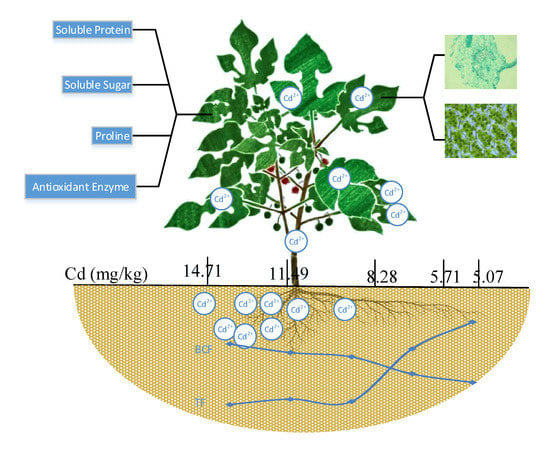
Morphological and Physiological Changes of Broussonetia papyrifera Seedlings in Cadmium Contaminated Soil
Abstract
1. Introduction
2. Results
2.1. Height Changes and the Characteristics of Leaf Phenotype
2.2. Changes in Biomass and Moisture Content (MC)
2.3. Cd Content in B. papyrifera Tissues
2.4. Anatomic Structure of Leaves
2.5. Physiological Characteristics of B. Papyrifera Seedlings
2.5.1. Changes in Soluble Protein Content, Soluble Sugar Content and Proline Content
2.5.2. Changes in MDA Content and Antioxidant Enzyme Activity
2.5.3. Changes in Chlorophyll Content
3. Discussion
4. Materials and Methods
4.1. Source of Seedlings and Cd Treatment
4.2. Determination of Growth Indicator and Cd Accumulation
4.3. Determination of Physiological Characteristics
4.4. The Observation of Leaf Anatomy
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABA | abscisic acid |
| BCF | bioconcentration factor |
| CAT | catalase |
| CBB | coomassie brilliant blue |
| DW | dry weight |
| FW | fresh weight |
| MDA | malondialdehyde |
| MC | moisture content |
| PTFE | polytetrafluoroethylene |
| POD | peroxidase |
| ROS | reactive oxygen species |
| SE | standard error |
| SOD | superoxide dismutase |
| TF | translocation factor |
| TBA | thiobarbituric acid |
References
- Wang, X.; Sato, T.; Xing, B.; Tao, S. Health risks of heavy metals to the general public in Tianjin, China via consumption of vegetables and fish. Sci. Total Environ. 2005, 350, 28–37. [Google Scholar] [CrossRef]
- Lee, Y.H.; Stuebing, R.B. Heavy metal contamination in the river toad, Bufo juxtasper (Inger), near a copper mine in East Malaysia. Bull. Environ. Contam. Toxicol. 1990, 45, 272–279. [Google Scholar] [CrossRef]
- Vangronsveld, J.; Assche, F.V.; Clijsters, H. Reclamation of a bare industrial area contaminated by non-ferrous metals: In situ metal immobilization and revegetation. Environ. Pollut. 1995, 87, 51–59. [Google Scholar] [CrossRef]
- Nriagu, J.O. A silent epidemic of environmental metal poisoning? Environ. Pollut. 1988, 50, 139–161. [Google Scholar] [CrossRef]
- Douay, F.; Roussel, H.; Pruvot, C.; Loriette, A.; Fourrier, H. Assessment of a remediation technique using the replacement of contaminated soils in kitchen gardens nearby a former lead smelter in Northern France. Sci. Total Environ. 2008, 401, 29–38. [Google Scholar] [CrossRef]
- Baczek-Kwinta, R.; Juzon, K.; Borek, M.; Antonkiewicz, J. Photosynthetic response of cabbage in cadmium-spiked soil. Photosynthetica 2019, 57, 731–739. [Google Scholar] [CrossRef]
- Hernández-Allica, J.; Becerril, J.M.; Zárate, O.; Garbisu, C. Assessment of the efficiency of a metal phytoextraction process with biological indicators of soil health. Plant Soil 2006, 281, 147–158. [Google Scholar] [CrossRef]
- Zhang, J.; Martinoia, E.; Lee, Y. Vacuolar transporters for cadmium and arsenic in plants and their applications in phytoremediation and crop development. Plant Cell Physiol. 2018, 59, 1317–1325. [Google Scholar] [CrossRef]
