Mechanisms of Antidiabetic Activity of Methanolic Extract of Punica granatum Leaves in Nicotinamide/Streptozotocin-Induced Type 2 Diabetes in Rats
Abstract
:1. Introduction
2. Results
2.1. Phytochemical Screening
2.2. High Performance Liquid Chromatography (HPLC)
2.3. Acute Toxicity Study of MEPGL
2.4. Effect of MEPGL on Body Weight
2.5. Effect of MEPGL on Blood Glucose and Plasma Insulin Levels
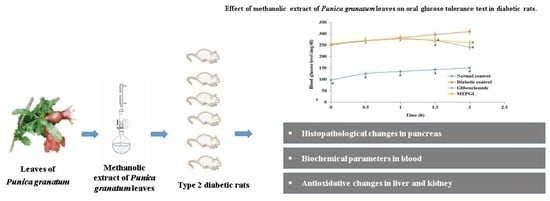
2.6. Effect of MEPGL on Oral Glucose Tolerance Test (OGTT)
2.7. Effect of MEPGL on Different Biochemical Parameters
2.8. Effect of MEPGL on Oxidant–Antioxidant Status in Liver and Kidney
2.9. Effect of MEPGL on Pancreatic Histopathology
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animals
4.3. Preparation of the MEPGL
4.4. HPLC Analysis
4.4.1. Preparation of Standards and Samples for HPLC
4.4.2. Chromatographic Conditions
4.5. Phytochemical Screening of MEPGL
4.6. Acute Toxicity Study
4.7. Induction of Type 2 DM
4.8. Experimental Design and Sample Collection
4.9. Biochemical Analysis
4.10. Histopathological Examination
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acharjee, S.; Ghosh, B.; Al-Dhubiab, B.E.; Nair, A.B. Understanding type 1 diabetes: Etiology and models. Can. J. Diabetes 2013, 37, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Chawla, A.; Chawla, R.; Jaggi, S. Microvasular and macrovascular complications in diabetes mellitus: Distinct or continuum? Indian J. Endocrinol. Metab. 2016, 20, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Volpe, C.M.O.; Villar-Delfino, P.H.; Dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes mellitus and oxidative stress—A concise review. Saudi. Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Nair, A.; Jhawat, V.; Mustaq, N.; Sharma, A.; Dhanawat, M.; Khan, S.A. Unwinding Complexities of Diabetic Alzheimer by Potent Novel Molecules. Am. J. Alzheimers Dis. Other Demen. 2020, 35. [Google Scholar] [CrossRef]
- Gerber, P.A.; Rutter, G.A. The Role of Oxidative Stress and Hypoxia in Pancreatic Beta-Cell Dysfunction in Diabetes Mellitus. Antioxid. Redox Signal. 2017, 26, 501–518. [Google Scholar] [CrossRef] [Green Version]
- Chaudhury, A.; Duvoor, C.; Reddy Dendi, V.S.; Kraleti, S.; Chada, A.; Ravilla, R.; Marco, A.; Shekhawat, N.S.; Montales, M.T.; Kuriakose, K.; et al. Clinical Review of Antidiabetic Drugs: Implications for Type 2 Diabetes Mellitus Management. Front. Endocrinol. 2017, 8, 6. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Ata, A.; V Anil Kumar, K.; Sharopov, F.; Ramírez-Alarcón, K.; Ruiz-Ortega, A.; Abdulmajid Ayatollahi, S.; Tsouh Fokou, P.V.; Kobarfard, F.; Amiruddin Zakaria, Z.; et al. Antidiabetic Potential of Medicinal Plants and Their Active Components. Biomolecules 2019, 9, 551. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, H.; Pandey, M.; Hua, C.K.; Mun, C.S.; Jing, J.K.; Kong, L.; Ern, L.Y.; Ashraf, N.A.; Kit, S.W.; Yee, T.S.; et al. An update on natural compounds in the remedy of diabetes mellitus: A systematic review. J. Tradit. Complementary Med. 2018, 8, 361–376. