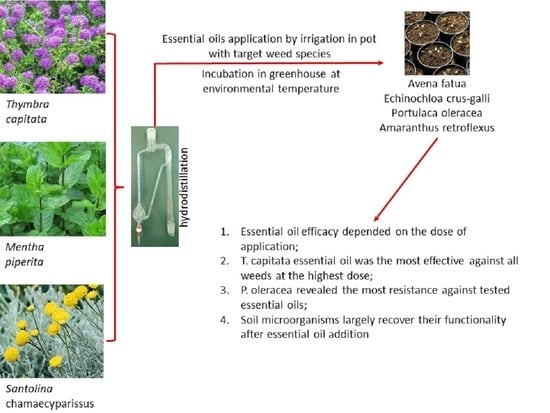
Potential Effects of Essential Oils Extracted from Mediterranean Aromatic Plants on Target Weeds and Soil Microorganisms
Abstract
:1. Introduction
2. Results
2.1. Essential Oils Composition
2.2. Effect of EOs on Target Plants
2.2.1. Effects of EOs on A. retroflexus
2.2.2. Effects of EOs on P. oleracea
2.2.3. Effects of EOs on A. fatua
2.2.4. Effects of EOs on E. crus-galli
2.2.5. Overall Efficacy of EOs
2.3. Effects of EOs on Soil Biochemical Properties and on the Main Microbial Groups
