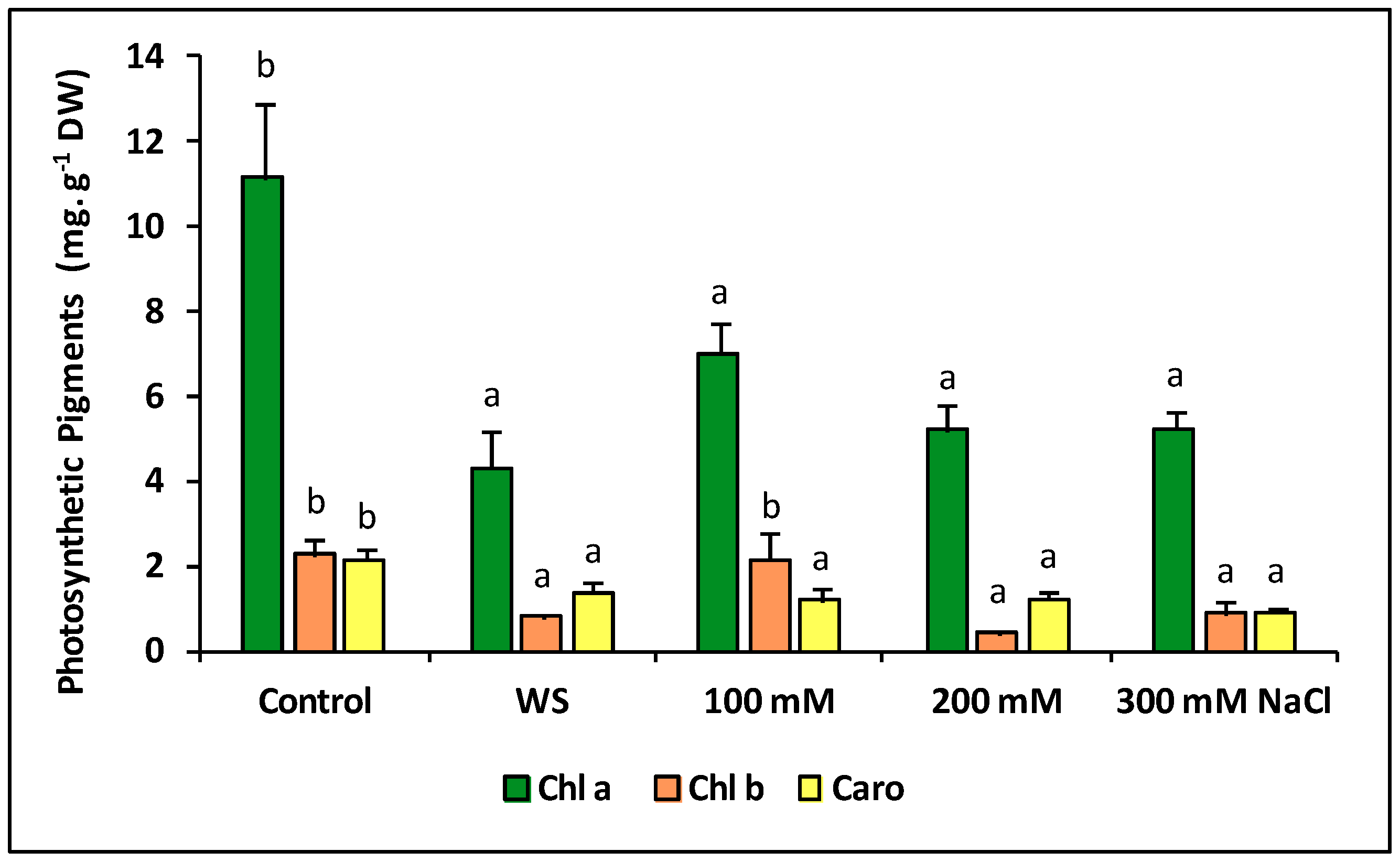
2.6. Photosynthetic Pigments
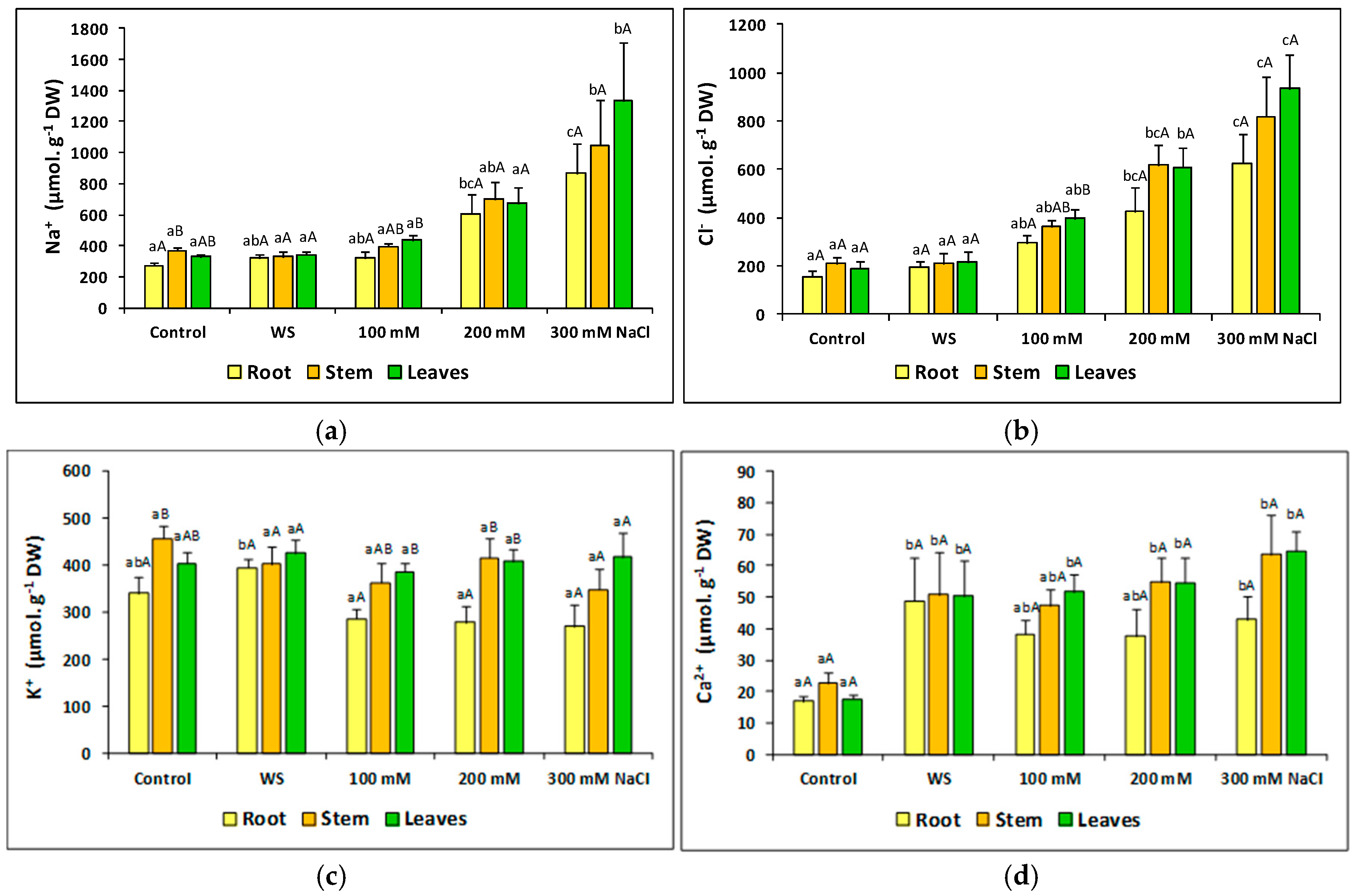
2.7. Ion Accumulation
2.8. Osmolytes, Oxidative Stress Markers and Antioxidants
2.9. Principal Component Analysis of Morphological and Biochemical Parameters Measured in Plants Grown under Experimental Greenhouse Conditions
3. Discussion
4. Materials and Methods
4.1. Study Area
4.2. Habitat Survey
4.3. Climatic Analysis
4.4. Soil Analyses
4.5. Plant Growth and Stress Treatments in the Greenhouse
4.6. Substrate Analysis
4.7. Plant Growth Parameters
4.8. Photosynthetic Pigments
4.9. Ion Quantification
4.10. Osmolyte Quantification
4.11. Oxidative Stress Markers and Non-Enzymatic Antioxidants
4.12. Antioxidant Enzymes Assays
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heywood, V.H. An outline of the impacts of climate change on endangered species in the Mediterranean region. Nat. Sicil. 2011, 35, 107–119. [Google Scholar]
- Laguna, E.; Gallego, P.P.F. Global environmental change in the unique flora: Endangered plant communities in the Valencia region. Mètode Rev. Difusió Investig. 2015, 6, 36–45. [Google Scholar] [CrossRef]
- Gómez-Mercado, F.; Lozano, S.D.H.; López-Carrique, E. Impacts of future climate scenarios on hypersaline habitats and their conservation interest. Biodivers. Conserv. 2017, 26, 2717–2734. [Google Scholar] [CrossRef]
- Grigore, M.N. Defining halophytes: A conceptual and historical approach in an ecological frame. In Halophytes and Climate Change: Adaptive Mechanisms and Potential Uses; CABI: Boston, MA, USA, 2019; pp. 3–18. [Google Scholar]