- Antonkiewicz, J.; Kolodziej, B.; Bielinska, E.J.; Witkowicz, R.; Tabor, S. Using jerusalem artichoke to extract heavy metals from municipal sewage sludge amended soil. Pol. J. Environ. Stud. 2018, 27, 513–527. [Google Scholar] [CrossRef]
- Ma, L.Q. A fern that hyperaccumulates arsenic. World Environ. 2001, 409, 579. [Google Scholar] [CrossRef]
- Chen, T.; Wei, C.; Huang, Z.; Huang, Q.; Lu, Q.; Fan, Z. Arsenic hyperaccumulator Pteris Vittata L. and its arsenic accumulation. Chin. Sci. Bull. 2002, 47, 902–905. [Google Scholar] [CrossRef]
- Chen, T.; Lei, M.; Wan, X.; Yang, J.; Zhou, X. Arsenic hyperaccumulator Pteris vittata L. and its aplication to the field. In Twenty Years of Research and Development on Soil Pollution and Remediation in China; Springer: Singapore, 2018; pp. 465–476. [Google Scholar] [CrossRef]
- Xue, S.; Chen, Y.; Lin, Q.; Xu, S.; Wang, Y. Phytolacca acinosa Roxb. (Phytolaccaceae): A new manganese hyperaccumulator plant from Southern China. Acta Ecol. Sin. 2003, 23, 935–937. [Google Scholar] [CrossRef]
- Xue, S.; Chen, Y.; Luo, Y.; Reeves, R.D.; Lin, Q. Manganese tolerance and hyperaccumulation of Phytolacca acinosa Roxb. Acta Pedol. Sin. 2004, 41, 889–895. [Google Scholar] [CrossRef]
- Liu, L.; Wu, L.; Li, N.; Luo, Y.; Li, S.; Li, Z.; Han, C.; Jiang, Y.; Christie, P. Rhizosphere concentrations of zinc and cadmium in a metal contaminated soil after repeated phytoextraction by Sedum plumbizincicola. Int. J. Phytoremediat. 2011, 13, 750–764. [Google Scholar] [CrossRef][Green Version]
- Liu, H.; Zhao, H.; Wu, L.; Liu, A.; Zhao, F.-J.; Xu, W. Heavy metal ATPase 3 (HMA3) confers cadmium hypertolerance on the cadmium/zinc hyperaccumulator Sedum plumbizincicola. New Phytol. 2017, 215, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Abid, M.; Zhang, Y.J.; Li, Z.; Bai, D.F.; Zhong, Y.P.; Fang, J.B. Effect of salt stress on growth, physiological and biochemical characters of four kiwifruit genotypes. Sci. Hortic. 2020, 271, 109473. [Google Scholar] [CrossRef]
- Nikolić, N.; Zorić, L.; Cvetković, I.; Pajević, S.; Borišev, M.; Orlović, S.; Pilipović, A. Assessment of cadmium tolerance and phytoextraction ability in young Populus deltoides L. and Populus × euramericana plants through morpho-anatomical and physiological responses to growth in cadmium enriched soil. iForest Biogeosci. For. 2017, 10, 635–644. [Google Scholar] [CrossRef]
- Zhou, J.; Jiang, Z.; Ma, J.; Yang, L.; Wei, Y. The effects of lead stress on photosynthetic function and chloroplast ultrastructure of Robinia pseudoacacia seedlings. Environ. Sci. Pollut. Res. 2017, 24, 10718–10726. [Google Scholar] [CrossRef]
- Nóia Júnior, R.d.S.; Amaral, G.C.; Pezzopane, J.E.M.; Fonseca, M.D.S.; Câmara da Silva, A.P.; Xavier, T.M.T. Ecophysiological acclimatization to cyclic water stress in Eucalyptus. J. For. Res. 2019, 31, 797–806. [Google Scholar] [CrossRef]
- Luo, y.y.; Wang, s.s.; Yan, j.; Tian, p. Effects of lead stress on anatomic structure of stems and leaves of Artemisia Sacrorum var. Messerschmidtiana. Bull. Soil Water Conserv. 2010, 30, 182–185. [Google Scholar] [CrossRef]
