Exogenous ACC Deaminase Is Key to Improving the Performance of Pasture Legume-Rhizobial Symbioses in the Presence of a High Manganese Concentration
Abstract
:1. Introduction
2. Results
2.1. Analysis of the acdS Gene Sequence from Pseudomonas sp. Q1
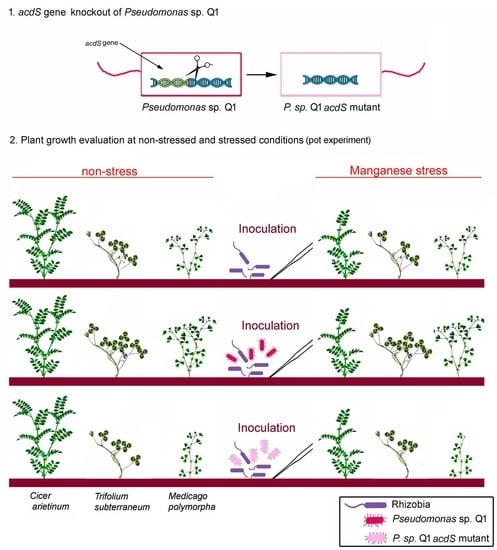
2.2. Confirmation of the ∆acdS Knockout Mutant and acdS-Complemented Strain Construction
2.3. Tolerance to a High Concentration of Mn
2.4. Benefits of the Q1 Strain and of Its ACC Deaminase Activity on the Symbiotic Rhizobial-Legume Model
2.4.1. Chickpea-Mesorhizobium Symbiosis
2.4.2. Subterranean Clover-Rhizobium and Burr Medic-Ensifer Symbioses
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Growth Conditions
4.2. DNA Methods and Construction of Pseudomonas sp. Q1 Derivatives
4.2.1. DNA Methods
4.2.2. Construction of Pseudomonas sp. Q1 ∆acdS Mutant Strain
4.2.3. Complementation of Pseudomonas sp. Q1 ∆acdS mutant
pGRG36-Tetr Vector Construction
Complementation
4.3. ACC Deaminase Activity Assay
4.4. Detection of acdS Genes by PCR
4.5. Compatibility between the Bacterial Endophyte Q1 or Its Derivatives and the Rhizobial Strains
4.6. Evaluation of Strains’ Tolerance to High Concentration of Mn
4.7. Effect of the Pseudomonas sp. Q1 and the ∆acdS Mutant Strains on the Rhizobia-Legume Symbioses
4.7.1. Bacterial Inoculum Preparation
4.7.2. Seed Surface Sterilization and Germination
4.7.3. Evaluation of the Minimum Concentration Levels of Mn with Significant Constraints for Legume Plants
4.7.4. Pot Experiments under Gnotobiotic Conditions
4.7.5. Hydroponic Assay
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sade, H.; Meriga, B.; Surapu, V.; Gadi, J.; Sunita, M.S.; Suravajhala, P.; Kavi Kishor, P.B. Toxicity and tolerance of aluminum in plants: Tailoring plants to suit to acid soils. Biometals 2016, 29, 187–210. [Google Scholar] [CrossRef]
- Kochian, L.V.; Piñeros, M.A.; Hoekenga, O.A. The physiology, genetics and molecular biology of plant aluminum resistance and toxicity. Plant Soil 2005, 274, 175–195. [Google Scholar] [CrossRef]
- Gupta, N.; Gaurav, S.; Kumar, A. Molecular basis of aluminium toxicity in plants: A review. Am. J. Plant Sci. 2013, 4, 21–37. [Google Scholar] [CrossRef] [Green Version]
- Bose, J.; Babourina, O.; Ma, Y.; Zhou, M.; Shabala, S.; Rengel, Z. Specificity of ion uptake and homeostasis maintenance during acid and aluminium stresses. In Aluminum Stress Adaptation in Plants. Signaling and Communication in Plants; Panda, S., Baluška, F., Eds.; Springer: Cham, Switzerland, 2015; Volume 24, pp. 229–251. [Google Scholar]
- von Uexküll, H.R.; Mutert, E. Global extent, development and economic impact of acid soils. Plant Soil 1995, 171, 1–15. [Google Scholar] [CrossRef]
- Alho, L.; Carvalho, M.; Brito, I.; Goss, M.J. The effect of arbuscular mycorrhiza fungal propagules on the growth of subterranean clover (Trifolium subterraneum L.) under Mn toxicity in ex situ experiments. Soil Use Manag. 2015, 31, 337–344. [Google Scholar] [CrossRef]
- Pinto-Correia, T.; Ribeiro, N.; Sá-Sousa, P. Introducing the montado, the cork and holm oak agroforestry system of Southern Portugal. Agrofor. Syst. 2011, 82, 99. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, M.; Goss, M.J.; Teixeira, D. Manganese toxicity in Portuguese cambisols derived from granitic rocks: Causes, limitations of soil analyses and possible solutions. Rev. Ciências Agrárias 2015, 38, 518–527. [Google Scholar] [CrossRef]
- Serrano, J.; Shahidian, S.; Marques da Silva, J.; Moral, F.; Carvajal-Ramirez, F.; Carreira, E.; Pereira, A.; Carvalho, M.D. Evaluation of the effect of dolomitic lime application on pastures—Case study in the Montado Mediterranean ecosystem. Sustainability 2020, 12, 3758. [Google Scholar] [CrossRef]