- Touchette, B.W.; Kneppers, M.K.; Eggert, C.M. Salt marsh plants: Biological overview and vulnerability to climate change. In Halophytes and Climate Change: Adaptive Mechanisms and Potential Uses; CABI Publishing: Boston, MA, USA, 2019; pp. 115–134. [Google Scholar]
- Laguna, E.; Deltoro, V.; Fos, S.; Pérez-Rovira, P.; Ballester, G.; Olivares, A.; Serra, L.; Pérez-Botella, J. Priority Habitats of the Valencian Community; Generalitat Valenciana: Valencia, Spain, 2003. [Google Scholar]
- Dufour, L. Diagnoses et observations critiques sur quelques plantes d’Espagne mal connues ou nouvelles. Bull. Soc. Bot. France 1860, 7, 221–227, 240–247, 323–328, 347–352, 426–433, 441–448. [Google Scholar] [CrossRef]
- Ferrer-Gallego, P.P.; Roselló, R.; Mateo, G.; Laguna, E.; Peris, J.B. Revised typifications of four Léon Dufour’s names. Flora Montiberica 2019, 74, 38–45. [Google Scholar]
- Ferrer-Gallego, P.P.; Laguna, E.; Mateo, G. Neotipificación de Thalictrum maritimum Dufour (Ranunculaceae), planta endémica y amenazada del este peninsular ibérico. Flora Montiberica 2015, 59, 83–87. [Google Scholar]
- Aguilella, A.; Fos, S.; Laguna, E. Catálogo Valenciano de Especies de Flora Amenazadas; Generalitat Valenciana: Valencia, Spain, 2010; pp. 300–301. [Google Scholar]
- Ballester, J.A.; Díes, B.; Hernández Muñoz, J.A.; Laguna, E.; Oltra, C.; Palop, S.; Urios, G. Parques Naturales de la Comunidad Valenciana/Natural Parks of the Valencian Community; Generalitat Valenciana: Valencia, Spain, 2003. [Google Scholar]
