Photosynthetic Responses of Canola to Exogenous Application or Endogenous Overproduction of 5-Aminolevulinic Acid (ALA) under Various Nitrogen Levels
Abstract
:1. Introduction
2. Results
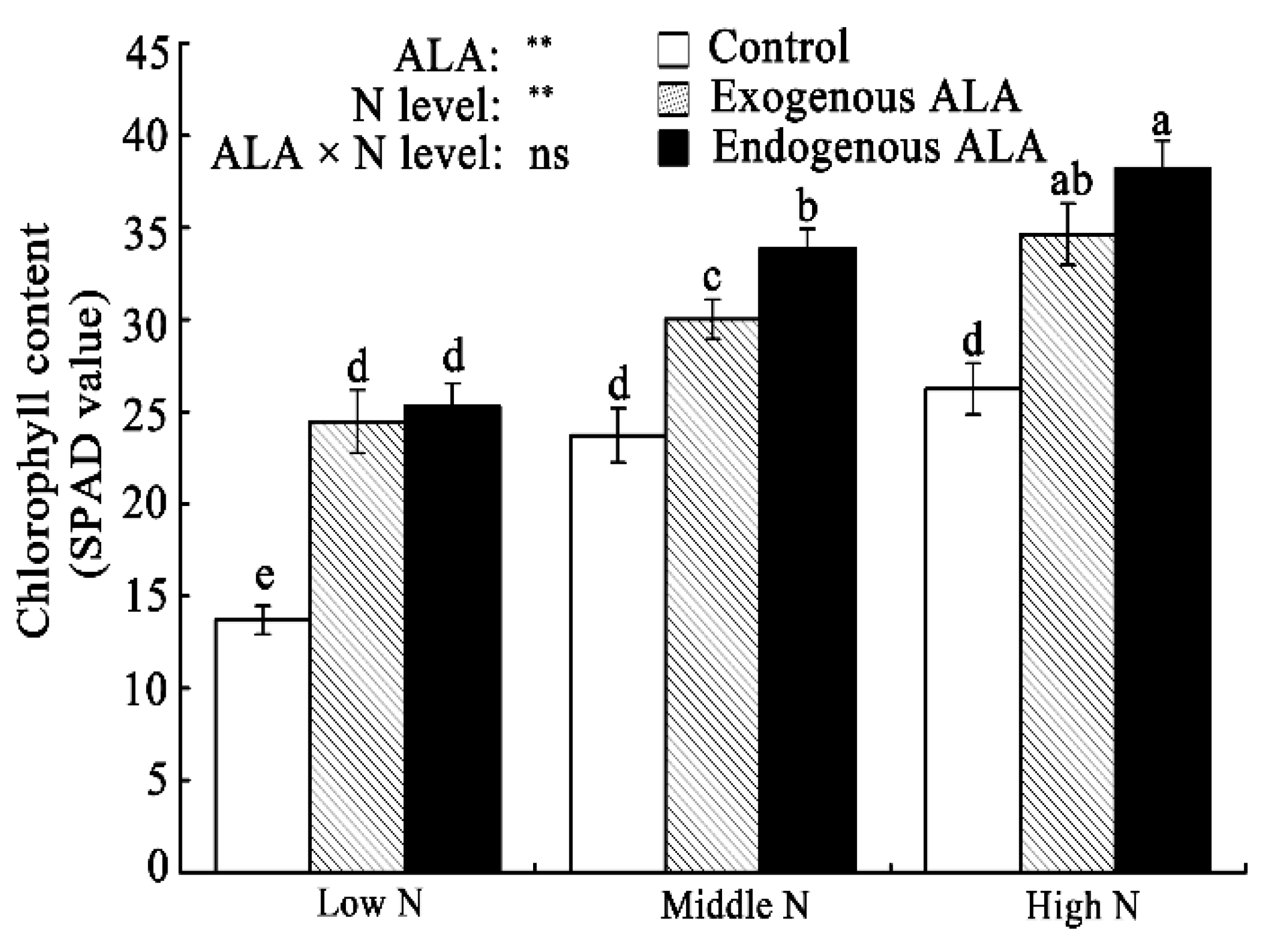
2.1. Chl Content
2.2. Photosynthetic Gas Exchange Characteristics
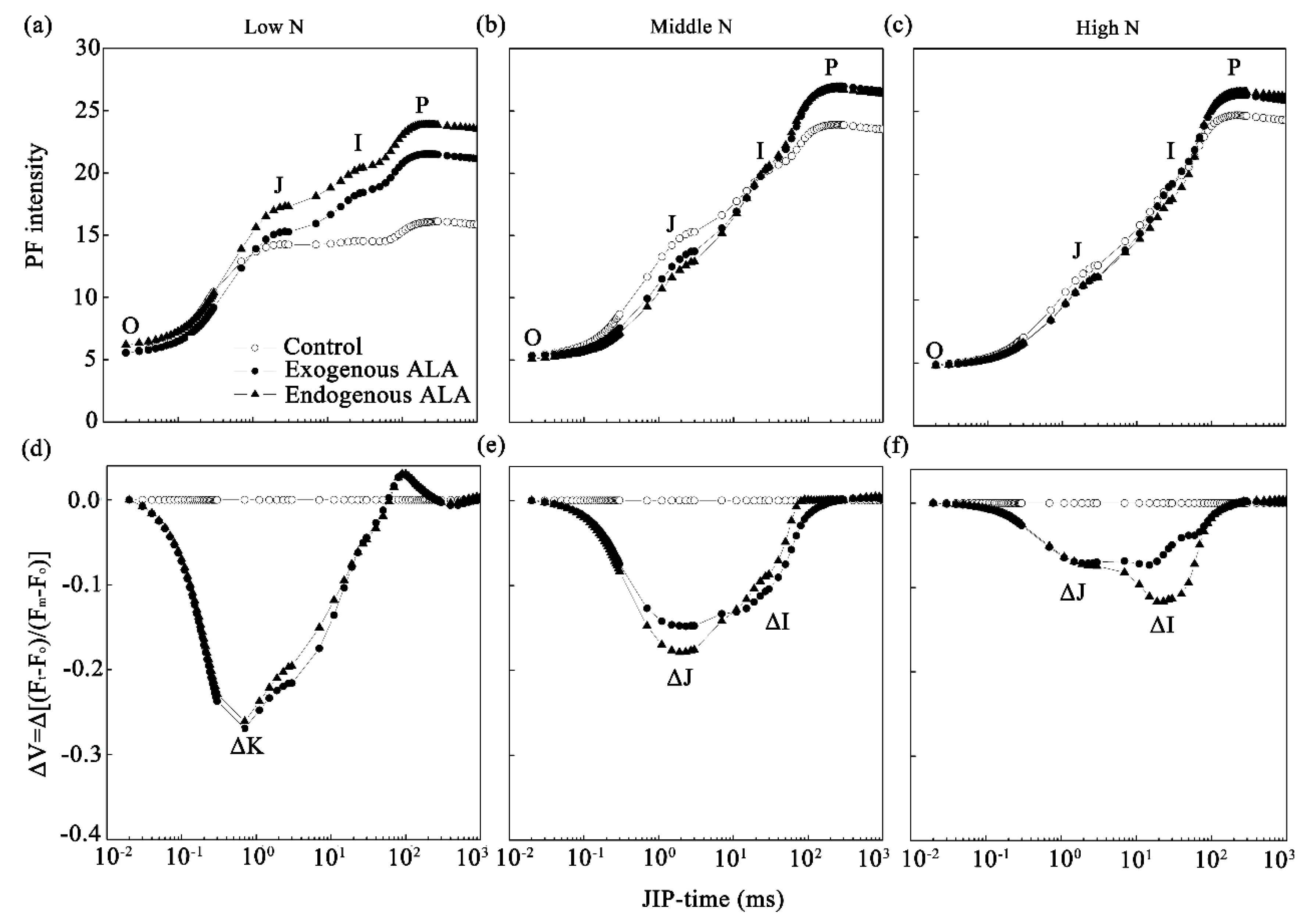
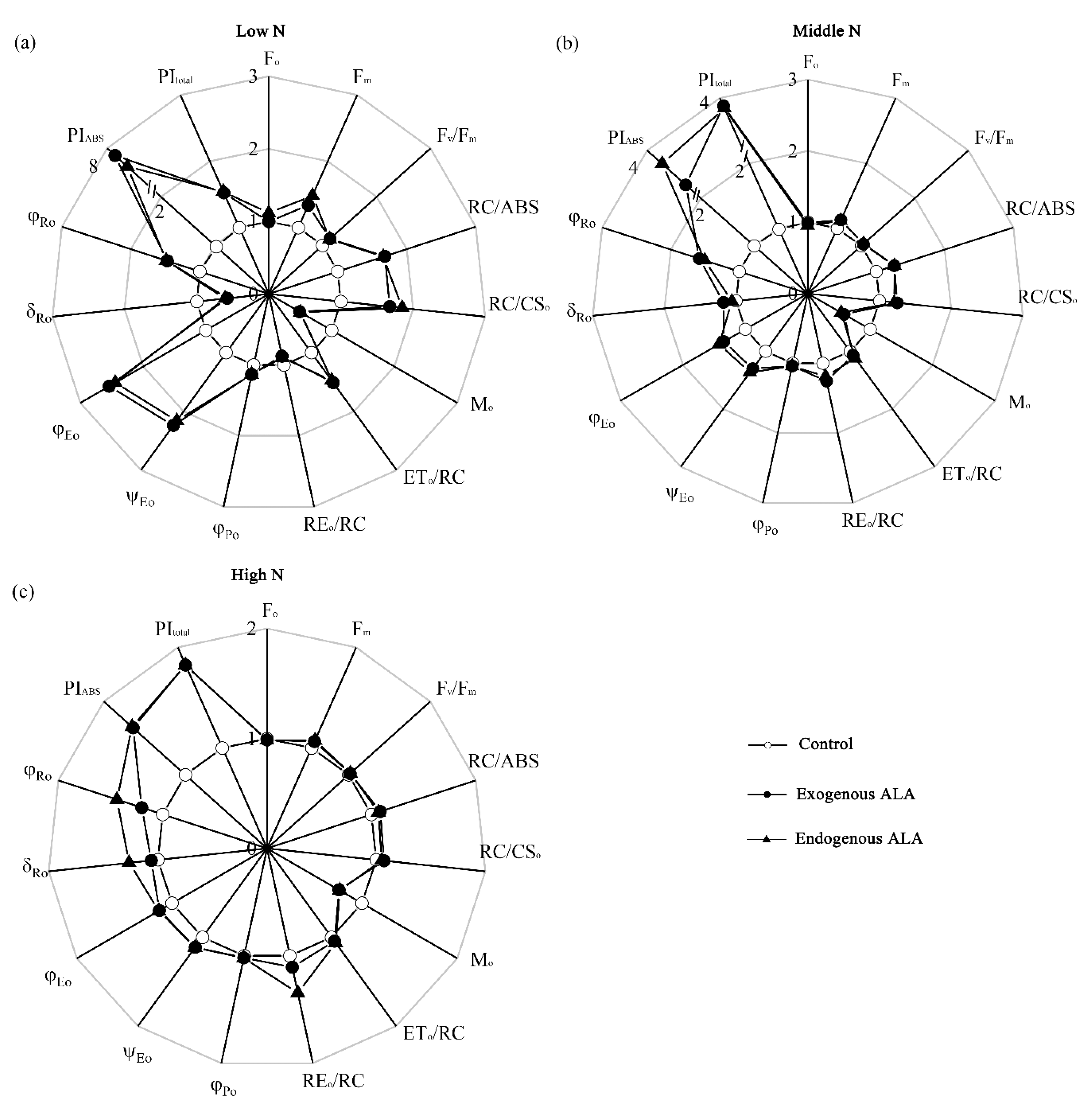
2.3. PF Curves and JIP-Test
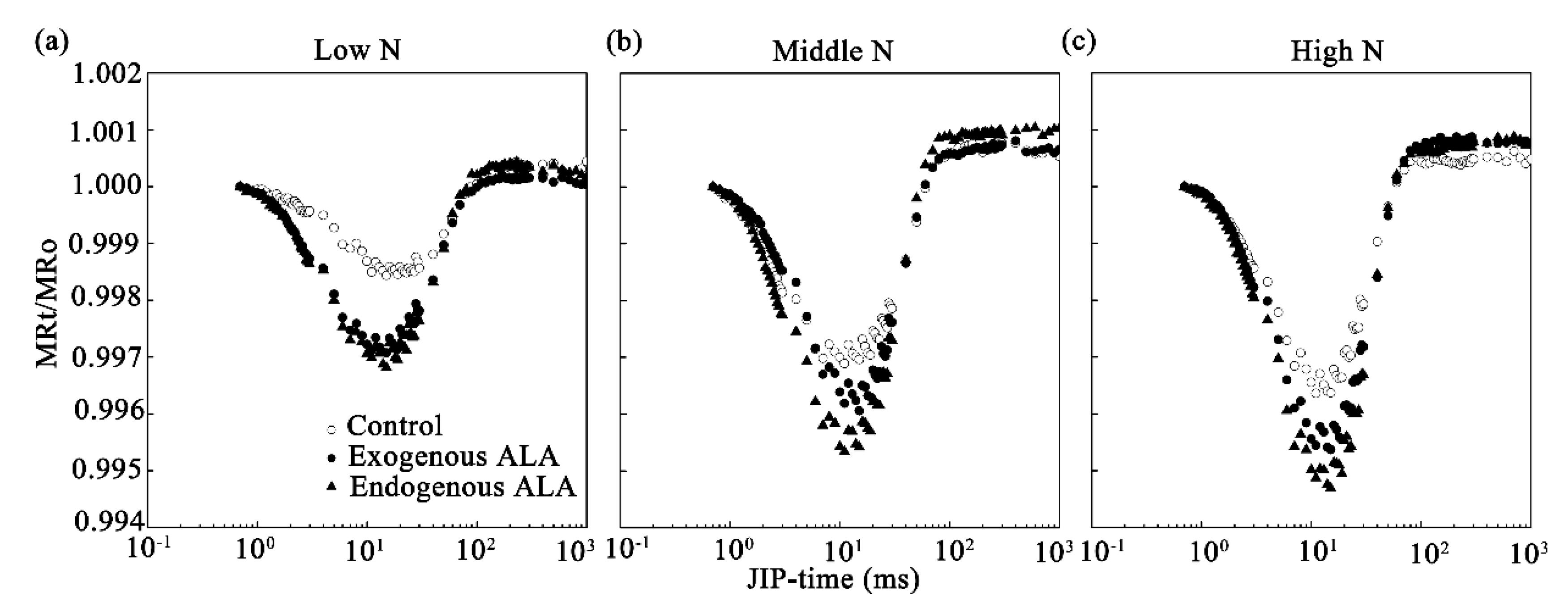
2.4. MR Curves and Related Parameters
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Nitrogen Supply and Exogenous ALA Addition
4.3. Measurement of Chl Content
4.4. Gas Exchange Analysis
4.5. Simultaneous Measurement of PF and MR Kinetics
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Martin, W.F.; Bryant, D.A.; Beatty, J.T. A physiological perspective on the origin and evolution of photosynthesis. FEMS Microbiol. Rev. 2018, 42, 205–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muthusamy, S.K.; Lenka, S.K.; Katiyar, A.; Chinnusamy, V.; Singh, A.K.; Bansal, K.C. Genome-wide identification and analysis of biotic and abiotic stress regulation of C4 photosynthetic pathway genes in rice. Appl. Biochem. Biotechnol. 2019, 187, 221–238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Hu, B.; Chu, C.C. Towards understanding the hierarchical nitrogen signalling network in plants. Curr. Opin. Plant Biol. 2020, 55, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Scharf, P.C.; Wiebold, W.J.; Lory, J.A. Corn yield response to nitrogen fertilizer timing and deficiency level. Agron. J. 2002, 94, 435–441. [Google Scholar] [CrossRef]
- Gong, X.W.; Li, J.; Ma, H.C.; Chen, G.H.; Dang, K.; Yang, P.; Wang, M.; Feng, B.L. Nitrogen deficiency induced a decrease in grain yield related to photosynthetic characteristics, carbon–nitrogen balance and nitrogen use efficiency in proso millet (Panicum miliaceum L.). Arch. Agron. Soil Sci. 2020, 66, 398–413. [Google Scholar] [CrossRef]