- Huang, H.; Zhao, Y.; Xu, Z.; Zhang, W.; Jiang, K. Physiological responses of Broussonetia papyrifera to manganese stress, a candidate plant for phytoremediation. Ecotoxicol. Environ. Saf. 2019, 181, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.P.; Zhu, J.; Wang, P.; Zeng, J.; Tan, R.; Yang, Y.Z.; Liu, Z.M. Effect of Cd on growth, physiological response, Cd subcellular distribution and chemical forms of Koelreuteria paniculata. Ecotoxicol. Environ. Saf. 2018, 160, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Sitko, K.; Rusinowski, S.; Kalaji, H.M.; Szopinski, M.; Malkowski, E. Photosynthetic Efficiency as Bioindicator of Environmental Pressure in A. halleri. Plant Physiol. 2017, 175, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Moradi, L.; Ehsanzadeh, P. Effects of Cd on photosynthesis and growth of safflower (Carthamus tinctorius L.) genotypes. Photosynthetica 2015, 53, 506–518. [Google Scholar] [CrossRef]
- González-Lorca, J.; Rivera-Hutinel, A.; Moncada, X.; Lobos, S.; Seelenfreund, D.; Seelenfreund, A. Ancient and modern introduction of Broussonetia papyrifera ([L.] Vent.; Moraceae) into the Pacific: Genetic, geographical and historical evidence. N. Z. J. Bot. 2015, 53, 75–89. [Google Scholar] [CrossRef]
- Peñailillo, J.; Olivares, G.; Moncada, X.; Payacán, C.; Chang, C.S.; Chung, K.F.; Matthews, P.J.; Seelenfreund, A.; Seelenfreund, D. Sex distribution of paper mulberry (Broussonetia papyrifera) in the Pacific. PLoS ONE 2016, 11, e0161148. [Google Scholar] [CrossRef]
- Zhang, X.; Qu, L.J.; Guo, X.Q. Study on structure and property of Broussonetia papyrifera (BP) fiber. Adv. Mater. Res. 2011, 146–147, 1593–1596. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Yu, C.; Yu, H. Research of degumming process, microstructure and properties of mulberry fiber. J. Donghua Univ. (Engl. Ed.) 2007, 24, 170–172. [Google Scholar]
- Moncada, X.; Payacán, C.; Arriaza, F.; Lobos, S.; Seelenfreund, D.; Seelenfreund, A. DNA extraction and amplification from contemporary polynesian bark-cloth. PLoS ONE 2013, 8, e56549. [Google Scholar] [CrossRef]
- Teodoro, P.E.; Peña-Ahumada, B.; Saldarriaga-Córdoba, M.; Kardailsky, O.; Moncada, X.; Moraga, M.; Matisoo-Smith, E.; Seelenfreund, D.; Seelenfreund, A. A tale of textiles: Genetic characterization of historical paper mulberry barkcloth from Oceania. PLoS ONE 2020, 15, e0233113. [Google Scholar] [CrossRef]
- Daud, M.; Khalid, N.; Iqbal, J.; Ahmad, S.; Zaidi, J.H. Potential of Broussonetia papyrifera leaves as biomonitors for atmospheric pollution: Use of INAA and AAS techniques. Radiochim. Acta 2006, 94, 871–877. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, C.S.; Yu, L.N.; Bi, J.; Liu, S.F.; Zhu, F.; Yang, Q.L. Chemical composition of Broussonetia Papyrifera flowers. Adv. Mater. Res. 2011, 236–238, 2581–2585. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, C.S.; Yu, L.N.; Bi, J.; Liu, S.F.; Zhu, F.; Yang, Q.L. Antioxidant activity and total phenolics of Broussonetia papyrifera flower extracts. Appl. Mech. Mater. 2012, 140, 263–267. [Google Scholar] [CrossRef]
- Pang, S.; Wang, G.; Lin, J.; Diao, Y.; Xu, R. Cytotoxic activity of the alkaloids from Broussonetia papyrifera fruits. Pharm. Biol. 2014, 52, 1315–1319. [Google Scholar] [CrossRef]
