The Epidemiology of Plant Virus Disease: Towards a New Synthesis
Abstract
:1. Introduction
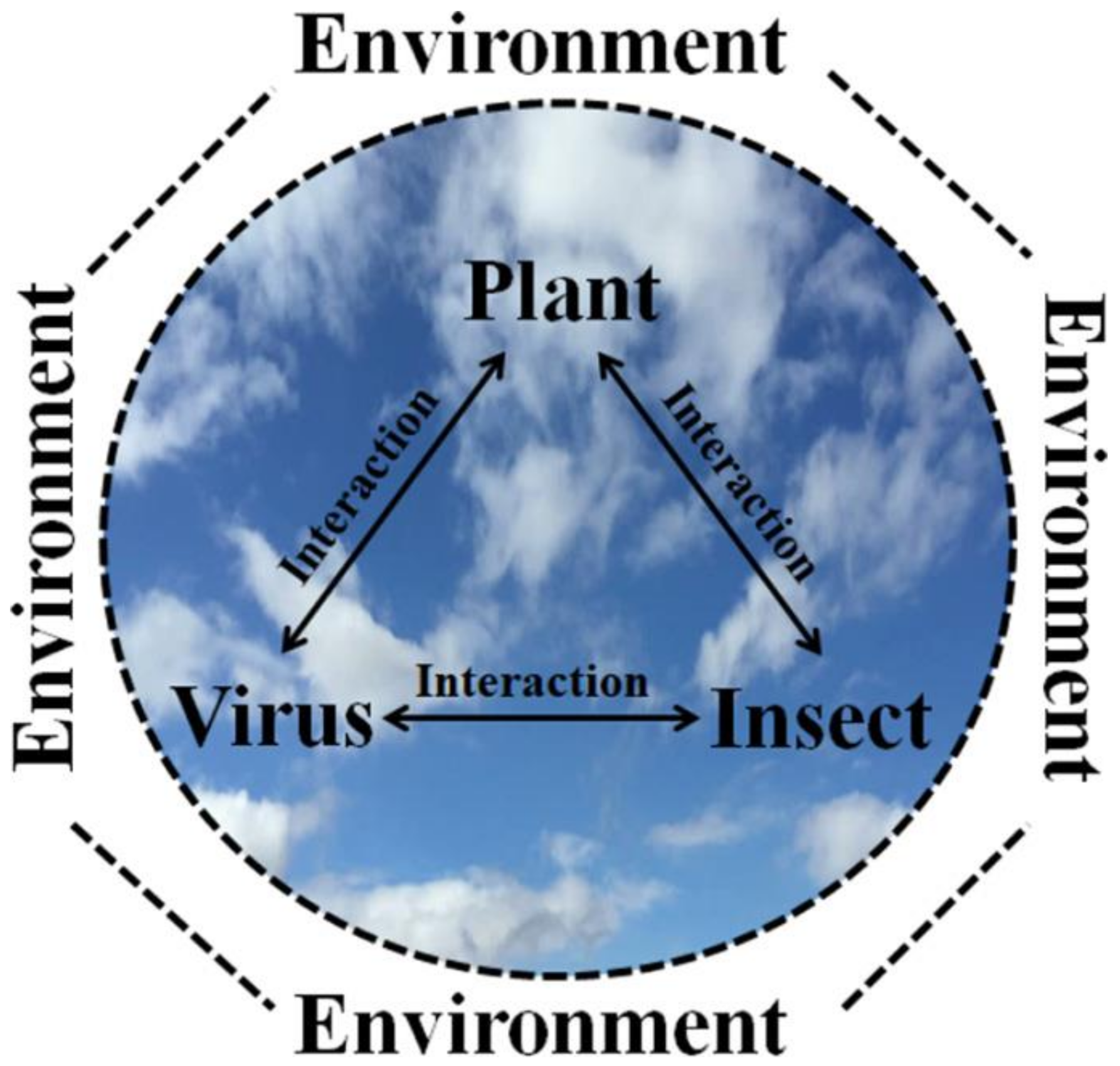
2. Plant Virus Epidemiology, Ecology and Evolution
2.1. Epidemiology and Evolution
2.2. Epidemiology and Ecology
2.3. Further Synthesis
3. Epidemiology and Disease Control
3.1. Epidemiological Analysis
3.2. The Basic Reproduction Number
3.3. Spatial Aspects of Epidemics
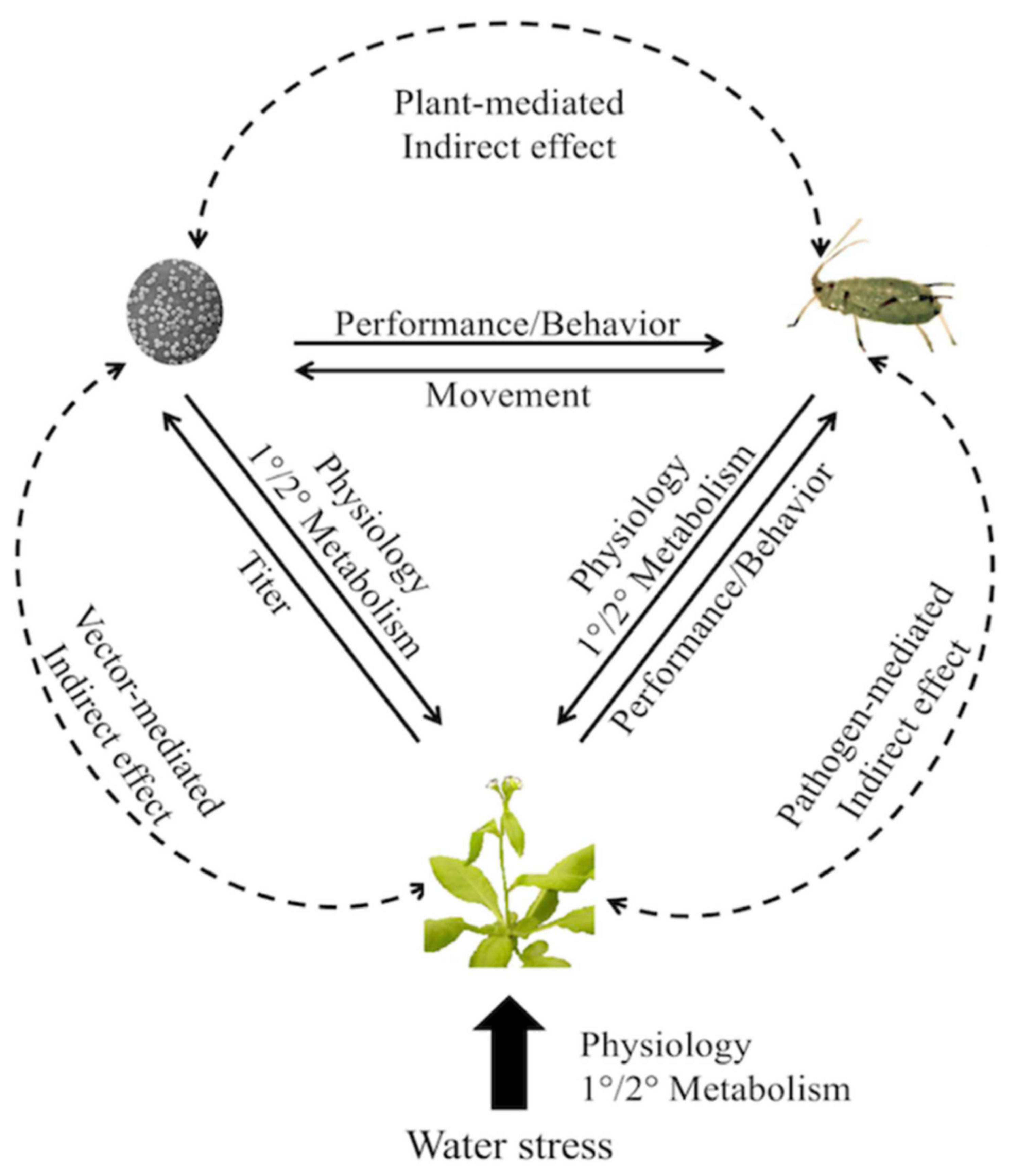
3.4. Environmental Drivers
3.5. Production Systems and Cycles
3.6. Phytosanitation and Rogueing
3.7. Host Resistance Deployment
3.8. Crop Heterogeneity
3.9. Combinations of Disease Control Measures
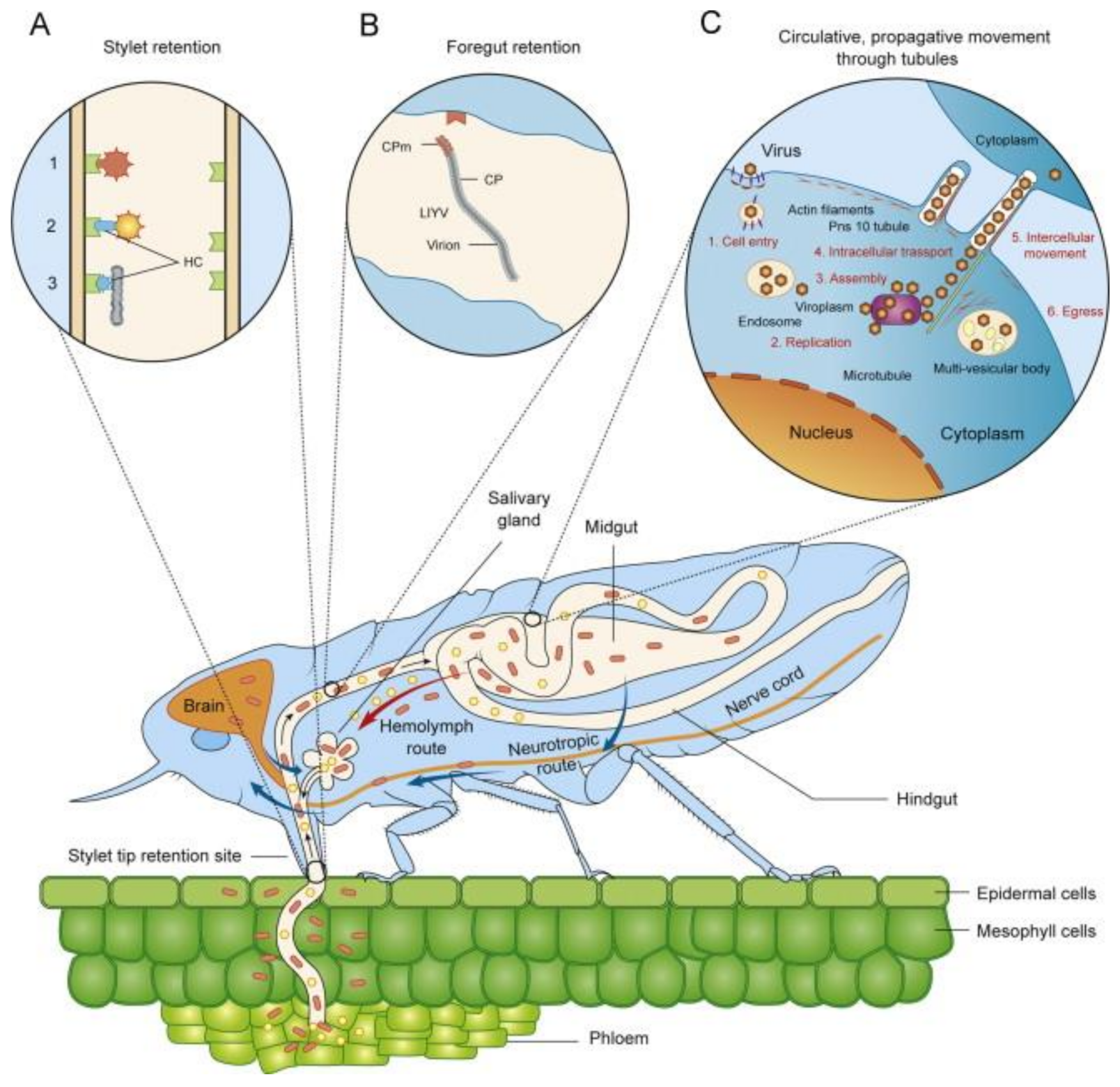
4. Transmission
- (i)
- horizontal transmission by arthropods;
- (ii)
- vertical transmission;
- (iii)
