Differences in Physiological and Biochemical Attributes of Wheat in Response to Single and Combined Salicylic Acid and Biochar Subjected to Limited Water Irrigation in Saline Sodic Soil
Abstract
:1. Introduction
2. Results
2.1. Soil Physicochemical Parameters
2.2. The Percent of Na+ and K+ Content in Leaves
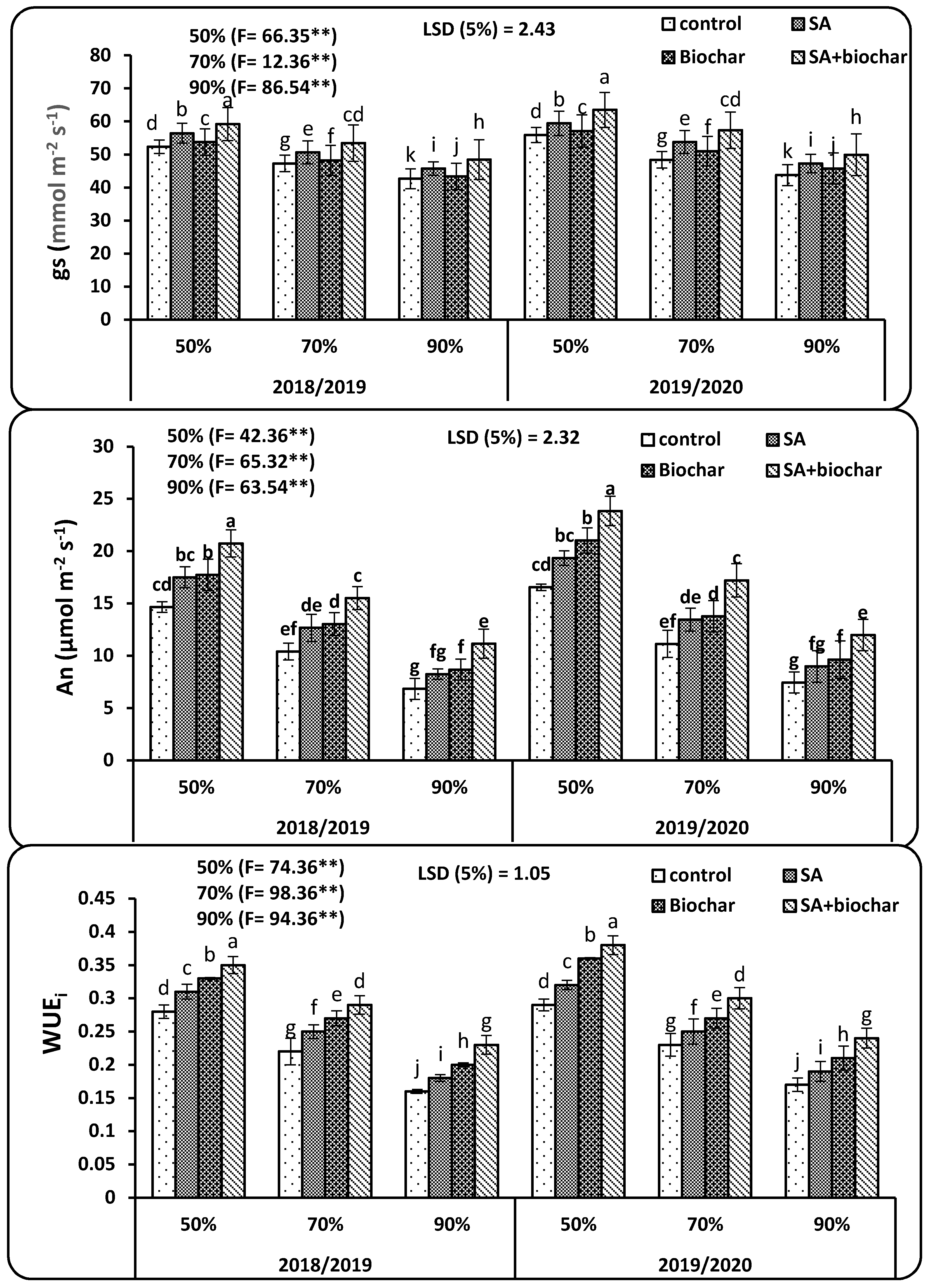
2.3. Physiological Properties of Wheat Plants
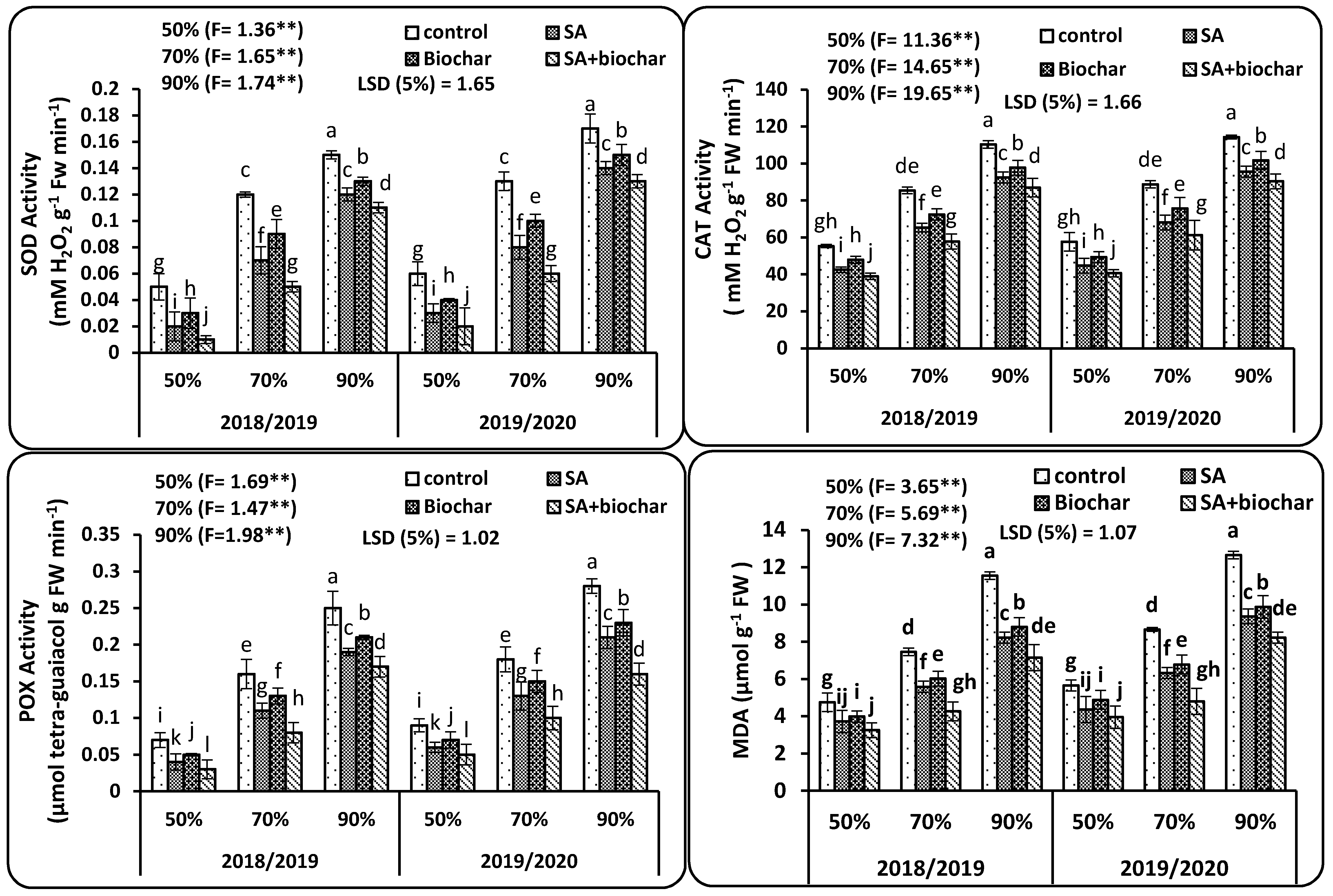
2.4. The Antioxidant Enzymatic Activity
Yield Related Traits and Productivity
2.5. Nutrient Uptake
3. Discussion
4. Materials and Methods
4.1. Study Site
4.2. Experimental Design and Crop Management
4.3. Preparation of Biochar and Salicylic Acid
4.4. Soil Physicochemical Properties
4.5. Physiological Measurements
Leaf Na+ and K+ Determination
4.6. Biochemical Analysis
Yield and Its Related Parameters
4.7. Nutrient Uptake
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Yang, J.; Liu, G.; Yao, R.; Yu, S. Impact of irrigation volume and water salinity on winter wheat productivity and soil salinity distribution. Agric. Water Manag. 2015, 149, 44–54. [Google Scholar] [CrossRef]
- Attia, A.; Rajan, N.; Xue, Q.; Nair, S.; Ibrahim, A.; Hays, D. Application of DSSAT-CERES-Wheat model to simulate winter wheat response to irrigation management in the Texas High Plains. Agric. Water Manag. 2016, 165, 50–60. [Google Scholar] [CrossRef]
- FAOSTAT. Food and agriculture organization of the United Nations statistics division. Available online: http://faostat.fao.org/site/567/DesktopDefault.aspx (accessed on 10 December 2019).
- Hafez, E.M.; Kobata, T. The effect of different nitrogen sources from urea and ammonium sulfate on the spikelet number in Egyptian spring wheat cultivars on well watered pot soils. Plant Prod. Sci. 2012, 15, 332–338. [Google Scholar] [CrossRef] [Green Version]
- MALR (Ministry of Agriculture and Land Reclamation). Sustainable Agricultural Development Strategy towards 2030; Ministry of Agriculture and Land Reclamation: Foreign Agricultural Relations: Cairo, Egypt, 2009. Available online: https://far-malr.gov.eg/pdf/en/Full%20SADS2030.pdf.
- FAO. The Future of Food and Agriculture: Trends and Challenges; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019. [Google Scholar]
- Tari, A.F. The effects of different deficit irrigation strategies on yield, quality, and water-use efficiencies of wheat under semi-arid conditions. Agric. Water Manag. 2016, 167, 1–10. [Google Scholar] [CrossRef]
- Boguszewska, D.; Zagdańska, B. ROS as signaling molecules and enzymes of plant response to unfavorable environmental conditions. In Oxidative Stress–Molecular Mechanisms and Biological Effects; InTech: Rijeka, Croatia, 2012; pp. 341–362. [Google Scholar] [CrossRef] [Green Version]
- Hafez, E.M.; Omara, A.; El-Esawi, M. Minimizing hazard impacts of soil salinity and water stress on wheat plants by integrated soil application of vermicompost and biochar. Physiologia Plantarum 2020. Accepted. [Google Scholar]
- Hafez, E.; Omara, A.E.D.; Ahmed, A. The Coupling Effects of Plant Growth Promoting Rhizobacteria and Salicylic Acid on Physiological Modifications, Yield Traits, and Productivity of Wheat under Water Deficient Conditions. Agronomy 2019, 9, 524. [Google Scholar] [CrossRef] [Green Version]
- Hafez, E.M.; Gharib, H.S. Effect of exogenous application of ascorbic acid on physiological and biochemical characteristics of wheat under water stress. Int. J. Plant Prod. 2016, 10, 579–596. [Google Scholar]
- Kamara, M.M.; Rehan, M.; Ibrahim, K.M.; Alsohim, A.S.; Elsharkawy, M.M.; Kheir, A.M.S.; Hafez, E.M.; El-Esawi, M.A. Genetic Diversity and Combining Ability of White Maize Inbred Lines under Different Plant Densities. Plants 2020, 9, 1140. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [Green Version]
