Trichostatin A Affects Developmental Reprogramming of Bread Wheat Microspores towards an Embryogenic Route
Abstract
1. Introduction
2. Results
2.1. Application of a Short TSA Treatment after a Cold or Mannitol Stress Treatment
2.2. Application of TSA Simultaneously with a Cold or a Mannitol Stress Treatment
2.3. Application of TSA as a Unique Induction Treatment
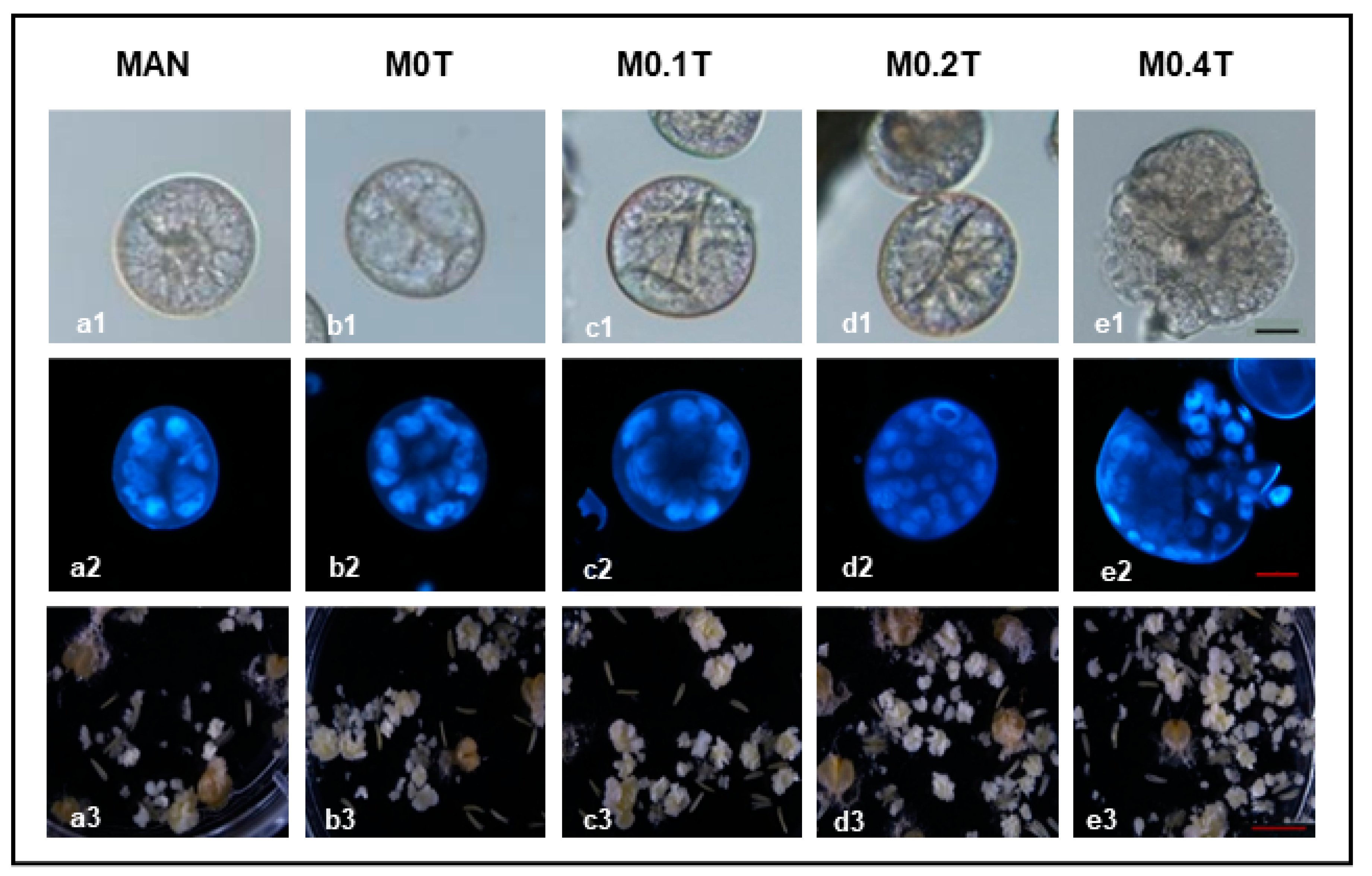
2.4. Microspore Morphological Changes Associated with ME Induction and TSA
2.5. Transcript Level Changes in Marker Genes for Early and Middle Stages of Microspore Embryogenesis
3. Discussion
3.1. TSA Application Simultaneously with Mannitol Treatment Increases Microspore Embryogenesis
3.2. TSA with Mannitol and Mannitol Treatments Trigger Similar Reprogramming Morphological Changes
3.3. TSA Modifies the Transcript Level of Marker Genes for Early Microspore Embryogenesis
4. Materials and Methods
4.1. Material, Growing Conditions of Donor Plants, and Harvesting of Spikes
4.2. Preparation of Ovary Preconditioned Medium and Ovary Coculture (OVPCM)
4.3. Application of the Histone Deacetylase Inhibitor Trichostatin A (TSA)
4.3.1. Application of TSA after Cold Stress Treatment
4.3.2. Application of TSA after Mannitol Stress Treatment
4.3.3. Application of TSA in Combination with Cold Stress Treatment
4.3.4. Application of TSA in Combination with Mannitol Stress Treatment
4.3.5. Application of TSA as a Unique Anther Treatment
4.4. Anther Culture
4.5. Stereoscopic and Microscopic Observation, DAPI Staining, and Ultrastructural Studies
4.6. Transcript Level Analysis by Quantitative RT-PCR
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BLAST | Basic local alignment search tool |
| CLD | Control cold treatment (7–9 days at 4 °C) |
| MAN | Control mannitol treatment (0.7 M mannitol at 25 °C for 5 days) |
| C0T | Cold treatment with 1% DMSO |
| C0.1T | Cold treatment with 0.1 µM TSA |
| C0.2T | Cold treatment with 0.2 µM TSA |
| C0.4T | Cold treatment with 0.4 µM TSA |
| C + 0T | Cold treatment plus 2 h with 1% DMSO |
| C + 0.1T | Cold treatment plus 2 h with 0.1 µM TSA |
| C + 0.2T | Cold treatment plus 2 h with 0.2 µM TSA |
| C + 0.4T | Cold treatment plus 2 h with 0.4 µM TSA |
| DAPI | 4′,6-Diamidine-2’-phenylindole dihydrochloridl |
| DH | Doubled haploid |
| EMB | Number of embryos/100 anthers |
| FM | Fresh microspores |
| GP | Number of green plants/100 anthers |
| GPDH | Number of green DH plants/100 anthers |
| ME | Microspore embryogenesis |
| M0T | Mannitol treatment with 1% DMSO |
| M0.1T | Mannitol treatment with 0.1 µM TSA |
| M0.2T | Mannitol treatment with 0.2 µM TSA |
| M0.4T | Mannitol treatment with 0.4 µM TSA |
| M + 0T | Mannitol treatment plus 2 h with 1% DMSO |
| M + 0.1T | Mannitol treatment plus 2 h with 0.1 µM TSA |
| M + 0.2T | Mannitol treatment plus 2 h with 0.2 µM TSA |
| M + 0.4T | Mannitol treatment plus 2 h with 0.4 µM TSA |
| PDH | Percentage of DH plants/total green plants analyzed |
| PEMB | Number of proembryos /100 anthers |
| PGP | Number of green plants/total plants |
| RNA | Ribonucleic acid |
| RT | Room temperature |
| SLM | “Star-like” morphology |
| TSA | Trichostatin A |
| 0T | 1% DMSO treatment for 2 h |
| 0.1T | 0.1 µM TSA treatment for 2 h |
| 0.2T | 0.2 µM TSA treatment for 2 h |
| 0.4T | 0.4 µM TSA treatment for 2 h |
References