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, S.; Chauhan, S.; Nair, A.; Sharma, P. Astilbin: A promising unexplored compound with multidimensional medicinal and health benefits. Pharmacol. Res. 2020, 158. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Özen, C.; Abu-Reidah, I.M.; Chigurupati, S.; Patra, J.K.; Horbanczuk, J.O.; Jóźwik, A.; Tzvetkov, N.T.; Uhrin, P.; Atanasov, A.G. Vasculoprotective Effects of Pomegranate (Punica granatum L.). Front. Pharmacol. 2018, 9, 544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaygannia, E.; Bahmani, M.; Zamanzad, B.; Rafieian-Kopaei, M. A Review Study on Punica granatum L. J. Evid. Based Complement. Altern. Med. 2016, 21, 221–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurenka, J.S. Therapeutic applications of pomegranate (Punica granatum L.): A review. Altern. Med. Rev. 2008, 13, 128–144. [Google Scholar]
- Khwairakpam, A.D.; Bordoloi, D.; Thakur, K.K.; Monisha, J.; Arfuso, F.; Sethi, G.; Mishra, S.; Kumar, A.P.; Kunnumakkara, A.B. Possible use of Punica granatum (Pomegranate) in cancer therapy. Pharmacol. Res. 2018, 133, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Thangavelu, A.; Elavarasu, S.; Sundaram, R.; Kumar, T.; Rajendran, D.; Prem, F. Ancient Seed for Modern Cure—Pomegranate Review of Therapeutic Applications in Periodontics. J. Pharm. Bioallied Sci. 2017, 9, S11–S14. [Google Scholar] [CrossRef]
- Mestry, S.N.; Dhodi, J.B.; Kumbhar, S.B.; Juvekar, A.R. Attenuation of diabetic nephropathy in streptozotocin-induced diabetic rats by Punica granatum Linn. leaves extract. J. Tradit. Complementary Med. 2017, 7, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Szkudelski, T. Streptozotocin-nicotinamide-induced diabetes in the rat. Characteristics of the experimental model. Exp. Biol. Med. 2012, 237, 481–490. [Google Scholar] [CrossRef]
- Alenzi, F.Q. Effect of nicotinamide on experimental induced diabetes. Iran. J. Allergy Asthma Immunol. 2009, 8, 11–18. [Google Scholar]
- Pan, S.Y.; Litscher, G.; Gao, S.H.; Zhou, S.F.; Yu, Z.L.; Chen, H.Q.; Zhang, S.F.; Tang, M.K.; Sun, J.N.; Ko, K.M. Historical perspective of traditional indigenous medical practices: The current renaissance and conservation of herbal resources. Evid. Based Complement. Altern. Med. 2014, 2014, 525340. [Google Scholar] [CrossRef]
- Kooti, W.; Farokhipour, M.; Asadzadeh, Z.; Ashtary-Larky, D.; Asadi-Samani, M. The role of medicinal plants in the treatment of diabetes: A systematic review. Electron Physician 2016, 8, 1832–1842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.; Al-Dhubiab, B.E.; Chattopadhyaya, I.; Nair, A.; Kumria, R.; Gupta, S. Assessment of pharmacokinetic interaction of spirulina with glitazone in a type 2 diabetes rat model. J. Med. Food 2013, 16, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Ahangarpour, A.; Oroojan, A.A.; Khorsandi, L.; Shabani, R.; Mojaddami, S. Preventive effects of betulinic acid on streptozotocinnicotinamide induced diabetic nephropathy in male mouse. J. Nephropathol. 2016, 5, 128–133. [Google Scholar] [CrossRef] [Green Version]