- Goss, M.J.; Carvalho, M.J.G.P.R. Manganese toxicity: The significance of magnesium for the sensitivity of wheat plants. Plant Soil 1992, 139, 91–98. [Google Scholar] [CrossRef]
- Andrew, C.; Hegarty, M. Comparative responses to manganese excess of eight tropical and four temperate pasture legume species. Aust. J. Agric. Res. 1969, 20, 687–696. [Google Scholar] [CrossRef]
- Hayes, R.C.; Dear, B.S.; Orchard, B.A.; Peoples, M.B.; Eberbach, P.L. Response of subterranean clover, balansa clover, and gland clover to lime when grown in mixtures on an acid soil. Aust. J. Agric. Res. 2008, 59, 824–835. [Google Scholar] [CrossRef]
- Evans, J.; Scott, B.J.; Lill, W.J. Manganese tolerance in subterranean clover (Trifolium subterraneum L.) genotypes grown with ammonium nitrate or symbiotic nitrogen. Plant Soil 1987, 97, 207–215. [Google Scholar] [CrossRef]
- DeHaan, L.R.; Russelle, M.P.; Sheaffer, C.C.; Ehlke, N.J. Kura clover and birdsfoot trefoil response to soil pH. Commun. Soil Sci. Plant Anal. 2002, 33, 1435–1449. [Google Scholar] [CrossRef] [Green Version]
- Santoyo, G.; Moreno-Hagelsieb, G.; Orozco-Mosqueda, M.D.C.; Glick, B.R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef]
- Pandey, P.K.; Samanta, R.; Yadav, R.N.S. Inside the plant: Addressing bacterial endophytes in biotic stress alleviation. Arch. Microbiol. 2019, 201, 415–429. [Google Scholar] [CrossRef]
- Tariq, M.; Hameed, S.; Yasmeen, T.; Ali, A. Non-rhizobial bacteria for improved nodulation and grain yield of mung bean [Vigna radiata (L.) Wilczek]. Afr. J. Biotechnol. 2012, 11, 15012–15019. [Google Scholar] [CrossRef]
- Brígido, C.; Menéndez, E.; Paço, A.; Glick, B.R.; Belo, A.; Félix, M.R.; Oliveira, S.; Carvalho, M. Mediterranean native leguminous plants: A reservoir of endophytic bacteria with potential to enhance chickpea growth under stress conditions. Microorganisms 2019, 7, 392. [Google Scholar] [CrossRef] [Green Version]
- Ju, W.L.; Liu, L.; Fang, L.C.; Cui, Y.X.; Duan, C.J.; Wu, H. Impact of co-inoculation with plant-growth-promoting rhizobacteria and rhizobium on the biochemical responses of alfalfa-soil system in copper contaminated soil. Ecotoxicol. Environ. Saf. 2019, 167, 218–226. [Google Scholar] [CrossRef]
- Safronova, V.I.; Piluzza, G.; Zinovkina, N.Y.; Kimeklis, A.K.; Belimov, A.A.; Bullitta, S. Relationships between pasture legumes, rhizobacteria and nodule bacteria in heavy metal polluted mine waste of SW Sardinia. Symbiosis 2012, 58, 149–159. [Google Scholar] [CrossRef]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Glick, B.R.; Todorovic, B.; Czarny, J.; Cheng, Z.; Duan, J.; McConkey, B. Promotion of plant growth by bacterial ACC deaminase. Crit. Rev. Plant Sci. 2007, 26, 227–242. [Google Scholar] [CrossRef]
- Belimov, A.A.; Safronova, V.I.; Sergeyeva, T.A.; Egorova, T.N.; Matveyeva, V.A.; Tsyganov, V.E.; Borisov, A.Y.; Tikhonovich, I.A.; Kluge, C.; Preisfeld, A.; et al. Characterization of plant growth promoting rhizobacteria isolated from polluted soils and containing 1-aminocyclopropane-1-carboxylate deaminase. Can. J. Microbiol. 2001, 47, 642–652. [Google Scholar] [CrossRef]
- Duan, J.; Muller, K.M.; Charles, T.C.; Vesely, S.; Glick, B.R. 1-aminocyclopropane-1-carboxylate (ACC) deaminase genes in rhizobia from southern Saskatchewan. Microb. Ecol. 2009, 57, 423–436. [Google Scholar] [CrossRef]