- Montserrat, P. Thalictrum L. In Flora Iberica, Lycopodiaceae-Papaveraceae; Castroviejo, S., Laínz, G., López González, G., Montserrat, P., Muñoz Garmendia, F., Paiva, J., Villar, L., Eds.; Real Jardín Botánico, C.S.I.C: Madrid, Spain, 1986; Volume 1, pp. 387–401. [Google Scholar]
- Ferrer-Gallego, P.P.; Ferrando, I.; Gago, C.; Laguna, E. Manual para la Conservación de Germoplasma y el Cultivo de la Flora Valenciana Amenazada, 2nd ed.; Generalitat Valenciana: Valencia, Spain, 2013; pp. 196–197. [Google Scholar]
- Gil, J.A.F.; Yabor, L.; Nadal, A.B.; Collado, F.; Ferrer-Gallego, P.; Vicente, O.; Boscaiu, M. A Methodological Approach for Testing the Viability of Seeds Stored in Short-Term Seed Banks. Not. Sci. Biol. 2017, 9, 563–570. [Google Scholar] [CrossRef]
- Lopéz, S.; Fabregat, C. Thalictrum maritimum Dufour. In Atlas y Libro Rojo de la Flora Vascular Amenazada de España. Adenda, 2006; Bañares, Á., Blanca, G., Güemes, J., Moreno, J.C., Ortiz, S., Eds.; Dirección General para la Biodiversidad y Sociedad Española de Biología de la Conservación de Plantas: Madrid, Spain, 2007; pp. 84–85. [Google Scholar]
- Moreno, J.C. The 2008 Red List of Spanish Vascular Flora; Ministerio de Medio Ambiente, Rural y Marino: Madrid, Spain, 2008. [Google Scholar]
- Curcó, A.M. Flora Vascular del Delta de l’Ebre. Col·lecció Tècnica, 1, 1st ed.; Generalitat de Catalunya, Departament de Medi Ambient i Habitatge, Parc Natural del Delta de l’Ebre: Barcelona, Spain, 2007. [Google Scholar]
- BDBCV (Banco de Datos de Biodiversidad de la Comunidad Valenciana). Conselleria de Medi Ambient, Aigua, Urbanisme i Habitatge, Generalitat Valenciana, Valencia. Available online: http://bdb.gva.es/ (accessed on 15 June 2020).