- Rani, R.; Dahiya, S.; Dhingra, D.; Dilbaghi, N.; Kaushik, A.; Kim, K.H.; Kumar, S. Antidiabetic activity enhancement in streptozotocin + nicotinamide-induced diabetic rats through combinational polymeric nanoformulation. Int. J. Nanomed. 2019, 14, 4383–4395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Yan, L.J. Streptozotocin-induced type 1 diabetes in rodents as a model for studying mitochondrial mechanisms of diabetic β cell glucotoxicity. Diabetes Metab. Syndr. Obes. 2015, 8, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [Green Version]
- Gharib, E.; Montasser Kouhsari, S. Study of the Antidiabetic Activity of Punica granatum L. Fruits Aqueous Extract on the Alloxan-Diabetic Wistar Rats. Iran J. Pharm. Res. 2019, 18, 358–368. [Google Scholar]
- Bajaj, S.; Khan, A. Antioxidants and diabetes. Indian J. Endocrinol. Metab. 2012, 16, S267. [Google Scholar]
- Yang, S.; Wang, S.; Yang, B.; Zheng, J.; Cai, Y.; Yang, Z. Weight loss before a diagnosis of type 2 diabetes mellitus is a risk factor for diabetes complications. Medicine 2016, 95, e5618. [Google Scholar] [CrossRef]
- Rines, A.K.; Sharabi, K.; Tavares, C.D.; Puigserver, P. Targeting hepatic glucose metabolism in the treatment of type 2 diabetes. Nat. Rev. Drug Discov. 2016, 15, 786–804. [Google Scholar] [CrossRef] [Green Version]
- Makris, K.; Spanou, L. Is there a relationship between mean blood glucose and glycated hemoglobin? J. Diabetes Sci. Technol. 2011, 5, 1572–1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacob, S.; Morsy, M.A.; Nair, A. An overview on the insulin preparations and devices. Indian J. Pharm. Educ. Res. 2018, 52, 550–557. [Google Scholar] [CrossRef] [Green Version]
- Gomathi, D.; Ravikumar, G.; Kalaiselvi, M.; Devaki, K.; Uma, C. Effect of Evolvulus alsinoides on lipid metabolism of streptozotocin induced diabetic rats. Asian Pac. J. Trop. Dis. 2013, 3, 184–188. [Google Scholar] [CrossRef] [Green Version]
- Teshome, G.; Ambachew, S.; Fasil, A.; Abebe, M. Prevalence of Liver Function Test Abnormality and Associated Factors in Type 2 Diabetes Mellitus: A Comparative Cross-Sectional Study. EJIFCC 2019, 30, 303–316. [Google Scholar]
- Qian, K.; Zhong, S.; Xie, K.; Yu, D.; Yang, R.; Gong, D.-W. Hepatic ALT isoenzymes are elevated in gluconeogenic conditions including diabetes and suppressed by insulin at the protein level. Diabetes Metab. Res. Rev. 2015, 31, 562–571. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.M.; Bonventre, J.V. Acute Kidney Injury and Progression of Diabetic Kidney Disease. Adv. Chronic Kidney Dis. 2018, 25, 166–180. [Google Scholar] [CrossRef]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Mel’nikova, T.I.; Porozov, Y.B.; Terentiev, A.A. Oxidative Stress and Advanced Lipoxidation and Glycation End Products (ALEs and AGEs) in Aging and Age-Related Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 3085756. [Google Scholar] [CrossRef] [Green Version]
- Ananthan, R.; Baskar, C.; NarmathaBai, V.; Pari, L.; Latha, M.; Ramkumar, K.M. Antidiabetic effect of Gymnema montanum leaves: Effect on lipid peroxidation induced oxidative stress in experimental diabetes. Pharmacol. Res. 2003, 48, 551–556. [Google Scholar] [CrossRef]