- interactions between horizontal and vertical transmission;
- (iv)
- transmissibility, virus accumulation and virulence
- (v)
- the effects of vector population dynamics, behaviour and feeding, and how these are affected by natural enemies;
- (vi)
- the conditional vector preferences for infected or healthy plants;
- (vii)
- the common occurrence of coinfection of plants by multiple virus species, strains or genomic segments.
4.1. Horizontal Transmission by Arthropod Vectors
4.2. Vertical Transmission
4.3. Interactions between Horizontal and Vertical Transmission
4.4. Transmissibility, Virus Accumulation and Virulence
5. Vector Behaviour
5.1. Population Dynamics and Dispersal
5.2. Feeding Preference and Behaviour
5.3. Tripartite and Tritrophic Interactions
6. Vector Preference
- (i)
- A vector might show a preference for a given plant species or genotype, even though many related species/genotypes may harbour populations of the vector. This then becomes an important consideration when this range of plant species/genotypes can be shown to be susceptible (at least in artificial inoculation studies) to the virus being vectored.
- (ii)
- Preference can also be shown for infected or healthy phenotypes of a given plant species through visual or olfactory cues.
- (iii)
- In an elaboration of this, vectors may switch preference from infected phenotypes when nonviruliferous, to healthy phenotypes when viruliferous. This form of vector preference has been termed conditional preference and is the subject of much current research based on the premise that it represents virus manipulation of the plant and vector.
6.1. Vector-Host Range Preferences
6.2. Host Phenotype Preference and Vector Performance
6.3. Conditional Vector Preference and Virus Manipulation
6.4. Other Recent Work
6.5. Future Opportunities
7. Co-Infection
7.1. Methodological Issues
7.2. One Vector Species
7.3. Two Vector Species
7.4. Many Vector Species
7.5. Co-Infection and Vector Preference
7.6. Co-Infection and Segmented Viruses
7.7. Future Opportunities
8. Concluding Comments
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Basic Reproductive Number with Conditional Vector Preference
References
- Jeger, M.J.; Stevenson, K.L.; Madden, L.V. Plant Disease Epidemiology; Oxford Bibliographies online. Available online: https://www.oxfordbibliographies.com/view/document/obo-9780199830060/obo-9780199830060-0166.xml (accessed on 11 January 2017).