- Soriano, M.; Li, H.; Boutilier, K. Microspore embryogenesis: Establishment of embryo identity and pattern in culture. Plant. Reprod. 2013, 26, 181–196. [Google Scholar] [CrossRef] [PubMed]
- González-Melendi, P.; Ramirez, C.; Testillano, P.S.; Kumlehn, J.; Risueño, M.C. Three dimensional confocal and electron microscopy imaging define the dynamics and mechanisms of diploidisation at early stages of barley microspore-derived embryogenesis. Planta 2005, 222, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Griggs, R.; Zheng, M.Y. Nuclear Fusion during Early Stage of Microspore Embryogenesis Indicates Chromosome Doubling in Wheat (Triticum aestivum). Am. J. Plant. Sci. 2016, 7, 489–499. [Google Scholar] [CrossRef][Green Version]
- Humphreys, D.G.; Knox, R.E. Doubled Haploid Breeding in Cereals. In Advances in Plant Breeding Strategies: Breeding, Biotechnology and Molecular Tools; Springer Nature: Gewerbestrasse, Switzerland, 2015; pp. 241–290. [Google Scholar]
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield Trends Are Insufficient to Double Global Crop Production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef] [PubMed]
- Lantos, C.; Weyen, J.; Orsini, J.M.; Gnad, H.; Schlieter, B.; Lein, V.; Kontowski, S.; Jacobi, A.; Mihály, R.; Broughton, S.; et al. Efficient application of in vitro anther culture for different European winter wheat (Triticum aestivum L.) breeding programmes. Plant. Breed. 2013, 132, 149–154. [Google Scholar] [CrossRef]
- Weigt, D.; Kiel, A.; Siatkowski, I.; Zyprych-Walczak, J.; Tomkowiak, A.; Kwiatek, M. Comparison of the Androgenic Response of Spring and Winter Wheat (Triticum aestivum L.). Plants 2019, 9, 49. [Google Scholar] [CrossRef]
- Devaux, P.; Cistué, L. Wheat Doubled Haploids: Production to Sequencing. What Makes Them So Appealing? In The World Wheat Book: A History of Wheat Breeding; Bonjean, A.P., Angus, W.J., van Ginkel, M., Eds.; Lavoisier Tec & Doc Publishers: Paris, France, 2016; pp. 885–938. [Google Scholar]
- Maraschin, S.D.F.; Caspers, M.; Potokina, E.; Wulfert, F.; Graner, A.; Spaink, H.P.; Wang, M. cDNA array analysis of stress-induced gene expression in barley androgenesis. Physiol. Plant. 2006, 127, 535–550. [Google Scholar] [CrossRef]
- Muñoz-Amatriain, M.; Svensson, J.T.; Castillo, A.M.; Cistué, L.; Close, T.J.; Vallés, M.P. Transcriptome analysis of barley anthers: Effect of mannitol treatment on microspore embryogenesis. Physiol. Plant. 2006, 127, 551–560. [Google Scholar] [CrossRef]
- Seifert, F.; Bössow, S.; Kumlehn, J.; Gnad, H.; Scholten, S. Analysis of wheat microspore embryogenesis induction by transcriptome and small RNA sequencing using the highly responsive cultivar “Svilena”. BMC Plant. Biol. 2016, 16, 1–16. [Google Scholar] [CrossRef]
- Bélanger, S.; Marchand, S.; Jacques, P.-E.; Meyers, B.; Belzile, F. Differential Expression Profiling of Microspores During the Early Stages of Isolated Microspore Culture Using the Responsive Barley Cultivar Gobernadora. G3 Genes Genom. Genet. 2018, 8, 1603–1614. [Google Scholar] [CrossRef]
- Ojolo, S.P.; Cao, S.; Priyadarshani, S.V.G.N.; Li, W.; Yan, M.; Aslam, M.; Zhao, H.; Qin, Y. Regulation of Plant Growth and Development: A Review From a Chromatin Remodeling Perspective. Front. Plant. Sci. 2018, 9, 1232. [Google Scholar] [CrossRef]
