Ontogeny and Anatomy of the Dimorphic Pitchers of Nepenthes rafflesiana Jack
Abstract
:1. Introduction
2. Results
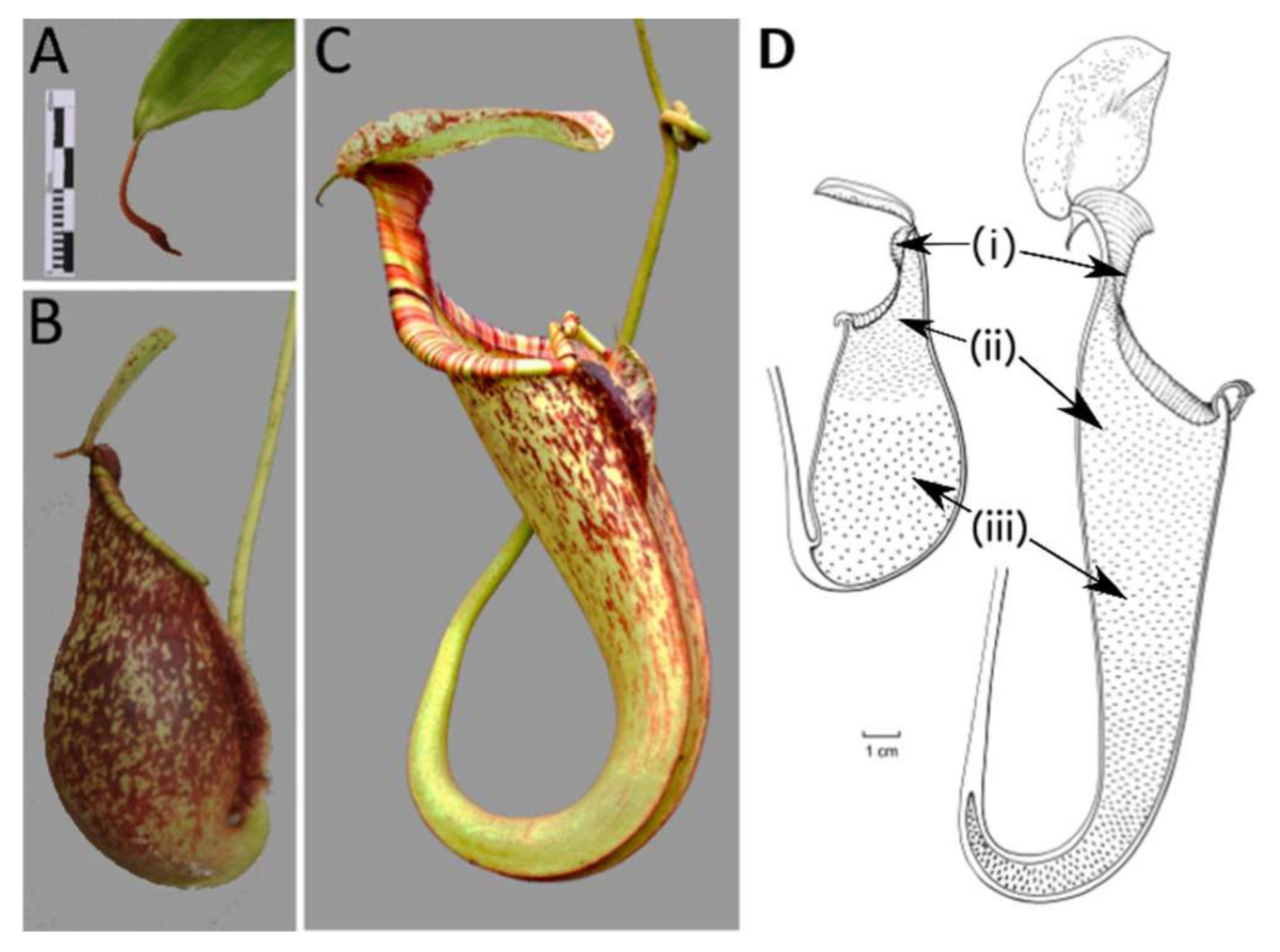
2.1. Developmental Phases
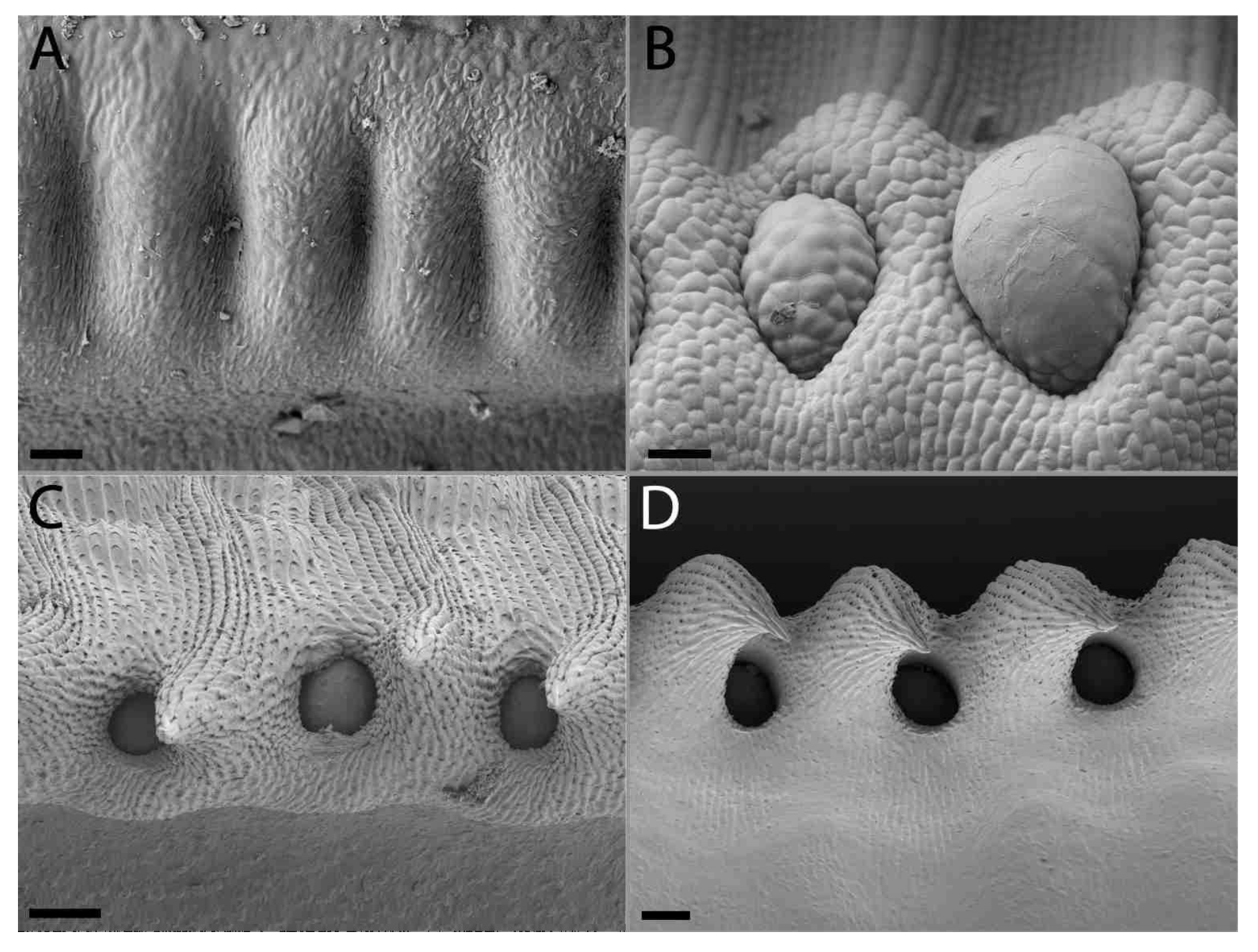
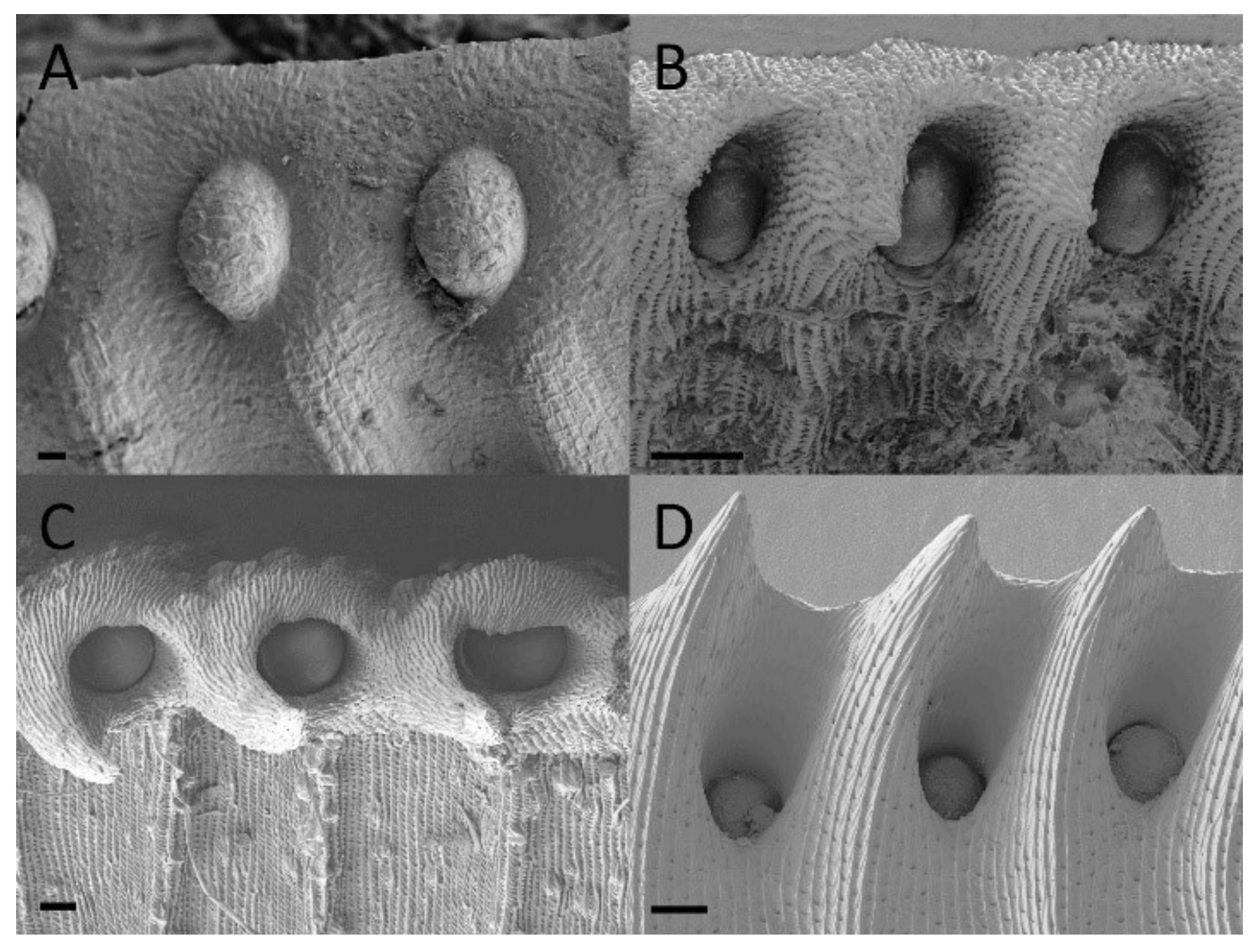
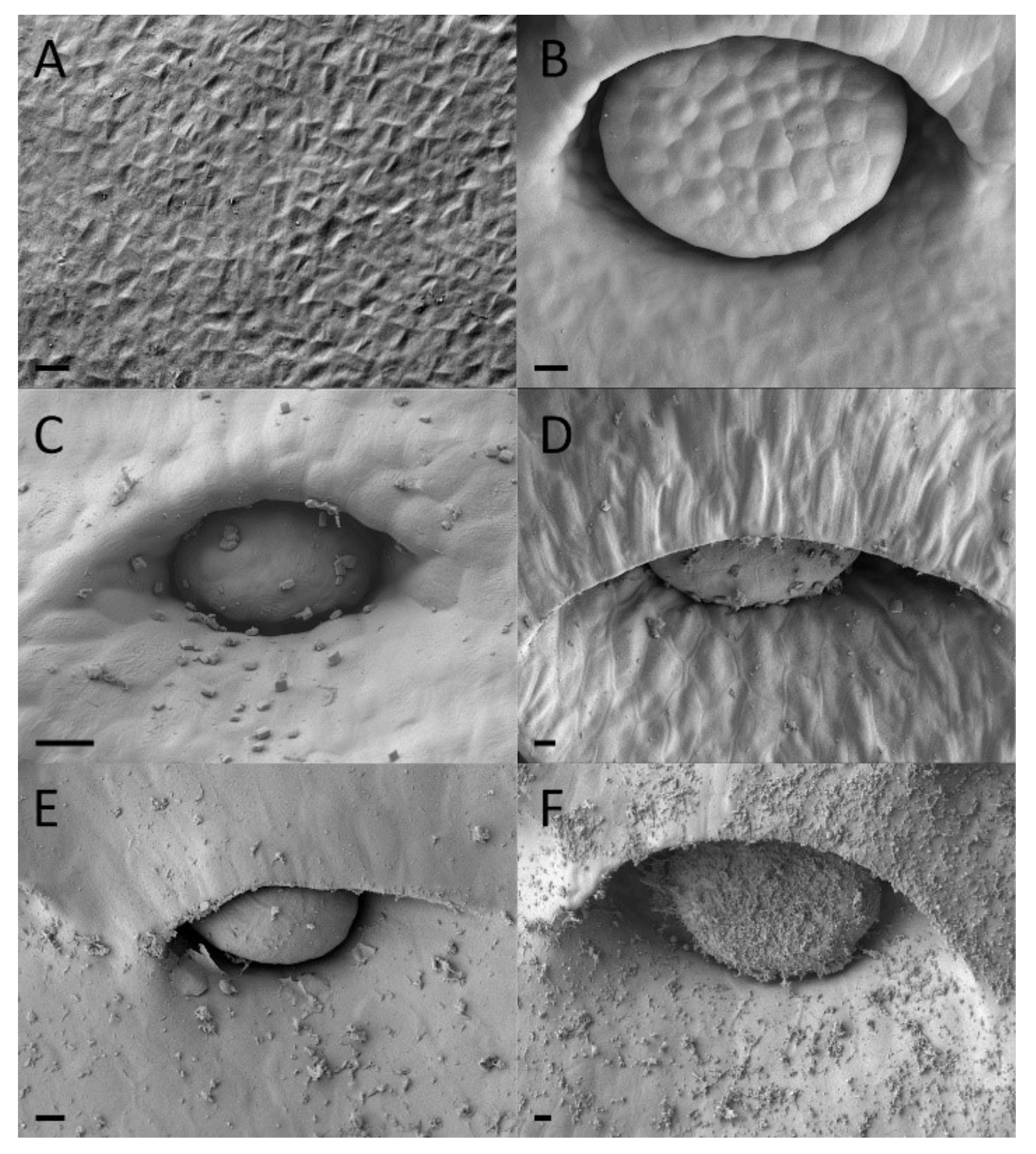
2.2. Extrafloral Nectary and Peristomal Teeth
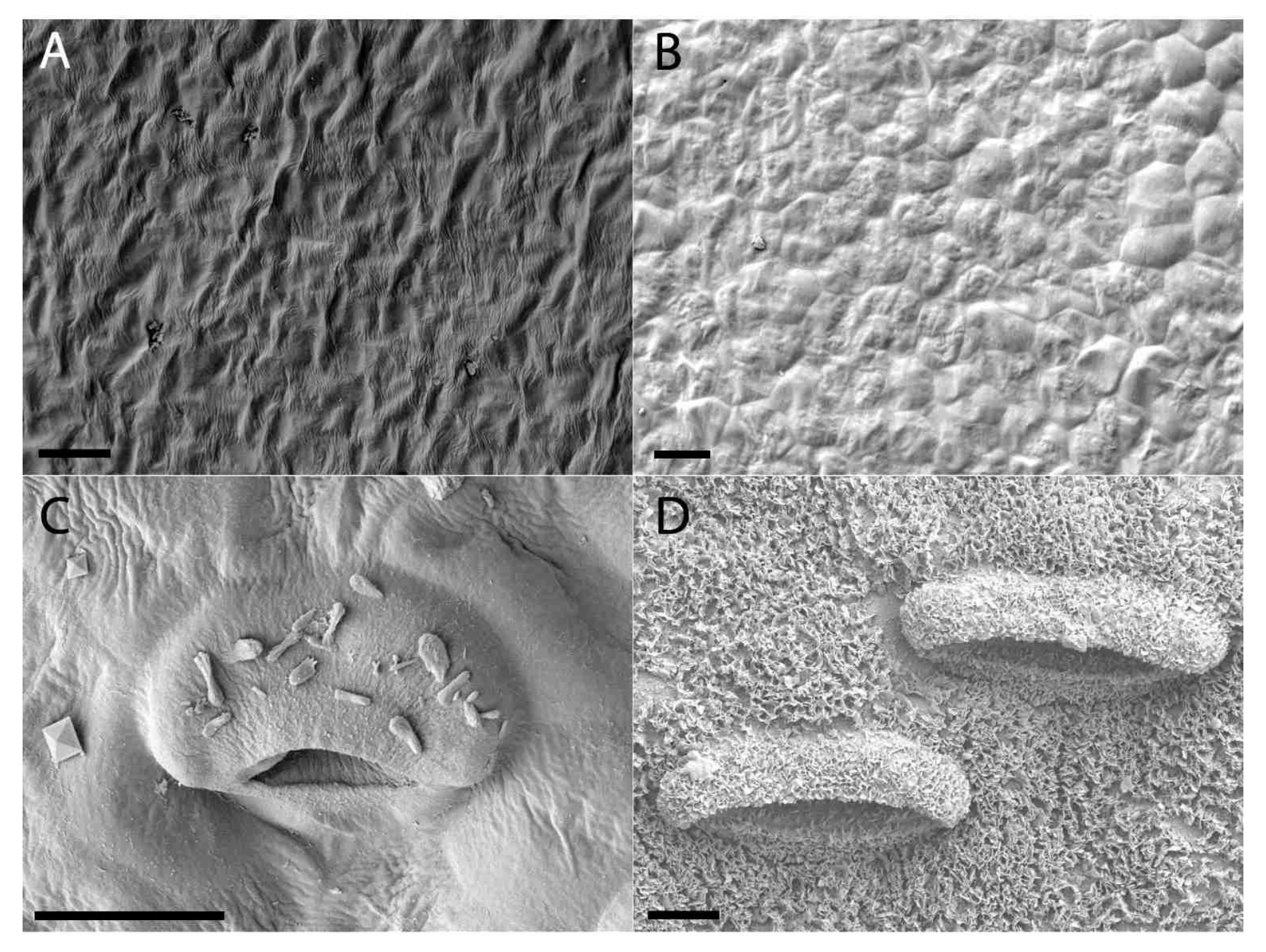
2.3. Digestive Glands
2.4. Waxy Scales and Lunate Cells
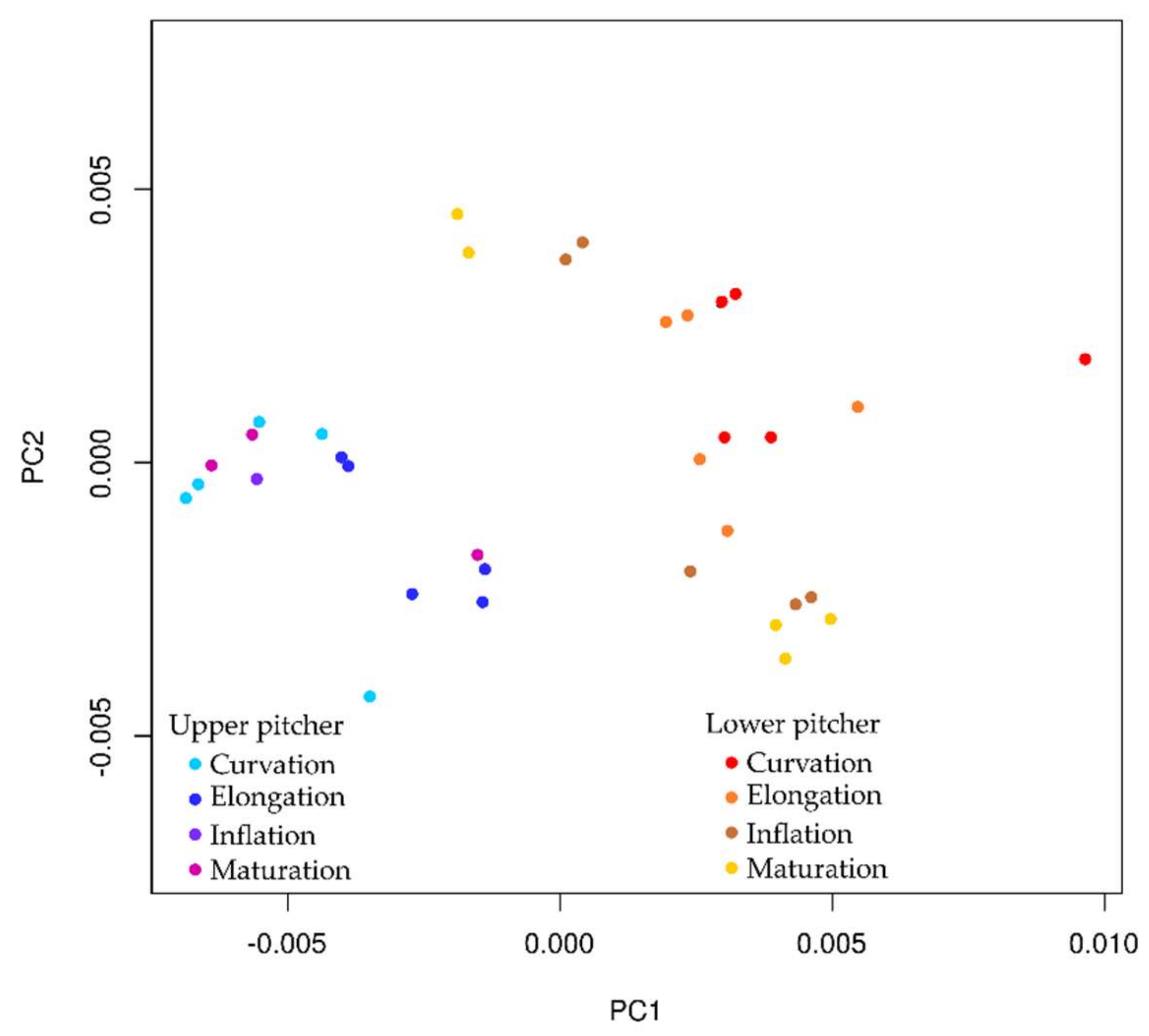
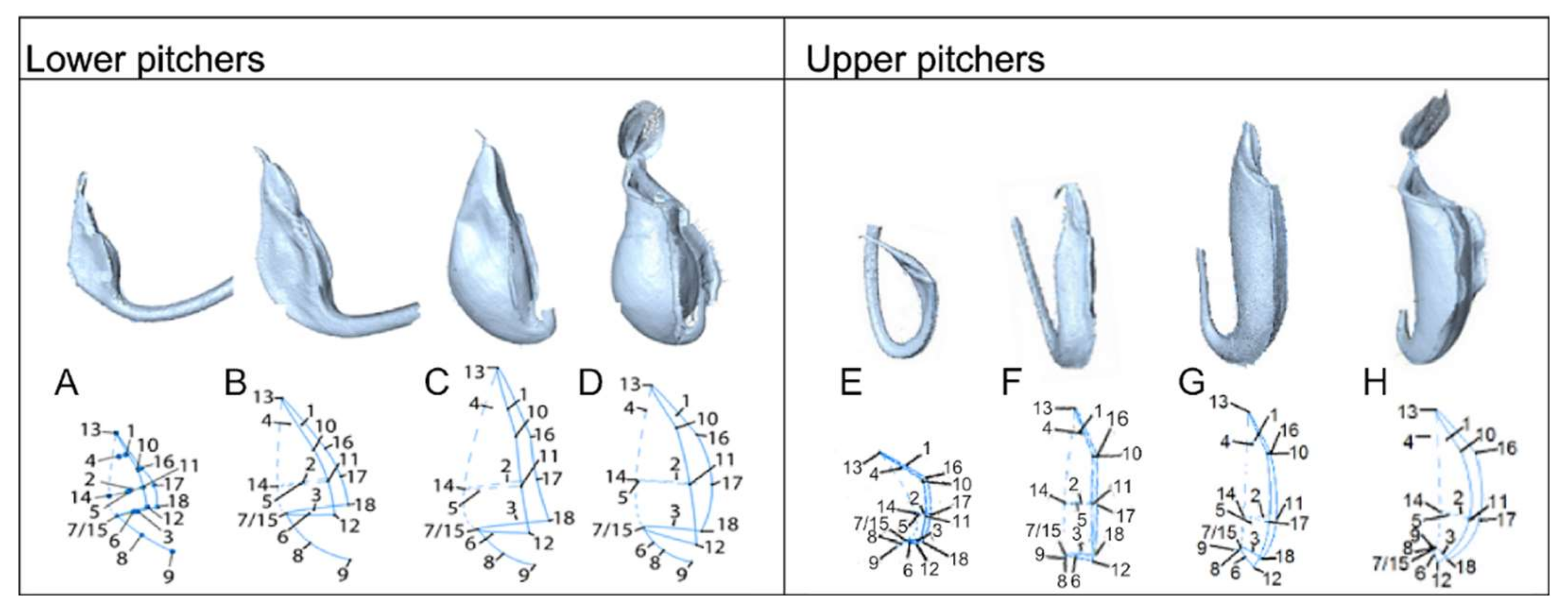
2.5. Ontogeny of Upper and Lower Pitchers
3. Discussion
3.1. Developmental Phases
3.2. Extrafloral Nectary and Peristomal Teeth
3.3. Digestive Glands
3.4. Waxy Scales and Lunate Cells
3.5. Ontogeny of Upper and Lower Pitchers
4. Materials and Methods
4.1. Study Species: Nepenthes Rafflesiana
4.2. 3D Surface Laser Scanning and Landmark-Based Geometric Morphometrics
4.3. Pipeline Created in Galaxy for Statistical Analysis of Pitchers
4.4. Scanning Electron Microscopy (SEM)
4.5. Light Microscopy (LM)
4.6. Detection of Sugars in Peristomal Fluid
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Juniper, B.; Robins, R.; Joel, D.M. The Carnivorous Plants; Academic Press: London, UK; San Diego, CA, USA, 1989; ISBN 9780123921703. [Google Scholar]
- Schulze, W.; Schulze, E.D.; Pate, J.S.; Gillison, A.N. The nitrogen supply from soils and insects during growth of the pitcher plants Nepenthes mirabilis, Cephalotus follicularis and Darlingtonia californica. Oecologia 1997, 112, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Cheek, M.; Jebb, M.; Nooteboom, H.P. Flora Malesiana; Series I; Spermatophyta = Flowering plants; Nepenthaceae; Nationaal Herbarium Nederland: Leiden, The Netherlands, 2001; Volume 15. [Google Scholar]