- Ma, W.B.; Guinel, F.C.; Glick, B.R. Rhizobium leguminosarum biovar viciae 1-aminocyclopropane-1-carboxylate deaminase promotes nodulation of pea plants. Appl. Environ. Microbiol. 2003, 69, 4396–4402. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, F.X.; Brígido, C.; Glick, B.R.; Oliveira, S. ACC deaminase genes are conserved among Mesorhizobium species able to nodulate the same host plant. FEMS Microbiol. Lett. 2012, 336, 26–37. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, F.X.; Brígido, C.; Glick, B.R.; Rossi, M.J. The role of rhizobial ACC deaminase in the nodulation process of leguminous plants. Int. J. Agron. 2016. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.B.; Charles, T.C.; Glick, B.R. Expression of an exogenous 1-aminocyclopropane-1-carboxylate deaminase gene in Sinorhizobium meliloti increases its ability to nodulate alfalfa. Appl. Environ. Microbiol. 2004, 70, 5891–5897. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, F.; Brígido, C.; Alho, L.; Glick, B.R.; Oliveira, S. Enhanced chickpea growth-promotion ability of a Mesorhizobium strain expressing an exogenous ACC deaminase gene. Plant Soil 2012, 353, 221–230. [Google Scholar] [CrossRef]
- Nascimento, F.X.; Brígido, C.; Glick, B.R.; Oliveira, S.; Alho, L. Mesorhizobium ciceri LMS-1 expressing an exogenous 1-aminocyclopropane-1-carboxylate (ACC) deaminase increases its nodulation abilities and chickpea plant resistance to soil constraints. Lett. Appl. Microbiol. 2012, 55, 15–21. [Google Scholar] [CrossRef]
- Brígido, C.; Nascimento, F.X.; Duan, J.; Glick, B.R.; Oliveira, S. Expression of an exogenous 1-aminocyclopropane-1-carboxylate deaminase gene in Mesorhizobium spp. reduces the negative effects of salt stress in chickpea. FEMS Microbiol. Lett. 2013, 349, 46–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belimov, A.A.; Zinovkina, N.Y.; Safronova, V.I.; Litvinsky, V.A.; Nosikov, V.V.; Zavalin, A.A.; Tikhonovich, I.A. Rhizobial ACC deaminase contributes to efficient symbiosis with pea (Pisum sativum L.) under single and combined cadmium and water deficit stress. Environ. Exp. Bot. 2019, 167. [Google Scholar] [CrossRef]
- Brígido, C.; Singh, S.; Menéndez, E.; Tavares, M.J.; Glick, B.R.; Félix, M.d.R.; Oliveira, S.; Carvalho, M. Diversity and functionality of culturable endophytic bacterial communities in chickpea plants. Plants 2019, 8, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brígido, C.; Robledo, M.; Menendez, E.; Mateos, P.F.; Oliveira, S. A ClpB chaperone knockout mutant of Mesorhizobium ciceri shows a delay in the root nodulation of chickpea plants. Mol. Plant Microbe Interact. 2012, 25, 1594–1604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da-Silva, J.R.; Alexandre, A.; Brígido, C.; Oliveira, S. Can stress response genes be used to improve the symbiotic performance of rhizobia? AIMS Microbiol. 2017, 3, 365–382. [Google Scholar] [CrossRef] [PubMed]
- De Marco, D.G.; Li, C.B.; Randall, P.J. Manganese toxicity in Trifolium balansae, T. resupinatum, T. subterraneum, Medicago murex, M. polymorpha, M. sativa, Lotus pedunculatus, and Ornithopus compressus: Relative tolerance and critical toxicity concentrations. Anim. Prod. Sci. 1995, 35, 367–374. [Google Scholar] [CrossRef]
- Nonnoi, F.; Chinnaswamy, A.; de la Torre, V.S.G.; de la Pena, T.C.; Lucas, M.M.; Pueyo, J.J. Metal tolerance of rhizobial strains isolated from nodules of herbaceous legumes (Medicago spp. and Trifolium spp.) growing in mercury-contaminated soils. Appl. Soil Ecol. 2012, 61, 49–59. [Google Scholar] [CrossRef] [Green Version]
- Brígido, C.; Glick, B.R. Phytoremediation using Rhizobia. In Phytoremediation: Management of Environmental Contaminants; Ansari, A.A., Gill, S.S., Gill, R., Lanza, G.R., Newman, L., Eds.; Springer International Publishing: Cham, Switzerland, 2015; Volume 2, pp. 95–114. [Google Scholar]