- Cruz, J.L.; Mosquim, P.R.; Pelacani, C.R.; Araújo, W.L.; DaMatta, F.M. Photosynthesis impairment in cassava leaves in response to nitrogen deficiency. Plant Soil 2003, 257, 417–423. [Google Scholar] [CrossRef]
- Vouillot, M.O.; Huet, P.; Boissard, P. Early detection of N deficiency in a wheat crop using physiological and radiometric methods. Agronomie 1998, 18, 117–130. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Zhang, J. Photosynthetic CO2 assimilation, chlorophyll fluorescence and photoinhibition as affected by nitrogen deficiency in maize plants. Plant Sci. 2000, 151, 135–143. [Google Scholar] [CrossRef]
- Wei, S.S.; Wang, X.Y.; Shi, D.Y.; Li, Y.H.; Zhang, J.W.; Liu, P.; Zhao, B.; Dong, S.T. The mechanisms of low nitrogen induced weakened photosynthesis in summer maize (Zea mays L.) under field conditions. Plant Physiol. Biochem. 2016, 105, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, B.; Geisseler, D.; Michel, K.; Joergensen, R.G.; Schulz, E.; Merbach, I.; Raupp, J.; Rauber, R.; Hu, K.; Niu, L.; et al. Effects of fertilization and soil management on crop yields and carbon stabilization in soils. A review. Agron. Sustain. Dev. 2011, 31, 361–372. [Google Scholar] [CrossRef] [Green Version]
- Vavilin, D.V.; Vermaas, W.F. Regulation of the tetrapyrrole biosynthetic pathway leading to heme and chlorophyll in plants and cyanobacteria. Physiol. Plant 2002, 115, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Hayashi, S.; Furusaki, S.; Shioya, S. 5-Aminolevulinic acid promotes callus growth and paclitaxel production in light-grown Taxus cuspidata suspension cultures. Eng. Life Sci. 2015, 15, 116–121. [Google Scholar] [CrossRef]
- Bindu, R.C.; Vivekanandan, M. Hormonal activities of 5-aminolevulinic acid in callus induction and micropropagation. Plant Growth Regul. 1998, 26, 15–18. [Google Scholar] [CrossRef]