- Xu, M.L.; Wang, L.; Hu, J.H.; Lee, S.K.; Wang, M.H. Antioxidant activities and related polyphenolic constituents of the methanol extract fractions from Broussonetia papyrifera stem bark and wood. Food Sci. Biotechnol. 2010, 19, 677–682. [Google Scholar] [CrossRef]
- Wu, W.T. Evaluation of anti-inflammatory effects of Broussonetia papyrifera stem bark. Indian J. Pharmacol. 2012, 44, 26–30. [Google Scholar] [CrossRef]
- Wu, L.; Xu, Z.; Zhang, W.; Ding, Y.; Tang, Y.; Zhao, Y. Potential distribution of Broussonetia papyrifera in China based on MaxEnt model. J. Central South Univ. For. Technol. 2018, 38, 40–45. [Google Scholar] [CrossRef]
- Saito, K.; Linquist, B.; Keobualapha, B.; Shiraiwa, T.; Horie, T. Broussonetia papyrifera (paper mulberry): Its growth, yield and potential as a fallow crop in slash-and-burn upland rice system of northern Laos. Agrofor. Syst. 2009, 76, 525–532. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.; Li, H.; Wu, G. Overexpression of AtNHX5 improves tolerance to both salt and drought stress in Broussonetia papyrifera (L.) Vent. Tree Physiol. 2011, 31, 349–357. [Google Scholar] [CrossRef]
- Zhang, M.; Fang, Y.; Ji, Y.; Jiang, Z.; Wang, L. Effects of salt stress on ion content, antioxidant enzymes and protein profile in different tissues of Broussonetia papyrifera. S. Afr. J. Bot. 2013, 85, 1–9. [Google Scholar] [CrossRef]
- Sun, J.; Peng, X.; Fan, W.; Tang, M.; Liu, J.; Shen, S. Functional analysis of BpDREB2 gene involved in salt and drought response from a woody plant Broussonetia papyrifera. Gene 2014, 535, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, J.; Xia, X.; Chu, J.; Wei, Y.; Shi, S.; Chang, E.; Yin, W.; Jiang, Z. The evaluation of heavy metal accumulation and application of a comprehensive bio-concentration index for woody species on contaminated sites in Hunan, China. Environ. Sci. Pollut. Res. 2014, 21, 5076–5085. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.L. Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 2002, 53, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vos, C.H.R.D.; Schat, H.; Waal, M.A.M.D.; Vooijs, R.; Ernst, W.H.O. Increased resistance to copper-induced damage of the root cell plasmalemma in copper tolerant Silene cucubalus. Physiol. Plant. 1991, 82, 523–528. [Google Scholar] [CrossRef]
- Petrov, V.D.; Van Breusegem, F. Hydrogen peroxide-a central hub for information flow in plant cells. AoB Plants 2012, 2012, pls014. [Google Scholar] [CrossRef]
- Kurusu, T.; Kuchitsu, K.; Tada, Y. Plant signaling networks involving Ca(2+) and Rboh/Nox-mediated ROS production under salinity stress. Front. Plant Sci. 2015, 6, 427. [Google Scholar] [CrossRef]
- Dietz, K.J.; Baier, M.; Krämer, K. Free Radicals and Reactive Oxygen Species as Mediators of Heavy Metal Toxicity in Plants; Springer: Berlin/Heidelberg, Germany, 1999; pp. 73–97. [Google Scholar]