- Meimberg, H.; Heubl, G. Introduction of a nuclear marker for phylogenetic analysis of Nepenthaceae. Plant Biol. 2006, 8, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Cheek, M.; Jebb, M. Recircumscription of the Nepenthes alata group (Caryophyllales: Nepenthaceae), in the Philippines, with four new species. Eur. J. Taxon. 2013, 69, 1–23. [Google Scholar] [CrossRef]
- Moran, J.A.; Booth, W.E.; Charles, J.K. Aspects of pitcher morphology and spectral characteristics of six bornean Nepenthes pitcher plant species: Implications for prey capture. Ann. Bot. 1999, 83, 521–528. [Google Scholar] [CrossRef] [Green Version]
- Moran, J.A.; Clarke, C.; Gowen, B.E. The use of light in prey capture by the tropical pitcher plant Nepenthes aristolochioides. Plant Signal. Behav. 2012, 7, 957–960. [Google Scholar] [CrossRef] [Green Version]
- Plancho, B.J. Sweet but dangerous: Nectaries in carnivorous plants. Acta Agrobot. 2007, 60, 31–37. [Google Scholar]
- Bauer, U.; Federle, W. The insect-trapping rim of Nepenthes pitchers: Surface structure and function. Plant Signal. Behav. 2009, 4, 1019–1023. [Google Scholar] [CrossRef] [Green Version]
- Chin, L.; Moran, J.A.; Clarke, C. Trap geometry in three giant montane pitcher plant species from Borneo is a function of tree shrew body size. New Phytol. 2010, 186, 461–470. [Google Scholar] [CrossRef]
- Di Giusto, B.; Bessière, J.-M.; Guéroult, M.; Lim, L.B.L.; Marshall, D.J.; Hossaert-McKey, M.; Gaume, L. Flower-scent mimicry masks a deadly trap in the carnivorous plant Nepenthes rafflesiana. J. Ecol. 2010, 98, 845–856. [Google Scholar] [CrossRef]
- Bohn, H.F.; Federle, W. Insect aquaplaning: Nepenthes pitcher plants capture prey with the peristome, a fully wettable water-lubricated anisotropic surface. PNAS 2004, 101, 14138–14143. [Google Scholar] [CrossRef] [Green Version]