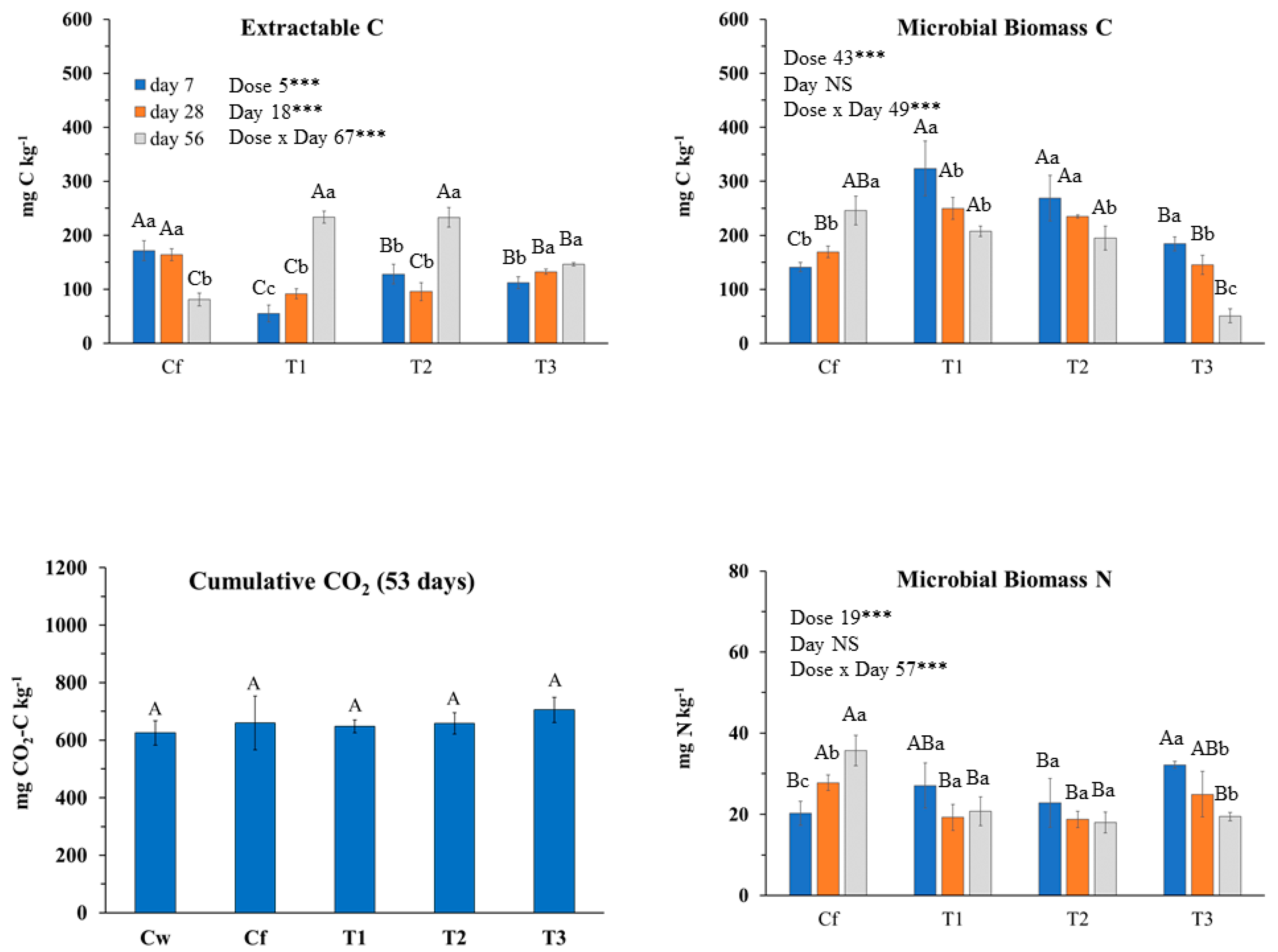
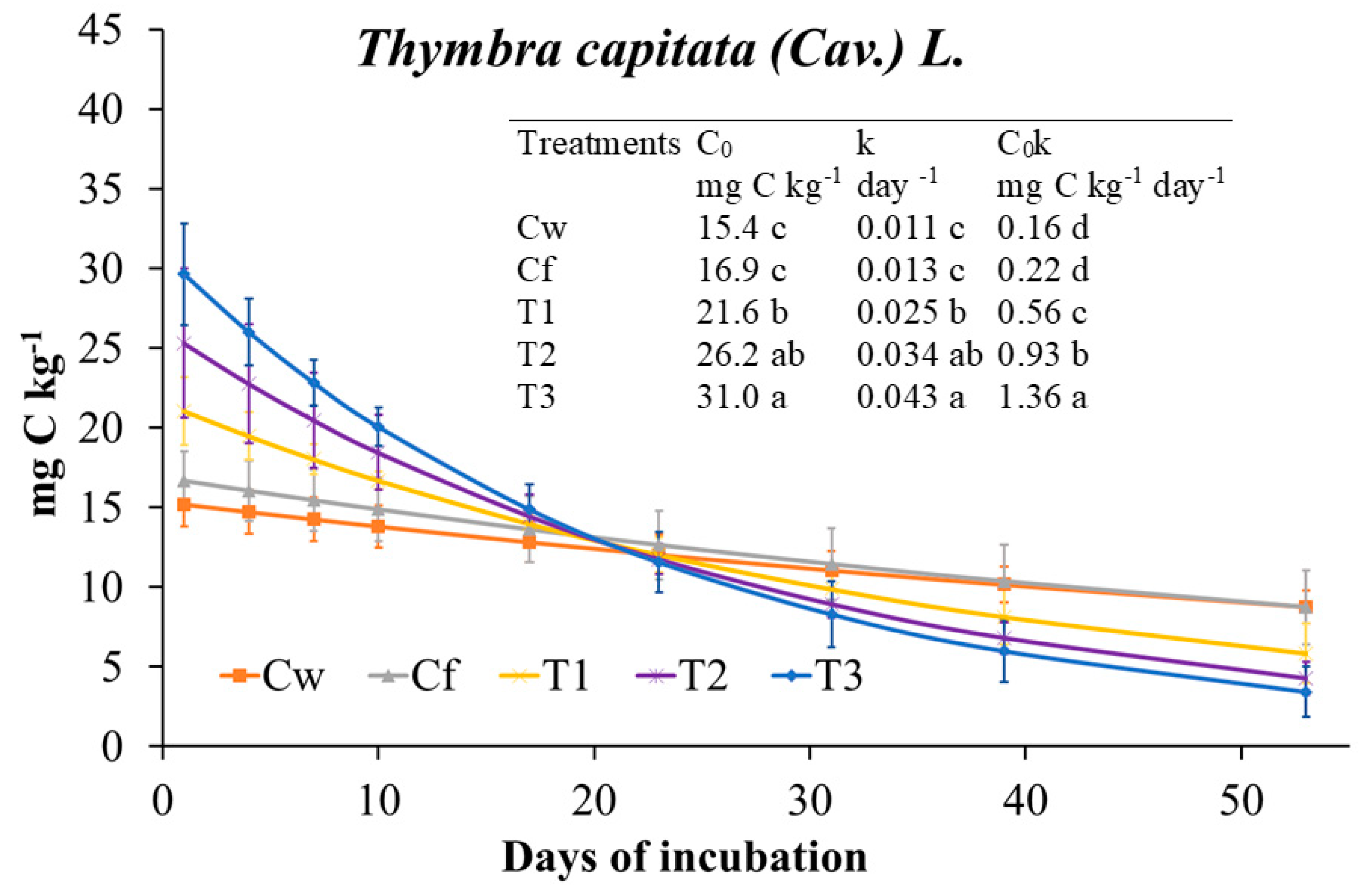
2.3.1. Effects of T. capitata EO on Soil Biochemical Properties and Main Microbial Groups
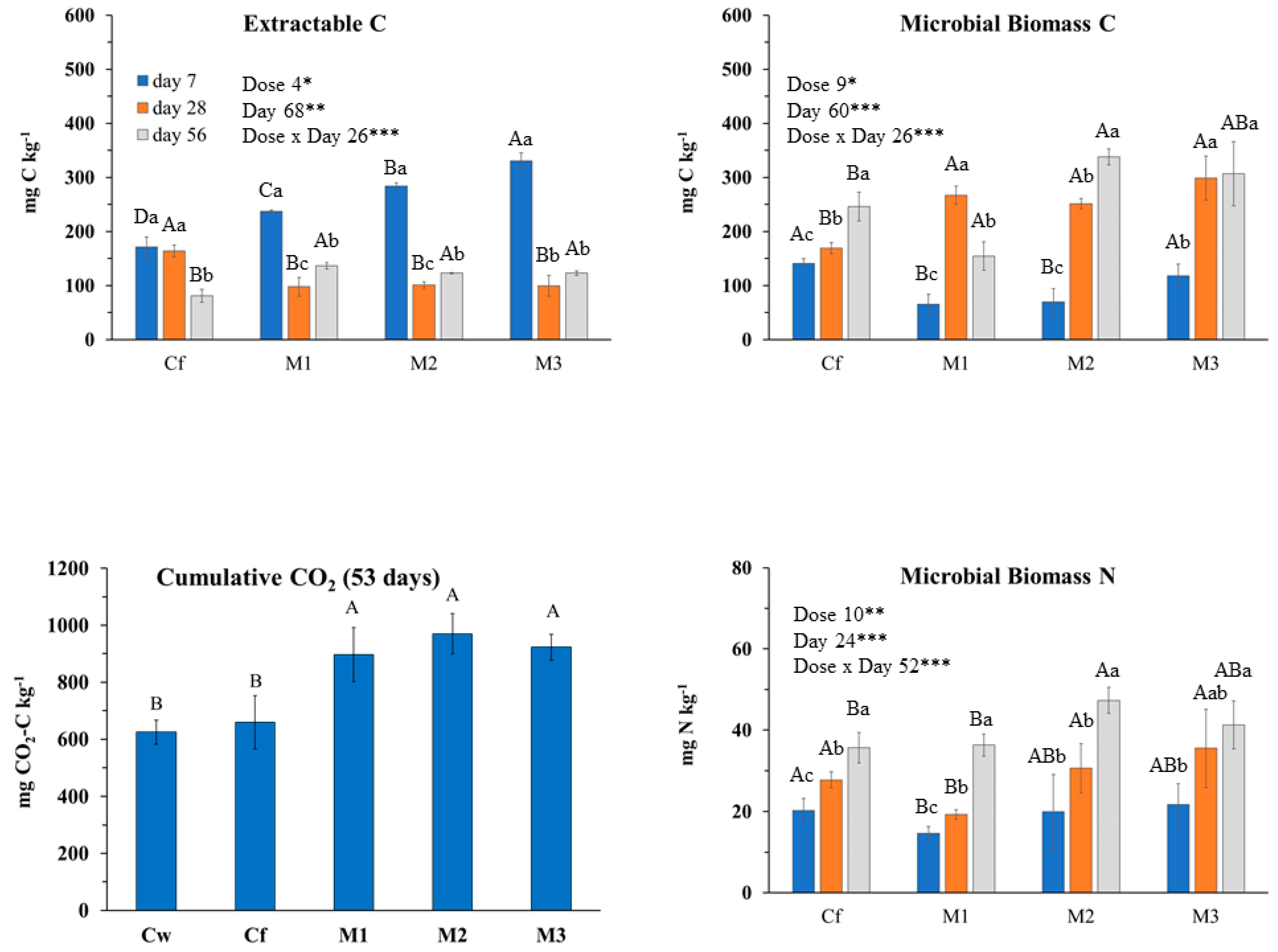
2.3.2. Effects of M. piperita EO on Soil Biochemical Properties and Main Microbial Groups
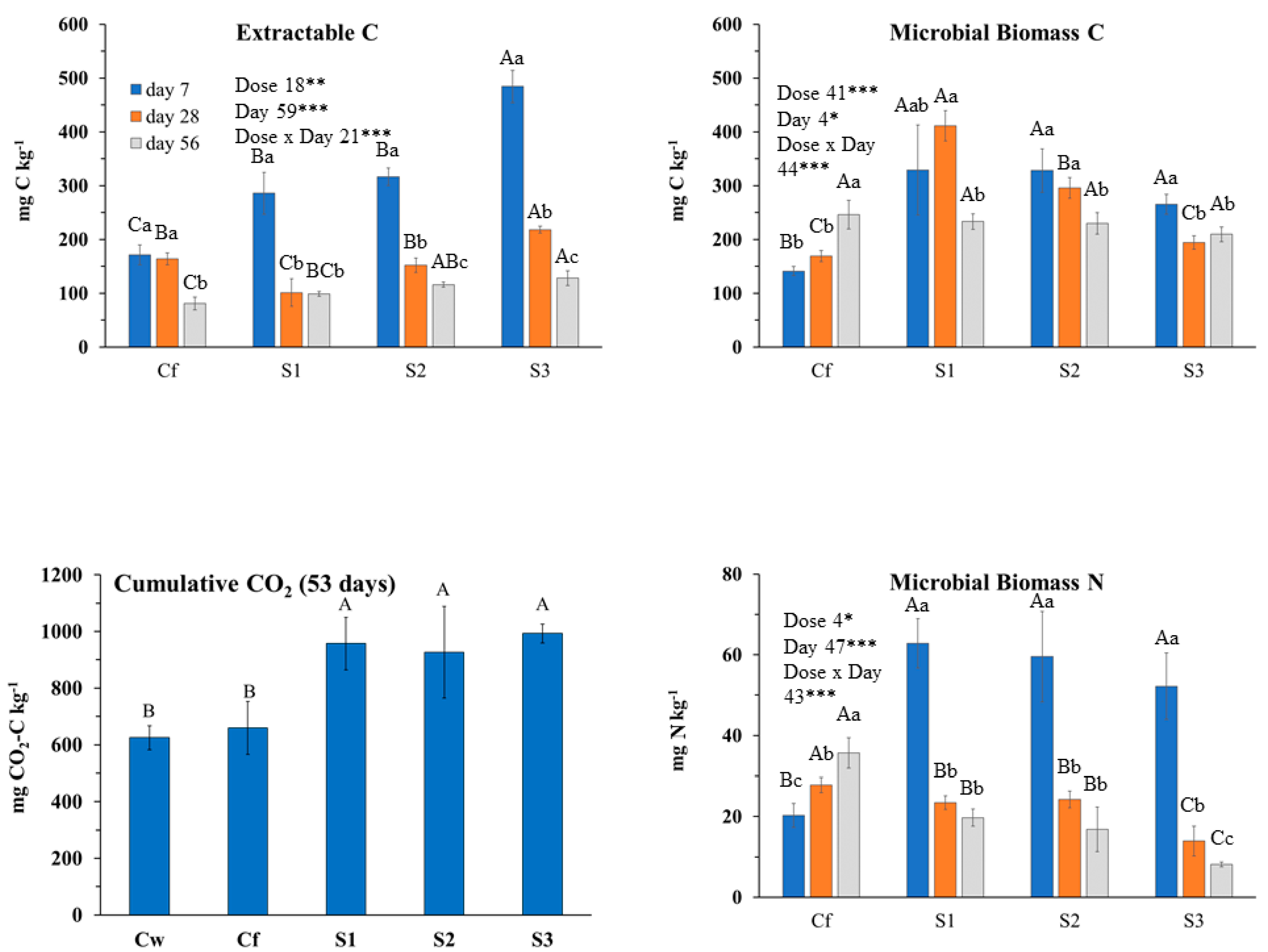
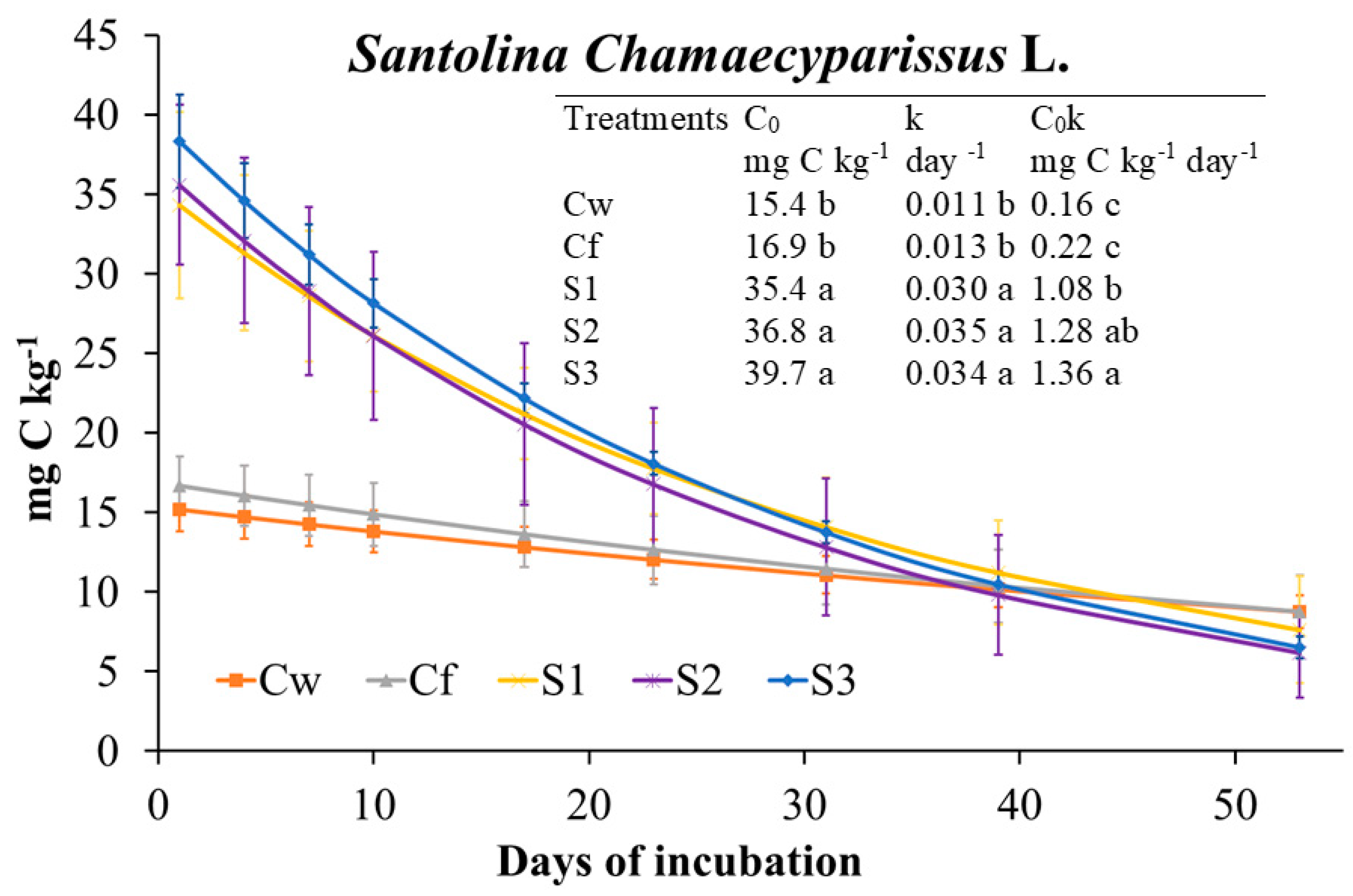
2.3.3. Effects of S. chamaecyparissus EO on Soil Biochemical Properties and Main Microbial Groups
3. Discussion
3.1. Herbicidal Activity of T. capitata, M. piperita and S. chamaecyparissus EOs
3.2. Changes in Soil Biochemical Properties and in Main Microbial Groups Following the Addition of EOs
3.2.1. T. capitata (THY) EO Effects
3.2.2. M. piperita (MNT) EO Effects
3.2.3. S. chamaecyparissus (SNT) EO Effects
4. Materials and Methods
4.1. Essential Oils
4.2. Herbicidal Activity of EOs against Target Weeds
- -
- T. capitata: 4 (T1), 8 (T2), 12 (T3) µL mL−1;
- -
- M. piperita: 12 (M1), 16 (M2), 20 (M3) µL mL−1;
- -
- S. chamaecyparissus: 12 (S1), 16 (S2), 20 (S3) µL mL−1.