- Carrillo-Castañeda, G.; Juárez Muñoz, J.; Ramón Peralta-Videa, J.; Gomez, E.; Gardea-Torresdey, J.L. Plant growth-promoting bacteria promote copper and iron translocation from root to shoot in alfalfa seedlings. J. Plant Nutr. 2003, 26, 1801–1814. [Google Scholar] [CrossRef]
- Gupta, D.K.; Rai, U.N.; Sinha, S.; Tripathi, R.D.; Nautiyal, B.D.; Rai, P.; Inouche, M. Role of Rhizobium (CA-1) inoculation in increasing growth and metal accumulation in Cicer arietinum L. growing under my-ash stress condition. Bull. Environ. Contam. Toxicol. 2004, 73, 424–431. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Merten, D.; Svatoš, A.; Büchel, G.; Kothe, E. Metal-induced oxidative stress impacting plant growth in contaminated soil is alleviated by microbial siderophores. Soil Biol. Biochem. 2009, 41, 154–162. [Google Scholar] [CrossRef]
- Rau, N.; Mishra, V.; Sharma, M.; Das, M.K.; Ahaluwalia, K.; Sharma, R.S. Evaluation of functional diversity in rhizobacterial taxa of a wild grass (Saccharum ravennae) colonizing abandoned fly ash dumps in Delhi urban ecosystem. Soil Biol. Biochem. 2009, 41, 813–821. [Google Scholar] [CrossRef]
- Brígido, C.; Glick, B.R.; Oliveira, S. Survey of plant growth-promoting mechanisms in native portuguese chickpea Mesorhizobium isolates. Microb. Ecol. 2017, 73, 900–915. [Google Scholar] [CrossRef]
- Srinivasan, M.; Holl, F.B.; Petersen, D.J. Nodulation of Phaseolus vulgaris by Rhizobium etli is enhanced by the presence of Bacillus. Can. J. Microbiol. 1997, 43, 1–8. [Google Scholar] [CrossRef]
- Mishra, P.K.; Mishra, S.; Selvakumar, G.; Bisht, J.K.; Kundu, S.; Gupta, H.S. Coinoculation of Bacillus thuringeinsis-KR1 with Rhizobium leguminosarum enhances plant growth and nodulation of pea (Pisum sativum L.) and lentil (Lens culinaris L.). World J. Microbiol. Biotechnol. 2009, 25, 753–761. [Google Scholar] [CrossRef]
- Sepúlveda-Caamaño, M.; Gerding, M.; Vargas, M.; Moya-Elizondo, E.; Oyarzúa, P.; Campos, J. Lentil (Lens culinaris L.) growth promoting rhizobacteria and their effect on nodulation in coinoculation with rhizobia. Arch. Agron Soil Sci. 2018, 64, 244–256. [Google Scholar] [CrossRef]
- Pandya, M.; Rajput, M.; Rajkumar, S. Exploring plant growth promoting potential of non rhizobial root nodules endophytes of Vigna radiata. Microbiology 2015, 84, 80–89. [Google Scholar] [CrossRef]
- Chaudhary, D.; Sindhu, S.S. Amelioration of salt stress in chickpea (Cicer arietinum L.) by coinculation of ACC deaminase-containing rhizospheric bacteria with Mesorhizobium strains. Legume Res. 2017, 40, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Sahur, A.; Ala, A.; Patandjengi, B.; Syam’un, E. Effect of seed inoculation with Actinomycetes and Rhizobium isolated from indigenous soybean and rhizosphere on nitrogen fixation, growth, and yield of soybean. Int. J. Agron. 2018, 2018, 4371623. [Google Scholar] [CrossRef] [Green Version]
- Puente, M.L.; Zawoznik, M.; de Sabando, M.L.; Perez, G.; Gualpa, J.L.; Carletti, S.M.; Cassán, F.D. Improvement of soybean grain nutritional quality under foliar inoculation with Azospirillum brasilense strain Az39. Symbiosis 2019, 77, 41–47. [Google Scholar] [CrossRef]
- Zhang, W.-H.; Chen, W.; He, L.-Y.; Wang, Q.; Sheng, X.-F. Characterization of Mn-resistant endophytic bacteria from Mn-hyperaccumulator Phytolacca americana and their impact on Mn accumulation of hybrid penisetum. Ecotoxicol. Environ. Saf. 2015, 120, 369–376. [Google Scholar] [CrossRef]
- Carlos, M.H.J.; Stefani, P.V.Y.; Janette, A.M.; Melani, M.S.S.; Gabriela, P.O. Assessing the effects of heavy metals in ACC deaminase and IAA production on plant growth-promoting bacteria. Microbiol. Res. 2016, 188, 53–61. [Google Scholar] [CrossRef]