- Lionello, P.; Scarascia, L. The relation between climate change in the Mediterranean region and global warming. Reg. Environ. Chang. 2018, 18, 1481–1493. [Google Scholar] [CrossRef]
- Braun-Blanquet, J.; Conard, H.S.; Fuller, G.D. Plant Sociology; the Study of Plant Communities; Authorized English Translation of Pflanzensoziologie; Smithsonian Institution; McGraw-Hill Book Co. Inc.: New York, NY, USA, 1932. [Google Scholar]
- Rivas-Martínez, S.; Fernández-González, F.; Loidi, J.; Lousa, M.; Penas, A. Syntaxonomical checklist of vascular plant communities of Spain and Portugal to association level. Itinera Geobot. 2001, 14, 5–341. [Google Scholar]
- Rivas-Martínez, S.; Díaz, T.E.; Fernández-González, F.; Izco, J.; Loidi, J.; Lousa, M.; Penas, A. Vascular plant communities of Spain and Portugal. Addenda to the syntaxonomical checklist of 2001. Itinera Geobot. 2002, 15, 5–922. [Google Scholar]
- Worldwide Bioclimatic Classification System, 1996−2020, S. Rivas-Martinez & S. Rivas-Saenz, Phytosociological Research Center, Spain. Available online: http://www.globalbioclimatics.org (accessed on 20 May 2020).
- The Agroclimatic Information System for Irrigation (SIAR, Sistema de Información Agroclimática para Regadío), Benifaió, Llíria and Moncada Agro-Meteorological Stations. Available online: http://eportal.miteco.gob.es/websiar/Inicio.aspx (accessed on 20 May 2020). (In Spanish).
- Lidón, A.; Boscaiu, M.; Collado, F.; Vicente, O. Soil requirements of three salt tolerant, endemic species from South-East Spain. Not. Bot. Horti Agrobo. 2009, 37, 64–70. [Google Scholar]
- Al Hassan, M.; Chaura, J.; López-Gresa, M.P.; Borsai, O.; Daniso, E.; Donat-Torres, M.P.; Mayoral, O.; Vicente, O.; Boscaiu, M. Native-Invasive Plants vs. Halophytes in Mediterranean Salt Marshes: Stress Tolerance Mechanisms in Two Related Species. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef]
- Koźmińska, A.; Al Hassan, M.; Hanus-Fajerska, E.; Naranjo, M.A.; Boscaiu, M.; Vicente, O. Comparative analysis of water deficit and salt tolerance mechanisms in Silene. South Afr. J. Bot. 2018, 117, 193–206. [Google Scholar] [CrossRef]
- González-Orenga, S.; Llinares, J.V.; Al Hassan, M.; Fita, A.; Collado, F.; Lisón, P.; Vicente, O.; Boscaiu, M. Physiological and morphological characterisation of Limonium species in their natural habitats: Insights into their abiotic stress responses. Plant Soil 2020, 449, 267–284. [Google Scholar] [CrossRef]
- Zheng, Q.; Liu, L.; Liu, Z.; Chen, J.; Zhao, G. Comparison of the response of ion distribution in the tissues and cells of the succulent plants Aloe vera and Salicornia europaea to saline stress. J. Plant Nutr. Soil Sci. 2009, 172, 875–883. [Google Scholar] [CrossRef]
- González-Orenga, S.; Ferrer-Gallego, P.P.; Laguna, E.; López-Gresa, M.P.; Donat-Torres, M.P.; Verdeguer, M.; Vicente, O.; Boscaiu, M. Insights on Salt Tolerance of Two Endemic Limonium Species from Spain. Metabolites 2019, 9, 294. [Google Scholar] [CrossRef]
- Flowers, T.J.; Yeo, A.R. Ion relation of salt tolerance. In Solute Transport in Plant Cells and Tissues; Baker, D.A., Hall, J.L., Eds.; Longman Scientific and Technical: Harlow, UK, 1988; pp. 392–413. [Google Scholar]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Flowers, T.J.; Troke, P.F.; Yeo, A.R. The Mechanism of Salt Tolerance in Halophytes. Annu. Rev. Plant Physiol. 1977, 28, 89–121. [Google Scholar] [CrossRef]
- Wyn Jones, R.; Storey, R.; Leigh, R.A.; Ahmad, N.; Pollard, A. A hypothesis on cytoplasmic osmoregulation. In Regulation of Cell Membrane Activities in Plants; Marre, E., Ciferri, O., Eds.; Elsevier: Amsterdam, The Netherlands, 1977; pp. 121–136. [Google Scholar]