- Assche, F.; Clijsters, H. Effects of metals on enzyme activity in plants. Plant Cell Environ. 1990, 13, 195–206. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, Q.; Ma, M.; Liu, Y.; Wang, W.; Ning, W. Cadmium tolerance, distribution, and accumulation in Taraxacum ohwianum Kitam. as a potential Cd-hyperaccumulator. Int. J. Phytoremed. 2019, 21, 541–549. [Google Scholar] [CrossRef]
- Hu, P.-J.; Gan, Y.-Y.; Tang, Y.-T.; Zhang, Q.-F.; Jiang, D.; Yao, N.; Qiu, R.-L. Cellular tolerance, accumulation and distribution of cadmium in leaves of hyperaccumulator Picris divaricata. Pedosphere 2012, 22, 497–507. [Google Scholar] [CrossRef]
- Qiu, Q.; Wang, Y.; Yang, Z.; Yuan, J. Effects of phosphorus supplied in soil on subcellular distribution and chemical forms of cadmium in two Chinese flowering cabbage (Brassica parachinensis L.) cultivars differing in cadmium accumulation. Food Chem. Toxicol. 2011, 49, 2260–2267. [Google Scholar] [CrossRef] [PubMed]
- Aibibu, N.; Liu, Y.; Zeng, G.; Wang, X.; Chen, B.; Song, H.; Xu, L. Cadmium accumulation in Vetiveria zizanioides and its effects on growth, physiological and biochemical characters. Bioresour. Technol. 2010, 101, 6297–6303. [Google Scholar] [CrossRef] [PubMed]
- Gadallah, M.A.A. Effects of indole-3-acetic acid and zinc on the growth, osmotic potential and soluble carbon and nitrogen components of soybean plants growing under water deficit. J. Arid Environ. 2000, 44, 451–467. [Google Scholar] [CrossRef]
- Reinheckel, T.; Noack, H.; Lorenz, S.; Wiswedel, I.; Augustin, W. Comparison of protein oxidation and aldehyde formation during oxidative stress in isolated mitochondria. Free Radic. Res. Commun. 1998, 29, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Chaoui, A.; Mazhoudi, S.; Ghorbal, M.H.; Ferjani, E.E. Cadmium and zinc induction of lipid peroxidation and effects on antioxidant enzyme activities in bean (Phaseolus vulgaris L.). Plant Sci. 1997, 127, 139–147. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.; Jiang, W.; Liu, D. Cadmium accumulation, activities of antioxidant enzymes, and malondialdehyde (MDA) content in Pistia stratiotes L. Environ. Sci. Pollut. Res. 2013, 20, 1117–1123. [Google Scholar] [CrossRef]
- Ge, W.; Jiao, Y.; Jiang, W.; Liu, D. Ultrastructural and photosynthetic response of Populus 107 leaves to cadmium stress. Pol. J. Environ. Stud. 2015, 24, 519–527. [Google Scholar] [CrossRef]
- Van den Ende, W.; Valluru, R. Sucrose, sucrosyl oligosaccharides, and oxidative stress: Scavenging and salvaging? J. Exp. Bot. 2009, 60, 9–18. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Zhang, Y.; Jiang, L.; Shao, H.B. Roles of plant soluble sugars and their responses to plant cold stress. Afr. J. Biotechnol. 2009, 8, 2004–2010. [Google Scholar] [CrossRef]
- Shao, X.; Zhu, Y.; Cao, S.; Wang, H.; Song, Y. Soluble sugar content and metabolism as related to the heat-induced chilling tolerance of loquat fruit during cold storage. Food Bioprocess Technol. 2012, 6, 3490–3498. [Google Scholar] [CrossRef]
- Couee, I.; Sulmon, C.; Gouesbet, G.; El Amrani, A. Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J. Exp. Bot. 2006, 57, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, V.; Balasubramanian, D. Proline is a protein-compatible hydrotrope. Langmuir 1995, 11, 2830–2833. [Google Scholar] [CrossRef]