- Dardanelli, M.S.; de Cordoba, F.J.F.; Espuny, M.R.; Carvajal, M.A.R.; Díaz, M.E.S.; Serrano, A.M.G.; Okon, Y.; Megías, M. Effect of Azospirillum brasilense coinoculated with Rhizobium on Phaseolus vulgaris flavonoids and Nod factor production under salt stress. Soil Biol. Biochem. 2008, 40, 2713–2721. [Google Scholar] [CrossRef]
- Martínez, R.; Espejo, A.; Sierra, M.; Ortiz-Bernad, I.; Correa, D.; Bedmar, E.; López-Jurado, M.; Porres, J.M. Co-inoculation of Halomonas maura and Ensifer meliloti to improve alfalfa yield in saline soils. Appl. Soil Ecol. 2015, 87, 81–86. [Google Scholar] [CrossRef]
- Hashem, A.; Abd_Allah, E.F.; Alqarawi, A.A.; Al-Huqail, A.A.; Wirth, S.; Egamberdieva, D. The interaction between arbuscular mycorrhizal fungi and endophytic bacteria enhances plant growth of Acacia gerrardii under salt stress. Front. Microbiol. 2016, 7, 1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egamberdieva, D.; Wirth, S.J.; Shurigin, V.V.; Hashem, A.; Abd Allah, E.F. Endophytic bacteria improve plant growth, symbiotic performance of chickpea (Cicer arietinum L.) and induce suppression of root rot caused by Fusarium solani under salt stress. Front. Microbiol. 2017, 8, 1887. [Google Scholar] [CrossRef] [PubMed]
- Mayak, S.; Tirosh, T.; Glick, B.R. Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol. Biochem. 2004, 42, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Fatnassi, I.C.; Chiboub, M.; Saadani, O.; Jebara, M.; Jebara, S.H. Impact of dual inoculation with Rhizobium and PGPR on growth and antioxidant status of Vicia faba L. under copper stress. Comptes Rendus Biol. 2015, 338, 241–254. [Google Scholar] [CrossRef]
- Fatnassi, I.C.; Chiboub, M.; Saadani, O.; Jebara, M.; Jebara, S.H. Phytostabilization of moderate copper contaminated soils using co-inoculation of Vicia faba with plant growth promoting bacteria. J. Basic Microbiol. 2015, 55, 303–311. [Google Scholar] [CrossRef]
- Jian, L.; Bai, X.; Zhang, H.; Song, X.; Li, Z. Promotion of growth and metal accumulation of alfalfa by coinoculation with Sinorhizobium and Agrobacterium under copper and zinc stress. PeerJ 2019, 7, e6875. [Google Scholar] [CrossRef] [Green Version]
- Safronova, V.I.; Stepanok, V.V.; Engqvist, G.L.; Alekseyev, Y.V.; Belimov, A.A. Root-associated bacteria containing 1-aminocyclopropane-1-carboxylate deaminase improve growth and nutrient uptake by pea genotypes cultivated in cadmium supplemented soil. Biol. Fertil. Soils. 2006, 42, 267–272. [Google Scholar] [CrossRef]
- Conforte, V.P.; Echeverria, M.; Sanchez, C.; Ugalde, R.A.; Menendez, A.B.; Lepek, V.C. Engineered ACC deaminase-expressing free-living cells of Mesorhizobium loti show increased nodulation efficiency and competitiveness on Lotus spp. J. Gen. Appl. Microbiol. 2010, 56, 331–338. [Google Scholar] [CrossRef] [Green Version]
- Kong, Z.Y.; Glick, B.R.; Duan, J.; Ding, S.L.; Tian, J.; McConkey, B.J.; Wei, G.H. Effects of 1-aminocyclopropane-1-carboxylate (ACC) deaminase-overproducing Sinorhizobium meliloti on plant growth and copper tolerance of Medicago lupulina. Plant Soil 2015, 391, 383–398. [Google Scholar] [CrossRef]
- Tittabutr, P.; Sripakdi, S.; Boonkerd, N.; Tanthanuch, W.; Minamisawa, K.; Teaumroong, N. Possible Role of 1-aminocyclopropane-1-carboxylate (ACC) deaminase activity of Sinorhizobium sp. BL3 on symbiosis with mung bean and determinate nodule senescence. Microbes Environ. 2015, 30, 310–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaharoona, B.; Arshad, M.; Zahir, Z. Effect of plant growth promoting rhizobacteria containing ACC-deaminase on maize (Zea mays L.) growth under axenic conditions and on nodulation in mung bean (Vigna radiata L.). Lett. Appl. Microbiol. 2006, 42, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, S.M.; Khalid, A.; Arshad, M.; Tahir, J.; Mahmood, T. Improving nodulation, growth and yield of Cicer arietinum L. through bacterial ACC-deaminase induced changes in root architecture. Eur. J. Soil Biol. 2010, 46, 342–347. [Google Scholar] [CrossRef]
