Pitfall Flower Development and Organ Identity of Ceropegia sandersonii (Apocynaceae-Asclepiadoideae)
Abstract
:1. Introduction
2. Results
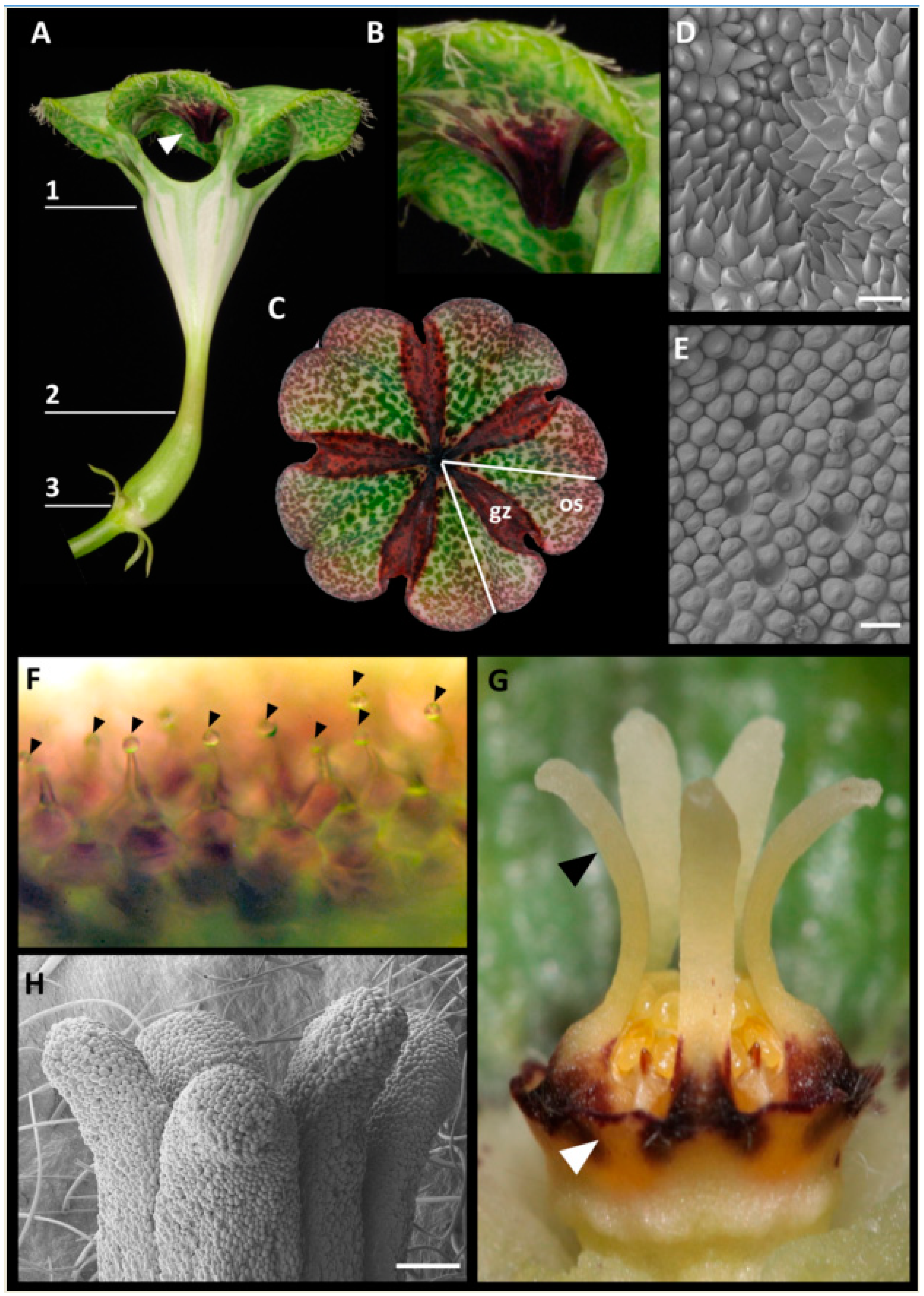
2.1. Anatomy of Mature Pitfall Flowers
2.2. Early Floral Bud and Gynostegium Development
2.3. Vascularization in Mature Flowers
2.4. Phylogenetic Analyses of Ceropegia sandersonii MADS-box Genes
2.5. Differential Gene Expression Analyses
2.6. RT-PCR Experiments with Selected MADS-Box Genes
3. Discussion
3.1. Development and Synorganization of the Highly Specialized Corolla and Corona
3.2. Con- and Postgenital Fusion of Floral Organs
3.3. Vascularization of Ceropegia Pitfall Flowers
3.4. MADS-Box Genes Driving Floral Organ Identity
4. Materials and Methods
4.1. Plant Material
4.2. Fixation of Flowers for Micromorphology (micro-CT, SEM)
4.3. Scanning Electron Microscopy (SEM)
4.4. Light Microscopy (LM)
4.5. 3D X-ray Micro-Computer Tomography (micro-CT)
4.6. RNA Isolation
4.7. Transcriptome Analyses and MADS-Box Gene Identification
4.8. Differential Expression Analyses of Identified MADS-Box Genes
4.9. Primer Design, cDNA Synthesis, and Semi-Quantitative Reverse Transcription PCR (RT-PCR)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cronquist, A. An Integrated System of Classification of Flowering Plants; Columbia University Press: New York, NY, USA, 1981. [Google Scholar]
- Cronquist, A. The Evolution and Classification of Flowering Plants, 2nd ed.; New York Botanical Garden: Bronx, NY, USA, 1988. [Google Scholar]
- Takhtajan, A. Diversity and Classification of Flowering Plants; Columbia University Press: New York, NY, USA, 1997. [Google Scholar]
- Sauquet, H.; Von Balthazar, M.; Magallón, S.; Doyle, J.A.; Endress, P.K.; Bailes, E.J.; De Morais, E.B.; Bull-Hereñu, K.; Carrive, L.; Chartier, M.; et al. The ancestral flower of angiosperms and its early diversification. Nat. Commun. 2017, 8, 16047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endress, P.K. Development and evolution of extreme synorganization in angiosperm flowers and diversity: A comparison of Apocynaceae and Orchidaceae. Ann. Bot. 2016, 117, 749–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ollerton, J.; Liede-Schumann, S.; Endress, E.M.; Meve, U.; Rech, A.R.; Shuttleworth, A.; Keller, A.H.; Fishbein, M.; O