- Bauer, U.; Bohn, H.F.; Federle, W. Harmless nectar source or deadly trap: Nepenthes pitchers are activated by rain, condensation and nectar. Proc. Biol. Sci. 2008, 275, 259–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaume, L.; Forterre, Y.A. Viscoelastic deadly fluid in carnivorous pitcher plants. PLoS ONE 2007, 2, e1185. [Google Scholar] [CrossRef] [PubMed]
- Di Giusto, B.; Grosbois, V.; Fargeas, E.; Marshall, D.J.; Gaume, L. Contribution of pitcher fragrance and fluid viscosity to high prey diversity in a Nepenthes carnivorous plant from Borneo. J. Biosci. 2008, 33, 121–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonhomme, V.; Pelloux-Prayer, H.; Jousselin, E.; Forterre, Y.; Labat, J.-J.; Gaume, L. Slippery or sticky? Functional diversity in the trapping strategy of Nepenthes carnivorous plants. New Phytol. 2011, 191, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Gaume, L.; Di Giusto, B. Adaptive significance and ontogenetic variability of the waxy zone in Nepenthes rafflesiana. Ann. Bot. 2009, 104, 1281–1291. [Google Scholar] [CrossRef]
- Scholz, I.; Bückins, M.; Dolge, L.; Erlinghagen, T.; Weth, A.; Hischen, F.; Mayer, J.; Hoffmann, S.; Riederer, M.; Riedel, M.; et al. Slippery surfaces of pitcher plants: Nepenthes wax crystals minimize insect attachment via microscopic surface roughness. J. Exp. Biol. 2010, 213, 1115–1125. [Google Scholar] [CrossRef] [Green Version]
- Gorb, E.V.; Baum, M.J.; Gorb, S.N. Development and regeneration ability of the wax coverage in Nepenthes alata pitchers: A cryo-SEM approach. Sci. Rep. 2013, 3, 3078. [Google Scholar] [CrossRef] [Green Version]
- Owen, T.P.; Lennon, K.A. Structure and development of the pitchers from the carnivorous plant Nepenthes alata (Nepenthaceae). Am. J. Bot. 1999, 86, 1382–1390. [Google Scholar] [CrossRef]