- Zahir, Z.A.; Zafar-ul-Hye, M.; Sajjad, S.; Naveed, M. Comparative effectiveness of Pseudomonas and Serratia sp. containing ACC-deaminase for coinoculation with Rhizobium leguminosarum to improve growth, nodulation, and yield of lentil. Biol. Fertil. Soils 2011, 47, 457–465. [Google Scholar] [CrossRef]
- Tittabutr, P.; Piromyou, P.; Longtonglang, A.; Noisa-Ngiam, R.; Boonkerd, N.; Teaumroong, N. Alleviation of the effect of environmental stresses using co-inoculation of mungbean by Bradyrhizobium and rhizobacteria containing stress-induced ACC deaminase enzyme. Soil Sci. Plant Nutr. 2013, 59, 559–571. [Google Scholar] [CrossRef] [Green Version]
- Shahzad, S.M.; Khalid, A.; Arif, M.S.; Riaz, M.; Ashraf, M.; Iqbal, Z.; Yasmeen, T. Co-inoculation integrated with P-enriched compost improved nodulation and growth of chickpea (Cicer arietinum L.) under irrigated and rainfed farming systems. Biol. Fertil. Soils 2014, 50, 1–12. [Google Scholar] [CrossRef]
- Soussou, S.; Brunel, B.; Pervent, M.; Van Tuinen, D.; Cleyet-Marel, J.-C.; Baudoin, E. Rhizobacterial Pseudomonas spp. strains harbouring acdS gene could enhance metallicolous legume nodulation in Zn/Pb/Cd mine tailings. Water Air Soil Pollut. 2017, 228, 142. [Google Scholar] [CrossRef]
- Cedeño-García, G.A.; Gerding, M.; Moraga, G.; Inostroza, L.; Fischer, S.; Sepúlveda-Caamaño, M.; Oyarzúa, P. Plant growth promoting rhizobacteria with ACC deaminase activity isolated from Mediterranean dryland areas in Chile: Effects on early nodulation in alfalfa. Chil. J. Agric. Res. 2018, 78, 360–369. [Google Scholar] [CrossRef] [Green Version]
- Guan, R.; Su, J.; Meng, X.; Li, S.; Liu, Y.; Xu, J.; Zhang, S. Multilayered regulation of ethylene induction plays a positive role in Arabidopsis resistance against Pseudomonas syringae. Plant Physiol. 2015, 169, 299. [Google Scholar] [CrossRef] [Green Version]
- Khatabi, B.; Schäfer, P. Ethylene in mutualistic symbioses. Plant Signal. Behav. 2012, 7, 1634–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hontzeas, N.; Saleh, S.S.; Glick, B.R. Changes in gene expression in canola roots induced by ACC-deaminase-containing plant-growth-promoting bacteria. Mol. Plant Microbe Interact. 2004, 17, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Gourion, B.; Berrabah, F.; Ratet, P.; Stacey, G. Rhizobium–legume symbioses: The crucial role of plant immunity. Trends Plant Sci. 2015, 20, 186–194. [Google Scholar] [CrossRef] [Green Version]
- Uchiumi, T.; Ohwada, T.; Itakura, M.; Mitsui, H.; Nukui, N.; Dawadi, P.; Kaneko, T.; Tabata, S.; Yokoyama, T.; Tejima, K.; et al. Expression islands clustered on the symbiosis island of the Mesorhizobium loti genome. J. Bacteriol. 2004, 186, 2439–2448. [Google Scholar] [CrossRef] [Green Version]
- Nukui, N.; Minamisawa, K.; Ayabe, S.-I.; Aoki, T. Expression of the 1-aminocyclopropane-1-carboxylic acid deaminase gene requires symbiotic nitrogen-fixing regulator gene nifA2 in Mesorhizobium loti MAFF303099. Appl. Environ. Microbiol. 2006, 72, 4964–4969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nascimento, F.X.; Rossi, M.J.; Soares, C.; McConkey, B.J.; Glick, B.R. New insights into 1-aminocyclopropane-1-carboxylate (acc) deaminase phylogeny, evolution and ecological significance. PLoS ONE 2014, 9, e99168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, J.; Wu, D.; Liang, Y.; Li, L.; Guo, Y. Disruption of acdS gene reduces plant growth promotion activity and maize saline stress resistance by Rahnella aquatilis HX2. J. Basic Microbiol. 2019, 59, 402–411. [Google Scholar] [CrossRef]