- Lyons, T.J.; Jenkins, A.J. Glycation, oxidation, and lipoxidation in the development of the complications of diabetes: A carbonyl stress hypothesis. Diabetes Rev. 1997, 5, 365–391. [Google Scholar]
- Wen, W.; Lin, Y.; Ti, Z. Antidiabetic, Antihyperlipidemic, Antioxidant, Anti-inflammatory Activities of Ethanolic Seed Extract of Annona reticulata L. in Streptozotocin Induced Diabetic Rats. Front. Endocrinol. 2019, 10, 716. [Google Scholar] [CrossRef]
- Erejuwa, O.O.; Sulaiman, S.A.; Wahab, M.S.; Salam, S.K.; Salleh, M.S.; Gurtu, S. Comparison of antioxidant effects of honey, glibenclamide, metformin, and their combinations in the kidneys of streptozotocin-induced diabetic rats. Int. J. Mol. Sci. 2011, 12, 829–843. [Google Scholar] [CrossRef] [Green Version]
- Morsy, M.A.; Abdel-Aziz, A.M.; Abdel-Hafez, S.M.N.; Venugopala, K.N.; Nair, A.B.; Abdel-Gaber, S.A. The Possible Contribution of P-Glycoprotein in the Protective Effect of Paeonol against Methotrexate-Induced Testicular Injury in Rats. Pharmaceuticals 2020, 13, 223. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Bhardwaj, P.; Sharma, P. Antioxidant and toxicological evaluation of Cassia sopherain streptozotocin-induced diabetic Wistar rats. Pharmacogn. Res. 2013, 5, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Oche, O.; Sani, I.; Chilaka, N.G.; Samuel, N.U.; Samuel, A. Pancreatic islet regeneration and some liver biochemical parameters of leaf extracts of Vitex doniana in normal and streptozotocin-induced diabetic albino rats. Asian Pac. J. Trop. Biomed. 2014, 4, 124–130. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Akkawi, M.; Abu-Lafi, S.; Abu-Remeleh, Q. Phytochemical screening of Pomegranate juice, peels, leaves and membranes water extracts and their effect on β-hematin formation, a comparative study. Pharm. Pharmacol. Int. J. 2019, 7, 193–200. [Google Scholar]
- Nawwar, M.A.; Hussein, S.A.; Merfort, I. Leaf phenolics of Punica granatum. Phytochemistry 1994, 37, 1175–1177. [Google Scholar] [CrossRef]
- Adefegha, S.A.; Oboh, G.; Ejakpovi, I.I.; Oyeleye, S.I. Antioxidant and antidiabetic effects of gallic and protocatechuic acids: A structure–function perspective. Comp. Clin. Path. 2015, 24, 1579–1585. [Google Scholar] [CrossRef]
- Guo, S.; Ren, X.; He, K.; Chen, X.; Zhang, S.; Roller, M.; Zheng, B.; Zheng, Q.; Ho, C.T.; Bai, N. The anti-diabetic effect of eight Lagerstroemia speciosa leaf extracts based on the contents of ellagitannins and ellagic acid derivatives. Food Funct. 2020, 11, 1560–1571. [Google Scholar] [CrossRef]
- Fatima, N.; Hafizur, R.M.; Hameed, A.; Ahmed, S.; Nisar, M.; Kabir, N. Ellagic acid in Emblica officinalis exerts anti-diabetic activity through the action on β-cells of pancreas. Eur. J. Nutr. 2017, 56, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Qin, W.; Wu, F.; Wang, S.; Pan, C.; Wang, L.; Zeng, B.; Ma, S.; Liang, J. Apigenin and naringenin regulate glucose and lipid metabolism, and ameliorate vascular dysfunction in type 2 diabetic rats. Eur. J. Pharmacol. 2016, 773, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis; Champman and HallL: London, UK, 1998. [Google Scholar]
