Plasticity of the Root System Architecture and Leaf Gas Exchange Parameters Are Important for Maintaining Bottle Gourd Responses under Water Deficit
Abstract
:1. Introduction
2. Results
2.1. Differences in Water Consumption of Bottle Gourd Genotypes
2.2. Analysis of Variance and Mean Comparison for Physiological Parameters, Biomass, and Root System Architecture Traits
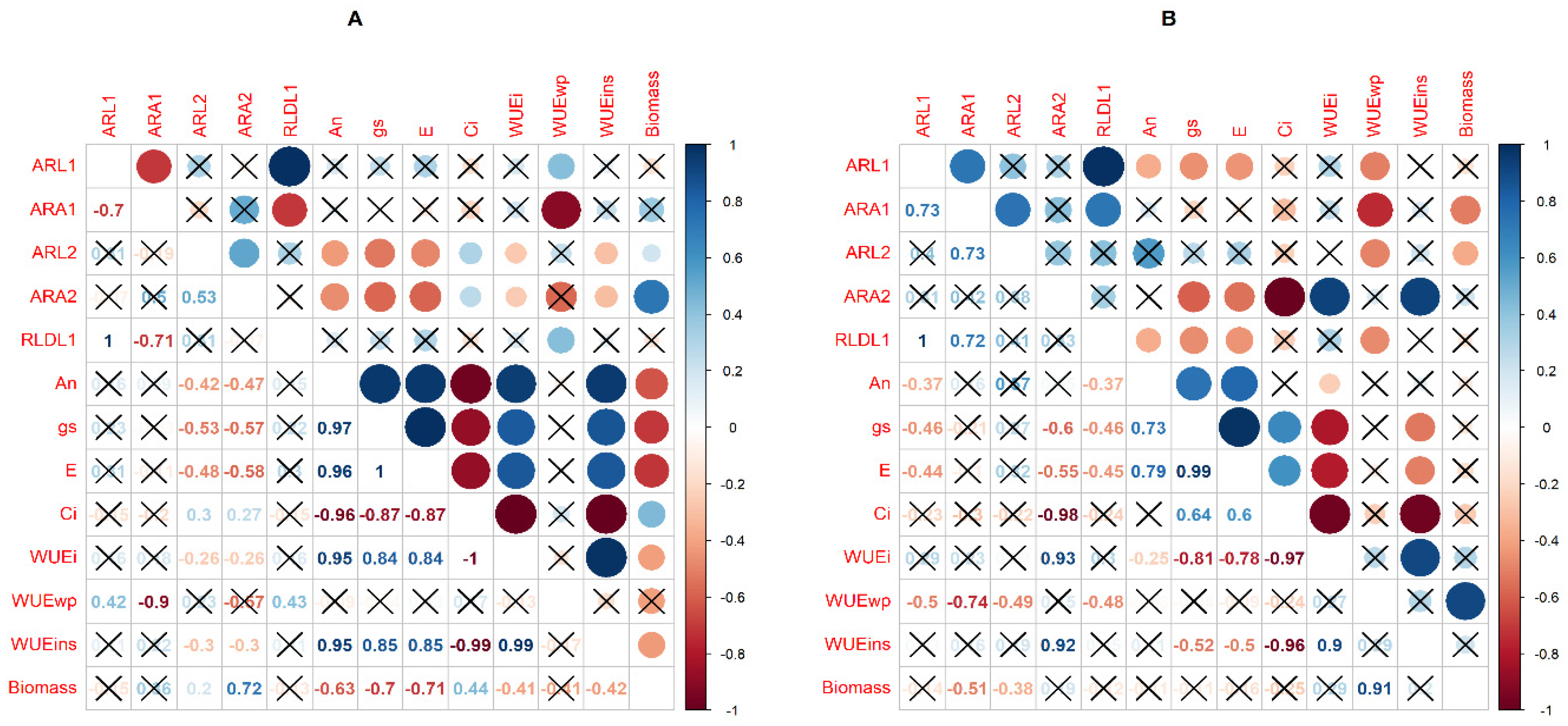
2.3. Correlations between Physiological and Root System Architecture Traits under Well-Watered and Water Deficit Conditions
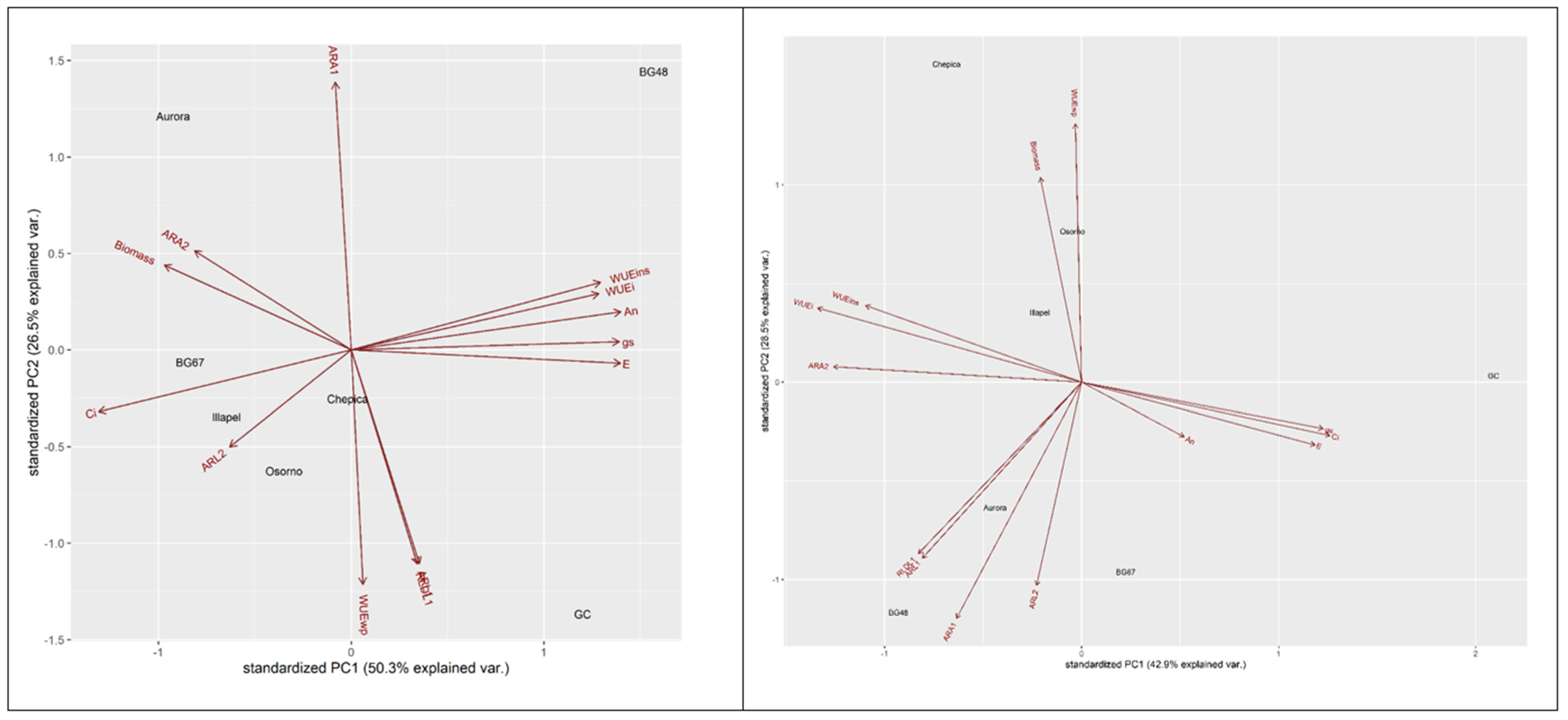
2.4. Principal Component Analysis for the Differentiation of Drought-Tolerant and Sensitive Bottle Gourd Genotypes
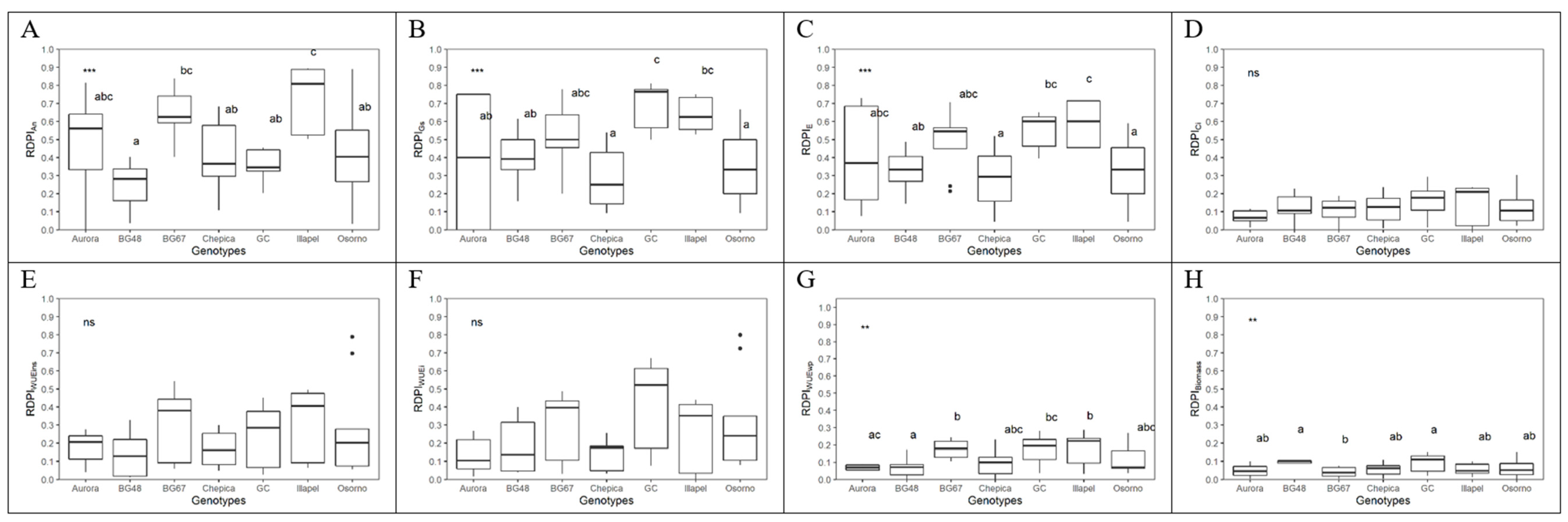
2.5. Morphological and Physiological Plasticity
3. Discussion
4. Material and Methods
4.1. Plant Material
4.2. Experimental Design and Growing Conditions
4.3. Water Deficit Treatment, Fractions of Transpirable Soil Water, and Transpiration Rate
4.4. Physiological Parameters and Biomass Production
4.5. Root Parameters and Image Processing
- -
- RLDL: root length density, based on the length of roots (cm root cm3 soil);
- -
- L: total length of root observed under the rhizobox (cm);
- -
- A: framework area observed in the rhizobox (60 × 40 = 2400 cm2);
- -
- D: depth of the rhizobox (2 cm).
4.6. Morphological and Physiological Plasticity Index
4.7. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. The Impact of Disasters on Agriculture and Food Security; FAO: Rome, Italy, 2015; p. 76. [Google Scholar]
- Dong, C.; MacDonald, G.; Okin, G.S.; Gillespie, T.W. Quantifying drought sensitivity of mediterranean climate vegetation to recent warming: A case study in Southern California. Remote Sens. 2019, 11, 2902. [Google Scholar] [CrossRef] [Green Version]
- Garreaud, R.D.; Boisier, J.P.; Rondanelli, R.; Montecinos, A.; Sepúlveda, H.H.; Veloso-Aguila, D. The Central Chile Mega Drought (2010–2018): A climate dynamics perspective. Int. J. Climatol. 2020, 40, 421–439. [Google Scholar] [CrossRef]