- Akram, N.A.; Ashraf, M. Regulation in plant stress tolerance by a potential plant growth regulator, 5-aminolevulinic acid. J. Plant Growth Regul. 2013, 32, 663–679. [Google Scholar] [CrossRef]
- Memon, S.A.; Hou, X.L.; Wang, L.J.; Li, Y. Promotive effect of 5-aminolevulinic acid on chlorophyll, antioxidative enzymes and photosynthesis of Pakchoi (Brassica campestris ssp. chinensis var. communis Tsen et Lee). Acta Physiol. Plant. 2009, 31, 51–57. [Google Scholar] [CrossRef]
- Murooka, Y.; Tanaka, T. 5-Aminolevulinic acid (5-ALA)—A multifunctional amino acid as a plant growth stimulator and stress tolerance factor. In Plant Adaptation to Environmental Change: Significance of Amino Acids and Their Derivatives; Anjum, N.A., Gill, S.S., Gill, R., Eds.; CABI Publishing: Wallingford, UK, 2014; pp. 18–34. [Google Scholar]
- Xie, L.; Wang, Z.H.; Cheng, X.H.; Gao, J.J.; Zhang, Z.P.; Wang, L.J. 5-Aminolevulinic acid promotes anthocyanin accumulation in Fuji apples. Plant Growth Regul. 2013, 69, 295–303. [Google Scholar] [CrossRef]
- Feng, X.X.; An, Y.Y.; Zheng, J.; Sun, M.; Wang, L.J. Proteomics and SSH analyses of ALA-promoted fruit coloration and evidence for the involvement of a MADS-box gene, MdMADS1. Front. Plant Sci. 2016, 7, 1615. [Google Scholar] [CrossRef] [Green Version]
- Hotta, Y.; Tanaka, T.; Takaoka, H.; Takeuchi, Y.; Konnai, M. Promotive effects of 5-aminolevulinic acid on the yield of several crops. Plant Growth Regul. 1997, 22, 109–114. [Google Scholar] [CrossRef]
- Sun, Y.P.; Liu, J.; Cao, R.X.; Huang, Y.J.; Hall, A.M.; Guo, C.B.; Wang, L.J. Effects of 5-aminolevulinic acid treatment on photosynthesis of strawberry. Photosynthetica 2017, 55, 276–284. [Google Scholar] [CrossRef]
- Youssef, T.; Awad, M.A. Mechanisms of enhancing photosynthetic gas exchange in date palm seedlings (Phoenix dactylifera L.) under salinity stress by a 5-aminolevulinic acid-based fertilizer. J. Plant Growth Regul. 2008, 27, 1–9. [Google Scholar] [CrossRef]