- Greenway, H.; Munns, R. Mechanisms of salt tolerance in nonhalophytes. Annu. Rev. Plant Physiol 1980, 31, 149–190. [Google Scholar]
- Wu, H.; Zhang, X.; Giraldo, J.P.; Shabala, S. It is not all about sodium: Revealing tissue specificity and signalling roles of potassium in plant responses to salt stress. Plant Soil 2018, 431, 1–17. [Google Scholar] [CrossRef]
- Brenes, M.; Solana, A.; Boscaiu, M.; Fita, A.; Vicente, O.; Calatayud, A.; Prohens, J.; Plazas, M. Physiological and Biochemical Responses to Salt Stress in Cultivated Eggplant (Solanum melongena L.) and in S. insanum L., a Close Wild Relative. Agronomy 2020, 10, 651. [Google Scholar] [CrossRef]
- Rengel, Z. The role of calcium in salt toxicity. Plant Cell Environ. 1992, 15, 625–632. [Google Scholar] [CrossRef]
- A Bressan, R.; Hasegawa, P.M.; Pardo, J.M. Plants use calcium to resolve salt stress. Trends Plant Sci. 1998, 3, 411–412. [Google Scholar] [CrossRef]
- Hepler, P.K. Calcium: A Central Regulator of Plant Growth and Development. Plant Cell 2005, 17, 2142–2155. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.; Pandey, G.K.; Tuteja, N. Calcium- and salt-stress signaling in plants: Shedding light on SOS pathway. Arch. Biochem. Biophys. 2008, 471, 146–158. [Google Scholar] [CrossRef] [PubMed]
- González-Orenga, S.; Al Hassan, M.; Llinares, J.V.; Lisón, P.; López-Gresa, M.P.; Verdeguer, M.; Vicente, O.; Boscaiu, M. Qualitative and Quantitative Differences in Osmolytes Accumulation and Antioxidant Activities in Response to Water Deficit in Four Mediterranean Limonium Species. Plants 2019, 8, 506. [Google Scholar] [CrossRef]
- Zhu, J.-K. Plant salt tolerance. Trends Plant Sci. 2001, 6, 66–71. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Chen, T.H.H.; Murata, N. Glycine betaine protects plants against abiotic stress: Mechanisms and biotechnological applications. Plant Cell Environ. 2011, 34, 1–2. [Google Scholar] [CrossRef]
- Szabados, L.; Savoure, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive Oxygen Species: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox Homeostasis and Antioxidant Signaling: A Metabolic Interface between Stress Perception and Physiological Responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef]
- Asada, K. Production and Scavenging of Reactive Oxygen Species in Chloroplasts and Their Functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Bose, J.; Rodrigo-Moreno, A.; Shabala, S. ROS homeostasis in halophytes in the context of salinity stress tolerance. J. Exp. Bot. 2013, 65, 1241–1257. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S. Salinity and programmed cell death: Unravelling mechanisms for ion specific signalling. J. Exp. Bot. 2009, 60, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V.; Shabala, S.; Davies, J.M. Spatial variation in H2O2 response of Arabidopsis thaliana root epidermal Ca2+ flux and plasma membrane Ca2+ channels. Plant J. 2007, 49, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Chen, S.; Zhao, Q.; Wang, T.; Yang, C.; Diaz, C.; Sun, G.; Dai, S. Physiological and Proteomic Analysis of Salinity Tolerance in Puccinellia tenuiflora. J. Proteome Res. 2011, 10, 3852–3870. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Das, P.; Parida, A.K.; Agarwal, P.K. Proteomics, metabolomics, and ionomics perspectives of salinity tolerance in halophytes. Front. Plant Sci. 2015, 6, 537. [Google Scholar] [CrossRef] [PubMed]