- Aldunce, P.; Araya, D.; Sapiain, R.; Ramos, I.; Lillo, G.; Urquiza, A.; Garreaud, R. Local perception of drought impacts in a changing climate: The mega-drought in central Chile. Sustainability 2017, 9, 2053. [Google Scholar] [CrossRef] [Green Version]
- Roco, L.; Engler, A.; Bravo-Ureta, B.E.; Jara-Rojas, R. Farmers’ perception of climate change in mediterranean Chile. Reg. Environ. Chang. 2015, 15, 867–879. [Google Scholar] [CrossRef]
- Lynch, J.P. Rightsizing root phenotypes for drought resistance. J. Exp. Bot. 2018, 69, 3279–3292. [Google Scholar] [CrossRef] [Green Version]
- Parkash, V.; Singh, S. A review on potential plant-basedwater stress indicators for vegetable crops. Sustainability 2020, 12, 3945. [Google Scholar] [CrossRef]
- Mashilo, J.; Odindo, A.O.; Shimelis, H.A.; Musenge, P.; Tesfay, S.Z.; Magwaza, L.S. Drought tolerance of selected bottle gourd [Lagenaria siceraria (Molina) Standl.] landraces assessed by leaf gas exchange and photosynthetic efficiency. Plant Physiol. Biochem. 2017, 120, 75–87. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Prasad, P.V.V.; Kumari, J.; Rengel, Z. Root length and root lipid composition contribute to drought tolerance of winter and spring wheat. Plant Soil. 2019, 439, 57–73. [Google Scholar] [CrossRef] [Green Version]
- Khasanova, A.; Lovell, J.T.; Bonnette, J.; Weng, X.; Jenkins, J.; Yoshinaga, Y.; Schmutz, J.; Juenger, T.E. The genetic architecture of shoot and root trait divergence between mesic and xeric ecotypes of a perennial grass. Front. Plant Sci. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Ahmadi, J.; Pour-Aboughadareh, A.; Fabriki-Ourang, S.; Mehrabi, A.A.; Siddique, K.H.M. Screening wheat germplasm for seedling root architectural traits under contrasting water regimes: Potential sources of variability for drought adaptation. Arch. Agron. Soil Sci. 2018, 64, 1351–1365. [Google Scholar] [CrossRef]