- Huang, G.Y.; Wang, Y.S. Physiological and biochemical responses in the leaves of two mangrove plant seedlings (Kandelia candel and Bruguiera gymnorrhiza) exposed to multiple heavy metals. J. Hazard. Mater. 2010, 182, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.K.; Gaur, J.P. Heavy-metal-induced proline accumulation and its role in ameliorating metal toxicity in Chlorella vulgaris. New Phytol. 2010, 143, 253–259. [Google Scholar] [CrossRef]
- Schat, H.; Sharma, S.S.; Vooijs, R. Heavy metal-induced accumulation of free proline in a metal-tolerant and a nontolerant ecotype of Silene vulgaris. Physiol. Plant. 1997, 101, 477–482. [Google Scholar] [CrossRef]
- Chen, C.T.; Chen, L.M.; Lin, C.C.; Kao, C.H. Regulation of proline accumulation in detached rice leaves exposed to excess copper. Plant Sci. 2001, 160, 283–290. [Google Scholar] [CrossRef]
- Sharma, S.S.; Dietz, K.J. The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J. Exp. Bot. 2006, 57, 711–726. [Google Scholar] [CrossRef]
- Slooten, L.; Capiau, K.; Camp, W.V.; Montagu, M.V.; Sybesma, C.D. Factors affecting the enhancement of oxidative stress tolerance in transgenic tobacco overexpressing manganese superoxide dismutase in the chloroplasts. Plant Physiol. 1995, 107, 737–750. [Google Scholar] [CrossRef]
- Dazy, M.; Masfaraud, J.F.; Ferard, J.F. Induction of oxidative stress biomarkers associated with heavy metal stress in Fontinalis antipyretica Hedw. Chemosphere 2009, 75, 297–302. [Google Scholar] [CrossRef]
- Qureshi, M.I.; Abdin, M.Z.; Qadir, S.; Iqbal, M. Lead-induced oxidative stress and metabolic alterations in Cassia angustifolia Vahl. Biol. Plant. 2007, 51, 121–128. [Google Scholar] [CrossRef]
- Mobin, M.; Khan, N.A. Photosynthetic activity, pigment composition and antioxidative response of two mustard (Brassica juncea) cultivars differing in photosynthetic capacity subjected to cadmium stress. J. Plant Physiol. 2007, 164, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, X.; Zeng, G.; Qu, D.; Gu, J.; Zhou, M.; Chai, L. Cadmium-induced oxidative stress and response of the ascorbate-glutathione cycle in Bechmeria nivea (L.) Gaud. Chemosphere 2007, 69, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.C.; Wei, Y.H. Comparative analysis of chlorophyll content on several sun plants and shade Plants. Hubei Agric. Sci. 2009, 48, 1923–1924. [Google Scholar] [CrossRef]
- Zhi, Y.; Zhou, Q.; Leng, X.; Zhao, C. Mechanism of remediation of cadmium-contaminated soil with low-energy plant Snapdragon. Front. Chem. 2020, 8, 222. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Nan, Z.; Wang, H.; Li, X.; Zhou, J.; Yao, X.; Jin, P. Effect of Cd stress on the bioavailability of Cd and other mineral nutrition elements in broad bean grown in a loess subsoil amended with municipal sludge compost. Environ. Sci. Pollut. Res. 2017, 25, 7418–7432. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, Y. Preliminary study on Cd accumulation characteristics in Sansevieria trifasciata Prain. Plant Divers. 2020, 42, 351–355. [Google Scholar] [CrossRef]