- Vincent, J.M. A Manual for the Practical Study of Root-Nodule Bacteria; Blackwell Scientific Publications: Oxford, UK, 1970; Volume 15, p. 164. [Google Scholar]
- Beringer, J.E. R factor transfer in Rhizobium leguminosarum. J. Gen. Microbiol. 1974, 84, 188–198. [Google Scholar] [CrossRef] [Green Version]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory: New York, NY, USA, 2001. [Google Scholar]
- Wei, H.L.; Chakravarthy, S.; Worley, J.N.; Collmer, A. Consequences of flagellin export through the type III secretion system of Pseudomonas syringae reveal a major difference in the innate immune systems of mammals and the model plant Nicotiana benthamiana. Cell. Microbiol. 2013, 15, 601–618. [Google Scholar] [CrossRef]
- Finan, T.M.; Kunkel, B.; De Vos, G.F.; Signer, E.R. Second symbiotic megaplasmid in Rhizobium meliloti carrying exopolysaccharide and thiamine synthesis genes. J. Bacteriol. 1986, 167, 66–72. [Google Scholar] [CrossRef] [Green Version]
- McKenzie, G.J.; Craig, N.L. Fast, easy and efficient: Site-specific insertion of transgenes into Enterobacterial chromosomes using Tn7 without need for selection of the insertion event. BMC Microbiol. 2006, 6, 39. [Google Scholar] [CrossRef] [Green Version]
- Dehio, C.; Meyer, M. Maintenance of broad-host-range incompatibility group P and group Q plasmids and transposition of Tn5 in Bartonella henselae following conjugal plasmid transfer from Escherichia coli. J. Bacteriol. 1997, 179, 538–540. [Google Scholar] [CrossRef] [Green Version]
- Heckman, K.L.; Pease, L.R. Gene splicing and mutagenesis by PCR-driven overlap extension. Nat. Protoc. 2007, 2, 924. [Google Scholar] [CrossRef]
- Schafer, A.; Tauch, A.; Jager, W.; Kalinowski, J.; Thierbach, G.; Puhler, A. Small mobilizable multipurpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19—Selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 1994, 145, 69–73. [Google Scholar] [CrossRef]
- Solovyev, V.; Salamov, A. Automatic Annotation of Microbial Genomes and Metagenomic Sequences. In Metagenomics and Its Applications in Agriculture, Biomedicine and Environmental Studies; Li, R.W., Ed.; Nova Science Publishers: Harpak, NY, USA, 2011. [Google Scholar]
- Naville, M.; Ghuillot-Gaudeffroy, A.; Marchais, A.; Gautheret, D. ARNold: A web tool for the prediction of Rho-independent transcription terminators. RNA Biol. 2011, 8, 11–13. [Google Scholar] [CrossRef] [Green Version]
- Penrose, D.M.; Glick, B.R. Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol. Plant 2003, 118, 10–15. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Harper, J.E.; Gibson, A.H. Differential nodulation tolerance to nitrate among legume species. Crop Sci. 1984, 24, 797–801. [Google Scholar] [CrossRef]
- Broughton, W.J.; Dilworth, M.J. Control of leghaemoglobin synthesis in snake beans. Biochem. J. 1971, 125, 1075–1080. [Google Scholar] [CrossRef] [Green Version]
- Menéndez, E.; Robledo, M.; Jiménez-Zurdo, J.I.; Velázquez, E.; Rivas, R.; Murray, J.D.; Mateos, P.F. Legumes display common and host-specific responses to the rhizobial cellulase CelC2 during primary symbiotic infection. Sci. Rep. 2019, 9, 13907. [Google Scholar] [CrossRef] [Green Version]







| Plasmids or Bacteria | Description | Reference or Source |
|---|---|---|
| Plasmids | ||
| pNZY28-blunt | Ampr, blunt ends | NZYtech |