- Jeger, M.J. Theory and plant epidemiology. Plant Pathol. 2000, 49, 651–658. [Google Scholar] [CrossRef]
- Dammann, O.; Gray, P.; Gressens, P.; Wolkenhauer, O.; Leviton, A. Systems epidemiology: What’s in a Name? Online J. Public Health Inform. 2014, 6, e198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garbelotto, M.; Gonthier, P. Variability and disturbances as key factors in forest pathology and plant health studies. Forests 2017, 8, 441. [Google Scholar] [CrossRef] [Green Version]
- Islam, W.; Noman, A.; Naveed, H.; Alamri, S.A.; Hashem, M.; Huang, Z.; Han, Y.H.; Chen, H.Y. Plant-insect vector-virus interactions under environmental change. Sci. Total Environ. 2020, 701, 135044. [Google Scholar] [CrossRef] [PubMed]
- Guitiérrez, S.; Michalakis, Y.; van Munster, M.; Blanc, S. Plant feeding by insect vectors can affect life cycle, population genetics and evolution of plant viruses. Funct. Ecol. 2013, 27, 610–622. [Google Scholar] [CrossRef] [Green Version]
- Jones, R.A.C.; Salam, M.U.; Maling, T.J.; Diggle, A.J.; Thackray, D.J. Principles of predicting plant virus disease epidemics. Annu. Rev. Phytopathol. 2010, 48, 179–203. [Google Scholar] [CrossRef]
- Thresh, J.M.; Fargette, D.; Jeger, M.J. Epidemiology of tropical plant viruses. In Virus and Virus-Like Diseases of Major Crops in Developing Countries; Loebenstein, G., Thottappilly, G., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; pp. 55–77. [Google Scholar]
- Jeger, M.J. Ecology and Epidemiology. In Handbook of Plant Virology; Khan, J.A., Dijkstra, J., Eds.; The Howarth Press: New York, NY, USA, 2006; pp. 171–183. [Google Scholar]
- Navas-Castillo, J.; Fiallo-Olivé, E. Plant virus diseases: Epidemiology. In eLS; John Wiley & Sons, Ltd.: Chichester, UK, 2017. [Google Scholar] [CrossRef]
- Jones, R.A.C. Trends in plant virus epidemiology: Opportunities from new or improved technology. Virus Res. 2014, 186, 3–19. [Google Scholar] [CrossRef]
- Jeger, M.J.; Madden, L.V.; van den Bosch, F. Plant virus epidemiology: Applications and prospects for mathematical modelling and analysis to improve understanding and disease control. Plant Dis. 2018, 102, 837–854. [Google Scholar] [CrossRef] [Green Version]
- Jeger, M.J.; Holt, J.; van den Bosch, F.; Madden, L.V. Epidemiology of insect-transmitted viruses: Modelling disease dynamics and control interventions. Physiol. Entomol. 2004, 29, 291–304. [Google Scholar] [CrossRef]
- Milgroom, M.G.; Peever, T. Population biology of plant pathogens: The synthesis of plant disease epidemiology and population genetics. Plant Dis. 2003, 86, 608–617. [Google Scholar] [CrossRef] [Green Version]
- Jeger, M.J.; Seal, S.E.; van den Bosch, F. Evolutionary epidemiology of plant virus disease. Adv. Plant Virus Res. 2006, 67, 164–203. [Google Scholar]
- Elena, S.F.; Fraile, A.; García-Arenal, F. Evolution and emergence of plant viruses. Adv. Virus Res. 2014, 88, 161–191. [Google Scholar] [PubMed] [Green Version]
- Roossinck, M.J. Mechanisms of plant virus evolution. Annu. Rev. Phytopathol. 1997, 35, 191–209. [Google Scholar] [CrossRef] [PubMed]
- Van der Kooi, J.; Ollerton, J. The origin of flowering plants and pollinators. Science 2020, 368, 1306–1308. [Google Scholar] [CrossRef]
- Peccoud, J.; Simon, J.-C.; von Dolen, C.; Coeur d’acier, A.; Plantegenest, M.; Vanlerbergue-Masutti, F.; Jousseline, E. Evolutionary history of aphid-plant associations and their role in aphid diversification. Comptes R. Biol. 2010, 333, 474–487. [Google Scholar] [CrossRef]