- Pecinka, A.; Chevalier, C.; Colas, I.; Kalantidis, K.; Varotto, S.; Krugman, T.; Michailidis, C.; Vallés, M.-P.; Muñoz, A.; Pradillo, M. Chromatin dynamics during interphase and cell division: Similarities and differences between model and crop plants. J. Exp. Bot. 2019, 71, 5205–5222. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Zhan, Z.; Jiang, D. Histone modifications and their regulatory roles in plant development and environmental memory. J. Genet. Genom. 2019, 46, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Testillano, P.S. Microspore embryogenesis: Targeting the determinant factors of stress-induced cell reprogramming for crop improvement. J. Exp. Bot. 2019, 70, 2965–2978. [Google Scholar] [CrossRef] [PubMed]
- Solís, M.-T.; El-Tantawy, A.-A.; Cano, V.; Risueño, M.C.; Testillano, P.S. 5-azacytidine promotes microspore embryogenesis initiation by decreasing global DNA methylation, but prevents subsequent embryo development in rapeseed and barley. Front. Plant. Sci. 2015, 6, 472. [Google Scholar] [CrossRef]
- Nowicka, A.; Juzoń, K.; Krzewska, M.; Dziurka, M.; Dubas, E.; Kopeć, P.; Zieliński, K.; Żur, I. Chemically-induced DNA de-methylation alters the effectiveness of microspore embryogenesis in triticale. Plant. Sci. 2019, 287, 110189. [Google Scholar] [CrossRef]
- Berenguer, E.; Bárány, I.; Solís, M.-T.; Pérez-Pérez, Y.; Risueño, M.C.; Testillano, P.S. Inhibition of Histone H3K9 Methylation by BIX-01294 Promotes Stress-Induced Microspore Totipotency and Enhances Embryogenesis Initiation. Front. Plant. Sci. 2017, 8, 1161. [Google Scholar] [CrossRef]
- Li, H.; Soriano, M.; Cordewener, J.; Muiño, J.M.; Riksen, T.; Fukuoka, H.; Angenent, G.C.; Boutilier, K. The Histone Deacetylase Inhibitor Trichostatin A Promotes Totipotency in the Male Gametophyte. Plant. Cell 2014, 26, 195–209. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Gao, Y.; Jiang, X.; Zhang, M.; Wu, H.; Liu, Z.; Feng, H. Effects of histone deacetylase inhibitors on microspore embryogenesis and plant regeneration in Pakchoi (Brassica rapa ssp. chinensis L.). Sci. Hortic. 2016, 209, 61–66. [Google Scholar] [CrossRef]
- Pandey, P.; Daghma, D.S.; Houben, A.; Kumlehn, J.; Melzer, M.; Rutten, T. Dynamics of post-translationally modified histones during barley pollen embryogenesis in the presence or absence of the epi-drug trichostatin A. Plant. Reprod. 2017, 30, 95–105. [Google Scholar] [CrossRef]
- Jiang, F.; Ryabova, D.; Diedhiou, J.; Hucl, P.; Randhawa, H.; Marillia, E.-F.; Foroud, N.A.; Eudes, F.; Kathiria, P. Trichostatin A increases embryo and green plant regeneration in wheat. Plant. Cell Rep. 2017, 36, 1701–1706. [Google Scholar] [CrossRef]
- Wang, H.M.; Enns, J.L.; Nelson, K.L.; Brost, J.M.; Orr, T.D.; Ferrie, A.M.R. Improving the efficiency of wheat microspore culture methodology: Evaluation of pretreatments, gradients, and epigenetic chemicals. Plant. Cell Tissue Organ. Cult. (PCTOC) 2019, 139, 589–599. [Google Scholar] [CrossRef]
- Sánchez-Díaz, R.A.; Castillo, A.M.; Vallés, M.P. Microspore embryogenesis in wheat: New marker genes for early, middle and late stages of embryo development. Plant. Reprod. 2013, 26, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.S.; Tuteja, N. Enhancement of androgenesis by abiotic stress and other pretreatments in major crop species. Plant. Sci. 2012, 182, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.Y.; Bieren, K.; Griggs, R. Developmental Dynamics of Wheat (Triticum aestivum L.) Microspores under Culture. Adv. Biosci. Biotechnol. 2015, 6, 693–701. [Google Scholar] [CrossRef]