4.3. Effects of EOs on Soil Microorganisms
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Villa, F.; Cappitelli, F.; Cortesi, P.; Kunova, A. Fungal biofilms: Targets for the development of novel strategies in plant disease management. Front. Microbiol. 2017, 8, 654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatcher, P.E.; Melander, B. Combining physical, cultural, and biological methods: Prospects for integrated non-chemical weed management strategies. Weed Res. 2003, 43, 303–322. [Google Scholar] [CrossRef]
- Hossard, L.; Guichard, L.; Pelosi, C.; Makowski, D. Lack of evidence for a decrease in synthetic pesticide use on the main arable crops in France. Sci. Total Environ. 2017, 575, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Kanissery, R.; Gairhe, B.; Kadyampakeni, D.; Batuman, O.; Alferez, F. Glyphosate: Its environmental persistence and impact on crop health and nutrition. Plants 2019, 8, 499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haney, R.L.; Senseman, S.A.; Hons, F.M.; Zuberer, D.A. Effect of glyphosate on soil microbial activity and biomass. Weed Sci. 2000, 48, 89–93. [Google Scholar] [CrossRef]
- Lancaster, S.H.; Hollister, E.B.; Senseman, S.A.; Gentry, T.J. Effects of repeated glyphosate applications on soil microbial community composition and the mineralization of glyphosate. Pest Manag. Sci. 2010, 66, 59–64. [Google Scholar] [CrossRef]
- Ntalli, N.; Zioga, D.; Argyropoulou, M.; Papatheodorou, E.; Menkissoglu-Spiroudi, U.; Monokrousos, N. Anise, parsley, and rocket as nematicidal soil amendments and their impact on non-target soil organisms. Appl. Soil Ecol. 2019, 143, 17–25. [Google Scholar] [CrossRef]
- Benvenuti, S.; Cioni, P.L.; Flamini, G.; Pardossi, A. Weeds for weed control: Asteraceae essential oils as natural herbicides. Weed Res. 2017, 57, 342–353. [Google Scholar] [CrossRef]
- Della Pepa, T.; Elshafie, H.S.; Capasso, R.; De Feo, V.; Camele, I.; Nazzaro, F.; Scognamiglio, M.R.; Caputo, L. Antimicrobial and phytotoxic activity of Origanum heracleoticum and O. majorana essential oils growing in cilento (Southern Italy). Molecules 2019, 24, 2576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Gawad, A.A.; Elshamy, A.; El Gendy, A.E.N.; Gaara, A.; Assaeed, A. Volatiles profiling, allelopathic activity, and antioxidant potentiality of Xanthium strumarium leaves essential oil from Egypt: Evidence from chemometrics analysis. Molecules 2019, 24, 584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araniti, F.; Graña, E.; Reigosa, M.J.; Sánchez-Moreiras, A.M.; Abenavoli, M.R. Individual and joint activity of terpenoids, isolated from Calamintha nepeta extract, on Arabidopsis thaliana. Nat. Prod. Res. 2013, 27, 2297–2303. [Google Scholar] [CrossRef] [PubMed]
- Xianfei, X.; Xiaoqiang, C.; Shunying, Z.; Guolin, Z. Chemical composition, and antimicrobial activity of essential oils of Chaenomeles speciosa from China. Food Chem. 2007, 100, 1312–1315. [Google Scholar] [CrossRef]
- Araniti, F.; Sánchez-Moreiras, A.M.; Graña, E.; Reigosa, M.J.; Abenavoli, M.R. Terpenoid trans-caryophyllene inhibits weed germination and induces plant water status alteration and oxidative damage in adult Arabidopsis. Plant Biol. 2017, 19, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Vasilakoglou, I.; Dhima, K.; Paschalidis, K.; Christos, R. Herbicidal potential on Lolium rigidum of nineteen major essential oil components and their synergy. J. Essent. Oil Res. 2013, 25, 1–10. [Google Scholar] [CrossRef]
- Verdeguer, M.; Castañeda, L.G.; Torres-Pagan, N.; Llorens-Molina, J.A.; Carrubba, A. Control of Erigeron bonariensis with Thymbra capitata, Mentha piperita, Eucalyptus camaldulensis, and Santolina chamaecyparissus Essential Oils. Molecules 2020, 25, 562. [Google Scholar] [CrossRef] [Green Version]
- Blázquez, M.A. Role of natural essential oils in sustainable agriculture and food preservation. J. Sci. Res. Rep. 2014, 3, 1843–1860. [Google Scholar] [CrossRef]
- Sadgrove, N.; Jones, G. A contemporary introduction to essential oils: Chemistry, bioactivity, and prospects for Australian agriculture. Agriculture 2015, 5, 48–102. [Google Scholar] [CrossRef] [Green Version]
- Araniti, F.; Landi, M.; Lupini, A.; Sunseri, F.; Guidi, L.; Abenavoli, M.R. Origanum vulgare essential oils inhibit glutamate and aspartate metabolism altering the photorespiratory pathway in Arabidopsis thaliana seedlings. J. Plant Physiol. 2018, 231, 297–309. [Google Scholar] [CrossRef]
- Zhang, D.J.; Zhang, J.; Yang, W.Q.; Wu, F.Z. Potential allelopathic effect of Eucalyptus grandis across a range of plantation ages. Ecol. Res. 2010, 25, 13–23. [Google Scholar] [CrossRef]
- Tworkoski, T. Herbicide effects of essential oils. Weed Sci. 2002, 50, 425–431. [Google Scholar] [CrossRef]
- Angelini, L.G.; Carpanese, G.; Cioni, P.L.; Morelli, I.; Macchia, M.; Flamini, G. Essential oils from Mediterranean Lamiaceae as weed germination inhibitors. J. Agric. Food Chem. 2003, 51, 6158–6164. [Google Scholar] [CrossRef] [PubMed]
- Pirbalouti, A.G.; Hashemi, M.; Ghahfarokhi, F.T. Essential oil and chemical compositions of wild and cultivated Thymus daenensis Celak and Thymus vulgaris L. Ind. Crops Prod. 2013, 48, 43–48. [Google Scholar] [CrossRef]
- XiaoHua, C.; FangYuan, Z.; Lei, Y. Chloroplast DNA molecular characterization and leaf volatiles analysis of mint (Mentha; Lamiaceae) populations in China. Ind. Crops Prod. 2012, 37, 270–274. [Google Scholar]
- Gharib, F.A.; da Silva, J.T. Composition, total phenolic content, and antioxidant activity of the essential oil of four Lamiaceae herbs. Med. Aromatic Plant Sci. Biotechnol. 2013, 7, 19–27. [Google Scholar]
- Okmen, A.S.; Okmen, G.; Arslan, A.; Vurkun, M. Antibacterial activities of Mentha piperita L. extracts against bacteria isolated from soccer player’s shoes and its antioxidant activities. Indian J. Pharm. Educ. Res. 2017, 51, 163–169. [Google Scholar] [CrossRef]
- Mahdavikia, F.; Saharkhiz, M.J. Phytotoxic activity of essential oil and water extract of peppermint (Mentha× piperita L. CV. Mitcham). J. Appl. Res. Med. Aromatic Plants 2015, 2, 146–153. [Google Scholar] [CrossRef]