- Ding, Z.; Kheir, A.M.S.; Ali, O.; Hafez, E.M.; Elshamey, E.A.; Zhou, Z.; Wang, B.; Lin, X.; Ge, Y.; Fahmy, A.E.; et al. Vermicompost and deep tillage system as an environmental method to improve saline-alkaline soils and wheat productivity. J. Environ. Manag. 2020, 277, 111388. [Google Scholar] [CrossRef]
- Chai, Q.; Gan, Y.T.; Zhao, C.; Xu, H.L.; Waskom, R.M.; Niu, Y.N.; Siddique, K.H.M. Regulated deficit irrigation for crop production under drought stress. Rev. Agron. Sustain. Dev. 2016, 36, 21. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Vinocur, B.; Altman, A. Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta 2003, 218, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Asadi, M.; Heidari, M.A.; Kazemi, M.; Filinejad, A.R. Salicylic acid induced changes in some physiological parameters in chickpea (Cicer arietinum L.) under salt stress. J. Agric. Sci. Technol. 2013, 9, 311–316. [Google Scholar]
- Hasanuzzaman, M.; Matin, M.; Fardus, J.; Hasanuzzaman, M.; Hossain, M.; Parvin, K. Foliar application of salicylic acid improves growth and yield attributes by upregulating the antioxidant defense system in Brassica campestris plants grown in lead-amended soils. Acta Agrobot. 2019, 72, 2. [Google Scholar] [CrossRef]
- Gunes, A.; Inal, A.; Alpaslan, M.; Cicek, N.; Guneri, E.; Eraslan, F. Effects of exogenously applied salicylic acid on the induction of multiple stress tolerance and mineral nutrition in maize (Zea mays L.). Arch. Agron. Soil Sci. 2007, 51, 687–695. [Google Scholar] [CrossRef]
- Kang, G.; Li, G.; Liu, G.; Xu, W.; Peng, X.; Wang, C.; Zhu, Y.; Guo, T. Exogenous salicylic acid enhances wheat drought tolerance by influence on the expression of genes related to ascorbate- glutathione cycle. Biol. Plants 2013, 57, 718–724. [Google Scholar] [CrossRef]
- Mutlu, S.; Ökke¸s, A.; Nalbanto˘glu, B.; Mete, E. Exogenous salicylic acid alleviates cold damage by regulating antioxidative system in two barley (Hordeum vulgare L.) cultivars. Front. Life Sci. 2016, 9, 99–109. [Google Scholar] [CrossRef] [Green Version]
- Hafez, E.; Farig, M. Efficacy of salicylic acid as a cofactor for ameliorating effects of water stress and enhancing wheat yield and water use efficiency in saline soil. Int. J. Plant Prod. 2019, 13, 163–176. [Google Scholar] [CrossRef]
- Rahmani, I.; Ahmadi, N.; Ghanati, F.; Sadeghi, M. Effects of salicylic acid applied pre-or post-transport on post-harvest characteristics and antioxidant enzyme activity of gladiolus cut flower spikes. N. Z. J. Crop Hortic. Sci. 2015, 43, 294–305. [Google Scholar] [CrossRef] [Green Version]
- Razmi, N.; Ebadi, A.; Daneshian, J.; Jahanbakhsh, S. Salicylic acid induced changes on antioxidant capacity, pigments and grain yield of soybean genotypes in water deficit condition. J. Plant Int. 2017, 12, 457–464. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Andersen, M.N.; Liu, F. Residual effects of biochar on improving growth, physiology and yield of wheat under salt stress. Agric. Water Manag. 158, 61–68. [CrossRef]
- Akhtar, S.S.; Andersen, M.N.; Liu, F. Biochar Mitigates Salinity Stress in Potato. J. Agron. Crop Sci. 2015, 201, 368–378. [Google Scholar] [CrossRef]
- Thi, N.; Xu, C.-Y.; Tahmasbian, I.; Che, R.; Xu, Z.; Zhou, X.; Wallace, H.M.; Bai, S.H. Effects of biochar on soil available inorganic nitrogen: A review and meta-analysis. Geoderma 2017, 288, 79–96. [Google Scholar]