- Meyer, R.S.; DuVal, A.E.; Jensen, H.R. Patterns and processes in crop domestication: An historical review and quantitative analysis of 203 global food crops. New Phytol. 2012, 196, 29–48. [Google Scholar] [CrossRef]
- Peyambari, M.; Warner, S.; Stoler, N.; Rainer, D.; Roossinck, M.J. A 1000-year-old RNA virus. J. Virol. 2019, 93, e01188-18. [Google Scholar]
- Roossinck, M.J. Evolutionary history of Cucumber Mosaic Virus deduced by phylogenetic analyses. J. Virol. 2002, 76, 3382–3387. [Google Scholar] [CrossRef] [Green Version]
- Santillan, F.W.; Fribourg, C.E.; Adams, I.P.; Gibbs, A.J.; Boonham, N.; Kehou, M.A.; Maina, S.; Jones, R.A.C. The biology and phylogenetics of Potato virus S from the Andean Region of South America. Plant Dis. 2018, 102, 869–885. [Google Scholar] [CrossRef] [Green Version]
- Duan, G.; Zhan, F.; Du, Z.; Ho, S.Y.W.; Gao, F. Europe was a hub for the global spread of Potato virus S in the 19th century. Virology 2018, 525, 200–204. [Google Scholar] [CrossRef]
- Fuentes, S.; Jones, R.A.C.; Matsuoka, H.; Ohshima, K.; Kreuze, J.; Gibbs, A.J. Potato virus Y; the Andean connection. Virus Evol. 2019, 5, vez037. [Google Scholar] [CrossRef] [PubMed]
- Roossinck, M.J. A new look at plant viruses and their potential beneficial roles in crops. Mol. Plant Pathol. 2015, 16, 331–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roossinck, M.J. Plants, viruses and the environment: Ecology and mutualism. Virology 2015, 479–480, 271–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, H.; Fukuhara, T.; Kitazawa, H.; Kormelink, R. Virus latency and the impact on plants. Front. Microbiol. 2019, 10, 2764. [Google Scholar] [CrossRef]
- Montes, N.; Alonso-Blanco, C.; García-Arenal, F. Cucumber mosaic virus infection as a potential selective pressure on Arabidopsis thaliana populations. PLoS Pathog. 2019, 15, e1007810. [Google Scholar] [CrossRef] [Green Version]
- Hamelin, F.M.; Hilker, F.M.; Sun, T.A.; Jeger, M.J.; Hajimorad, R.; Allen, L.J.S.; Prendeville, H.R. The evolution of parasitic and mutualistic plant-virus symbioses through transmission-virulence trade-offs. Virus Res. 2017, 241, 77–87. [Google Scholar] [CrossRef]
- Lefeuvre, P.; Martin, D.P.; Elena, S.F.; Shepherd, D.N.; Roumagnac, P.; Varsani, A. Evolution and ecology of plant viruses. Nat. Rev. Microbiol. 2019, 17, 632–644. [Google Scholar] [CrossRef]
- Fabre, F.; Bruchou, C.; Palloix, A.; Moury, B. Key determinants of resistance durability to plant viruses: Insights from a model linking within- and between host dynamics. Virus Res. 2009, 141, 140–149. [Google Scholar] [CrossRef]
- Malmstrom, C.M.; Melcher, U.; Bosque-Pérez, N.A. The expanding field of plant virus ecology: Historical foundations, knowledge gaps, and research directions. Virus Res. 2011, 159, 84–94. [Google Scholar] [CrossRef]
- Plantegenest, M.; Le May, C.; Fabre, F. Landscape epidemiology of plant diseases. J. R. Soc. Interface 2007, 4, 963–972. [Google Scholar] [CrossRef] [Green Version]
- Chisholm, P.J.; Busch, J.W.; Crowder, D.W. Effects of life history and ecology on virus evolutionary potential. Virus Res. 2019, 265, 1–9. [Google Scholar] [CrossRef] [PubMed]