- Wang, L.J.; Sun, Y.P.; Zhang, Z.P.; Kang, L. Effects of 5-aminolevulinic acid (ALA) on photosynthesis and chlorophyll fluorescence of watermelon seedlings grown under low light and low temperature conditions. Acta Hortic. 2010, 856, 159–166. [Google Scholar] [CrossRef]
- Nishihara, E.; Kondo, K.; Parvez, M.M.; Takahashi, K.; Watanabe, K.; Tanaka, K. Role of 5-aminolevulinic acid (ALA) on active oxygen-scavenging system in NaCl-treated spinach (Spinacia oleracea). J. Plant Physiol. 2003, 160, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.P.; Zhang, Z.P.; Wang, L.J. Promotion of 5-aminolevulinic acid treatment on leaf photosynthesis is related with increase of antioxidant enzyme activity in watermelon seedlings grown under shade condition. Photosynthetica 2009, 47, 347–354. [Google Scholar] [CrossRef]
- Sun, X.E.; Feng, X.X.; Li, C.; Zhang, Z.P.; Wang, L.J. Study on salt tolerance with YHem1 transgenic canola (Brassica napus). Physiol. Plant. 2015, 154, 223–242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.P.; Yao, Q.H.; Wang, L.J. Expression of yeast Hem1 controlled by Arabidopsis HemA1 promoter enhances leaf photosynthesis in transgenic tobacco. Mol. Biol. Rep. 2011, 38, 4369–4379. [Google Scholar] [CrossRef]
- Wei, Z.Y.; Zhang, Z.P.; Lee, M.R.; Sun, Y.P.; Wang, L.J. Effect of 5-aminolevulinic acid on leaf senescence and nitrogen metabolism of pakchoi under different nitrate levels. J. Plant Nutr. 2012, 35, 49–63. [Google Scholar] [CrossRef]
- Iwai, K.; Saito, A.; van Leeuwen, J.; Tanaka, T.; Takeuchi, Y. A new functional fertilizer containing 5-aminolevulinic acid promoted hydroponically-grown vegetables in the Netherlands. Acta Hort. 2005, 697, 351–355. [Google Scholar] [CrossRef] [Green Version]
- Toth, S.Z.; Schansker, G.; Strasser, R.J. A non-invasive assay of the plastoquinone pool redox state based on the OJIP-transient. Photosynth. Res. 2007, 93, 193–203. [Google Scholar] [CrossRef] [Green Version]