- Ozgur, R.; Uzilday, B.; Sekmen, A.H.; Turkan, I. Reactive oxygen species regulation and antioxidant defence in halophytes. Funct. Plant Biol. 2013, 40, 832–847. [Google Scholar] [CrossRef]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Riadh, K.; Gopi, R.; Manivannan, P.; Inès, J.; Al-Juburi, H.J.; Chang-Xing, Z.; Hong-Bo, S.; Panneerselvam, R. Antioxidant defense responses: Physiological plasticity in higher plants under abiotic constraints. Acta Physiol. Plant. 2009, 31, 427–436. [Google Scholar] [CrossRef]
- Szőllősi, R. Superoxide Dismutase (SOD) and Abiotic Stress Tolerance in Plants. In Oxidative Damage to Plants; Elsevier: Amsterdam, The Netherlands, 2014; pp. 89–129. [Google Scholar]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef]
- Arbona, V.; Flors, V.; Jacas, J.; García-Agustín, P.; Gómez-Cadenas, A. Enzymatic and non-enzymatic antioxidant responses of Carrizo citrange, a salt-sensitive citrus rootstock, to different levels of salinity. Plant Cell Physiol. 2003, 44, 388–394. [Google Scholar] [CrossRef]
- Al Hassan, M.; Chaura, J.; Donat-Torres, M.P.; Boscaiu, M.; Vicente, O. Antioxidant responses under salinity and drought in three closely related wild monocots with different ecological optima. AoB PLANTS 2017, 9, plx009. [Google Scholar] [CrossRef]
- Kozminska, A.; Al Hassan, M.; Kumar, D.; Oprica, L.; Martinelli, F.; Grigore, M.-N.; Vicente, O.; Boscaiu, M. Characterizing the effects of salt stress in Calendula officinalis L. J. Appl. Bot. Food Qual. 2017, 90, 323–329. [Google Scholar]
- Bautista, I.; Boscaiu, M.; Lidón, A.; Llinares, J.V.; Lull, C.; Donat-Torres, M.P.; Mayoral, O.; Vicente, O. Environmentally induced changes in antioxidant phenolic compounds levels in wild plants. Acta Physiol. Plant. 2015, 38, 9. [Google Scholar] [CrossRef]
- Gil, R.; Bautista, I.; Boscaiu, M.; Lidón, A.; Wankhade, S.; Sánchez, H.; Llinares, J.; Vicente, O. Responses of five Mediterranean halophytes to seasonal changes in environmental conditions. AoB Plants 2014, 6, plu049. [Google Scholar] [CrossRef] [PubMed]
- Soria, J.M. Past, present and future of la Albufera of Valencia Natural Park. Limnetica 2006, 25, 135–142. [Google Scholar]
- Mansanet, J. Flora y Vegetación de la Dehesa de La Albufera. Inéd. Departamento de Botánica. Facultad de Ciencias; Universidad de Valencia: Valencia, Spain, 1979. [Google Scholar]
- Euro+Med (2006+): Euro+Med PlantBase—The Information Resource for Euro-Mediterranean Plant Diversity. Available online: http://ww2.bgbm.org/EuroPlusMed/ (accessed on 15 June 2020).
- Bouyoucos, G.J. Hydrometer method improved for making particle size analysis of soils. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An Examination of The Degtjareff Method for Determining Soil Organic Matter, and a Proposed Modification of the Chromic Acid Titration Method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Loeppert, R.H.; Suarez, D.L. Carbonate and gypsum. In Methods of Soil Analysis. Part 3. Chemical Methods; SSSA Book Series No. 5; Bartels, J.M., Ed.; John Wiley & Sons: Bigham, WI, USA, 1996; pp. 437–474. [Google Scholar]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Weimberg, R. Solute adjustments in leaves of two species of wheat at two different stages of growth in response to salinity. Physiol. Plant. 1987, 70, 381–388. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Hodges, D.M.; Delong, J.M.; Forney, C.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Taulavuori, E.; Hellström, E.; Taulavuori, K.; Laine, K. Comparison of two methods used to analyse lipid peroxidation from Vaccinium myrtillus (L.) during snow removal, reacclimation and cold acclimation. J. Exp. Bot. 2001, 52, 2375–2380. [Google Scholar] [CrossRef]