- Rolli, E.; Marieschi, M.; Maietti, S.; Sacchetti, G.; Bruni, R. Comparative phytotoxicity of 25 essential oils on pre-and post-emergence development of Solanum lycopersicum L.: A multivariate approach. Ind. Crops Prod. 2014, 60, 280–290. [Google Scholar] [CrossRef]
- Lawrence, B.M.; Tucker, A.O. The genus Thymus as a source of commercial products. In Thyme, the Genus Thymus; Stahl-Biskup, E., Sáez, F., Eds.; Taylor & Francis: London, UK, 2002; pp. 252–262. [Google Scholar]
- Morales, R. The history, botany, and taxonomy of the genus Thymus. In Thyme, the Genus Thymus; Stahl-Biskup, E., Sáez, F., Eds.; Taylor & Francis: London, UK, 2002; pp. 1–43. [Google Scholar]
- Barros, L.; Heleno, S.A.; Carvalho, A.M.; Ferreira, I.C. Lamiaceae often used in Portuguese folk medicine as a source of powerful antioxidants: Vitamins and phenolics. LWT-Food Sci. Technol. 2010, 43, 544–550. [Google Scholar] [CrossRef]
- Solomakos, N.; Govaris, A.; Koidis, P.; Botsoglou, N. The antimicrobial effect of thyme essential oil, nisin, and their combination against Listeria monocytogenes in minced beef during refrigerated storage. Food Microbiol. 2008, 25, 120–127. [Google Scholar] [CrossRef]
- Bozin, B.; Mimica-Dukic, N.; Simin, N.; Anackov, G. Characterization of the volatile composition of essential oils of some Lamiaceae spices and the antimicrobial and antioxidant activities of the entire oils. J. Agric. Food Chem. 2006, 54, 1822–1828. [Google Scholar] [CrossRef]
- Gagliano Candela, R.; Maggi, F.; Lazzara, G.; Rosselli, S.; Bruno, M. The Essential Oil of Thymbra capitata and its application as a biocide on stone and derived surfaces. Plants 2019, 8, 300. [Google Scholar] [CrossRef] [Green Version]
- Küçükbay, F.Z.; Kuyumcu, E.; Çelen, S.; Azaz, A.D.; Arabacı, T. Chemical composition of the essential oils of three Thymus Taxa from Turkey with antimicrobial and antioxidant activities. Rec. Nat. Prod. 2014, 8, 252–262. [Google Scholar]
- Verdeguer, M. Fitotoxicidad de aceites Esenciales y Extractos Acuosos de Plantas Mediterráneas para el Control de Arvenses. Ph.D. Thesis, Universitat Politècnica de València, Valencia, Spain, 2011. [Google Scholar]
- Verdeguer, M.; Torres-Pagan, N.; Muñoz, M.; Jouini, A.; García-Plasencia, S.; Chinchilla, P.; Berbegal, M.; Salamone, A.; Agnello, S.; Carrubba, A.; et al. Herbicidal Activity of Thymbra capitata (L.) Cav. Essential Oil. Molecules 2020, 25, 2832. [Google Scholar] [CrossRef] [PubMed]
- Cuellar, M.J.; Giner, R.M.; Recio, M.C.; Just, M.J.; Máñez, S.; Cerda, S.; Ríos, J.L. Screening of antiinflammatory medicinal plants used in traditional medicine against skin diseases. Phytother. Res. 1998, 12, 18–23. [Google Scholar] [CrossRef]
- Grosso, C.; Coelho, J.A.; Urieta, J.S.; Palavra, A.M.; Barroso, J.G. Herbicidal activity of volatiles from coriander, winter savory, cotton lavender, and thyme isolated by hydrodistillation and supercritical fluid extraction. J. Agric. Food Chem. 2010, 58, 11007–11013. [Google Scholar] [CrossRef]
- Vokou, D.; Liotiri, S. Stimulation of soil microbial activity by essential oils. Chemoecology 1999, 9, 41–45. [Google Scholar] [CrossRef]
- Vokou, D.; Chalkos, D.; Karamanlidou, G.; Yiangou, M. Activation of soil respiration and shift of the microbial population balance in soil as a response to Lavandula stoechas essential oil. J. Chem. Ecol. 2002, 28, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Khare, P.; Srivastava, S.; Nigam, N.; Singh, A.K.; Singh, S. Impact of essential oils of E. citriodora, O. basilicum and M. arvensis on three different weeds and soil microbial activities. Environ. Technol. Innov. 2019, 14, 100343. [Google Scholar] [CrossRef]
- Verdeguer, M.; Blazquez, M.A.; Boira, H. Phytotoxic effects of Lantana camara, Eucalyptus camaldulensis and Eriocephalus africanus essential oils in weeds of Mediterranean summer crops. Biochem. System. Ecol. 2009, 37, 362–369. [Google Scholar] [CrossRef]
- Anderson, T.H. Microbial eco-physiological indicators to assess soil quality. Agric. Ecosyst. Environ. 2003, 98, 285–293. [Google Scholar] [CrossRef]
- Kaur, A.; Chaudhary, A.; Kaur, A.; Choudhary, R.; Kaushik, R. Phospholipid fatty acid—A biondicator of environment monitoring and assessment in soil ecosystem. Curr. Sci. 2005, 89, 1103–1112. [Google Scholar]
- Laudicina, V.A.; Dennis, P.G.; Palazzolo, E.; Badalucco, L. Key Biochemical Attributes to Assess Soil Ecosystem Sustainability. In Environmental Protection Strategies for Sustainable Development. Strategies for Sustainability; Malik, A., Grohmann, E., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 193–227. [Google Scholar]
- Heap, I. The International Herbicide-Resistant Weed Database. 2020. Available online: www.weedscience.org (accessed on 28 September 2020).
- Juliano, L.M.; Casimero, M.C.; Llewellyn, R. Multiple herbicide resistance in barnyardgrass (Echinochloa crus-galli) in direct-seeded rice in the Philippines. Int. J. Pest Manag. 2010, 56, 299–307. [Google Scholar] [CrossRef]
- Malassi, R.N. Herbicide effects on cuticle ultrastructure in Eleusine indica and Portulaca oleracea. Biocell 2006, 30, 51–56. [Google Scholar] [CrossRef]
- Norsworthy, J.K.; Smith, J.P. Tolerance of leafy greens to preemergence and postemergence herbicides. Weed Technol. 2005, 19, 724–730. [Google Scholar] [CrossRef]
- Riar, D.S.; Norsworthy, J.K.; Srivastava, V.; Nandula, V.; Bond, J.A.; Scott, R.C. Physiological and molecular basis of acetolactate synthase-inhibiting herbicide resistance in barnyardgrass (Echinochloa crus-galli). J. Agric. Food Chem. 2013, 61, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Avila, L.A.; Lee, D.J.; Senseman, S.A.; McCauley, G.N.; Chandler, J.M.; Cothren, J.T. Assessment of acetolactate synthase (ALS) tolerance to imazethapyr in red rice ecotypes (Oryza spp) and imidazolinone tolerant/resistant rice (Oryza sativa) varieties. Pest Manag. Sci. 2005, 61, 171–178. [Google Scholar] [CrossRef]
- Liu, R. Survey, Ecology and Management of Problematic Weeds in Rice Production in Texas. Ph.D. Thesis, Texas A & M University, College Station, TX, USA, 2018. [Google Scholar]