- Strasser, R.J.; Stirbet, A.D. Estimation of the energetic connectivity of PS II centres in plants using the fluorescence rise O–J–I–P; Fitting of experimental data to three different PS II models. Math. Comput. Simulat. 2001, 56, 451–462. [Google Scholar] [CrossRef]
- An, Y.; Lin, Q.; Wang, L. ALA pretreatment improves waterlogging tolerance of fig plants. PLoS ONE 2016, 11, e0147202. [Google Scholar] [CrossRef]
- Lazar, D. Modelling of light-induced chlorophyll a fluorescence rise (O-J-I-P transient) and changes in 820 nm-transmittance signal of photosynthesis. Photosynthetica 2009, 47, 483–498. [Google Scholar] [CrossRef]
- Schansker, G.; Srivastava, A.; Govindjee; Strasser, R.J. Characterization of the 820-nm transmission signal paralleling the chlorophyll a fluorescence rise (OJIP) in pea leaves. Funct. Plant Biol. 2003, 30, 785–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strasser, R.J.; Schansker, G.; Srivastava, A.; Govindjee. Simultaneous measurement of photosystem I and photosystem II probed by modulated transmission at 820 nm and by chlorophyll a fluorescence in the sub ms to second time range. In Proceedings of the 12th International Congress on Photosynthesis; CSIRO Publishing: Melbourne, Australia, 2001. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M.; Qiang, S.; Goltsev, V. Simultaneous in vivo recording of prompt and delayed fluorescence and 820-nm reflection changes during drying and after rehydration of the resurrection plant Haberlea rhodopensis. Biochim. Biophys. Acta 2010, 1797, 1313–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, L.B.; Caldwell, C.C.; Jiang, Y.F. Photosynthesis and growth of camelina and canola in response to water deficit and applied nitrogen. Crop Sci. 2018, 58, 393–401. [Google Scholar] [CrossRef]
- Seepaul, R.; George, S.; Wright, D.L. Comparative response of Brassica carinata and B. napus vegetative growth, development and photosynthesis to nitrogen nutrition. Ind. Crop Prod. 2016, 94, 872–883. [Google Scholar] [CrossRef]