- Leng, L.; Huang, H.; Li, H.; Li, J.; Zhou, W. Biochar stability assessment methods: A review. Sci. Total Environ. 2019, 647, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.F.; Refay, Y.; Al-Suhaibani, N.; Al-Ashkar, I.; El-Hendawy, S.; Hafez, E.M. Integrative Effects of Rice-Straw Biochar and Silicon on Oil and Seed Quality, Yield and Physiological Traits of Helianthus annuus L. Grown under Water Deficit Stress. Agronomy 2019, 9, 637. [Google Scholar] [CrossRef] [Green Version]
- Yang, A.; Akhtar, S.S.; Li, L.; Fu, Q.; Li, Q.; Naeem, M.A.; He, X.; Zhang, Z.; Jacobsen, S.-E. Biochar Mitigates Combined Effects of Drought and Salinity Stress in Quinoa. Agronomy 2020, 10, 912. [Google Scholar] [CrossRef]
- Zheng, W.; Sharma, B.K.; Rajagopalan, N. Using Biochar as a Soil Amendment for Sustainable Agriculture; USA Field Report; Illinois Department of Agriculture: Springfield, IL, USA, 2010. [Google Scholar]
- Hafez, E.M.; Alsohim, A.S.; Farig, M.; Omara, A.E.D.; Rashwan, E.; Kamara, M.M. Synergistic Effect of Biochar and Plant Growth Promoting Rhizobacteria on Alleviation of Water Deficit in Rice Plants under Salt-Affected Soil. Agronomy 2019, 12, 847. [Google Scholar] [CrossRef] [Green Version]
- Dahlawi, S.; Naeem, A.; Rengel, Z.; Naidu, R. Biochar application for the remediation of salt-affected soils: Challenges and opportunities. Sci. Total Environ. 2018, 625, 320–335. [Google Scholar]
- Hussain, M.; Farooq, M.; Nawaz, A.; Al-Sadi, A.M.; Solaiman, Z.M.; Alghamdi, S.S.; Ammara, U.; Ok, Y.S.; Siddique, K.H.M. Biochar for crop production: Potential benefits and risks. J. Soils Sediments 2017, 17, 685–716. [Google Scholar] [CrossRef]
- Duarte, D.J.; Glaser, B.; Cerri, P.; Eduardo, C. Effect of Biochar Particle Size on Physical, Hydrological and Chemical Properties of Loamy and Sandy Tropical Soils. Agronomy 2019, 9, 165. [Google Scholar] [CrossRef] [Green Version]
- Haider, G.; Steffens, D.; Moser, G.; Müller, C.; Kammann, C.I. Biochar reduced nitrate leaching and improved soil moisture content without yield improvements in a four-year field study. Agric. Ecosyst. Environ. 2017, 237, 80–94. [Google Scholar] [CrossRef]
- Hayat, Q.; Hayat, S.; Irfan, M.; Ahmad, A. Effect of exogenous salicylic acid under changing environment: A review. Environ. Exp. Bot. 2010, 68, 14–25. [Google Scholar] [CrossRef]
- Dzvene, A.R.; Chiduza, C.; Mnkeni, P.N.S.; Peter, P.C. Characterization of livestock biochars and their effect on selected soil properties and maize early growth stage in soils of Eastern Cape province, South Africa. S. Afr. J. Plant Soil 2019, 36, 199–209. [Google Scholar] [CrossRef]
- Omondi, M.O.; Xia, X.; Nahayo, A.; Liu, X.; Korai, P.K.; Pan, G. Quantification of biochar effects on soil hydrological properties using meta-analysis of literature data. Geoderma 2016, 274, 28–34. [Google Scholar] [CrossRef]
- Ijaz, M.; Tahir, M.; Shahid, M.; Ul-Allah, S.; Sattar, A.; Sher, A.; Mahmood, K.M. Combined application of biochar and PGPR consortia for sustainable production of wheat under semiarid conditions with a reduced dose of synthetic fertilizer. Braz. J. Microbiol. 2019, 50, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Yu, O.Y.; Harper, M.; Hoepfl, M.; Domermuth, D. Characterization of biochar and its effects on the water holding capacity of loamy sand soil: Comparison of hemlock biochar and switchblade grass biochar characteristics. Environ. Prog. Sustain. Energy 2017, 36, 1474–1479. [Google Scholar] [CrossRef]
- Jini, D.; Joseph, B. Physiological mechanism of salicylic acid for alleviation of salt stress in rice. Rice Sci. 2017, 24, 97–108. [Google Scholar] [CrossRef]
- Jones, H.G. Stomatal control of photosynthesis and transpiration. J. Exp. Bot. 1998, 49, 387–398. [Google Scholar] [CrossRef]