- Cistué, L.; Ramos, A.; Castillo, A.M. Influence of anther pretreatment and culture medium composition on the production of barley doubled haploids from model and low responding cultivars. Plant. Cell Tissue Organ. Cult. 1998, 55, 159–166. [Google Scholar] [CrossRef]
- Yoshida, M.; Kijima, M.; Akita, M.; Beppu, T. Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J. Biol. Chem. 1990, 265, 17174–17179. [Google Scholar] [PubMed]
- Mazzio, E.A.; Soliman, K.F.A. Whole-transcriptomic Profile of SK-MEL-3 Melanoma Cells Treated with the Histone Deacetylase Inhibitor: Trichostatin A. Cancer Genom. Proteom. 2018, 15, 349–364. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, L.; Li, J.; Huang, J.; Wen, R.; Ma, L.; Zhou, D.; Li, L. Trichostatin A and 5-azacytidine both cause an increase in global histone H4 acetylation and a decrease in global DNA and H3K9 methylation during mitosis in maize. BMC Plant. Biol. 2010, 10, 1–11. [Google Scholar] [CrossRef]
- Ma, X.; Lv, S.; Zhang, C.; Yang, C. Histone deacetylases and their functions in plants. Plant. Cell Rep. 2013, 32, 465–478. [Google Scholar] [CrossRef]
- Asif, M.; Eudes, F.; Goyal, A.; Amundsen, E.; Randhawa, H.; Spaner, D. Organelle antioxidants improve microspore embryogenesis in wheat and triticale. Vitr. Cell. Dev. Biol. Anim. 2013, 49, 489–497. [Google Scholar] [CrossRef]
- Zang, X.H.; Yu, X.-Z.; Yu, X.-Z.; Yue, D.-M. Phytotoxicity of dimethyl sulfoxide (DMSO) to rice seedlings. Int. J. Environ. Sci. Technol. 2015, 13, 607–614. [Google Scholar] [CrossRef]
- Hooghvorst, I.; Ribas, P.; Nogués, S. Chromosome doubling of androgenic haploid plantlets of rice (Oryza sativa) using antimitotic compounds. Plant. Breed. 2020, 139, 754–761. [Google Scholar] [CrossRef]
- Echávarri, B.; Cistué, L. Enhancement in androgenesis efficiency in barley (Hordeum vulgare L.) and bread wheat (Triticum aestivum L.) by the addition of dimethyl sulfoxide to the mannitol pretreatment medium. Plant. Cell Tissue Organ. Cult. 2015, 125, 11–22. [Google Scholar] [CrossRef]
- Wakayama, T.; Yanagimachi, R. Effect of cytokinesis inhibitors, DMSO and the timing of oocyte activation on mouse cloning using cumulus cell nuclei. Reproduction 2001, 122, 49–60. [Google Scholar] [CrossRef]
- Verheijen, M.; Lienhard, M.; Schrooders, Y.; Clayton, O.; Nudischer, R.; Boerno, S.; Timmermann, B.; Selevsek, N.; Schlapbach, R.; Gmuender, H.; et al. DMSO induces drastic changes in human cellular processes and epigenetic landscape in vitro. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Nguyen, H.T.H.; Bouteau, F.; Mazars, C.; Kuse, M.; Kawano, T. Enhanced elevations of hypo-osmotic shock-induced cytosolic and nucleic calcium concentrations in tobacco cells by pretreatment with dimethyl sulfoxide. Biosci. Biotechnol. Biochem. 2018, 83, 318–321. [Google Scholar] [CrossRef]
- Cistué, L.; Vallés, M.P.; Echávarri, B.; Sanz, J.M.; Castillo, A.M. Production of Barley Doubled Haploids by Anther and Microspore Culture. In In Vitro Application in Crop Improvement; Mujib, A., Cho, M.J., Predieri, S., Banerjee, S., Eds.; Science Publishers: Enfield, NH, USA, 2004; pp. 1–17. [Google Scholar]