- Owen, T.P.; Lennon, K.A.; Santo, M.J.; Anderson, A.N. Pathways for nutrient transport in the pitchers of the carnivorous plant Nepenthes alata. Ann. Bot. 1999, 84, 459–466. [Google Scholar] [CrossRef] [Green Version]
- Thornhill, A.H.; Harper, I.S.; Hallam, N.D. The development of the digestive glands and enzymes in the pitchers of three Nepenthes species: N. alata, N. tobaica, and N. ventricosa (Nepenthaceae). Int. J. Plant Sci. 2008, 169, 615–624. [Google Scholar] [CrossRef]
- Solereder, H. Systamatic Anatomy of the Dicotyledons: A Handbook for Laboratories of Pure and Applied Botany; Scott, D.H., Ed.; Claredon Press: Oxford, UK, 1908; Volume II, ISBN 9780874216561. [Google Scholar]
- Takeuchi, Y.; Chaffron, S.; Salcher, M.M.; Shimizu-Inatsugi, R.; Kobayashi, M.J.; Diway, B.; Von Mering, C.; Pernthaler, J.; Shimizu, K.K. Bacterial diversity and composition in the fluid of pitcher plants of the genus Nepenthes. Syst. Appl. Microbiol. 2015, 38, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Moran, J.A.; Gray, L.K.; Clarke, C.; Chin, L. Capture mechanism in Palaeotropical pitcher plants (Nepenthaceae) is constrained by climate. Ann. Bot. 2013, 112, 1279–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaume, L.; Bazile, V.; Huguin, M.; Bonhomme, V. Different pitcher shapes and trapping syndromes explain resource partitioning in Nepenthes species. Ecol. Evol. 2016, 6, 1378–1392. [Google Scholar] [CrossRef] [PubMed]
- Bauer, U.; Clemente, C.J.; Renner, T.; Federle, W. Form follows function: Morphological diversification and alternative trapping strategies in carnivorous Nepenthes pitcher plants. J. Evol. Biol. 2012, 25, 90–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorb, E.V.; Gorb, S.N. The effect of surface anisotropy in the slippery zone of Nepenthes alata pitchers on beetle attachment. Beilstein J. Nanotechnol. 2011, 2, 302–310. [Google Scholar] [CrossRef] [Green Version]