- Li, T.; Hu, Y.; Du, X.; Tang, H.; Shen, C. Salicylic acid alleviates the adverse effects of salt stress in Torreya grandis cv. Merrillii seedlings by activating photosynthesis and enhancing antioxidant systems. PLoS ONE 2014, 9, 92. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Malik, M.A.; Farooq, M.; Ashraf, M.Y.; Cheema, A. Improving Drought tolerance by exogenous application of glycinebetaine and salicylic acid in sunflower. J. Agron. Crop Sci. 2008, 194, 193–199. [Google Scholar] [CrossRef]
- Hafez, E.M.; Seleiman, M.F. Response of barley quality traits, yield and antioxidant enzymes to water-stress and chemical inducers. Int. J. Plant Prod. 2017, 11, 477–490. [Google Scholar]
- Arfan, M.; Athar, H.R.; Ashraf, M. Does exogenous application of salicylic acid through the rooting medium modulate growth and photosynthetic capacity in two differently adapted spring wheat cultivars under salt stress? J. Plant Physiol. 2007, 6, 685–694. [Google Scholar] [CrossRef]
- Hafez, E.M.; Ragab, A.Y.; Kobata, T. Water-Use Efficiency and Ammonium-N Source Applied of Wheat under Irrigated and Desiccated Conditions. Int. J. Plant Soil Sci. 2014, 3, 1302–1316. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirasteh-Anosheh, H.; Ranjbar, G.; Emam, Y.; Ashraf, M. Salicylic-acid-induced recovery ability in salt-stressed Hordeum vulgare plants. Turk. J. Bot. 2014, 38, 112–121. [Google Scholar] [CrossRef]
- Gharib, H.; Hafez, E.; El Sabagh, A. Optimized Potential of Utilization Efficiency and Productivity in Wheat by Integrated Chemical Nitrogen Fertilization and Stimulative Compounds. Cercetari Agronomice Moldova 2016, 49, 5–20. [Google Scholar] [CrossRef] [Green Version]
- Bistgani, Z.E.; Siadat, S.A.; Bakhshandeh, A.; Pirbalouti, A.G.; Hashemi, M. Morpho-physiological and phytochemical traits of (Thymus daenensis Celak.) in response to deficit irrigation and chitosan application. Acta Physiol. Plant 2017, 39, 231. [Google Scholar] [CrossRef]
- Hafez, E.M. Influence of salicylic acid on ion distribution, enzymatic activity and some agromorphological characteristics of wheat under salt-affected soil. Egyptian J. Agron. 2016, 38, 455–469. [Google Scholar] [CrossRef] [Green Version]
- Xie, W.J.; Wu, L.F.; Zhang, Y.P.; Wu, T.; Li, X.P.; Ouyang, Z. Effects of straw application on coastal saline topsoil salinity and wheat yield trend. Soil Till. Res. 2017, 169, 1–6. [Google Scholar] [CrossRef]
- Kheir, A.S.; Abou elsoud, H.M.; Hafez, E.M.; Ali, O.A. Integrated effect of nano-Zn, nano-Si, and drainage using crop straw-filled ditches on saline sodic soil properties and rice productivity. Arab. J. Geosci. 2019, 12, 471. [Google Scholar] [CrossRef]
- Seilsepour, M.; Rashidi, M. Prediction of soil cation exchange capacity based on some soil physical and chemical properties. World Appl. Sci. J. 2008, 3, 200–205. [Google Scholar]
- U.S.D.A. Diagnosis and Improvement of Saline and Alkali Soils. Agriculture Handbook No. 60; Richards, L.A., Ed.; U.S. Department of Agriculture: Washington, DC, USA, 1954; Volume 120, p. 800. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Sanchez, F.J.; de Andrés, E.F.; Tenorio, J.L.; Ayerbe, L. Growth of epicotyls, turgor maintenance and osmotic adjustment in pea plants (Pisum sativum L.) subjected to water stress. Field Crops Res. 2004, 86, 81–90. [Google Scholar] [CrossRef]
- Izanloo, A.; Anthony, G.; Thorsten, S. Different mechanisms of adaptation to cyclic water stress in two South Australian bread wheat cultivars. J. Exp. Bot. 2008, 59, 3327–3346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishiyama, R.; Watanabe, Y.; Fujita, Y.; Le, D.T.; Kojima, M.; Werner, T.; Vankova, R.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Kakimoto, T.; et al. Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. Plant Cell 2011, 23, 2169–2183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall: Englewood Cliffs, NJ, USA, 1958. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen Peroxide Is Scavenged by Ascorbate-Specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Aebi, H.E. Catalase. In Methods of Enzymatic Analysis, 3rd ed.; Verlag Chemie: Weinheim, Germany, 1983; pp. 273–286. [Google Scholar]