- Cui, Y.; Ling, Y.; Zhou, J.; Li, X. Interference of the Histone Deacetylase Inhibits Pollen Germination and Pollen Tube Growth in Picea wilsonii Mast. PLoS ONE 2015, 10, e0145661. [Google Scholar] [CrossRef]
- Parra-Vega, V.; Corral-Martínez, P.; Rivas-Sendra, A.; Seguí-Simarro, J.M. Induction of embryogenesis in Brassica napus micro-spores produces a callosic subintinal layer and abnormal cell walls with altered levels of callose and cellulose. Front. Plant. Sci. 2015, 25, 1018. [Google Scholar]
- Gervais, C.; Newcomb, W.; Simmonds, D.H. Rearrangement of the actin filament and microtubule cytoskeleton during induction of microspore embryogenesis in Brassica napus L. cv. Topas. Protoplasma 2000, 213, 194–202. [Google Scholar] [CrossRef]
- Indrianto, A.; Barinova, I.; Touraev, A.; Heberle-Bors, E. Tracking individual wheat microspores in vitro: Identification of embryogenic microspores and body axis formation in the embryo. Planta 2001, 212, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Raina, S.K.; Irfan, S.T. High-frequency embryogenesis and plantlet regeneration from isolated microspores of indica rice. Plant. Cell Rep. 1998, 17, 957–962. [Google Scholar] [CrossRef]
- Touraev, A.; Ilham, A.; Vicente, O.; Heberle-Bors, E. Stress induced microspore embryogenesis in tobacco: An optimized system for molecular studies. Plant. Cell Rep. 1996, 15, 561–565. [Google Scholar] [CrossRef]
- Maraschin, S.F.; Vennik, M.; Lamers, G.E.M.; Spaink, H.P.; Wang, M. Time-lapse tracking of barley androgenesis reveals position-determined cell death within pro-embryos. Planta 2004, 220, 531–540. [Google Scholar] [CrossRef]
- Daghma, D.E.S.; Hensel, G.; Rutten, T.; Melzer, M.; Kumlehn, J. Cellular dynamics during early barley pollen embryogenesis revealed by time-lapse imaging. Front. Plant. Sci. 2014, 5, 675. [Google Scholar] [CrossRef] [PubMed]
- Testillano, P.S.; Coronado, M.; Seguí-Simarro, J.M.; Domenech, J.; González-Melendi, P.; Raška, I.; Risueño, M. Defined Nuclear Changes Accompany the Reprogramming of the Microspore to Embryogenesis. J. Struct. Biol. 2000, 129, 223–232. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, K.; Bhalla, P.L. Ultrastructure of microsporogenesis and microgametogenesis in Brachypodium distachyon. Protoplasma 2015, 252, 1575–1586. [Google Scholar] [CrossRef] [PubMed]
- Garrido, D.; Vicente, O.; Heberle-Bors, E.; Garrido, D.; Rodriguez-García, M.I. Cellular changes during the acquisition of embryogenic potential in isolated pollen grains of Nicotiana tabacum. Protoplasma 1995, 186, 220–230. [Google Scholar] [CrossRef]
- Seguí-Simarro, J.M.; Corral-Martinez, P.; Corredor, E.; Raška, I.; Testillano, P.S.; Risueño, M.C. A change of developmental program induces the remodeling of the interchromatin domain during microspore embryogenesis in Brassica napus L. J. Plant. Physiol. 2011, 168, 746–757. [Google Scholar] [CrossRef] [PubMed]
- Love, A.J.; Yu, C.; Petukhova, N.V.; Kalinina, N.O.; Chen, J.; Taliansky, M. Cajal bodies and their role in plant stress and disease responses. RNA Biol. 2016, 14, 779–790. [Google Scholar] [CrossRef]
- Baranova, E.N.; Chaban, I.A.; Kononenko, N.V.; Khaliluev, M.R.; Christov, N.K.; Gulevich, A.A.; Todorovska, E.G. Ultrastructural organization of the domains in the cell nucleus of dicotyledonous and monocotyledonous plants under abiotic stress. Russ. Agric. Sci. 2017, 43, 199–206. [Google Scholar] [CrossRef]