- Bauer, U.; Willmes, C.; Federle, W. Effect of pitcher age on trapping efficiency and natural prey capture in carnivorous Nepenthes rafflesiana plants. Ann. Bot. 2009, 103, 1219–1226. [Google Scholar] [CrossRef]
- Clarke, C.M.; Bauer, U.; Lee, C.C.; Tuen, A.A.; Rembold, K.; Moran, J.A. Tree shrew lavatories: A novel nitrogen sequestration strategy in a tropical pitcher plant. Biol. Lett. 2009, 5, 632–635. [Google Scholar] [CrossRef] [Green Version]
- Moran, J.A.; Clarke, C.M.; Hawkins, B.J. From carnivore to detritivore? Isotopic evidence for leaf litter utilization by the tropical pitcher plant Nepenthes ampullaria. Int. J. Plant Sci. 2003, 164, 635–639. [Google Scholar] [CrossRef] [Green Version]
- Pavlovič, A.; Slováková, L.; Šantrůček, J. Nutritional benefit from leaf litter utilization in the pitcher plant Nepenthes ampullaria. Plant. Cell Environ. 2011, 34, 1865–1873. [Google Scholar] [CrossRef]
- Bauer, U.; Di Guisto, B. With a flick of the lid: A novel trapping mechanism in Nepenthes gracilis pitcher plants. PLoS ONE 2012, 7, e38951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rembold, K.; Fischer, E.; Wetzel, M.A.; Barthlott, W. Prey composition of the pitcher plant Nepenthes madagascariensis. J. Trop. Ecol. 2010, 26, 365–372. [Google Scholar] [CrossRef]
- Bauer, U.; Grafe, T.U.; Federle, W. Evidence for alternative trapping strategies in two forms of the pitcher plant, Nepenthes rafflesiana. J. Exp. Bot. 2011, 62, 3683–3692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moran, J.A. Pitcher dimorphism, composition prey and the mechanisms of prey attraction in the pitcher plant Nepenthes rafflesiana in Borneo. J. Ecol. 1996, 84, 515–525. [Google Scholar] [CrossRef]