- Cochavi, A.; Rachmilevitch, S.; Bel, G. The effect of irrigation regimes on plum (Prunus cerasifera) root system development dynamics. Plant Biosyst. 2019, 153, 529–537. [Google Scholar] [CrossRef]
- Price, A.H.; Steele, K.A.; Gorham, J.; Bridges, J.M.; Moore, B.J.; Evans, J.L.; Richardson, P.; Jones, R.G.W. Upland rice grown in soil-filled chambers and exposed to contrasting water-deficit regimes. I. Root distribution, water use and plant water status. Field Crop Res. 2002, 76, 11–24. [Google Scholar] [CrossRef]
- Polania, J.; Rao, I.M.; Cajiao, C.; Grajales, M.; Rivera, M.; Velasquez, F.; Raatz, B.; Beebe, S.E. Shoot and root traits contribute to drought resistance in recombinant inbred lines of MD 23–24 × SEA 5 of common bean. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Espinoza, L.H.; Barrios-Masias, F.H. Physiological and anatomical changes in tomato roots in response to low water stress. Sci. Hortic. 2020, 265, 109208. [Google Scholar] [CrossRef]
- Di Iorio, A.; Montagnoli, A.; Scippa, G.S.; Chiatante, D. Fine root growth of Quercus pubescens seedlings after drought stress and fire disturbance. Environ. Exp. Bot. 2011, 74, 272–279. [Google Scholar] [CrossRef]
- Hund, A.; Ruta, N.; Liedgens, M. Rooting depth and water use efficiency of tropical maize inbred lines, differing in drought tolerance. Plant Soil 2009, 318, 311–325. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Lynch, J.P. Reduced crown root number improves water acquisition under water deficit stress in maize (Zea mays L.). J. Exp. Bot. 2016, 67, 4545–4557. [Google Scholar] [CrossRef] [Green Version]
- Sithole, N.; Modi, A.T. Responses of selected bottle gourd [Lagenaria siceraria (Molina Standly)] landraces to water stress. Acta Agric. Scand. Sect. B Soil Plant Sci. 2015, 65, 350–356. [Google Scholar] [CrossRef]
- Abenavoli, M.R.; Leone, M.; Sunseri, F.; Bacchi, M.; Sorgonà, A. Root Phenotyping for Drought Tolerance in Bean Landraces from Calabria (Italy). J. Agron. Crop Sci. 2016, 202, 1–12. [Google Scholar] [CrossRef]