- Corral-Martínez, P.; Parra-Vega, V.; Seguí-Simarro, J.M. Novel features of Brassica napus embryogenic microspores revealed by high pressure freezing and freeze substitution: Evidence for massive autophagy and excretion-based cytoplasmic cleaning. J. Exp. Bot. 2013, 64, 3061–3075. [Google Scholar] [CrossRef]
- Bonet-García, F.J.; Olmedilla, A. Structural changes during early embryogenesis in wheat pollen. Protoplasma 2000, 211, 94–102. [Google Scholar] [CrossRef]
- Bárány, I.; Berenguer, E.; Solís, M.-T.; Pérez-Pérez, Y.; Santamaria, M.E.; Crespo, J.L.; Risueño, M.C.; Diaz, I.; Testillano, P.S. Autophagy is activated and involved in cell death with participation of cathepsins during stress-induced microspore embryogenesis in barley. J. Exp. Bot. 2018, 69, 1387–1402. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-L.; Xie, L.-F.; Mao, H.-Z.; Puah, C.S.; Yang, W.-C.; Jiang, L.; Sundaresan, V.; Ye, D. TAPETUM DETERMINANT1 Is Required for Cell Specialization in the Arabidopsis Anther. Plant. Cell 2003, 15, 2792–2804. [Google Scholar] [CrossRef] [PubMed]
- Leljak-Levanić, D.; Juranić, M.; Sprunck, S. De novo zygotic transcription in wheat (Triticum aestivum L.) includes genes encoding small putative secreted peptides and a protein involved in proteasomal degradation. Plant. Reprod. 2013, 26, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wijeratne, A.J.; Tang, C.; Zhang, T.; Fenelon, R.E.; Owen, H.A.; Zhao, D. Ectopic expression of TAPETUM DETERMINANT1 affects ovule development in Arabidopsis. J. Exp. Bot. 2015, 67, 1311–1326. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Xia, Q.; Xie, W.; Dumonceaux, T.J.; Zou, J.; Datla, R.; Selvaraj, G. Male gametophyte development in bread wheat (Triticum aestivum L.): Molecular, cellular, and biochemical analyses of a sporophytic contribution to pollen wall ontogeny. Plant. J. 2002, 30, 613–623. [Google Scholar] [CrossRef]
- Rowland, O.; Domergue, F. Plant fatty acyl reductases: Enzymes generating fatty alcohols for protective layers with potential for industrial applications. Plant. Sci. 2012, 62, 28–38. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Sun, Y.; Wang, Y.; Li, T.; Chai, G.; Jiang, W.; Shan, L.; Li, C.; Xiao, E.; et al. FAR5, a fatty acyl-coenzyme A reductase, is involved in primary alcohol biosynthesis of the leaf blade cuticular wax in wheat (Triticum aestivum L.). J. Exp. Bot. 2014, 66, 1165–1178. [Google Scholar] [CrossRef]
- Wójcikowska, B.; Botor, M.; Morończyk, J.; Wójcik, A.M.; Nodzyński, T.; Karcz, J.; Gaj, M.D. Trichostatin A Triggers an Embryogenic Transition in Arabidopsis Explants via an Auxin-Related Pathway. Front. Plant. Sci. 2018, 9, 1353. [Google Scholar] [CrossRef] [PubMed]
- Cummins, I.; O’Hagan, D.; Jablonkai, I.; Cole, D.J.; Hehn, A.; Werck-Reichhart, D.; Edwards, R. Cloning, characterization and regulation of a family of phi class glutathione transferases from wheat. Plant. Mol. Biol. 2003, 52, 591–603. [Google Scholar] [CrossRef]