- Chaimovitsh, D.; Abu-Abied, M.; Belausov, E.; Rubin, B.; Dudai, N.; Sadot, E. Microtubules are an intracellular target of the plant terpene citral. Plant J. 2010, 61, 399–408. [Google Scholar] [CrossRef]
- Duke, S.O.; Oliva, A.; Macias, F.; Galindo, J.; Molinillo, J.; Cutler, H. Mode of action of phytotoxic terpenoids. In Allelopathy: Chemistry and Mode of Action of Allelochemicals; CRC Press: Boca Raton, FL, USA, 2004; pp. 201–217. [Google Scholar]
- Araniti, F.; Grana, E.; Krasuska, U.; Bogatek, R.; Reigosa, M.J.; Abenavoli, M.R.; Sanchez-Moreiras, A.M. Loss of gravitropism in farnesene-treated arabidopsis is due to microtubule malformations related to hormonal and ROS unbalance. PLoS ONE 2016, 11, e0160202. [Google Scholar] [CrossRef] [Green Version]
- Graña, E.; Sotelo, T.; Díaz-Tielas, C.; Araniti, F.; Krasuska, U.; Bogatek, R.; Reigosa, M.J.; Sánchez-Moreiras, A.M. Citral induces auxin and ethylene-mediated malformations and arrests cell division in Arabidopsis thaliana roots. J. Chem. Ecol. 2013, 39, 271–282. [Google Scholar] [CrossRef] [Green Version]
- Karousou, R.; Koureas, D.N.; Kokkini, S. Essential oil composition is related to the natural habitats: Coridothymus capitatus and Satureja thymbra in NATURA 2000 sites of Crete. Phytochemistry 2005, 66, 2668–2673. [Google Scholar] [CrossRef]
- Bounatirou, S.; Smiti, S.; Miguel, M.G.; Faleiro, L.; Rejeb, M.N.; Neffati, M.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G. Chemical composition, antioxidant and antibacterial activities of the essential oils isolated from Tunisian Thymus capitatus Hoff. et Link. Food Chem. 2007, 105, 146–155. [Google Scholar] [CrossRef]
- Miceli, A.; Negro, C.; Tommasi, L. Essential oil variability in Thymbra capitata (L.) Cav. growing wild in Southern Apulia (Italy). Biochem. Syst. Ecol. 2006, 34, 528–553. [Google Scholar] [CrossRef]
- Garg, S.N.; Gupta, D.; Mehta, V.K.; Kumar, S. Volatile constituents of the essential oil of Santolina chamaecyparissus Linn, from the Southern hills of India. J. Essent. Oil Res. 2001, 13, 234–235. [Google Scholar] [CrossRef]
- Brahmi, F.; Khodir, M.; Mohamed, C.; Pierre, D. Chemical composition and biological activities of Mentha species. In Aromatic and Medicinal Plants—Back to Nature; El-Shemy, H.A., Ed.; InTech: London, UK, 2017; pp. 47–80. [Google Scholar]
- García-Plasencia, S. Actividad Herbicida del Aceite Esencial de Thymus capitatus (L.) Hoffmanns. et Link. y Efectividad en Función de Distintos Métodos de Aplicación. Master’s Thesis, Universitat Politècnica de València, Valencia, Spain, 2013. [Google Scholar]
- Hanana, M.; Mansour, M.B.; Algabr, M.; Amri, I.; Gargouri, S.; Romane, A.; Jamoussi, B.; Hamrouni, L. Potential use of essential oils from four Tunisian species of Lamiaceae: Biological alternative for fungal and weed control. Rec. Nat. Prod. 2017, 11, 258–269. [Google Scholar]
- Katz, D.A.; Sneh, B.; Friedman, J. The allelopathic potential of Coridothymus capitatus L. (Labiatae). Preliminary studies on the roles of the shrub in the inhibition of annuals germination and/or to promote allelopathically active actinomycetes. Plant Soil 1987, 98, 53–66. [Google Scholar] [CrossRef]
- Saoud, I.; Hamrouni, L.; Gargouri, S.; Amri, I.; Hanana, M.; Fezzani, T. Chemical composition weed killer and antifungal activities of Tunisian thyme (Thymus capitatus Hoff. et Link.) essential oils. Acta Aliment. Hung. 2013, 42, 417–427. [Google Scholar] [CrossRef]
- Kordali, S.; Cakir, A.; Ozer, H.; Cakmakci, R.; Kesdek, M.; Mete, E. Antifungal, phytotoxic and insecticidal properties of essential oil isolated from Turkish Origanum acutidens and its three components, carvacrol, thymol and p-cymene. Bioresour. Technol. 2008, 99, 8788–8795. [Google Scholar] [CrossRef]
- De Assis Alves, T.; Pinheiro, P.F.; Praça-Fontes, M.M.; Andrade-Vieira, L.F.; Corrêa, K.B.; de Assis Alves, T.; da Cruz, F.A.; Lacerda Júnior, V.; Ferreira, A.; Soares, T.C.B. Toxicity of thymol, carvacrol and their respective phenoxyacetic acids in Lactuca sativa and Sorghum bicolor. Ind. Crops Prod. 2018, 114, 59–67. [Google Scholar] [CrossRef]
- Campiglia, E.; Mancinelli, R.; Cavalieri, A.; Caporali, F. Use of essential oils of cinnamon (Cinnamomum zeylanicum L.), lavender (Lavandula spp.) and peppermint (Mentha x piperita L.) for weed control. Ital. J. Agron. 2007, 58, 171–175. [Google Scholar] [CrossRef]
- Maffei, M.; Camusso, W.; Sacco, S. Effect of Mentha × piperita essential oil and monoterpenes on cucumber root membrane potential. Phytochemistry 2001, 58, 703–707. [Google Scholar] [CrossRef]
- Mucciarelli, M.; Camusso, W.; Bertea, C.M.; Bossi, S.; Maffei, M. Effect of (+)-pulegone and other oil components of Mentha×piperita on cucumber respiration. Phytochemistry 2001, 57, 91–98. [Google Scholar] [CrossRef]
- De Elguea-Culebras, G.O.; Sanchez-Vioque, R.; Berruga, M.I.; Herraiz-Penalver, D.; Gonzalez-Coloma, A.; Andres, M.F.; Santana-Meridas, O. Biocidal potential and chemical composition of industrial essential oils from Hyssopus officinalis, Lavandula × intermedia var. super, and Santolina chamaecyparissus. Chem. Biodivers. 2018, 15, 1–9. [Google Scholar] [CrossRef]
- Antisari, L.V.; Laudicina, V.A.; Gatti, A.; Carbone, S.; Badalucco, L.; Vianello, G. Soil microbial biomass carbon and fatty acid composition of earthworm Lumbricus rubellus after exposure to engineered nanoparticles. Biol. Fertil. Soils 2015, 51, 261–269. [Google Scholar] [CrossRef]
- Monokrousos, N.; Papatheodorou, E.M.M.; Diamantopoulos, J.D.D.; Stamou, G.P.P. Temporal and spatial variability of soil chemical and biological variables in a Mediterranean shrubland. For. Ecol. Manag. 2004, 202, 83–91. [Google Scholar] [CrossRef]
- Papatheodorou, E.M.; Monokrousos, N.; Chalkos, D.; Karris, G.; Argyropoulou, M.L.; Diamantopoulos, J. Biological and biochemical parameters distinguishing soil microsites under different mediterranean shrub species. In 7. International Meeting on Soils with Mediterranean Type of Climate (Selected Papers); Zdruli, P., Steduto, P., Eds.; 2002; pp. 37–44. [Google Scholar]