- Cetner, M.D.; Kalaji, H.M.; Goltsev, V.; Aleksandrov, V.; Kowalczyk, K.; Borucki, W.; Jajoo, A. Effects of nitrogen-deficiency on efficiency of light-harvesting apparatus in radish. Plant Physiol. Biochem. 2017, 119, 81–92. [Google Scholar] [CrossRef]
- Zhao, L.S.; Li, K.; Wang, Q.M.; Song, X.Y.; Su, H.N.; Xie, B.B.; Zhang, X.Y.; Huang, F.; Chen, X.L.; Zhou, B.C.; et al. Nitrogen starvation impacts the photosynthetic performance of porphyridium cruentum as revealed by chlorophyll a fluorescence. Sci Rep. 2017, 7, 8542. [Google Scholar] [CrossRef]
- Abdallah, O.; Vasilij, G.; Strasser, R.J.; Rajagopal, S. Temperature effects on pea plants probed by simultaneous measurements of the kinetics of prompt fluorescence, delayed fluorescence and modulated 820 nm reflection. PLoS ONE 2013, 8, e59433. [Google Scholar]
- Hotta, Y.; Tanaka, T.; Luo, B.; Takeuchi, Y.; Konnai, M. Improvement of cold resistance in rice seedlings by 5-aminolevulinic acid. J. Pestic. Sci. 1998, 23, 29–33. [Google Scholar] [CrossRef] [Green Version]
- Memon, S.A.; Wang, L.J.; Hou, X.L. Effect of 5-aminolevulinic acid (ALA) on antioxidative enzymes, chlorophyll content and photosynthesis of pakchoi (Brassica campestris ssp. chinensis) under salt stress. Middle East J. Sci. Res. 2013, 18, 899–906. [Google Scholar]
- Wang, L.J.; Jiang, W.B.; Huang, B.J. Promotion of 5-aminolevulinic acid on photosynthesis of melon (Cucumis melo) seedlings under low light and chilling stress conditions. Physiol. Plant. 2004, 121, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, D.M.; Gao, Y.; Yu, B.; Xia, C.X.; Bai, J.G. Pretreatment with 5-aminolevulinic acid mitigates heat stress of cucumber leaves. Biol. Plant. 2012, 56, 780–784. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Yao, Q.H.; Wang, L.J. Expression of yeast Hem1 gene controlled by Arabidopsis HemA1 promoter improves salt tolerance in Arabidopsis plants. BMB Rep. 2010, 43, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Hotta, Y.; Tanaka, T.; Takaoka, H.; Takeuchi, Y.; Konnai, M. New physiological effects of 5-aminolevulinic acid in plants: The increase of photosynthesis, chlorophyll content, and plant growth. Biosci. Biotechnol. Biochem. 1997, 61, 2025–2028. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Q.; Kang, L.; Wang, L.J. Effect of 5-aminolevulinic acid (ALA) on photosynthesis and its relationship with antioxidant enzymes of strawberry leaves. Acta. Bot. Boreal. Occident. Sin. 2006, 26, 57–62. [Google Scholar]
- Tanaka, Y.; Tanaka, A.; Tsuji, H. Effects of 5-aminolevulinic acid on the accumulation of chlorophyll-b and apoproteins of the light-harvesting chlorophyll a/b-protein complex of photosystem-II. Plant Cell Physiol. 1993, 34, 465–472. [Google Scholar]
- Dinç, E.; Ceppi, M.G.; Tóth, S.Z.; Bottka, S.; Schansker, G. The Chl a fluorescence intensity is remarkably insensitive to changes in the chlorophyll content of the leaf as long as the chl a/b ratio remains unaffected. Biochim. Biophys. Acta. 2012, 1817, 770–779. [Google Scholar] [CrossRef] [Green Version]
- Ye, X.; Chen, X.F.; Deng, C.L.; Yang, L.T.; Lai, N.W.; Guo, J.X.; Chen, L.S. Magnesium-deficiency effects on pigments, photosynthesis and photosynthetic electron transport of leaves, and nutrients of leaf blades and veins in Citrus sinensis seedlings. Plants 2019, 8, 389. [Google Scholar] [CrossRef] [Green Version]
- Ceppi, M.G.; Oukarroum, A.; Çiçek, N.; Strasser, R.J.; Schansker, G. The IP amplitude of the fluorescence rise OJIP is sensitive to changes in the photosystem I content of leaves: A study on plants exposed to magnesium and sulfate deficiencies, drought stress and salt stress. Physiol. Plant. 2012, 144, 277–288. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Oukarroum, A.; Alexandrov, V.; Kouzmanova, M.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Allakhverdiev, S.I.; Goltsev, V. Identification of nutrient deficiency in maize and tomato plants by in vivo chlorophyll a fluorescence measurements. Plant Physiol. Biochem. 2014, 81, 16–25. [Google Scholar] [CrossRef]