- Dou, X.; Dai, H.; Skuza, L.; Wei, S. Bidens pilosa L. hyperaccumulating Cd with different species in soil and the role of EDTA on the hyperaccumulation. Environ. Sci. Pollut. Res. Int. 2019, 26, 25668–25675. [Google Scholar] [CrossRef]
- Toth, G.; Hermann, T.; Da Silva, M.R.; Montanarella, L. Heavy metals in agricultural soils of the European Union with implications for food safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [CrossRef]
- Adagunodo, T.A.; Sunmonu, L.A.; Emetere, M.E. Heavy metals’ data in soils for agricultural activities. Data Brief 2018, 18, 1847–1855. [Google Scholar] [CrossRef]
- Hou, C.; Xu, S. Analysis on the key influence factors of paraffin section’s quality. Chin. Agric. Sci. Bull. 2009, 25, 94–98. [Google Scholar]





| Cd Concentrations (mg/kg) | Root | Stem | Leaf † | ||||
|---|---|---|---|---|---|---|---|
| FW (g) | DW (g) | MC (%) | FW (g) | DW (g) | MC (%) | DW (g) | |
| 5.07 | 28.72 ± 2.84 a | 6.30 ± 0.57 a | 78.02 ± 0.00 a | 13.65 ± 1.50 a | 4.91 ± 0.21 a | 63.00 ± 0.09 a | 5.32 ± 0.57 a |
| 5.71 | 16.51 ± 0.45 b | 3.85 ± 0.14 ab | 76.70 ± 0.01 a | 15.89 ± 3.47 a | 6.01 ± 1.29 a | 62.05 ± 0.02 a | 6.00 ± 0.80 a |
| 8.28 | 13.20 ± 0.56 b | 3.02 ± 0.22 b | 77.03 ± 0.04 a | 11.39 ± 2.04 a | 4.48 ± 0.81 a | 60.45 ± 0.04 a | 4.52 ± 1.00 a |
| 11.49 | 17.56 ± 3.37 b | 3.94 ± 0.81 b | 77.74 ± 0.02 a | 12.22 ± 0.90 a | 4.15 ± 0.27 a | 66.00 ± 0.01 a | 4.28 ± 0.28 a |
| 14.71 | 16.33 ± 2.12 b | 3.53 ± 0.51 b | 78.50 ± 0.01 a | 11.46 ± 1.61 a | 4.37 ± 0.73 a | 62.14 ± 0.03 a | 3.87 ± 0.82 a |
| Cd Concentrations (mg/kg) | Cd Concentration (mg/kg DW) | BCF | TF | ||
|---|---|---|---|---|---|
| Root | Stem | Leaf | |||
| 5.07 | 0.15 ± 0.04 b | 0.60 ± 0.10 bc | 1.20 ± 0.10 c | 0.12 ± 0.01 b | 6.87 ± 1.74 a |
| 5.71 | 0.26 ± 0.10 b | 0.41 ± 0.04 c | 1.43 ± 0.15 bc | 0.14 ± 0.00 b | 4.81 ± 1.88 a |
| 8.28 | 1.58 ± 0.28 ab | 0.68 ± 0.07 b | 1.65 ± 0.14 b | 0.18 ± 0.02 ab | 0.78 ± 0.14 b |
| 11.49 | 1.43 ± 0.04 ab | 1.31 ± 0.04 a | 1.46 ± 0.07 bc | 0.19 ± 0.01 a | 0.97 ± 0.04 b |
| 14.71 | 4.28 ± 1.69 a | 1.43 ± 0.04 a | 2.10 ± 0.04 a | 0.21 ± 0.02 a | 0.56 ± 0.21 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Zhao, Y.; Xu, Z.; Huang, H.; Zhou, J.; Yang, G. Morphological and Physiological Changes of Broussonetia papyrifera Seedlings in Cadmium Contaminated Soil. Plants 2020, 9, 1698. https://doi.org/10.3390/plants9121698
Zhang W, Zhao Y, Xu Z, Huang H, Zhou J, Yang G. Morphological and Physiological Changes of Broussonetia papyrifera Seedlings in Cadmium Contaminated Soil. Plants. 2020; 9(12):1698. https://doi.org/10.3390/plants9121698
Chicago/Turabian StyleZhang, Wan, Yunlin Zhao, Zhenggang Xu, Huimin Huang, Jiakang Zhou, and Guiyan Yang. 2020. "Morphological and Physiological Changes of Broussonetia papyrifera Seedlings in Cadmium Contaminated Soil" Plants 9, no. 12: 1698. https://doi.org/10.3390/plants9121698
APA StyleZhang, W., Zhao, Y., Xu, Z., Huang, H., Zhou, J., & Yang, G. (2020). Morphological and Physiological Changes of Broussonetia papyrifera Seedlings in Cadmium Contaminated Soil. Plants, 9(12), 1698. https://doi.org/10.3390/plants9121698