- Balogun, F.O.; Tom Ashafa, A.O. Acute and Subchronic Oral Toxicity Evaluation of Aqueous Root Extract of Dicoma anomala Sond. in Wistar Rats. Evid. Based Complement. Alternat. Med. 2016, 2016, 3509323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aydιn, A.; Aktay, G.; Yesilada, E. A Guidance Manual for the Toxicity Assessment of Traditional Herbal Medicines. Nat. Prod. Commun. 2016, 11, 1763–1773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morsy, M.A.; Ashour, O.M.; Fouad, A.A.; Abdel-Gaber, S.A. Gastroprotective effects of the insulin sensitizers rosiglitazone and metformin against indomethacin-induced gastric ulcers in Type 2 diabetic rats. Clin. Exp. Pharmacol. Physiol. 2010, 37, 173–177. [Google Scholar] [CrossRef]
- Tahara, A.; Matsuyama-Yokono, A.; Nakano, R.; Someya, Y.; Shibasaki, M. Hypoglycaemic effects of antidiabetic drugs in streptozotocin-nicotinamide-induced mildly diabetic and streptozotocin-induced severely diabetic rats. Basic Clin. Pharmacol. Toxicol. 2008, 103, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Cam, M.C.; McNeill, J.H. A sensitive radioimmunoassay optimized for reproducible measurement of rat plasma insulin. J. Pharmacol. Toxicol. Methods 1996, 35, 111–119. [Google Scholar] [CrossRef]
- Kakkar, P.; Das, B.; Viswanathan, P.N. A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys. 1984, 21, 130–132. [Google Scholar]
- Sinha, A.K. Colorimetric assay of catalase. Anal. Biochem. 1972, 47, 389–394. [Google Scholar] [CrossRef]
- Rotruck, J.T.; Pope, A.L.; Ganther, H.E.; Swanson, A.B.; Hafeman, D.G.; Hoekstra, W.G. Selenium: Biochemical role as a component of glutathione peroxidase. Science 1973, 179, 588–590. [Google Scholar] [CrossRef]
- Boyne, A.F.; Ellman, G.L. A methodology for analysis of tissue sulfhydryl components. Anal. Biochem. 1972, 46, 639–653. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]



| Chemical Test | Observation | Inference |
|---|---|---|
| Alkaloids | ||
| (i) Dragendorff test | Reddish precipitate were not observed | Alkaloids absent |
| (ii) Hager test | Yellow precipitate were not observed | Alkaloids absent |
| (iii) Mayer test | A milky coloration was not observed | Alkaloids absent |
| Tannins | ||
| (i) Bromine water test | The bromine water was decolorized | Tannins present |
| (ii) Ferric chloride test | A blue black precipitate was observed | Tannins present |
| Glycosides | ||
| (i) General test for glucoside | Formation of yellow color | Glycosides present |
| Carbohydrates | ||
| (i) Molisch test | Formation of red color at the interphase of two layers | Carbohydrates present |
| (ii) Fehling test | Not Formation of a red precipitate | Reducing sugar present |
| Saponins | ||
| (i) Froth test | No significant frothing was obtained | Saponins absent |
| Steroids | ||
| (i) Acetic anhydride test | A bluish green interphase was not obtained | Steroids absent |