Alvarado-Cárdenas, L.; Amorim, F.W.; et al. The diversity and evolution of pollination systems in large plant clades: Apocynaceae as a case study. Ann. Bot. 2019, 123, 311–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endress, P.K. Patterns of floral construction in ontogeny and phylogeny. Biol. J. Linn. Soc. 1990, 39, 153–175. [Google Scholar] [CrossRef]
- Endress, P.K. Diversity and Evolutionary Biology of Tropical Flowers; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Rudall, P.J.; Bateman, R.M. Roles of synorganisation, zygomorphy and heterotopy in floral evolution: The gynostemium and labellum of orchids and other lilioid monocots. Biol. Rev. 2002, 77, 403–441. [Google Scholar] [CrossRef]
- Kunze, H. Morphology and evolution of the corolla and corona in the Apocynaceae s.l. Bot. Jahrb. Syst. 2005, 126, 347–383. [Google Scholar] [CrossRef]
- Walker, D.B. Postgenital carpel fusion in Catharanthus roseus (Apocynaceae). I. Light and scanning electron microscopic study of gynoecial ontogeny. Am. J. Bot. 1975, 62, 457–467. [Google Scholar] [CrossRef]
- Verbeke, J. Fusion events during floral morphogenesis. Annu. Rev. Plant Biol. 1992, 43, 583–598. [Google Scholar] [CrossRef]
- Liede, S.; Kunze, H. A descriptive system for corona analysis in Asclepiadaceae and Periplocaceae. Plant Syst. Evol. 1993, 185, 275–284. [Google Scholar] [CrossRef]
- Hofmann, U.; Specht, A.K. Der morphologische Charakter der Asclepiadaceen corona. Beitr. Biol. Pflanz. 1986, 61, 79–85. (In German) [Google Scholar]
- Rao, V.; Ganguli, A. The floral anatomy of some Asclepiadaceae. Proc. Indian Acad. Sci. B 1963, 57, 15–44. [Google Scholar]
- Kunze, H. Morphogenese und Synorganisation des Bestäubungsapparates einiger Asclepiadaceen. Beitr. Biol. Pflanz. 1981, 56, 133–170. (In German) [Google Scholar]
- Reese, G. Structure of the highly specialized carrion-flowers of stapeliads. Cact. Succ. J. 1973, 45, 18–29. [Google Scholar]
- Vogel, S. Die Bestäubung der Kesselfallen-Blüten von Ceropegia. Beitr. Biol. Pflanz. 1961, 36, 159–237. (In German) [Google Scholar]
- Delpino, F. Ulteriori osservazioni e considerazioni sulla dicogamia nel regno vegetale. Atti Soc. Ital. Sci. Nat. 1869, 1, 214–218. (In Italian) [Google Scholar]
- Knuth, P. Handbuch der Blütenbiologie; Engelmann: Leipzig, Germany, 1898–1905.