- Valladares, F.; Sanchez-Gomez, D.; Zavala, M.A. Quantitative estimation of phenotypic plasticity: Bridging the gap between the evolutionary concept and its ecological applications. J. Ecol. 2006, 94, 1103–1116. [Google Scholar] [CrossRef]
- Marchiori, P.E.R.; Machado, E.C.; Sales, C.R.G.; Espinoza-Núñez, E.; Magalhães Filho, J.R.; Souza, G.M.; Pires, R.C.M.; Ribeiro, R.V. Physiological plasticity is important for maintaining sugarcane growth under water deficit. Front. Plant Sci. 2017, 8, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valladares, F.; Sánchez-Gómez, D. Ecophysiological traits associated with drought in Mediterranean tree seedlings: Individual responses versus interspecific trends in eleven species. Plant Biol. 2006, 8, 688–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adu, M.O. Causal shoot and root system traits to variability and plasticity in juvenile cassava (Manihot esculenta Crantz) plants in response to reduced soil moisture. Physiol. Mol. Biol. Plants 2020, 26, 1799–1814. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Sorgonà, A.; Lupini, A.; Araniti, F.; Stevanato, P.; Cacco, G.; Abenavoli, R.M. Morpho-physiological responses of sugar beet (Beta vulgaris L.) genotypes to drought stress. Acta Physiol. Plant. 2013, 35, 853–865. [Google Scholar] [CrossRef]
- Mashilo, J.; Shimelis, H.; Odindo, A. Yield-based selection indices for drought tolerance evaluation in selected bottle gourd [Lagenaria siceraria (Molina) Standl.] landraces. Acta Agric. Scand. Sect. B Soil Plant Sci. 2017, 67. [Google Scholar] [CrossRef]
- Adiredjo, A.L.; Navaud, O.; Muños, S.; Langlade, N.B.; Lamaze, T.; Grieu, P. Genetic control of water use efficiency and leaf carbon isotope discrimination in sunflower (Helianthus annuus L.) subjected to two drought scenarios. PLoS ONE. 2014, 9, e101218. [Google Scholar] [CrossRef] [Green Version]