- Wang, R.; Ma, J.; Zhang, Q.; Wu, C.; Zhao, H.; Wu, Y.; Yang, G.; He, G. Genome-wide identification and expression profiling of glutathione transferase gene family under multiple stresses and hormone treatments in wheat (Triticum aestivum L.). BMC Genom. 2019, 20, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Faik, A.; Abouzouhair, J.; Sarhan, F. Putative fasciclin-like arabinogalactan-proteins (FLA) in wheat (Triticum aestivum) and rice (Oryza sativa): Identification and bioinformatic analyses. Mol. Genet. Genomics 2006, 276, 478–494. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.R.; Wang, F.; Dirpaul, J.M.; Zhou, N.; Polowick, P.L.; Ferrie, A.M.; Krochko, J.E. Transcript Profiling and Identification of Molecular Markers for Early Microspore Embryogenesis in Brassica napus. Plant. Physiol. 2007, 144, 134–154. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, S.-Y.; Liu, H.-H.; Zhang, X.-L.; Zeng, A.-S.; Wang, J.-J.; Hou, X.-L.; Li, Y. cDNA-AFLP analysis of differentially expressed genes during microspore embryogenesis in non-heading Chinese cabbage. Vitr. Cell. Dev. Biol. Anim. 2020, 56, 18–28. [Google Scholar] [CrossRef]
- El-Tantawy, A.-A.; Solís, M.-T.; Risueño, M.; Testillano, P.S. Changes in DNA Methylation Levels and Nuclear Distribution Patterns after Microspore Reprogramming to Embryogenesis in Barley. Cytogenet. Genome Res. 2014, 143, 200–208. [Google Scholar] [CrossRef]
- Soriano, M.; Cistué, L.; Vallés, M.P.; Castillo, A.M. Effects of colchicine on anther and microspore culture of bread wheat (Triticum aestivum L.). Plant. Cell Tissue Organ. Cult. 2007, 91, 225–234. [Google Scholar] [CrossRef]
- Castillo, A.M.; Sánchez-Díaz, R.A.; Vallés, M.P. Effect of ovary induction on bread wheat anther culture: Ovary genotype and developmental stage, and candidate gene association. Front. Plant. Sci. 2015, 6, 402. [Google Scholar] [CrossRef]
- Hu, T.; Kasha, K.J. Improvement of isolated microspore culture of wheat (Triticum aestivum L.) trough ovary co-culture. Plant. Cell Rep. 1997, 16, 520–525. [Google Scholar] [CrossRef]
- Hunter, C.P. Plant Regeneration from Microspores of Barley, Hordeum vulgare L. Ph.D. Thesis, Wye College University of London, London, UK, 1988. [Google Scholar]
- Soriano, M.; Cistué, L.; Castillo, A.M. Enhanced induction of microspore embryogenesis after n-butanol treatment in wheat (Triticum aestivum L.) anther culture. Plant. Cell Rep. 2008, 27, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.J. Monoploid Production by Chromosome Elimination. In Applied and Fundamental Aspects of Plant Cell, Tissue and Organ Culture; Reinert, J., Bajaj, Y.P.S., Eds.; Springer: Berlin, Germany, 1977; pp. 299–330. [Google Scholar]
- Castillo, A.M.; Vallés, M.P.; Cistué, L. Comparison of anther and isolated microspore cultures in barley. Effects of culture density and regeneration medium. Euphytica 2000, 113, 1–8. [Google Scholar] [CrossRef]
- Paolacci, A.R.; Tanzarella, O.A.; Porceddu, E.; Ciaffi, M. Identification and validation of reference genes for quantitative RT-PCR normalization in wheat. BMC Mol. Biol. 2009, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-∆∆Ct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]



| PEMB * | EMB * | GP * | PGP (%) | PDH (%) | GPDH * | |
|---|---|---|---|---|---|---|
| (A) CLD + 2hTSA | ||||||
| CLD | 67.8 a† | 14.5 a | 2.1 a | 19.7 a | 66.7 a | 1.4 a |
| C+0T | 82.9 a | 12.2 a | 1.7 a | 32.4 a | 69.9 a | 1.2 a |
| C+0.1T | 88.6 a | 15.8 a | 1.1 a | 22.0 a | 70.2 a | 0.8 a |
| C+0.2T | 66.5 a | 12.4 a | 1.2 a | 12.5 a | 83.3 a | 1.0 a |
| C+0.4T | 78.7 a | 15.2 a | 2.2 a | 13.1 a | 79.3 a | 1.8 a |