- Vogel, S. Über die “Uvula” von Ceropegia sandersonii Hook. f: Zugleich über einen merkwürdigen Fall postgenitaler Verwachsung. Beitr. Biol. Pflanz. 1960, 35, 395–412. (In German) [Google Scholar]
- Vogel, S. Kesselfallen-Blumen. Umsch. Wiss. Tech. 1965, 65, 12–17. (In German) [Google Scholar]
- Heiduk, A.; Brake, I.; Tolasch, T.; Frank, J.; Jurgens, A.; Meve, U.; Dötterl, S. Scent chemistry and pollinator attraction in the deceptive trap flowers of Ceropegia dolichophylla. South Afr. J. Bot. 2010, 76, 762–769. [Google Scholar] [CrossRef] [Green Version]
- Heiduk, A.; Kong, H.; Brake, I.; Von Tschirnhaus, M.; Tolasch, T.; Tröger, A.G.; Wittenberg, E.; Francke, W.; Meve, U.; Dötterl, S. Deceptive Ceropegia dolichophylla fools its kleptoparasitic fly pollinators with exceptional floral scent. Front. Ecol. Evol. 2015, 3, 66. [Google Scholar] [CrossRef] [Green Version]
- Heiduk, A.; Brake, I.; Von Tschirnhaus, M.; Göhl, M.; Jürgens, A.; Johnson, S.D.; Meve, U.; Dötterl, S. Ceropegia sandersonii mimics attacked honeybees to attract kleptoparasitic flies for pollination. Curr. Biol. 2016, 26, 2787–2793. [Google Scholar] [CrossRef]
- Heiduk, A.; Brake, I.; Tschirnhaus, M.V.; Haenni, J.-P.; Miller, R.; Hash, J.; Prieto-Benítez, S.; Jürgens, A.; Johnson, S.D.; Schulz, S.; et al. Floral scent and pollinators of Ceropegia trap flowers. Flora 2017, 232, 169–182. [Google Scholar] [CrossRef]
- Heiduk, A.; Haenni, J.-P.; Meve, U.; Schulz, S.; Dötterl, S. Flower scent of Ceropegia stenantha: Electrophysiological activity and synthesis of novel components. J. Comp. Physiol. A 2019, 205, 301–310. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, J.C.; Ferreira, M.J.P.; Demarco, D. Colleters in Asclepiadoideae (Apocynaceae): Protection of meristems against desiccation and new functions assigned. Int. J. Plant Sci. 2017, 178, 465–477. [Google Scholar] [CrossRef]
- Vogel, S. Duftdrüsen im Dienste der Bestäubung: Über Bau und Funktion der Osmophoren. Abh. Math. Naturwiss. Kl. 1962, 10, 599–763. (In German) [Google Scholar]
- Auttama, P.; McKey, D.; Kidyoo, A. Flowering phenology and trap pollination of the rare endemic plant Ceropegia thaithongiae in montane forest of northern Thailand. Botany 2018, 96, 601–620. [Google Scholar] [CrossRef]
- Kunze, H. Structure and function in asclepiad pollination. Plant Syst. Evol. 1991, 176, 227–253. [Google Scholar] [CrossRef]
- Monteiro, M.M.; Demarco, D. Corona development and floral nectaries of Asclepiadeae (Asclepiadoideae, Apocynaceae). Acta Bot. Bras. 2017, 31, 420–432. [Google Scholar] [CrossRef] [Green Version]
- Fishbein, M. Evolutionary innovation and diversification in the flowers of Asclepiadaceae. Ann. Mo. Bot. Gard. 2001, 88, 603–623. [Google Scholar] [CrossRef]
- Dirks-Mulder, A.; Butôt, R.; Van Schaik, P.; Wijnands, J.W.P.M.; Berg, R.V.D.; Krol, L.; Doebar, S.; Van Kooperen, K.; De Boer, H.J.; Kramer, E.M.; et al. Exploring the evolutionary origin of floral organs of Erycina pusilla, an emerging orchid model system. BMC Evol. Biol. 2017, 17, 89. [Google Scholar] [CrossRef] [Green Version]
- Pramanik, D.; Dorst, N.; Meesters, N.; Spaans, M.; Smets, E.; Welten, M.; Gravendeel, B. Evolution and development of three highly specialized floral structures of bee-pollinated Phalaenopsis species. EvoDevo 2020, 11, 1–20. [Google Scholar] [CrossRef]
- Puri, V.; Shiam, R. Studies in Floral Anatomy. VIII. Vascular anatomy of the flower of certain species of the Asclepiadaceae with special reference to corona. Agra Univ. J. Res. 1966, 15, 189–216. [Google Scholar]