- Aleksandrov, V.; Krasteva, V.; Paunov, M.; Chepisheva, M.; Kousmanova, M.; Kalaji, H.M.; Goltsev, V. Deficiency of some nutrient elements in bean and maize plants analyzed by luminescent method. Bulg. J. Agric. Sci. 2014, 20, 24–30. [Google Scholar]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Lukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Strauss, A.J.; Kruger, G.H.; Strasser, R.J.; van Heerden, P.D. The role of low soil temperature in the inhibition of growth and PSII function during dark chilling in soybean genotypes of contrasting tolerance. Physiol. Plant. 2010, 131, 89–105. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, X.; An, Y.; Gao, J.; Wang, L. Photosynthetic Responses of Canola to Exogenous Application or Endogenous Overproduction of 5-Aminolevulinic Acid (ALA) under Various Nitrogen Levels. Plants 2020, 9, 1419. https://doi.org/10.3390/plants9111419
Feng X, An Y, Gao J, Wang L. Photosynthetic Responses of Canola to Exogenous Application or Endogenous Overproduction of 5-Aminolevulinic Acid (ALA) under Various Nitrogen Levels. Plants. 2020; 9(11):1419. https://doi.org/10.3390/plants9111419
Chicago/Turabian StyleFeng, Xinxin, Yuyan An, Jingjing Gao, and Liangju Wang. 2020. "Photosynthetic Responses of Canola to Exogenous Application or Endogenous Overproduction of 5-Aminolevulinic Acid (ALA) under Various Nitrogen Levels" Plants 9, no. 11: 1419. https://doi.org/10.3390/plants9111419
APA StyleFeng, X., An, Y., Gao, J., & Wang, L. (2020). Photosynthetic Responses of Canola to Exogenous Application or Endogenous Overproduction of 5-Aminolevulinic Acid (ALA) under Various Nitrogen Levels. Plants, 9(11), 1419. https://doi.org/10.3390/plants9111419