| (ii) Liebermann-Burchard test | Pink to red color not formed | Steroids absent |
| Flavonoids | ||
| (i) Magnesium ribbon test | Effervescence was occurred | Flavonoids present |
| (ii) Shinoda test | Magenta color was appear | Flavonoids present |
| Phytosterols | ||
| (i) Salkowski test | Black coloration was observed | Phytosterols present |
| Day | Body Weight (g) | ||||||
|---|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | Group 7 | |
| 1st day | 206 ± 5.5 | 198 ± 3.0 | 210 ± 4.5 | 212 ± 5.5 | 207 ± 6.0 | 205 ± 5.0 | 200 ± 5.5 |
| 22nd day | 245 ± 4.5 a | 180 ± 5.0 | 240 ± 5.0 a | 205 ± 6.5 | 219 ± 5.5 a | 227 ± 6.5 a | 239 ± 6.0 a |
| 45th day | 290 ± 6.0 a | 155 ± 5.5 | 284 ± 7.0 a | 209 ± 6.0 a | 233 ± 5.0 a | 251 ± 6.5 a | 268 ± 5.0 a |
| Group | Blood Glucose (mg/dL) | Plasma Insulin (μU/mL) | ||||
|---|---|---|---|---|---|---|
| 1st Day | 22nd Day | 45th Day | 1st Day | 22nd Day | 45th Day | |
| Group 1 | 91.3 ± 3.10 | 92.0 ± 3.60 | 92.4 ± 3.90 | 17.8 ± 0.73 | 17.1 ± 0.80 | 18.5 ± 0.61 |
| Group 2 | 271 ± 3.20 a | 310 ± 4.90 a | 353 ± 4.10 ab | 9.21 ± 0.92 a | 6.43 ± 0.81 a | 5.31 ± 0.76 a |
| Group 3 | 280 ± 3.80 a | 190 ± 4.10 ab | 98.6 ± 3.80 b | 8.90 ± 0.70 a | 12.5 ± 0.67 ab | 16.6 ± 0.79 b |
| Group 4 | 277 ± 3.0 a | 247 ± 4.50 ac | 222 ± 4.30 abc | 9.89 ± 1.03 a | 9.32 ± 0.91 ac | 9.65 ± 1.03 ab |
| Group 5 | 282 ± 3.40 a | 233 ± 4.30 abc | 190 ± 5.20 abc | 8.97 ± 1.07 a | 9.79 ± 1.04 abc | 10.9 ± 1.13 abc |
| Group 6 | 278 ± 3.20 a | 212 ± 5.10 abc | 142 ± 4.70 abc | 9.03 ± 1.11 a | 10.4 ± 1.19 ab | 12.2 ± 1.08 ab |
| Group 7 | 283 ± 3.80 a | 188 ± 4.70 ab | 107 ± 3.80 b | 8.80 ± 1.28 a | 12.0 ± 1.14 ab | 14.7 ± 1.16 ab |
| Parameters | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | Group 7 |
|---|---|---|---|---|---|---|---|
| Hemoglobin (mg/dL) | 13.2 ± 3.2 | 8.57 ± 2.60 a | 12.1 ± 2.90 b | 9.42 ± 1.30 a | 10.0 ± 1.50 | 11.3 ± 1.70 | 12.1 ± 1.30 b |
| HbA1c (%) | 5.67 ± 0.70 | 13.1 ± 2.40 a | 6.91 ± 1.09 b | 13.0 ± 0.97 ac | 11.6 ± 0.89 | 9.30 ± 0.90 | 7.15 ± 1.10 b |
| ALT (U/L) | 27.2 ± 2.80 | 54.5 ± 3.80 a | 29.7 ± 3.70 b | 46.6 ± 3.20 ac | 39.8 ± 3.90 | 32.7 ± 3.70 b | 28.3 ± 3.80 b |
| AST (U/L) | 45.3 ± 4.0 | 87.9 ± 5.70 a | 48.2 ± 4.60 b | 80.5 ± 5.90 ac | 64.7 ± 4.90 b | 53.4 ± 5.50 b | 49.7 ± 5.10 b |
| Alkaline phosphatase (U/L) | 124 ± 5.10 | 280 ± 5.50 a | 143 ± 7.10 b | 260 ± 4.30 ac | 202 ± 4.90 abc | 161 ± 5.10 ab | 138 ± 4.70 b |
| Urea (mg/dL) | 32.0 ± 3.0 | 78.9 ± 3.20 a | 39.6 ± 2.40 b | 77.6 ± 2.60 ac | 63.4 ± 2.20 abc | 51.3 ± 1.70 abc | 41.2 ± 2.50 b |
| Creatinine (mg/dL) | 0.98 ± 0.24 | 2.85 ± 0.21 a | 1.03 ± 0.30 b | 2.19 ± 0.30 | 1.83 ± 0.40 | 1.39 ± 0.50 | 1.11 ± 0.60 b |
| Total protein (g/dL) | 7.90 ± 0.70 | 4.80 ± 0.35 a | 8.00 ± 0.82 b | 5.80 ± 0.46 | 6.60 ± 0.71 | 7.50 ± 0.53 | 8.20 ± 0.88 b |
| Total cholesterol (mg/dL) | 135 ± 6.10 | 263 ± 6.70 a | 180 ± 6.30 b | 240 ± 5.0 ac | 199 ± 7.10 abc | 171 ± 4.20 ab | 150 ± 5.30 b |
| Triglycerides (mg/dL) | 78.4 ± 2.70 | 166 ± 4.10 a | 87.6 ± 3.40 b | 150 ± 3.60 abc | 130 ± 3.40 abc | 110 ± 3.10 abc | 86.3 ± 3.90 b |