- Helander, I.M.; Alakomi, H.L.; Latva-Kala, K.; Mattila-Sandholm, T.; Pol, I.; Smid, E.J.; Gorris, L.G.M.; von Wright, A. Characterization of the action of selected essential oil components on gram-negative bacteria. J. Food Chem. 1998, 46, 3590–3595. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, F.; Ji, B.P.; Pei, S.S.; Xu, N. The antibacterial mechanism of carvacrol and thymol against Escherichia coli. Lett. Appl. Microbiol. 2008, 47, 174–179. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.; Landi, L.; Pietramellara, G.; Renella, G. Microbial diversity and soil functions. Eur. J. Soil Sci. 2003, 54, 655–670. [Google Scholar] [CrossRef]
- Antisari, L.V.; Carbone, S.; Gatti, A.; Ferrando, S.; Nacucchi, M.; De Pascalis, F.; Gambardella, C.; Badalucco, L.; Laudicina, V.A. Effect of cobalt and silver nanoparticles and ions on Lumbricus rubellus health and on microbial community of earthworm faeces and soil. Appl. Soil Ecol. 2016, 10, 62–71. [Google Scholar] [CrossRef]
- Wardle, D.A.; Ghani, A. A tale of two theories, a chronosequence and a bioindicator of soil quality. Soil Biol. Biochem. 2018, 121, A3–A7. [Google Scholar] [CrossRef]
- İşcan, G.; Kïrïmer, N.; Kürkcüoǧlu, M.; Başer, H.C.; Demïrcï, F. Antimicrobial Screening of Mentha piperita Essential Oils. J. Agric. Food Chem. 2002, 50, 3943–3946. [Google Scholar] [CrossRef]
- Zakarya, D.; Fkih-Tetouani, S.; Hajji, F. Chemical composition antimicrobial activity relationship of Eucalyptus essential oils. Plantes Méd. Phytothérapie 1993, 26, 331–339. [Google Scholar]
- Allison, S.D.; Martiny, J.B. Resistance, resilience, and redundancy in microbial communities. Proc. Natl. Acad. Sci. USA 2008, 105, 11512–11519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khubeiz, M.J.; Mansour, G. In vitro antifungal, antimicrobial properties and chemical composition of Santolina chamaecyparissus essential oil in Syria. Int. J. Toxicol. Pharm. Res. 2016, 8, 372–378. [Google Scholar]
- Morcia, C.; Malnati, M.; Terzi, V. In vitro antifungal activity of terpinen-4-ol, eugenol, carvone, 1, 8-cineole (eucalyptol) and thymol against mycotoxigenic plant pathogens. Food Addit. Contam. A 2012, 29, 415–422. [Google Scholar]
- Viljoen, A.; Van Vuuren, S.; Ernst, E.; Klepser, M.; Demirci, B.; Başer, H.; Van Wyk, B.E. Osmitopsis asteriscoides (Asteraceae)-the antimicrobial activity and essential oil composition of a Cape-Dutch remedy. J. Ethnopharmacol. 2003, 88, 137–143. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Liptzin, D. C:N:P stoichiometry in soil: Is there a ‘‘Redfield ratio’’ for the microbial biomass? Biogeochemistry 2007, 85, 235–252. [Google Scholar] [CrossRef]
- Badalamenti, E.; Gristina, L.; Laudicina, V.A.; Novara, A.; Pasta, S.; La Mantia, T. The impact of Carpobrotus cfr. acinaciformis (L.) L. Bolus on soil nutrients, microbial communities structure and native plant communities in Mediterranean ecosystems. Plant Soil 2016, 409, 19–34. [Google Scholar] [CrossRef]
- Potter, T.L.; Truman, C.C.; Strickland, T.C.; Bosch, D.D.; Webster, T.M. Herbicide incorporation by irrigation and tillage impact on runoff loss. J. Environ. Qual. 2008, 37, 839–847. [Google Scholar] [CrossRef] [Green Version]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. Microbial biomass measurements in forest soils: The use of the chloroform fumigation-incubation method in strongly acid soils. Soil Biol. Biochem. 1987, 19, 697–702. [Google Scholar] [CrossRef]
- Laudicina, V.A.; Palazzolo, E.; Badalucco, L. Natural organic compounds in soil solution: Potential role as soil quality indicators. Curr. Org. Chem. 2013, 17, 2991–2997. [Google Scholar] [CrossRef]
- Joergensen, R.G.; Brookes, P.C. Ninhydrin-reactive nitrogen measurements of microbial biomass in 0.5 M K2SO4 soil extracts. Soil Biol. Biochem. 1990, 22, 1023–1027. [Google Scholar] [CrossRef]
- Ioppolo, A.; Laudicina, V.A.; Badalucco, L.; Saiano, F.; Palazzolo, E. Wastewaters from citrus processing industry as natural biostimulants for soil microbial community. J. Environ. Manag. 2020, 273, 111137. [Google Scholar] [CrossRef]
- Wu, Y.; Ding, N.; Wang, G.; Xu, J.; Wu, J.; Brookes, P.C. Effects of different soil weights, storage times and extraction methods on soil phospholipid fatty acid analyses. Geoderma 2009, 150, 171–178. [Google Scholar] [CrossRef]

| Group of Chemical Compounds | T. capitata | M. piperita | S. chamaecyparissus |
|---|---|---|---|
| Monoterpene hydrocarbons | 22.54 (9) | 1.95 (10) | 9.30 (8) |
| Oxygenated monoterpenes | 73.98 (6) | 95.35 (16) | 39.32 (14) |
| Sesquiterpene hydrocarbons | 3.14 (1) | 2.22 (5) | 21.78 (8) |
| Oxygenated sesquiterpenes | 0.14 (1) | 0.00 (0) | 15.64 (6) |
| Others | 0.00 (0) | 0.14 (4) | 12.91(3) |
| Treatment/Dose | E | APL cm | RL cm | TL cm | FW g | DW g | DL |
|---|---|---|---|---|---|---|---|
| Cw | 0 d | 9.9 a | 7.4 a | 17.3 a | 0.48 a | 0.09 a | 0.2 c |
| Cf | 0 d | 11.4 a | 6.5 a | 17.9 a | 0.46 a | 0.08 a | 0.1 c |
| T1 | 30 c | 5.9 b | 2.6 b | 8.5 b | 0.32 ab | 0.04 b | 1.3 b |
| T2 | 100 a | 0 e | 0 c | 0 d | 0 d | 0 c | 3.0 a |
| T3 | 100 a | 0 e | 0 c | 0 d | 0 d | 0 c | 3.0 a |
| M1 | 50 bc | 5 bc | 2.9 b | 8.1 b | 0.27 bc | 0.04 b | 1.6 b |
| M2 | 100 a | 0 e | 0 c | 0 d | 0 d | 0 c | 3.0 a |
| M3 | 100 a | 0 e | 0 c | 0 d | 0 d | 0 c | 3.0 a |
| S1 | - | - | - | - | - | - | - |
| S2 | 60 b | 2.9 cd | 1.7 bc | 4.5 bc | 0.13 cd | 0.02 bc | 2.0 b |
| S3 | 90 a | 0.8 de | 0.4 c | 1.2 cd | 0.03 d | 0 c | 2.8 a |
| Treatment/Dose | E | APL cm | RL cm | TL cm | FW g | DW g | DL |
|---|---|---|---|---|---|---|---|
| Cw | 0 c | 9.3 a | 10.9 a | 19.1 a | 1.6 a | 0.20 a | 0 c |
| Cf | 0 c | 8.9 a | 11.3 a | 20.2 a | 1.5 a | 0.17 a | 0 c |
| T1 | 0 c | 8.3 ab | 8.5 abc | 16.9 ab | 1.3 ab | 0.14 ab | 0 c |
| T2 | 40 b | 4.9 cd | 5.5 cd | 10.4 de | 0.7 c | 0.07 c | 1.2 b |
| T3 | 90 a | 0.7 e | 0.7 e | 1.3 f | 0.1 d | 0 e | 2.7 a |
| M1 | 30 bc | 6.0 bc | 6.1 bcd | 12.1 bcd | 1.0 bc | 0.10 bc | 0.9 bc |
| M2 | 40 b | 5.1 cd | 5.3 cd | 10.7 cde | 0.7 c | 0.08 c | 1.2 b |