- Blainski, A.; Lopes, G.C.; Mello, J.C.P. Application and Analysis of the Folin Ciocalteu Method for the Determination of the Total Phenolic Content from Limonium Brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Beyer, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Connell, J.P.; Mullet, J.E. Pea Chloroplast Glutathione Reductase: Purification and Characterization. Plant Physiol. 1986, 82, 351–356. [Google Scholar] [CrossRef] [PubMed]


| Relevé nº | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Area (m2) | 25 | 100 | 90 | 100 | 8 | 100 | 100 | 60 | 100 | 100 | |
| Vegetation coverage (%) | 100 | 95 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
| Moisture (%) | 40.2 | 59.4 | 60.5 | 58.3 | 32.0 | 50.7 | 39.9 | 20.6 | 56.3 | 51.9 | |
| Conductivity (EC, dS m−1) | 3.7 | 2.1 | 1.9 | 2.1 | 12.2 | 3.8 | 1.3 | 3.0 | 1.6 | 2.4 | |
| Species | Vegetation Class | ||||||||||
| Juncetea maritimi | |||||||||||
| Thalictrum maritimum | 3 | 3 | + | 3 | 1 | 1 | 2 | 2 | 4 | 2 | |
| Centaurea dracunculifolia | 2 | + | 1 | ||||||||
| Dorycnium gracile | + | 1 | 2 | 2 | |||||||
| Elymus elongatus | 1 | + | |||||||||
| Juncus acutus | 2 | ||||||||||
| Juncus maritimus | 2 | 2 | 3 | 2 | 1 | 1 | 1 | ||||
| Linum maritimum | + | + | 1 | + | 1 | ||||||
| Plantago crassifolia | + | 1 | |||||||||
| Samolus valerandi | + | + | 1 | ||||||||
| Schoenus nigricans | 3 | 2 | 2 | 1 | 2 | 2 | 1 | 2 | |||
| Scirpioides holoschoenus | 2 | ||||||||||
| Spartina patens | 3 | 5 | 3 | 2 | |||||||
| Phragmito-Magnocaricetea | |||||||||||
| Cladium mariscus | + | + | 4 | 3 | 2 | ||||||
| Lythrum salicaria | 1 | 1 | 1 | ||||||||
| Phragmites australis subsp. hrysanthus | 3 | 3 | 4 | 1 | + | + | 1 | 2 | 1 | ||
| Phragmites australis subsp. australis | + | + | + | 1 | 1 | ||||||
| Molinio-Arrhenatheretea | |||||||||||
| Sonchus maritimus | + | 1 | 1 | 1 | |||||||
| Nerio-Tamaricetea | |||||||||||
| Tripidium ravennae | + | + | + | 1 | 1 | + | |||||
| Imperata cylindrica | + | ||||||||||
| Quercetea ilicis | |||||||||||
| Phillyrea angustifolia | + | + | |||||||||
| Pistacia lentiscus | + | + | 1 | 1 | |||||||
| Smilax aspera | + | ||||||||||
| Stellarietea mediae | |||||||||||
| Lagurus ovatus | 1 | + | + | + | + |
| Communities | Relevé nº |
|---|---|
| Juncetalia maritimi | 1, 2, 3, 4 |
| Hydrocotylo-Mariscetum serrati | 8, 9, 10 |
| Spartino-Juncetum maritimi subass. spartinetosum | 5, 6, 7 |
| Year | Mean T (°C) | Max T (°C) | Min T (°C) | Mean Hum (%) | Max Hum (%) | Min Hum (%) | Rainfall (mm) | Eto (mm) |
|---|---|---|---|---|---|---|---|---|
| 2001 | 17.98 | 29.24 | 7.88 | 65.38 | 93.35 | 19.41 | 313.80 | 1119.18 |
| 2002 | 17.44 | 29.09 | 7.43 | 67.93 | 94.27 | 21.22 | 322.70 | 1091.91 |
| 2003 | 17.60 | 29.47 | 6.54 | 67.65 | 93.35 | 18.84 | 289.00 | 1155.10 |
| 2004 | 17.63 | 28.95 | 7.13 | 69.57 | 96.33 | 18.40 | 594.40 | 1006.45 |
| 2005 | 16.45 | 29.50 | 4.48 | 68.32 | 95.89 | 16.36 | 377.60 | 1117.97 |
| 2006 | 17.53 | 29.04 | 6.93 | 69.13 | 95.68 | 18.00 | 464.40 | 1189.38 |
| 2007 | 16.81 | 29.60 | 5.90 | 68.13 | 95.31 | 16.26 | 894.40 | 1164.50 |
| 2008 | 16.88 | 29.45 | 5.91 | 68.35 | 95.67 | 17.74 | 674.40 | 1194.10 |
| 2009 | 17.34 | 29.85 | 6.26 | 68.60 | 97.16 | 19.03 | 446.20 | 1215.26 |
| 2010 | 16.78 | 29.69 | 5.54 | 68.31 | 97.06 | 19.32 | 565.00 | 1206.22 |
| 2011 | 17.57 | 30.45 | 6.87 | 70.32 | 96.52 | 18.75 | 472.00 | 1166.73 |
| 2012 | 17.31 | 30.46 | 5.13 | 67.58 | 98.34 | 18.41 | 503.61 | 1208.25 |
| 2013 | 17.55 | 29.92 | 6.23 | 63.26 | 95.27 | 16.95 | 263.80 | 1245.42 |
| 2014 | 18.32 | 30.81 | 8.02 | 65.32 | 95.90 | 15.54 | 224.40 | 1278.22 |
| 2015 | 17.76 | 30.88 | 7.10 | 70.02 | 98.56 | 17.34 | 401.26 | 1169.08 |