| HDL (mg/dL) | 49.9 ± 3.10 | 24.8 ± 3.90 a | 39.9 ± 3.20 b | 21.7 ± 3.30 ac | 28.7 ± 2.90 a | 36.5 ± 4.20 | 42.5 ± 4.40 b |
| Group | Liver | ||||
|---|---|---|---|---|---|
| SOD (unit/mg Protein) | CAT (μmol/min/mg Protein) | GPx (μmol/min/mg Protein) | GSH (mM/100 mg Tissue) | MDA (μmol/100 mg Tissue) | |
| Group 1 | 9.06 ± 1.12 | 91.8 ± 3.11 | 11.3 ± 1.09 | 59.6 ± 2.25 | 1.07 ± 0.54 |
| Group 2 | 4.90 ± 0.86 a | 42.4 ± 2.23 a | 5.44 ± 0.90 a | 25.6 ± 1.88 a | 2.12 ± 0.60 a |
| Group 3 | 5.89 ± 1.76 a | 63.1 ± 4.65 ab | 8.24 ± 2.31 ab | 44.3 ± 2.50 a | 1.97 ± 0.32 a |
| Group 4 | 5.24 ± 0.45 a | 39.6 ± 2.56 a | 6.75 ± 1.34 a | 30.6 ± 2.00 a | 1.91 ± 0.68 a |
| Group 5 | 6.50 ± 0.32 ab | 50.4 ± 3.67 a | 7.87 ± 1.58 a | 36.5 ± 2.56 a | 1.65 ± 0.51 a |
| Group 6 | 7.27 ± 0.40 ab | 67.7 ± 3.93 ab | 8.70 ± 1.55 ab | 41.3 ± 2.78 ab | 1.37 ± 0.76 ab |
| Group 7 | 8.86 ± 0.37 b | 83.2 ± 3.56 b | 10.7 ± 1.93 b | 50.4 ± 2.52 b | 1.14 ± 0.45 b |
| Group | Kidney | ||||
|---|---|---|---|---|---|
| SOD (unit/mg Protein) | CAT (μmol/min/mg Protein) | GPx (μmol/min/mg Protein) | GSH (mM/100 mg Tissue) | MDA (μmol/100 mg Tissue) | |
| Group 1 | 15.2 ± 0.72 | 70.6 ± 3.61 | 12.4 ± 1.98 | 35.3 ± 1.95 | 1.90 ± 0.56 |
| Group 2 | 9.11 ± 0.59 a | 45.4 ± 3.95 a | 6.7 ± 1.08 a | 21.4 ± 1.19 a | 3.03 ± 0.93 a |
| Group 3 | 12.8 ± 0.87 b | 60.5 ± 4.11 b | 10.5 ± 0.92 b | 32.2 ± 1.78 b | 2.80 ± 0.31 a |
| Group 4 | 10.8 ± 0.79 a | 46.5 ± 3.43 a | 8.00 ± 1.09 a | 23.9 ± 1.28 a | 2.94 ± 0.76 a |
| Group 5 | 11.2 ± 0.81 ab | 61.8 ± 3.18 a | 8.05 ± 1.10 a | 27.9 ± 1.56 ab | 2.80 ± 0.83 a |
| Group 6 | 12.8 ± 0.87 b | 66.6 ± 3.72 b | 10.1 ± 1.89 b | 30.9 ± 1.96 b | 2.76 ± 0.48 ab |
| Group 7 | 14.6 ± 0.62 b | 70.5 ± 4.17 b | 12.2 ± 1.65 b | 33.8 ± 1.87 b | 2.01 ± 0.59 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pottathil, S.; Nain, P.; Morsy, M.A.; Kaur, J.; Al-Dhubiab, B.E.; Jaiswal, S.; Nair, A.B. Mechanisms of Antidiabetic Activity of Methanolic Extract of Punica granatum Leaves in Nicotinamide/Streptozotocin-Induced Type 2 Diabetes in Rats. Plants 2020, 9, 1609. https://doi.org/10.3390/plants9111609
Pottathil S, Nain P, Morsy MA, Kaur J, Al-Dhubiab BE, Jaiswal S, Nair AB. Mechanisms of Antidiabetic Activity of Methanolic Extract of Punica granatum Leaves in Nicotinamide/Streptozotocin-Induced Type 2 Diabetes in Rats. Plants. 2020; 9(11):1609. https://doi.org/10.3390/plants9111609
Chicago/Turabian StylePottathil, Shinu, Parminder Nain, Mohamed A. Morsy, Jaspreet Kaur, Bandar E. Al-Dhubiab, Sandhya Jaiswal, and Anroop B. Nair. 2020. "Mechanisms of Antidiabetic Activity of Methanolic Extract of Punica granatum Leaves in Nicotinamide/Streptozotocin-Induced Type 2 Diabetes in Rats" Plants 9, no. 11: 1609. https://doi.org/10.3390/plants9111609
APA StylePottathil, S., Nain, P., Morsy, M. A., Kaur, J., Al-Dhubiab, B. E., Jaiswal, S., & Nair, A. B. (2020). Mechanisms of Antidiabetic Activity of Methanolic Extract of Punica granatum Leaves in Nicotinamide/Streptozotocin-Induced Type 2 Diabetes in Rats. Plants, 9(11), 1609. https://doi.org/10.3390/plants9111609