| M3 | 40 b | 3.0 de | 2.8 de | 5.7 ef | 0.6 cd | 0.05 ce | 1.2 b |
| S1 | 0 c | 8.0 ab | 8.4 abc | 16.4 abc | 1.6 ab | 0.16 a | 0 c |
| S2 | 10 bc | 7.9 ab | 8.7 abc | 16.7 abc | 1.6 ab | 0.16 a | 0.3 bc |
| S3 | 30 bc | 5.8 bc | 6.1 bcd | 11.9 bcd | 0.9 bc | 0.08 bc | 0.9 bc |
| Treatment/Dose | E | APL cm | RL cm | TL cm | FW g | DW g | DL |
|---|---|---|---|---|---|---|---|
| Cw | 0 e | 28.0 a | 18.1 a | 46.1 a | 1.02 b | 0.19 b | 0.15 d |
| Cf | 0 e | 28.5 a | 17.7 a | 46.2 a | 1.45 a | 0.23 a | 0.15 d |
| T1 | 80 abc | 6.0 cd | 3.3 c | 10.4 cd | 0.20 d | 0.02 d | 3.2 ab |
| T2 | 90 ab | 2.4 cd | 1.8 c | 4.2 cd | 0.09 d | 0.02 d | 3.6 ab |
| T3 | 100 a | 0 d | 0 c | 0 d | 0 d | 0 d | 4.0 a |
| M1 | 70 bc | 5.0 cd | 4.1 c | 9.2 cd | 0.09 d | 0 d | 3.0 b |
| M2 | 90 ab | 1.4 d | 0.5 c | 1.9 cd | 0.01 d | 0 d | 3.7 ab |
| M3 | 100 a | 0 d | 0 c | 0 d | 0 d | 0 d | 4.0 a |
| S1 | 30 d | 16.3 b | 9.3 b | 25.6 b | 0.57 c | 0.09 b | 1.4 c |
| S2 | 60 c | 8.8 c | 3.7 c | 12.6 c | 0.16 d | 0.02 d | 2.9 b |
| S3 | 60 c | 5.0 cd | 3.5 c | 8.5 cd | 0.06 d | 0.01 d | 3.1 ab |
| Treatment/Dose | E | APL cm | RL cm | TL cm | FW g | DW g | DL |
|---|---|---|---|---|---|---|---|
| Cw | 0 d | 28.6 a | 20.8 a | 49.5 a | 1.20 a | 0.15 a | 0.10 f |
| Cf | 0 d | 27.3 ab | 19.9 a | 47.2 a | 0.90 ab | 0.11 ab | 0.10 f |
| T1 | 10 cd | 26.1 ab | 17.2 ab | 43.3 ab | 0.91 ab | 0.10 ab | 0.9 e |
| T2 | 50 b | 14.6 cd | 9.9 cd | 24.5 cd | 0.40 cde | 0.06 bcde | 2.4 bc |
| T3 | 100 a | 0 f | 0 f | 0 f | 0 f | 0 f | 4.0 a |
| M1 | 40 bc | 10.4 cde | 7.8 cde | 18.5 cde | 0.21 def | 0.03 def | 1.8 cd |
| M2 | 50 b | 6.1 def | 5.6 def | 11.7 def | 0.16 ef | 0.02 ef | 3.7 ab |
| M3 | 90 a | 2.3 ef | 2.5 ef | 4.8 ef | 0.21 f | 0 f | 4.0 a |
| S1 | 10 cd | 19.2 bc | 13.1 bc | 32.3 bc | 0.70 bc | 0.1 bc | 0.8 de |
| S2 | 30 bcd | 17.2 c | 12.3 bc | 29.5 bc | 0.54 cd | 0.1 bcd | 1.8 cd |
| S3 | 40 bc | 12.2 cd | 6.9 cde | 19.1 cde | 0.32 def | 0.04 cdef | 2.3 bc |
| Species | Efficacy |
|---|---|
| Amaranthus retroflexus | 64 a |
| Portulaca oleracea | 25 c |
| Avena fatua | 52 a |
| Echinochloa crus-galli | 38 b |
| Treatment/Dose | Efficacy |
| Cw | 0 g |
| Cf | 0 g |
| T1 | 30 ef |
| T2 | 70 bc |
| T3 | 97 a |
| M1 | 47 d |
| M2 | 70 bc |
| M3 | 82 ab |
| S1 | 13 fg |
| S2 | 40 de |
| S3 | 55 cd |
| Treatment/Dose | Day | qCO2 | Bacteria | Fungi | BacG+ | BacG− | F/B | BacG+/BacG− |
|---|---|---|---|---|---|---|---|---|
| Cf | 7 | 4.2 a | 76 a | 13 b | 32 a | 44 a | 0.17 a | 0.72 a |
| Cf | 28 | 2.8 b | 85 a | 19 a | 43 a | 41 a | 0.22 a | 1.06 a |
| Cf | 56 | 1.5 c | 82 a | 17 ab | 47 a | 36 b | 0.23 a | 1.28 a |
| THY1 | 7 | 2.4 Ca | 67 Ba | 15 Ab | 30 ABa | 37 Ba | 0.22 Bb | 0.81 Aa |
| 28 | 1.8 Bb | 69 Aa | 16 Aab | 30 Aa | 39 Aa | 0.23 Ab | 0.77 Aab | |
| 56 | 1.2 Bc | 16 Ab | 20 Aa | 6 Ab | 9 Ab | 1.31 Aa | 0.71 Bb | |
| THY2 | 7 | 3.2 Ba | 55 Ba | 12 Ba | 24 Ba | 31 Ba | 0.22 Bb | 0.91 Ab |
| 28 | 1.8 Bb | 64 Aa | 13 Ba | 27 Aa | 36 Aa | 0.20 Ab | 0.74 Ab | |
| 56 | 0.9 Bc | 32 Ab | 13 ABa | 19 Aa | 13 Aa | 1.04 Aa | 1.55 Aa | |
| THY3 | 7 | 5.2 Aa | 97 Aa | 3 Cb | 35 Aa | 61 Aa | 0.03 Ba | 0.57 Aa |
| 28 | 2.7 Ab | 46 Bb | 4 Cb | 20 Bb | 27 Ba | 0.08 Bab | 0.74 Aa | |
| 56 | 2.9 Ab | 60 Aab | 9 Ba | 20 Ab | 40 Aa | 0.18 Ba | 0.69 Ba | |
| Day | 59 *** | 16 ** | 12 * | NS | 22 ** | 34 ** | 14 * | |
| Dose | 22 ** | 19 ** | 72 *** | 27 ** | 14 * | 19 ** | 22 ** | |
| Day × Dose | 14 * | 29 ** | 6 * | 26 ** | 24 ** | 31 ** | 29 ** | |
| MNT1 | 7 | 15.0 Aa | 72 Aa | 7 Bb | 15 ABc | 58 Aa | 0.11 Bb | 0.28 Ac |
| 28 | 2.3 Bb | 73 Aa | 10 Bab | 32 Ab | 41 Ab | 0.13 Cab | 0.77 Ab | |
| 56 | 1.9 Ab | 58 Aa | 10 Ba | 39 Aa | 20 ABc | 0.18 Bb | 2.56 ABa | |
| MNT2 | 7 | 15.8 Aa | 59 Aa | 11 Ab | 13 Bc | 46 Aa | 0.20 Aa | 0.31 Ac |
| 28 | 2.5 Bb | 62 Ba | 12 ABab | 30 ABb | 32 Bb | 0.20 Ba | 0.96 Ab | |
| 56 | 0.8 Bc | 55 Aa | 13 Ba | 41 Aa | 15 Bc | 0.24 Ba | 2.81 Aa | |
| MNT3 | 7 | 10.3 Ba | 66 Aa | 14 Ab | 18 Ac | 48 Aa | 0.21 Ab | 0.39 Ac |
| 28 | 2.0 Bb | 61 Bb | 15 Ab | 26 Bb | 34 Bab | 0.25 Ab | 0.76 Ab | |
| 56 | 0.9 Bc | 62 Aab | 23 Aa | 31 Aa | 31 Ab | 0.37 Aa | 1.02 Ba | |
| Day | 69 *** | NS | 18 ** | 51 *** | 53 *** | 24 ** | 52 *** | |
| Dose | 7 * | 44 *** | 54 *** | 23 ** | 5 * | 45 *** | 5 * | |
| Day × Dose | 22 ** | NS | 15 * | NS | 12 * | 8 * | 25 ** | |
| SNT1 | 7 | 3.9 Ba | 81 Aa | 27 Aa | 4 Bc | 78 Aa | 0.33 Aa | 0.05 Bc |
| 28 | 1.6 Cb | 93 Aa | 23 Ab | 44 Ab | 49 Ab | 0.24 Ab | 0.93 Ab | |
| 56 | 1.4 Ab | 74 ABa | 21 Ab | 68 Aa | 6 Bc | 0.30 Aab | 11.29 Aa | |
| SNT2 | 7 | 3.7 Ba | 80 Ac | 19 Ba | 10 Ac | 70 Aa | 0.23 ABa | 0.15 Ab |
| 28 | 2.0 Bb | 90 Ab | 17 Bab | 39 ABb | 51 Ab | 0.19 Bab | 0.76 Ab | |
| 56 | 1.1 Ac | 100 Aa | 16 Bb | 62 Aa | 38 Ac | 0.16 Bb | 1.73 Ca | |
| SNT3 | 7 | 4.9 Aa | 66 Ac | 9 Ca | 10 Ac | 56 Ba | 0.14 Ba | 0.18 Ab |
| 28 | 3.3 Ab | 86 Aa | 3 Cb | 36 Bb | 50 Aa | 0.03 Cb | 0.72 Ab | |
| 56 | 1.3 Ac | 56 Bb | 2 Cb | 48 Aa | 8 Bb | 0.03 Cb | 5.85 Ba | |
| Day | 81 *** | 14 * | NS | 72 *** | 61 *** | 5 * | 40 *** | |
| Dose | 7 * | 25 ** | 82 *** | NS | 6 * | 72 *** | 13 * | |
| Day × Dose | NS | 25 ** | 10 * | 16 ** | 28 ** | 13 * | 44 *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jouini, A.; Verdeguer, M.; Pinton, S.; Araniti, F.; Palazzolo, E.; Badalucco, L.; Laudicina, V.A. Potential Effects of Essential Oils Extracted from Mediterranean Aromatic Plants on Target Weeds and Soil Microorganisms. Plants 2020, 9, 1289. https://doi.org/10.3390/plants9101289
Jouini A, Verdeguer M, Pinton S, Araniti F, Palazzolo E, Badalucco L, Laudicina VA. Potential Effects of Essential Oils Extracted from Mediterranean Aromatic Plants on Target Weeds and Soil Microorganisms. Plants. 2020; 9(10):1289. https://doi.org/10.3390/plants9101289
Chicago/Turabian StyleJouini, Amira, Mercedes Verdeguer, Samuele Pinton, Fabrizio Araniti, Eristanna Palazzolo, Luigi Badalucco, and Vito Armando Laudicina. 2020. "Potential Effects of Essential Oils Extracted from Mediterranean Aromatic Plants on Target Weeds and Soil Microorganisms" Plants 9, no. 10: 1289. https://doi.org/10.3390/plants9101289