- Viscosi, V.; Fortini, P.; Slice, D.E.; Loy, A.; Blasi, C. Geometric morphometric analyses of leaf variation in four oak species of the subgenus Quercus (Fagaceae). Plant Biosyst. 2009, 143, 575–587. [Google Scholar] [CrossRef]
- Van der Niet, T.; Zollikofer, C.P.E.; De León, M.S.P.; Johnson, S.D.; Linder, H.P. Three-dimensional geometric morphometrics for studying floral shape variation. Trends Plant Sci. 2010, 15, 423–426. [Google Scholar] [CrossRef] [Green Version]
- Paulus, S. Measuring crops in 3D: Using geometry for plant phenotyping. Plant Methods 2019, 15, 1–13. [Google Scholar] [CrossRef]
- Vázquez-Arellano, M.; Griepentrog, H.W.; Reiser, D.; Paraforos, D.S. 3-D imaging systems for agricultural applications—A review. Sensors 2016, 618. [Google Scholar] [CrossRef] [Green Version]
- Vassilyev, A.E. The nectaries of the peristome in the closed pitchers of Nepenthes khasiana (Nepenthaceae) secrete polysacharide slime. Bot. Zhurnal 2007, 92, 1554–1568. [Google Scholar]
- Plancho, B.; Światek, P.; Wistuba, A. The giant extra-floral nectaries of carnivorous Heliamphora folliculata: Architecture and ultrastructure. Acta Biol. Crac. 2007, 49, 91–104. [Google Scholar]
- Vogel, S. Remarkable nectaries: Structure, ecology, organophyletic perspectives: II. Nectarioles. Flora 1998, 193, 1–29. [Google Scholar] [CrossRef]
- Vassilyev, A.E.; Muravnik, L.E. The nectaries of the lid in closed pitchers of Nepenthes khasiana (Nepenthaceae) secrete a digestive fluid. Bot. Zhurnal 2007, 92, 1141–1144. [Google Scholar]
- Bennett, K.F.; Ellison, A.M. Nectar, not colour, may lure insects to their death. Biol. Lett. 2009, 5, 469–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fahn, A. Plant Anatomy, 4th ed.; Pergamon Press: Oxford, UK, 1990; ISBN 0080374905. [Google Scholar]