- Casadebaig, P.; Debaeke, P.; Lecoeur, J. Thresholds for leaf expansion and transpiration response to soil water deficit in a range of sunflower genotypes. Eur. J. Agron. 2008, 28, 646–654. [Google Scholar] [CrossRef]
- Chiatante, D.; Tognetti, R.; Scippa, G.S.; Congiu, T.; Baesso, B.; Terzaghi, M.; Montagnoli, A. Interspecific variation in functional traits of oak seedlings (Quercus ilex, Quercus trojana, Quercus virgiliana) grown under artificial drought and fire conditions. J. Plant Res. 2015, 128, 595–611. [Google Scholar] [CrossRef]
- Opazo, I.; Toro, G.; Solis, S.; Salvatierra, A.; Franck, N.; Albornoz, F.; Pimentel, P. Late reduction on transpiration is an important trait for water deficit tolerance in interspecific Prunus rootstock hybrids. Theor. Exp. Plant Physiol. 2019, 31, 493–506. [Google Scholar] [CrossRef]
- Mo, Y.; Yang, R.; Liu, L.; Gu, X.; Yang, X.; Wang, Y.; Zhang, X.; Li, H. Growth, photosynthesis and adaptive responses of wild and domesticated watermelon genotypes to drought stress and subsequent re-watering. Plant Growth Regul. 2016, 79, 229–241. [Google Scholar] [CrossRef]
- Ors, S.; Ekinci, M.; Yildirim, E.; Sahin, U. Changes in gas exchange capacity and selected physiological properties of squash seedlings (Cucurbita pepo L.) under well-watered and drought stress conditions. Arch. Agron. Soil Sci. 2016, 62, 1700–1710. [Google Scholar] [CrossRef]
- Cornic, G. Drought stress inhibits photosynthesis by decreasing stomatal aperture—Not by affecting ATP synthesis. Trends Plant Sci. 2000. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.; Sun, J.; Zhang, Z.; Ren, H.; Sui, X. Rubisco gene expression and photosynthetic characteristics of cucumber seedlings in response to water deficit. Sci. Hortic. 2013, 161, 81–87. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Loreto, F.; Cornic, G.; Sharkey, T.D. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol. 2004, 6, 269–279. [Google Scholar] [CrossRef]