- Kara, M.; Mishra, D. Catalase, peroxidase, polyphenoloxidase activities during since leaf senescence. Plant Physiol. 1976, 54, 315–319. [Google Scholar] [CrossRef] [Green Version]
- Hammerschmidt, R.; Nuckles, E.M.; Ku´c, J. Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol. Plant Pathol. 1982, 20, 73–82. [Google Scholar] [CrossRef]
- Davenport, S.B.; Gallego, S.M.; Benavides, M.P.; Tomaro, M.L. Behavior of antioxidant defense system in the adaptive response to salt stress in (Helianthus annuus L.) cell. Plant Growth Reg. 2003, 40, 81–88. [Google Scholar] [CrossRef]
- Association of Official Agricultural Chemists. Official Methods of Analysis; Association of Official Agricultural Chemists: Washington, DC, USA, 1975; p. 832. [Google Scholar]
| Year | Treatments (Ts) | pH ¥ | EC § | ESP # | Na+¤ | K+ | Ca2+ | Mg2+ | |
|---|---|---|---|---|---|---|---|---|---|
| Water Ts | Soil and Foliar Ts | (dS m−1) | (%) | (meq L−1) | (meq L−1) | (meq L−1) | (meq L−1) | ||
| 2018/2019 | 50% DAM | Control | 8.03 ±0.00gh | 3.57 ±0.03ef | 11.03 ±0.22ef | 16.77 ±0.85ef | 0.42 ±0.02cd | 14.88 ±0.05d | 6.90 ±0.04d |
| SA † | 8.04 ±0.01h | 3.55 ±0.06fg | 10.97 ±0.12fg | 15.92 ±1.05fg | 0.43 ±0.05c | 15.10 ±0.09cd | 7.09 ±0.03cd | ||
| BC ‡ | 8.00 ±0.01i | 3.20 ±0.04g | 8.01 ±0.33g | 12.84 ±0.02g | 0.46 ±0.04b | 17.49 ±0.18b | 7.28 ±0.02b | ||
| SA+BC | 7.98 ±0.01j | 3.04 ±0.08h | 7.54 ±0.11h | 10.24 ±0.11h | 0.48 ±0.03a | 19.32 ±0.22a | 7.41 ±0.01a | ||
| 70% DAM | Control | 8.10 ±0.03cd | 3.95 ±0.07c | 15.23 ±0.36c | 20.55 ±1.08c | 0.37 ±0.00ef | 10.57 ±0.15fg | 6.69 ±0.09fg | |
| SA | 8.08 ±0.03e | 3.88 ±0.04d | 15.05 ±0.35d | 19.78 ±1.07d | 0.38 ±0.01e | 10.95 ±0.18f | 6.72 ±0.06f | ||
| BC | 8.05 ±0.02f | 3.64 ±0.05e | 12.87 ±0.24e | 17.23 ±0.99e | 0.41 ±0.82d | 13.45 ±0.08de | 6.97 ±0.05de | ||
| SA+BC | 8.02 ±0.01g | 3.52 ±0.02f | 10.91 ±0.15f | 16.07 ±0.87f | 0.43 ±0.03c | 15.85 ±0.07c | 7.02 ±0.08c | ||
| 90% DAM | Control | 8.17 ±0.02a | 4.74 ±0.06a | 22.93 ±0.23a | 27.44 ±0.98a | 0.33 ±0.02hi | 6.80 ±0.12hi | 6.08 ±0.02hi | |
| SA † | 8.16 ±0.02b | 4.68 ±0.05ab | 21.49 ±0.35ab | 26.64 ±0.88ab | 0.34 ±0.01h | 7.08 ±0.18h | 6.19 ±0.03h | ||
| BC ‡ | 8.11 ±0.01c | 4.15 ±0.03b | 17.92 ±0.24b | 21.84 ±0.75b | 0.36 ±0.03g | 9.16 ±0.23g | 6.54 ±0.08g | ||
| SA+BC | 8.09 ± 0.02d | 3.89 ±0.08cd | 15.19 ±0.15cd | 19.94 ±1.05cd | 0.38 ±0.02f | 11.24 ±0.25e | 6.72 ±0.07e | ||
| 2019/2020 | 50% DAM | Control | 8.01 ±0.00gh | 3.54 ±0.03ef | 10.99 ±0.22ef | 16.72 ±0.85ef | 0.44 ±0.02cd | 14.94 ±0.05d | 6.96 ±0.04d |
| SA † | 8.02 ±0.01h | 3.52 ±0.06fg | 10.93 ±0.12fg | 15.87 ±1.05fg | 0.45 ±0.05c | 15.16 ±0.09cd | 7.15 ±0.03cd | ||
| BC ‡ | 7.98 ±0.01i | 3.17 ±0.04g | 7.97 ±0.33g | 12.79 ±0.02g | 0.48 ±0.04b | 17.55 ±0.18b | 7.34 ±0.02b | ||
| SA+BC | 7.87 ±0.01j | 3.01 ±0.08h | 7.50 ±0.11h | 10.19 ±0.11h | 0.50 ±0.03a | 19.38 ±0.22a | 7.47 ±0.01a | ||
| 70% DAM | Control | 8.08 ±0.03cd | 3.92 ±0.07c | 15.19 ±0.36c | 20.50 ±1.08c | 0.39 ±0.00ef | 10.63 ±0.15fg | 6.75 ±0.09fg | |
| SA | 8.06 ±0.03e | 3.85 ±0.04d | 15.01 ±0.35d | 19.73 ±1.07d | 0.40 ±0.01e | 11.01 ±0.18f | 6.78 ±0.06f | ||
| BC | 8.03 ±0.02f | 3.61 ±0.05e | 12.83 ±0.24e | 17.17 ±0.99e | 0.43 ±0.82d | 13.51 ±0.08de | 7.03 ±0.05de | ||