| pJET1.2/blunt | Ampr, blunt ends | ThermoFisher Scientific |
| pT18mobsacB | plasmid suicide; Kmr, sacBs | [83] |
| pRK600 | Helper plasmid pRK2013, npt::Tn9, Cmr | [84] |
| pGRG36 | Ampr, arabinose-inducible promoter, RP4 conjugal transfer origin, pSC101 temperature-sensitive origin, presenting tnsABCD genes for Tn7 transposition | [85] |
| pGRG36-Tetr | pGRG36 derivative, Tetr | This work |
| pNZY28-∆acdS | regions flanking acdS in pNZY28 | This work |
| pJET1.2-acdS | acdS gene and its promoter and terminator regions | This work |
| pT18mobsacB-∆acdS | regions flanking acdS in pT18mobsacB | This work |
| pGRG36-Tetr-acdS | acdS gene and its promoter region of Pseudomonas sp. Q1 strain in pGRG36‑Tetr | This work |
| Pseudomonas sp. | ||
| Q1 | Pseudomonas sp. Q1, acdS+ | [18] |
| Q1 ∆acdS | Derivative of strain Q1, acdS- | This work |
| Q1 ∆acdS+ complemented | Derivative of strain Q1 ∆acdS, acdS+ with a copy of acdS gene and its promoter and terminator region | This work |
| Rhizobia | ||
| LMS-1 | M. ciceri, acdS+, nif+, nod+, Nodulates chickpea (Cicer arietinum) | [26,34] |
| ATCC 14480T | R. leguminosarum bv. trifolii, Nodulates red clover (Trifolium praetense) and white clover (Trifolium repens) | ATCC® Microbiome Standards |
| ATCC 9930T | E. meliloti, Nodulates (Medicago sativa) and sweet clover (Melilotus alba) | ATCC® Microbiome Standards |
| Escherichia coli | ||
| DH5α | Host for cloning | [82] |
| WM3064 | Host for conjugation, presence of RP4 (tra) in the chromosome | [86] |
| MT616 | Strain containing helper plasmid pRK600 | [84] |
| Primer Names | Primer Sequence (5′→3′) | Application |
|---|---|---|
| Tet-SdaI-Fw Tet-SdaI-Rv | CCTGCAGGGGACAAGGGAAAACGCAAG CCTGCAGGCCGTCAGCGTTTTGTAATGG | tetA gene and its promoter and terminator regions (2624 bp) |
| acdS-Q1-Fa acdS-Q1-Rb | TCAAGAGGTTGACGGGTTCT CTGGACGCGAGCGTGAAGGGCGTGATGGGAGA | acdS upstream region (948bp) |
| acdS-Q1-Fc acdS-Q1-Rd | TCTCCCATCACGCCCTTCACGCTCGCGTCCAG AACATCGCAAAGACGTAGGG | acdS downstream region (898bp) |
| acdS-Q1-Fa comp_acds_Q1 Rv | TCAAGAGGTTGACGGGTTCT AATGCCCTCGATTGCCGGA | acdS gene and its promoter and terminator regions (1242 bp) |
| RL_acdS_all_Fw RL_acdS_all_Rv | AACGCTATCCGCTCACCTT ATCCCCTGCATCGACTTTC | acdS gene fragment (886 bp) from R. leguminosarum bv trifolii ATCC 14480T |
| EM_acdS_all_Fw EM_acdS_all_Rv | CAGCCGTCCCTGTAGTAATAGC GAAAAGTTCGAACGCTACCC | acdS gene fragment (1006 bp) from Ensifer meliloti ATCC 9930T |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paço, A.; da-Silva, J.R.; Torres, D.P.; Glick, B.R.; Brígido, C. Exogenous ACC Deaminase Is Key to Improving the Performance of Pasture Legume-Rhizobial Symbioses in the Presence of a High Manganese Concentration. Plants 2020, 9, 1630. https://doi.org/10.3390/plants9121630
Paço A, da-Silva JR, Torres DP, Glick BR, Brígido C. Exogenous ACC Deaminase Is Key to Improving the Performance of Pasture Legume-Rhizobial Symbioses in the Presence of a High Manganese Concentration. Plants. 2020; 9(12):1630. https://doi.org/10.3390/plants9121630
Chicago/Turabian StylePaço, Ana, José Rodrigo da-Silva, Denise Pereira Torres, Bernard R. Glick, and Clarisse Brígido. 2020. "Exogenous ACC Deaminase Is Key to Improving the Performance of Pasture Legume-Rhizobial Symbioses in the Presence of a High Manganese Concentration" Plants 9, no. 12: 1630. https://doi.org/10.3390/plants9121630
APA StylePaço, A., da-Silva, J. R., Torres, D. P., Glick, B. R., & Brígido, C. (2020). Exogenous ACC Deaminase Is Key to Improving the Performance of Pasture Legume-Rhizobial Symbioses in the Presence of a High Manganese Concentration. Plants, 9(12), 1630. https://doi.org/10.3390/plants9121630