- Singh, S.K.; Raja Reddy, K. Regulation of photosynthesis, fluorescence, stomatal conductance and water-use efficiency of cowpea (Vigna unguiculata [L.] Walp.) under drought. J. Photochem. Photobiol. B Biol. 2011, 105, 40–50. [Google Scholar] [CrossRef]
- De Carvalho, R.C.; Cunha, A.; da Silva, J.M. Photosynthesis by six Portuguese maize cultivars during drought stress and recovery. Acta Physiol. Plant. 2011, 33, 359–374. [Google Scholar] [CrossRef] [Green Version]
- Guan, X.K.; Song, L.; Wang, T.C.; Turner, N.C.; Li, F.M. Effect of Drought on the Gas Exchange, Chlorophyll Fluorescence and Yield of Six Different-Era Spring Wheat Cultivars. J. Agron. Crop Sci. 2015, 201, 253–266. [Google Scholar] [CrossRef]
- El-Sharkawy, M.A.; Cock, J.H. Response of cassava to water stress. Plant Soil 1987, 100, 345–360. [Google Scholar] [CrossRef]
- York, L.M.; Nord, E.A.; Lynch, J.P. Integration of root phenes for soil resource acquisition. Front. Plant Sci. 2013, 4. [Google Scholar] [CrossRef] [Green Version]
- Ritchie, J. Soil water availability. Plant Soil 1981, 58, 327–338. [Google Scholar] [CrossRef]
- Medrano, H.; Tomás, M.; Martorell, S.; Flexas, J.; Hernández, E.; Rosselló, J.; Pou, A.; Escalona, J.-M.; Bota, J. From leaf to whole-plant water use efficiency (WUE) in complex canopies: Limitations of leaf WUE as a selection target. Crop J. 2015, 3, 220–228. [Google Scholar] [CrossRef] [Green Version]
- Zobel, R.W.; Waisel, Y. A plant root system architectural taxonomy: A framework for root nomenclature. Plant Biosyst. 2010, 144, 507–512. [Google Scholar] [CrossRef]
- Johnson, M.G.; Tingey, D.T.; Phillips, D.L.; Storm, M.J. Advancing fine root research with minirhizotrons. Environ. Exp. Bot. 2001, 45, 263–289. [Google Scholar] [CrossRef]
- Shah, T.M.; Imran, M.; Atta, B.M.; Ashraf, M.Y.; Hameed, A.; Waqar, I.; Shafiq, M.; Hussain, H.; Naveed, M.A.; Maqbool, M.A. Selection and screening of drought tolerant high yielding chickpea genotypes based on physio-biochemical indices and multi-environmental yield trials. BMC Plant Biol. 2020, 20, 1–16. [Google Scholar] [CrossRef] [PubMed]