| SA+BC | 8.00 ±0.01g | 3.49 ±0.02f | 10.87 ±0.15f | 16.02 ±0.87f | 0.45 ±0.03c | 15.91 ±0.07c | 7.08 ±0.08c | ||
| 90% DAM | Control | 8.15 ±0.02a | 4.71 ±0.06a | 22.89 ±0.23a | 27.39 ±0.98a | 0.35 ±0.02hi | 6.86 ±0.12hi | 6.14 ±0.02hi | |
| SA † | 8.14 ±0.02b | 4.65 ±0.05ab | 21.45 ±0.35ab | 26.59 ±0.88ab | 0.36 ±0.01h | 7.14 ±0.18h | 6.25 ±0.03h | ||
| BC ‡ | 8.09 ±0.01c | 4.12 ±0.03b | 17.88 ±0.24b | 21.79 ±0.75b | 0.38 ±0.03g | 9.22 ±0.23g | 6.60 ±0.08g | ||
| SA+BC | 8.07 ±0.02d | 3.86 ±0.08cd | 15.15 ±0.15cd | 19.89 ±1.05cd | 0.40 ±0.02f | 11.30 ±0.25e | 6.78 ±0.07e | ||
| F-test | |||||||||
| W | *** | *** | *** | *** | *** | *** | *** | ||
| SF | *** | *** | *** | *** | *** | *** | *** | ||
| W × SF | *** | *** | *** | ns | ns | ns | *** | ||
| 2018/2019 | 2019/2020 | ||||
|---|---|---|---|---|---|
| Water Treatments | Soil and Foliar Treatments | Na+ (%) | K+ (%) | Na+ (%) | K+ (%) |
| 50% DAM | Control | 1.79 ± 0.02e | 1.19 ± 0.01cd | 1.81 ± 0.01ef | 1.15 ± 0.03cd |
| SA † | 1.63 ± 0.02f | 1.32 ± 0.02bc | 1.75 ± 0.02g | 1.27 ± 0.02bc | |
| BC ‡ | 1.59 ± 0.01g | 1.38 ± 0.02b | 1.69 ± 0.01gh | 1.32 ± 0.01b | |
| SA + BC | 1.38 ± 0.02h | 1.49 ± 0.02a | 1.52 ± 0.02h | 1.39 ± 0.01a | |
| 70% DAM | Control | 2.25 ± 0.01c | 0.95 ± 0.01g | 2.21 ± 0.02c | 0.89 ± 0.00ef |
| SA | 2.09 ± 0.02d | 1.08 ± 0.02ef | 2.02 ± 0.02d | 0.98 ± 0.01de | |
| BC | 2.02 ± 0.02de | 1.17 ± 0.02e | 1.88 ± 0.03e | 1.08 ± 0.02d | |
| SA + BC | 1.76 ± 0.03ef | 1.22 ± 0.01c | 1.79 ± 0.03f | 1.17 ± 0.02c | |
| Control | 2.86 ± 0.03a | 0.71 ± 0.01j | 2.74 ± 0.01a | 0.65 ± 0.01h | |
| 90% DAM | SA | 2.67 ± 0.01b | 0.82 ± 0.02i | 2.35 ± 0.02b | 0.73 ± 0.02g |
| BC | 2.52 ± 0.02bc | 0.88 ± 0.02h | 2.27 ± 0.02bc | 0.81 ± 0.02f | |
| SA + BC | 2.13 ± 0.01cd | 0.99 ± 0.02f | 2.19 ± 0.03cd | 0.92 ± 0.01e | |
| F-test | |||||
| W | *** | *** | *** | *** | |
| SF | *** | *** | *** | *** | |
| W × SF | ns | *** | *** | *** | |
| Year | Water Treatments | Soil and Foliar Treatments | Chlorophyll a (mg g−1 FW) | Chlorophyll b (mg g−1 FW) | Proline (µ mol g−1 FW) | RWC # (%) | EL ¥ (%) |
|---|---|---|---|---|---|---|---|
| 2018/2019 | 50% DAM | Control | 1.32 ± 0.02c | 0.55 ± 0.03cd | 7.44 ± 0.03e | 88.55 ± 1.54de | 19.25 ± 1.18h |
| SA † | 1.52 ± 0.05b | 0.67 ± 0.04b | 7.28 ± 0.02fg | 93.25 ± 1.74b | 15.48 ± 1.02k | ||
| BC ‡ | 1.48 ± 0.04bc | 0.64 ± 0.04bc | 7.36 ± 0.03f | 91.05 ± 1.65c | 16.65 ± 1.05j | ||
| SA + BC | 1.68 ± 0.03a | 0.76 ± 0.04a | 7.17 ± 0.01h | 94.98 ± 1.85a | 13.47 ± 1.22l | ||
| 70% DAM | Control | 1.13 ± 0.01g | 0.37 ± 0.00ef | 8.54 ± 0.02c | 82.95 ± 1.48gh | 27.14 ± 1.12e | |
| SA | 1.26 ± 0.03e | 0.51 ± 0.02d | 7.55 ± 0.01de | 87.47 ± 1.95e | 20.65 ± 1.13g | ||
| BC | 1.23 ± 0.02f | 0.48 ± 0.01de | 8.12 ± 0.01d | 85.58 ± 1.65f | 21.89 ± 1.15f | ||
| SA + BC | 1.37 ± 0.02d | 0.58 ± 0.03c | 7.35 ± 0.00ef | 89.09 ± 1.14d | 17.48 ± 1.16i | ||
| 90% DAM | Control | 1.01 ± 0.02k | 0.24 ± 0.00h | 12.47 ± 0.01a | 75.42 ± 1.58j | 45.63 ± 1.02a | |
| SA | 1.09 ± 0.03i | 0.34 ± 0.01f | 9.32 ± 0.03bc | 80.85 ± 1.36h | 32.74 ± 1.05c | ||
| BC | 1.05 ± 0.01j | 0.31 ± 0.01g | 9.45 ± 0.02b | 78.06 ± 1.47i | 36.55 ± 1.09b | ||
| SA + BC | 1.18 ± 0.01h | 0.39 ± 0.02e | 8.45 ± 0.02cd | 84.25 ± 1.65g | 25.83 ± 1.08d | ||
| 2019/2020 | 50% DAM | Control | 1.43 ± 0.01d | 0.69 ± 0.04c | 7.23 ± 0.04e | 85.01 ± 1.95de | 20.69 ± 1.05h |
| SA † | 1.55 ± 0.05b | 0.81 ± 0.03ab | 6.65 ± 0.02g | 88.36 ± 1.45b | 16.58 ± 1.07k | ||
| BC ‡ | 1.51 ± 0.03bc | 0.78 ± 0.04b | 6.87 ± 0.03f | 85.97 ± 1.65c | 17.74 ± 1.13j | ||
| SA + BC | 1.63 ± 0.05a | 0.93 ± 0.02a | 6.14 ± 0.01h | 91.66 ± 1.53a | 14.98 ± 1.11l | ||
| 70% DAM | Control | 1.23 ± 0.03gh | 0.46 ± 0.03f | 8.42 ± 0.02c | 80.22 ± 1.12g | 28.74 ± 1.12e | |