| 2016 | 17.85 | 29.66 | 6.46 | 68.66 | 98.42 | 20.57 | 259.57 | 1218.41 |
| 2017 | 17.59 | 29.97 | 6.77 | 68.51 | 97.63 | 18.44 | 307.26 | 1238.82 |
| 2018 | 17.60 | 29.55 | 6.85 | 68.06 | 97.09 | 22.58 | 684.02 | 1225.71 |
| 2019 | 17.79 | 31.41 | 7.25 | 66.59 | 97.58 | 19.49 | 427.00 | 1243.83 |
| Mean | 17.46 | 29.84 | 6.56 | 67.88 | 96.28 | 18.56 | 446.57 | 1181.82 |
| Parameter | 0–10 cm Depth | 10–20 cm Depth |
|---|---|---|
| Sand (%) | 95.00 ± 0.28 | 93.56 ± 1.05 |
| Silt (%) | 3.50 ± 0.19 | 4.49 ± 0.74 |
| Clay (%) | 1.49 ± 0.08 | 1.92 ± 0.31 |
| Apparent density (g cm−3) | 1.09 ± 0.09 | 1.15 ± 0.07 |
| Porosity (%) | 58.86 ± 3.82 | 56.60 ± 2.64 |
| Carbonates (%) | 22.51 ± 4.69 | 28.23 ± 9.58 |
| Organic Matter (%) | 1.96 ± 0.75 | 1.29 ± 0.21 |
| pH | 7.30 ± 0.30 | 7.39 ± 0.22 |
| EC (dS m−1) | 5.01 ± 3.45 | 3.06 ± 1.32 |
| Na+ (meq L−1) | 46.36 ± 6.77 | 42.67 ± 4.62 |
| K+ (meq L−1) | 1.55 ± 0.56 | 1.26 ± 0.22 |
| Cl− (meq L−1) | 20.55 ±5.37 | 28.18 ± 2.22 |
| Ca2+ (meq L−1) | 7.51 ± 2.21 | 6.43 ± 0.70 |
| Mg2+ (meq L−1) | 5.48 ± 3.32 | 3.93 ± 0.81 |
| Biochemical Trait | Control | Water Stress | 100 mM NaCl | 200 mM NaCl | 300 mM NaCl |
|---|---|---|---|---|---|
| Pro (µmol g−1 DW) | 13.3 ± 1.2 a | 20.7 ± 2.6 b | 20.7 ± 2.5 b | 15.9 ± 0.8 ab | 20.5 ± 2.1 b |
| TSS (mg eq. Gluc g−1 DW) | 352.2 ± 80.7 bc | 233.1 ± 37.9 a | 265.5 ± 6.0 ab | 418.7 ± 46.0 c | 401.0 ± 38.5 bc |
| MDA (nmol g−1 DW) | 365.1 ± 52.9 a | 268.6 ± 50.7 a | 323.4 ± 28.2 a | 296.1 ± 23.5 a | 255.0 ± 16.7 a |
| TPC (mg eq GA g−1 DW) | 26.6 ± 5.6 b | 21.1 ± 2.1 ab | 19.0 ± 1.4 ab | 19.9 ± 2.2 ab | 17.5 ± 1.7 a |
| TF (mg eq C g−1 DW) | 10.7 ± 1.5 c | 8.1 ± 1.7 bc | 5.8 ± 0.6 ab | 4.0 ± 1.2 a | 4.1 ± 0.2 a |
| SOD (U g−1 protein) | 13.2 ± 1.2 a | 46.9 ± 8.8 c | 23.6 ± 5.4 a | 33.0 ± 3.6 b | 30.3 ± 4.1 b |
| CAT (U g−1 protein) | 228.6 ± 26.4 ab | 158.3 ± 87.3 a | 314.6 ± 26.9 b | 272.8 ± 33.3 ab | 544.5 ± 31.9 c |
| GR (U. g−1 protein) | 1543.9 ± 342.0 a | 1057.5 ± 249.0 a | 1624.9 ± 330.0 a | 2854.5 ± 137.0 b | 1793.9 ± 354.0 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Orenga, S.; Trif, C.; Donat-Torres, M.P.; Llinares, J.V.; Collado, F.; Ferrer-Gallego, P.P.; Laguna, E.; Boscaiu, M.; Vicente, O. Responses to Increased Salinity and Severe Drought in the Eastern Iberian Endemic Species Thalictrum maritimum (Ranunculaceae), Threatened by Climate Change. Plants 2020, 9, 1251. https://doi.org/10.3390/plants9101251
González-Orenga S, Trif C, Donat-Torres MP, Llinares JV, Collado F, Ferrer-Gallego PP, Laguna E, Boscaiu M, Vicente O. Responses to Increased Salinity and Severe Drought in the Eastern Iberian Endemic Species Thalictrum maritimum (Ranunculaceae), Threatened by Climate Change. Plants. 2020; 9(10):1251. https://doi.org/10.3390/plants9101251
Chicago/Turabian StyleGonzález-Orenga, Sara, Calin Trif, Mͣ Pilar Donat-Torres, Josep V. Llinares, Francisco Collado, P. Pablo Ferrer-Gallego, Emilio Laguna, Monica Boscaiu, and Oscar Vicente. 2020. "Responses to Increased Salinity and Severe Drought in the Eastern Iberian Endemic Species Thalictrum maritimum (Ranunculaceae), Threatened by Climate Change" Plants 9, no. 10: 1251. https://doi.org/10.3390/plants9101251
APA StyleGonzález-Orenga, S., Trif, C., Donat-Torres, M. P., Llinares, J. V., Collado, F., Ferrer-Gallego, P. P., Laguna, E., Boscaiu, M., & Vicente, O. (2020). Responses to Increased Salinity and Severe Drought in the Eastern Iberian Endemic Species Thalictrum maritimum (Ranunculaceae), Threatened by Climate Change. Plants, 9(10), 1251. https://doi.org/10.3390/plants9101251