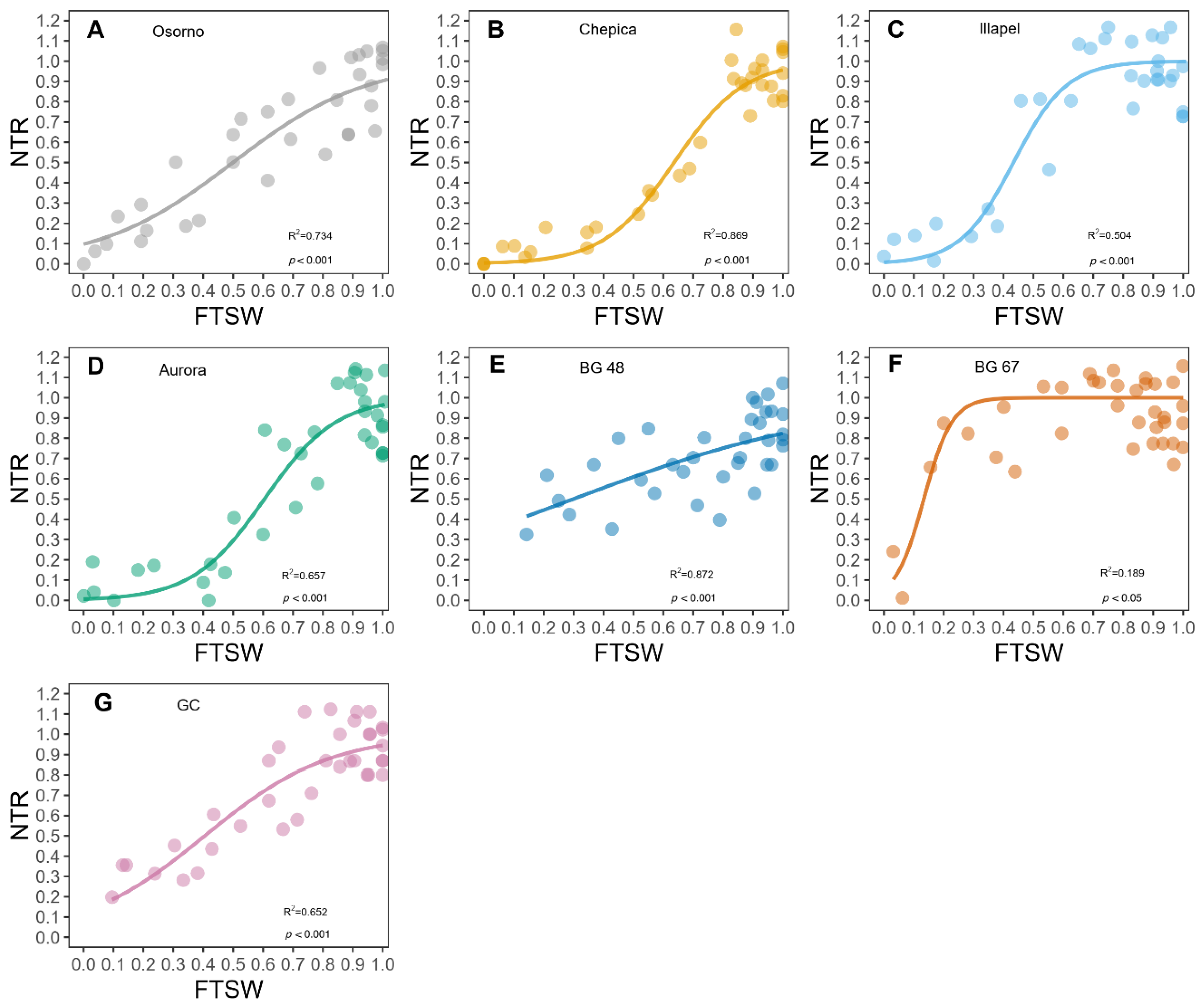


| Genotype | FTSWt | S.E | * |
|---|---|---|---|
| BG-48 | 0.824 | 0.08 | a |
| Chepica | 0.800 | 0.02 | b |
| Osorno | 0.777 | 0.10 | c |
| Aurora | 0.760 | 0.04 | c |
| GC | 0.696 | 0.10 | d |
| Illapel | 0.474 | 0.02 | e |
| BG-67 | 0.368 | 0.03 | f |
| Source of Variation | Significance (Physiological Traits) | |||||||
|---|---|---|---|---|---|---|---|---|
| An | gs | E | Ci | WUEi | WUEins | WUEwp | Biomass | |
| Genotype (G) | ** | ** | ** | ns | ns | ns | ns | ns |
| Water regime (W) | ** | ** | ** | ns | ns | ns | ** | ** |
| G*W | ns | ** | ** | ns | ** | ns | ns | ns |
| CV (%) | 35.8 | 60.7 | 40.5 | 18.0 | 34.1 | 30.7 | 15.4 | 7.5 |
| Significance (root system architecture traits) | ||||||||
| RLDL | ARL | ARA | RLDL1 | ARL1 | ARA1 | ARL2 | ARA2 | |
| Genotype (G) | ** | ** | ** | ns | ns | ** | ns | ns |
| Water regime (W) | ns | ns | ns | ns | ns | ** | ns | ns |
| G*W | ns | ns | ** | ** | ** | ns | ** | ** |
| CV (%) | 16.2 | 16.2 | 5.3 | 14.9 | 14.9 | 7.2 | 19.8 | 7.5 |
| Genotype | ARL1 (cm) | ARL2 (cm) | RLDL1 (cm/cm3) | ARA2 (°) |
|---|---|---|---|---|
| Osorno | −27.9 ns | 0.07 ** | −0.01 ns | 1.91 ns |
| Chepica | 86.3 ns | 2.5 ns | 0.02 ns | 3.04 ns |
| Illapel | 2.85 ns | −0.27 ns | 0 ns | −0.43 ns |
| Aurora | −62.5 ns | −4.83 ns | −0.01 ns | 5.86 * |
| BG-48 | 44.1 ** | −7.92 *** | 0.01 * | 1.11 * |
| BG-67 | 40.4 ns | −3.84 ns | 0.01 ns | −1.06 ns |
| GC | −47.3 ** | 7.7 ns | −0.01 * | −0.01 ns |
| Traits | Water Deficit (Eigenvectors) | Well-Watered (Eigenvectors) | ||||
|---|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC1 | PC2 | PC3 | |
| RLDL1 | 0.09 | −0.42 | 0.38 | −0.25 | −0.32 | −0.21 |
| ARL1 | 0.09 | −0.42 | 0.38 | −0.24 | −0.33 | −0.22 |
| ARA1 | −0.02 | 0.53 | 0.12 | −0.19 | −0.44 | 0.09 |
| ARL2 | −0.17 | −0.19 | 0.40 | −0.07 | −0.37 | 0.37 |
| ARA2 | −0.22 | 0.19 | 0.52 | −0.38 | 0.03 | 0.28 |