| SA | 1.36 ± 0.02e | 0.64 ± 0.04d | 7.78 ± 0.01de | 84.12 ± 1.14e | 21.45 ± 1.15g | ||
| BC | 1.31 ± 0.03f | 0.59 ± 0.03e | 8.07 ± 0.02d | 82.05 ± 1.85f | 22.63 ± 1.14f | ||
| SA + BC | 1.48 ± 0.02c | 0.71 ± 0.04cd | 7.02 ± 0.02ef | 86.47 ± 1.75d | 18.21 ± 1.10i | ||
| 90% DAM | Control | 1.05 ± 0.01j | 0.28 ± 0.01i | 12.42 ± 0.03a | 76.25 ± 1.74j | 46.21 ± 1.09a | |
| SA | 1.22 ± 0.02h | 0.40 ± 0.02h | 9.36 ± 0.04bc | 79.23 ± 1.85h | 33.75 ± 1.08c | ||
| BC | 1.14 ± 0.02i | 0.37 ± 0.01hi | 9.44 ± 0.03b | 77.05 ± 1.96i | 37.22 ± 1.05b | ||
| SA + BC | 1.28 ± 0.03g | 0.49 ± 0.01g | 8.22 ± 0.04cd | 82.66 ± 1.32fg | 26.45 ± 1.11d | ||
| F-test | |||||||
| W | *** | *** | *** | *** | ** | ||
| SF | *** | *** | *** | *** | *** | ||
| W × SF | *** | *** | *** | *** | ns | ||
| Year | Treatments (Ts) | Grains Per Spike | 1000-Grain Weight | Grain Yield | N Uptake | P Uptake | K Uptake | |
|---|---|---|---|---|---|---|---|---|
| Water Ts | Soil and Foliar Ts | (n) | (g) | (ton/ha) | (kg ha−1) | (kg ha−1) | (kg ha−1) | |
| 2018/2019 | 50% DAM | Control | 50.75 ± 1.21d | 52.50 ± 0.75d | 4.74 ± 0.04cd | 86.5 ± 1.53e | 47.6 ± 1.15e | 130.5 ± 2.74e |
| SA † | 53.66 ± 1.20c | 53.98 ± 0.84bc | 5.02 ± 0.03bc | 93.8 ± 1.57c | 54.1 ± 1.14c | 140.7 ± 2.95c | ||
| BC ‡ | 55.69 ± 1.22b | 54.99 ± 0.25b | 5.38 ± 0.05b | 95.9 ± 1.66b | 59.5 ± 1.22b | 147.8 ± 2.68b | ||
| SA+BC | 57.96 ± 1.25a | 56.44 ± 0.36a | 6.67 ± 0.08a | 103.8 ± 1.62a | 62.8 ± 1.25a | 163.7 ± 2.11a | ||
| 70% DAM | Control | 45.24 ± 1.24h | 48.65 ± 0.22g | 3.95 ± 0.03ef | 69.4 ± 1.44i | 35.0 ± 1.23gh | 102.4 ± 3.65h | |
| SA | 47.66 ± 1.1.18f | 50.32 ± 0.54ef | 4.34 ± 0.07d | 76.4 ± 1.45g | 38.4 ± 1.18f | 117.6 ± 2.36fg | ||
| BC | 49.47 ± 1.19ef | 51.66 ± 0.48e | 4.54 ± 0.08d | 80.3 ± 1.48f | 39.2 ± 1.18f | 120.9 ± 3.22f | ||
| SA+BC | 51.23 ± 1.15c | 53.21 ± 0.65c | 4.88 ± 0.06c | 88.9 ± 1.52d | 50.0 ± 1.19d | 133.5 ± 3.45d | ||
| 90% DAM | Control | 39.65 ± 1.25h | 45.24 ± 0.50i | 2.99 ± 0.06h | 45.7 ± 1.56k | 21.6 ± 1.12j | 79.3 ± 2.35k | |
| SA | 42.96 ± 1.26gh | 46.89 ± 0.65h | 3.42 ± 0.05g | 61.0 ± 1.58j | 28.3 ± 1.22ij | 90.5 ± 2.36j | ||
| BC | 44.35 ± 1.22g | 47.03 ± 0.62h | 3.78 ± 0.02f | 62.3 ± 1.59j | 30.4 ± 1.25i | 94.5 ± 2.45i | ||
| SA+BC | 46.78 ± 1.25e | 48.99 ± 0.84f | 4.05 ± 0.01e | 72.4 ± 1.58h | 36.5 ± 1.24g | 104.4 ± 2.44g | ||
| 2019/2020 | 50% DAM | Control | 49.65 ± 1.17d | 53.75 ± 0.74d | 5.18 ± 0.06cd | 81.3 ± 1.69e | 50.4 ± 1.21d | 136.7 ± 2.45de |
| SA † | 52.75 ± 1.14c | 55.74 ± 0.48bc | 5.54 ± 0.04bc | 89.4 ± 1.45c | 55.7 ± 1.19c | 150.9 ± 2.65c | ||
| BC ‡ | 54.25 ± 1.12b | 56.48 ± 0.65b | 5.87 ± 0.03b | 95.4 ± 1.55b | 57.7 ± 1.18b | 159.4 ± 2.55b | ||
| SA+BC | 56.74 ± 1.06a | 57.98 ± 0.24a | 6.23 ± 0.02a | 111.0 ± 1.89a | 62.4 ± 1.18a | 174.4 ± 2.85a | ||
| 70% DAM | Control | 45.88 ± 1.09g | 49.07 ± 0.25ef | 3.92 ± 0.05f | 64.8 ± 1.47i | 38.0 ± 1.17g | 105.5 ± 2.36g | |
| SA | 47.99 ± 1.05e | 51.74 ± 0.14de | 4.36 ± 0.04d | 70.4 ± 1.56g | 44.5 ± 1.23ef | 119.7 ± 2.24ef | ||
| BC | 48.87 ± 1.07e | 52.42 ± 0.23d | 4.87 ± 0.01d | 77.9 ± 1.44f | 45.9 ± 1.25e | 122.4 ± 2.65e | ||
| SA+BC | 50.75 ± 1.18c | 54.44 ± 0.65c | 5.33 ± 0.05c | 83.4 ± 1.58d | 52.4 ± 1.24cd | 139.7 ± 2.15d | ||
| 90% DAM | Control | 39.89 ± 1.11i | 44.55 ± 0.86g | 3.02 ± 0.07i | 47.2 ± 1.63k | 22.4 ± 1.24j | 80.4 ± 2.65i | |
| SA | 41.25 ± 1.15g | 46.25 ± 0.47fg | 3.43 ± 0.06gh | 58.3 ± 1.60jk | 30.7 ± 1.20i | 93.5 ± 2.45hi | ||
| BC | 43.22 ± 1.18gh | 47.02 ± 0.63f | 3.78 ± 0.03g | 60.8 ± 1.55j | 34.5 ± 1.14h | 96.1 ± 2.75h | ||
| SA+BC | 46.22 ± 1.19f | 49.78 ± 0.54e | 4.09 ± 0.02e | 66.7 ± 1.54h | 40.8 ± 1.18f | 108.5 ± 2.45f | ||
| F-test | ||||||||