- Tepfer, S.S. Floral Anatomy and Ontogeny in Aquilegia formosa var. truncata and Ranunculus repens. Univ. Calif. Publ. Bot. 1953, 25, 513–648. [Google Scholar]
- Sporne, K.R. Some aspects of floral vascular systems. Proc. Linn. Soc. London B 1958, 169, 75–84. [Google Scholar] [CrossRef]
- Esau, K. Primary vascular differentiation in plants. Biol. Rev. 1954, 29, 46–86. [Google Scholar] [CrossRef]
- Coombs, G.; Dold, A.P.; Peter, C.I. Generalized fly-pollination in Ceropegia ampliata (Apocynaceae-Asclepiadoideae): The role of trapping hairs in pollen export and receipt. Plant Syst. Evol. 2011, 296, 137–148. [Google Scholar] [CrossRef]
- Kunze, H. Corona and nectar system in Asclepiadinae (Asclepiadaceae). Flora 1997, 192, 175–183. [Google Scholar] [CrossRef]
- Christ, P.; Schnepf, E. The nectaries of Cynanchum vincetoxicum (Asclepiadaceae). Isr. J. Plant Sci. 1985, 34, 79–90. [Google Scholar]
- Theißen, G.; Melzer, R.; Rümpler, F. MADS-domain transcription factors and the floral quartet model of flower development: Linking plant development and evolution. Development 2016, 143, 3259–3271. [Google Scholar] [CrossRef] [Green Version]
- Irish, V.F.; Litt, A. Flower development and evolution: Gene duplication, diversification and redeployment. Curr. Opin. Genet. Dev. 2005, 15, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Theißen, G.; Saedler, H. Plant biology: Floral quartets. Nature 2001, 409, 469–472. [Google Scholar] [CrossRef]
- de Oliveira, R.R.; Cesarino, I.; Mazzafera, P.; Dornelas, M.C. Flower development in Coffea arabica L.: New insights into MADS-box genes. Plant Reprod. 2014, 27, 79–94. [Google Scholar] [CrossRef]
- Dyer, R.A. Ceropegia, Brachystelma and Riocreuxia in Southern Africa; AA Balkema: Rotterdam, The Netherlands, 1983. [Google Scholar]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Godzik, A. Cd-hit: A fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 2006, 22, 1658–1659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [Green Version]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guindon, S.; Dufayard, J.-F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- RStudio Team. Integrated Development for R. RStudio, PBC, Boston. 2019. Available online: http://www.rstudio.com/ (accessed on 3 January 2020).






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heiduk, A.; Pramanik, D.; Spaans, M.; Gast, L.; Dorst, N.; van Heuven, B.J.; Gravendeel, B. Pitfall Flower Development and Organ Identity of Ceropegia sandersonii (Apocynaceae-Asclepiadoideae). Plants 2020, 9, 1767. https://doi.org/10.3390/plants9121767
Heiduk A, Pramanik D, Spaans M, Gast L, Dorst N, van Heuven BJ, Gravendeel B. Pitfall Flower Development and Organ Identity of Ceropegia sandersonii (Apocynaceae-Asclepiadoideae). Plants. 2020; 9(12):1767. https://doi.org/10.3390/plants9121767
Chicago/Turabian StyleHeiduk, Annemarie, Dewi Pramanik, Marlies Spaans, Loes Gast, Nemi Dorst, Bertie Joan van Heuven, and Barbara Gravendeel. 2020. "Pitfall Flower Development and Organ Identity of Ceropegia sandersonii (Apocynaceae-Asclepiadoideae)" Plants 9, no. 12: 1767. https://doi.org/10.3390/plants9121767
APA StyleHeiduk, A., Pramanik, D., Spaans, M., Gast, L., Dorst, N., van Heuven, B. J., & Gravendeel, B. (2020). Pitfall Flower Development and Organ Identity of Ceropegia sandersonii (Apocynaceae-Asclepiadoideae). Plants, 9(12), 1767. https://doi.org/10.3390/plants9121767