| An | 0.39 | 0.08 | 0.05 | 0.16 | −0.10 | 0.56 |
| gs | 0.38 | 0.02 | −0.04 | 0.37 | −0.08 | 0.27 |
| E | 0.39 | −0.03 | −0.01 | 0.35 | −0.12 | 0.29 |
| Ci | −0.36 | −0.12 | −0.19 | 0.37 | −0.09 | −0.26 |
| WUEi | 0.35 | 0.11 | 0.20 | −0.40 | 0.14 | 0.11 |
| WUEwp | 0.01 | −0.46 | −0.24 | −0.01 | 0.48 | 0.11 |
| WUEins | 0.36 | 0.13 | 0.17 | −0.33 | 0.14 | 0.33 |
| Biomass | −0.27 | 0.17 | 0.31 | −0.06 | 0.38 | 0.03 |
| Eigenvalues | 2.56 | 1.86 | 1.35 | 2.36 | 1.92 | 1.57 |
| Proportion of total variance (%) | 0.50 | 0.26 | 0.14 | 0.43 | 0.29 | 0.19 |
| Cumulative variance (%) | 0.50 | 0.76 | 0.91 | 0.43 | 0.72 | 0.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zacarias Rafael, D.; Arriagada, O.; Toro, G.; Mashilo, J.; Mora-Poblete, F.; Contreras-Soto, R.I. Plasticity of the Root System Architecture and Leaf Gas Exchange Parameters Are Important for Maintaining Bottle Gourd Responses under Water Deficit. Plants 2020, 9, 1697. https://doi.org/10.3390/plants9121697
Zacarias Rafael D, Arriagada O, Toro G, Mashilo J, Mora-Poblete F, Contreras-Soto RI. Plasticity of the Root System Architecture and Leaf Gas Exchange Parameters Are Important for Maintaining Bottle Gourd Responses under Water Deficit. Plants. 2020; 9(12):1697. https://doi.org/10.3390/plants9121697
Chicago/Turabian StyleZacarias Rafael, Dinoclaudio, Osvin Arriagada, Guillermo Toro, Jacob Mashilo, Freddy Mora-Poblete, and Rodrigo Iván Contreras-Soto. 2020. "Plasticity of the Root System Architecture and Leaf Gas Exchange Parameters Are Important for Maintaining Bottle Gourd Responses under Water Deficit" Plants 9, no. 12: 1697. https://doi.org/10.3390/plants9121697
APA StyleZacarias Rafael, D., Arriagada, O., Toro, G., Mashilo, J., Mora-Poblete, F., & Contreras-Soto, R. I. (2020). Plasticity of the Root System Architecture and Leaf Gas Exchange Parameters Are Important for Maintaining Bottle Gourd Responses under Water Deficit. Plants, 9(12), 1697. https://doi.org/10.3390/plants9121697