| W | *** | *** | *** | *** | *** | *** | ||
| SF | *** | ** | *** | *** | ns | ** | ||
| W × SF | *** | ** | *** | ** | * | ** | ||
| Year Month | 2018/2019 | 2019/2020 | ||||||
| Temperature (°C) | Preceptation (mm) | RH ώ (%) | Temperature (°C) | Preceptation (mm) | RH ώ (%) | |||
| max | min | max | min | |||||
| Dec | 25.9 | 12.7 | 1.08 | 33.3 | 22.7 | 11.2 | 0.62 | 30.1 |
| Jan | 24.5 | 11.4 | 2.07 | 45.4 | 19.8 | 10.0 | 2.24 | 41.7 |
| Feb | 22.7 | 10.1 | 5.35 | 43.5 | 21.2 | 9.3 | 5.78 | 40.5 |
| Mar | 24.3 | 12.9 | 0.65 | 42.9 | 23.2 | 11.2 | 0.51 | 43.7 |
| April | 25.2 | 13.7 | 0.00 | 50.8 | 26.1 | 15.5 | 0.00 | 50.6 |
| May | 28.8 | 17.6 | 0.00 | 60.7 | 29.1 | 16.7 | 0.00 | 62.5 |
| Character | 2018/2019 | 2019/2020 |
|---|---|---|
| pH (1:2.5 soil:water suspension) | 8.17 ± 0.03 † | 8.11 ± 0.01 |
| Electrical conductivity (EC, dS m−1) ¥ | 4.24 ± 0.02 | 4.09 ± 0.01 |
| Soil organic matter (g kg−1) | 10.9 ± 0.02 | 12.2 ± 0.02 |
| ESP # (%) | 19.88 ± 0.39 | 17.37 ± 0.11 |
| Particle size distribution (%) | ||
| Sand | 28.34 ± 1.75 | 25.32 ± 1.75 |
| Silt | 23.45 ± 2.03 | 26.44 ± 1.55 |
| Clay | 48.21 ± 2.14 | 48.24 ± 2.12 |
| Texture grade | clayey | clayey |
| Soluble cations (meq L−1) ¥ | ||
| Ca2+ | 7.93 ± 0.84 | 8.75 ± 0.74 |
| Mg2+ | 4.02 ± 1.08 | 3.95 ± 1.21 |
| Na+ | 24.02 ± 2.02 | 20.56 ± 3.02 |
| K+ | 0.52 ± 0.01 | 0.48 ± 0.11 |
| SAR ® (%) | 9.22 ± 0.02 | 8.35 ± 0.13 |
| Soluble anions (meq L−1) ¥ | ||
| CO3− − | nd ‡ | nd |
| HCO3− | 4.01 ± 0.44 | 4.03 ± 0.58 |
| Cl− | 25.89 ± 1.33 | 21.98 ± 1.24 |
| SO4− − | 16.29 ± 3.10 | 12.97 ± 3.09 |
| Available macronutrients (mg kg−1) | ||
| N | 9.98 ± 0.54 | 11.44 ± 1.44 |
| P | 8.97 ± 1.26 | 9.74 ± 1.32 |
| K | 367 ± 25.38 | 392 ± 24.45 |
| Year | Soil Depth (cm) | FC © (%) | PWP £ (%) | ASW ∞ (%) | BD ± (g cm−3) |
|---|---|---|---|---|---|
| 2018/2019 | 0–20 | 41.29 ± 0.01 | 21.24 ± 0.03 | 20.05 ± 0.03 | 1.42 ± 0.02 |
| 20–40 | 38.48 ± 0.02 | 21.55 ± 0.02 | 16.93 ± 0.02 | 1.44 ± 0.04 | |
| 40–60 | 37.67 ± 0.03 | 20.45 ± 0.04 | 17.22 ± 0.04 | 1.47 ± 0.03 | |
| 2019/2020 | 0–20 | 42.16 ± 0.06 | 18.63 ± 0.03 | 23.53 ± 0.01 | 1.38 ± 0.05 |
| 20–40 | 41.35 ± 0.05 | 19.75 ± 0.03 | 21.60 ± 0.02 | 1.39 ± 0.03 | |
| 40–60 | 41.54 ± 0.04 | 19.89 ± 0.02 | 21.65 ± 0.05 | 1.43 ± 0.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hafez, E.M.; Kheir, A.M.S.; Badawy, S.A.; Rashwan, E.; Farig, M.; Osman, H.S. Differences in Physiological and Biochemical Attributes of Wheat in Response to Single and Combined Salicylic Acid and Biochar Subjected to Limited Water Irrigation in Saline Sodic Soil. Plants 2020, 9, 1346. https://doi.org/10.3390/plants9101346
Hafez EM, Kheir AMS, Badawy SA, Rashwan E, Farig M, Osman HS. Differences in Physiological and Biochemical Attributes of Wheat in Response to Single and Combined Salicylic Acid and Biochar Subjected to Limited Water Irrigation in Saline Sodic Soil. Plants. 2020; 9(10):1346. https://doi.org/10.3390/plants9101346
Chicago/Turabian StyleHafez, Emad M., Ahmed M. S. Kheir, Shimaa A. Badawy, Emadeldeen Rashwan, Mohamed Farig, and Hany S. Osman. 2020. "Differences in Physiological and Biochemical Attributes of Wheat in Response to Single and Combined Salicylic Acid and Biochar Subjected to Limited Water Irrigation in Saline Sodic Soil" Plants 9, no. 10: 1346. https://doi.org/10.3390/plants9101346
APA StyleHafez, E. M., Kheir, A. M. S., Badawy, S. A., Rashwan, E., Farig, M., & Osman, H. S. (2020). Differences in Physiological and Biochemical Attributes of Wheat in Response to Single and Combined Salicylic Acid and Biochar Subjected to Limited Water Irrigation in Saline Sodic Soil. Plants, 9(10), 1346. https://doi.org/10.3390/plants9101346