- Vogel, S.; Renner, S.S.; Bhatti, J.S.; Kothekar, V.S. The Role of Scent Glands in Pollination: On the Structure and Function of Osmophores; Smithsonian Institution Libraries: Wahsington, DC, USA, 1990. [Google Scholar]
- Fahn, A. Secretory Tissues in Plants; Academic Press: London, UK, 1979. [Google Scholar]
- Roshchina, V.V.; Roshchina, V.D. External secretion. In The Excretory Function of Higher Plants; Springer: Berlin/Heidelberg, Germany, 1993; pp. 67–130. [Google Scholar]
- Vassilyev, A.E. Ultrastructure and subcellular mechanisms of digestive gland functioning in the carnivorous plant Nepenthes khasiana (Nepenthaceae). Bot. Zhurnal 2006, 91, 1883–1891. [Google Scholar]
- Pinthong, K.; Chaveerach, A.; Tanee, T.; Sudmoon, R.; Mokkamul, P. Differential expressed protein in developing stages of Nepenthes gracilis Korth. pitcher. Pak. J. Biol. Sci. 2009, 12, 526–529. [Google Scholar] [PubMed] [Green Version]
- Zakaria, W.; Adibah, W.N.; Loke, K.-K.; Goh, H.-H.; Mohd Noor, N. RNA-seq analysis for plant carnivory gene discovery in Nepenthes x ventrata. Genomics Data 2016, 7, 18–19. [Google Scholar] [CrossRef] [Green Version]
- Meimberg, H.; Wistuba, A.; Dittrich, P.; Heubl, G. Molecular phylogeny of Nepenthaceae based on cladistic analysis of plastid trnK intron sequence data. Plant Biol. 2001, 3, 164–175. [Google Scholar] [CrossRef]
- Clarke, C.; Wong, K.M. Nepenthes of Borneo; Natural History Publications in association with Science and Technology Unit, Sabah: Kota Kinabalu, Malaysia, 1997. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: https://www.R-project.org/.
- Adams, D.; Collyer, M.; Kaliontzopoulou, A. Geomorph: Software for Geometric Morphometric Analyses. R Package Version 3.2.1. 2020. Available online: https://cran.r-project.org/package=geomorph (accessed on 4 August 2020).