| (B) MAN + 2hTSA | ||||||
| MAN | 273.7 a | 33.8 a | 11.9 a | 70.0 a | 22.8 ab | 2.7 ab |
| M+0T | 226.4 a | 26.4 a | 7.9 a | 61.3 a | 17.3 b | 1.4 b |
| M+0.1T | 262.1 a | 32.6 a | 12.5 a | 67.2 a | 34.1 a | 4.3 a |
| M+0.2T | 274.2 a | 30.8 a | 8.6 a | 66.4 a | 28.0 ab | 2.4 ab |
| M+0.4T | 254.7 a | 30.9 a | 8.3 a | 51.3 a | 12.5 b | 1.0 b |
| (C) CLDTSA | ||||||
| CLD | 183.0 b | 46.7 b | 1.6 b | 12.0 a | 81.6 ab | 1.3 b |
| C0T | 198.8 b | 47.9 b | 1.2 b | 9.4 a | 76.8 ab | 0.9 b |
| C0.1T | 222.7 ab | 58.3 ab | 3.2 ab | 17.0 a | 86.8 a | 2.7 a |
| C0.2T | 236.1 a | 64.2 a | 2.6 ab | 15.0 a | 67.2 ab | 1.7 ab |
| C0.4T | 313.5 a | 79.8 a | 5.2 a | 20.3 a | 63.5 b | 3.3 a |
| (D) MANTSA | ||||||
| MAN | 192.4 c | 48.6 c | 18.3 b | 65.2 a | 32.1 b | 5.9 c |
| M0T | 215.5 c | 66.4 bc | 26.9 ab | 66.7 a | 54.5 a | 14.7 b |
| M0.1T | 435.8 b | 100.5 ab | 33.1 ab | 69.8 a | 57.1 a | 18.9 ab |
| M0.2T | 563.3 ab | 103.5 a | 39.9 a | 66.0 a | 50.3 a | 20.1 ab |
| M0.4T | 646.4 a | 111.8 a | 40.0 a | 70.4 a | 62.4 a | 24.9 a |
| (E) TSA | ||||||
| MAN | 257.1 a | 50.5 a | 9.4 a | 63.9 a | 31.2 b | 2.9 b |
| 0T | 171.7 a | 38.1 a | 5.4 b | 32.1 c | 65.6 a | 3.5 b |
| 0.1T | 185.3 a | 44.3 a | 9.1 a | 46.0 ab | 74.6 a | 6.8 a |
| 0.2T | 170.9 a | 40.0 a | 7.2 ab | 38.2 bc | 85.3 a | 6.1 a |
| 0.4T | 196.7 a | 40.1 a | 9.7 a | 35.1 c | 76.2 a | 7.4 a |
| Cultivar | PEMB * | EMB * | GP * | PGP (%) | PDH (%) | GPDH * |
|---|---|---|---|---|---|---|
| Pavon | ||||||
| MAN | 211.7 b† | 59.2 b | 25.9 b | 73.7 a | 28.0 b | 7.2 b |
| M0T | 185.8 b | 70.7 ab | 33.1 ab | 66.3 a | 52.7 a | 17.8 ab |
| M0.1T | 402.9 ab | 113.4 a | 43.8 ab | 75.9 a | 56.2 a | 24.6 ab |
| M0.2T | 563.0 a | 115.8 a | 52.3 ab | 75.5 a | 44.0 ab | 23.0 ab |
| M0.4T | 516.1 a | 120.3 a | 52.1 a | 71.0 a | 58.9 a | 30.7 a |
| Caramba | ||||||
| MAN | 162.2 c | 30.4 c | 5.1 b | 49.4 a | 53.2 b | 2.7 b |
| M0T | 287.1 bc | 55.7 bc | 12.0 ab | 67.7 a | 56.5 b | 6.8 ab |
| M0.1T | 499.5 ab | 78.9 ab | 13.5 ab | 59.4 a | 60.0 b | 8.1 ab |
| M0.2T | 564.0 ab | 74.9 ab | 11.4 ab | 45.0 a | 79.3 a | 9.0 ab |
| M0.4T | 890.3 a | 97.7 a | 18.2 a | 69.5 a | 72.6 ab | 13.2 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo, A.M.; Valero-Rubira, I.; Burrell, M.Á.; Allué, S.; Costar, M.A.; Vallés, M.P. Trichostatin A Affects Developmental Reprogramming of Bread Wheat Microspores towards an Embryogenic Route. Plants 2020, 9, 1442. https://doi.org/10.3390/plants9111442
Castillo AM, Valero-Rubira I, Burrell MÁ, Allué S, Costar MA, Vallés MP. Trichostatin A Affects Developmental Reprogramming of Bread Wheat Microspores towards an Embryogenic Route. Plants. 2020; 9(11):1442. https://doi.org/10.3390/plants9111442
Chicago/Turabian StyleCastillo, Ana María, Isabel Valero-Rubira, María Ángela Burrell, Sandra Allué, María Asunción Costar, and María Pilar Vallés. 2020. "Trichostatin A Affects Developmental Reprogramming of Bread Wheat Microspores towards an Embryogenic Route" Plants 9, no. 11: 1442. https://doi.org/10.3390/plants9111442
APA StyleCastillo, A. M., Valero-Rubira, I., Burrell, M. Á., Allué, S., Costar, M. A., & Vallés, M. P. (2020). Trichostatin A Affects Developmental Reprogramming of Bread Wheat Microspores towards an Embryogenic Route. Plants, 9(11), 1442. https://doi.org/10.3390/plants9111442