- Kendall, D. A survey of the staistical theory of shape. Stat. Sci. 1989, 4, 87–120. [Google Scholar] [CrossRef]
- Wold, S.; Esbensen, K.; Geladi, P. Principal Component Analysis. Chemom. Intelligen Lab. Syst. 1987, 2, 37–52. [Google Scholar] [CrossRef]
- Adlassnig, W.; Peroutka, M.; Lendl, T. Traps of carnivorous pitcher plants as a habitat: Composition of the fluid, biodiversity and mutualistic activities. Ann. Bot. 2011, 107, 181–194. [Google Scholar] [CrossRef]
- Fehling, H. Die quantitative Bestimmung von Zucker und Stärkmehl mittelst Kupfervitriol. Ann. Chem. Pharm. 1849, 72, 106–113. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwallier, R.; van Wely, V.; Baak, M.; Vos, R.; van Heuven, B.J.; Smets, E.; van Vugt, R.R.; Gravendeel, B. Ontogeny and Anatomy of the Dimorphic Pitchers of Nepenthes rafflesiana Jack. Plants 2020, 9, 1603. https://doi.org/10.3390/plants9111603
Schwallier R, van Wely V, Baak M, Vos R, van Heuven BJ, Smets E, van Vugt RR, Gravendeel B. Ontogeny and Anatomy of the Dimorphic Pitchers of Nepenthes rafflesiana Jack. Plants. 2020; 9(11):1603. https://doi.org/10.3390/plants9111603
Chicago/Turabian StyleSchwallier, Rachel, Valeri van Wely, Mirna Baak, Rutger Vos, Bertie Joan van Heuven, Erik Smets, Rogier R. van Vugt, and Barbara Gravendeel. 2020. "Ontogeny and Anatomy of the Dimorphic Pitchers of Nepenthes rafflesiana Jack" Plants 9, no. 11: 1603. https://doi.org/10.3390/plants9111603
APA StyleSchwallier, R., van Wely, V., Baak, M., Vos, R., van Heuven, B. J., Smets, E., van Vugt, R. R., & Gravendeel, B. (2020). Ontogeny and Anatomy of the Dimorphic Pitchers of Nepenthes rafflesiana Jack. Plants, 9(11), 1603. https://doi.org/10.3390/plants9111603