- McLeish, M.J.; Fraile, A.; García-Arenal, F. Evolution of plant–virus interactions: Host range and virus emergence. Curr. Opin. Virol. 2019, 34, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Pagán, I. The diversity, evolution and epidemiology of plant viruses: A phylogenetic view. Infect. Gen. Evol. 2018, 65, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Gols, R.; Benrey, B. Crop domestication and its impact on naturally selected trophic interactions. Annu. Rev. Entomol. 2015, 60, 35–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacDiarmid, R.; Rodoni, B.; Melcher, U.; Ochoa-Corona, F.; Roossinck, M. Biosecurity implications of new technology and discovery in plant virus research. PLoS Pathog. 2013, 9, e1003337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, H.M.; Mauck, K.E.; Whitfield, A.E.; Garrett, K.A.; Malmstrom, C.M. Plant-virus interactions and the agro-ecological interface. Eur. J. Plant Pathol. 2014, 138, 529–547. [Google Scholar] [CrossRef]
- Burdon, J.J.; Thrall, P.H. What have we learned from studies of wild plant-pathogen associations?—The dynamic interplay of time, space and life-history. Eur. J. Plant Pathol. 2014, 138, 417–429. [Google Scholar] [CrossRef]
- Tack, A.J.M.; Laine, A.-L. Spatial eco-evolutionary feedback in plant-pathogen interactions. Eur. J. Plant Pathol. 2014, 138, 667–677. [Google Scholar] [CrossRef]
- Thompson, R.N.; Brooks-Pollock, E. Detection, forecasting and control of infectious disease epidemics: Modelling outbreaks in humans, animals and plants. Philos. Trans. R. Soc. B 2019, 374, 20190038. [Google Scholar] [CrossRef]
- Jeger, M.J. Analysis of disease progress as a basis for evaluation of disease management practices. Annu. Rev. Phytopathol. 2004, 42, 61–82. [Google Scholar] [CrossRef]
- Cunniffe, N.J.; Laranjeira, F.F.; Neri, F.M.; DeSimone, R.E.; Gilligan, C.A. Cost-effective control of plant disease when epidemiological knowledge is incomplete: Modelling Bahia bark scaling of citrus. PLoS Comp. Biol. 2014, 10, e1003753. [Google Scholar] [CrossRef] [PubMed]
- Purcell, A. Paradigms: Examples from the bacterium Xylella fastidiosa. Annu. Rev. Phytopathol. 2013, 51, 339–356. [Google Scholar] [CrossRef] [PubMed]
- Gottwald, T.R.; Wierenga, E.; Luo, W.; Parnell, S. Epidemiology of Plum pox ‘D’ strain in Canada and the USA. Can. J. Plant Pathol. 2013, 35, 442–457. [Google Scholar] [CrossRef]
- Dallot, S.; Gottwald, T.; Labonne, G.; Quiot, J.-B. Factors affecting the spread of Plum pox virus strain M in peach orchards subjected to roguing in France. Phytopathology 2004, 94, 1390–1398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osada, Y.; Yamakita, T.; Shoda-Kagaya, E.; Liebhold, A.M.; Yamanaka, T. Disentangling the drivers of invasion spread in a vector-borne tree disease. J. Anim. Ecol. 2018, 8, 1512–1524. [Google Scholar] [CrossRef] [PubMed]
- Varghese, A.; Drovandi, C.; Mira, A.; Mengersen, K. Estimating a novel stochastic model for within-field disease dynamics of banana bunchy top virus via approximate Bayesian computation. PLoS Comput. Biol. 2020, 16, e1007878. [Google Scholar] [CrossRef]
- Van den Driessche, P. Reproduction numbers of infectious disease models. Inf. Dis. Model. 2017, 2, 288–303. [Google Scholar] [CrossRef]
- Roberts, M.G.; Heesterbeek, J.A.P. Model-consistent estimation of the basic reproduction number from the incidence of an emerging infection. J. Math. Biol. 2007, 55, 803–816. [Google Scholar] [CrossRef] [Green Version]
- Segarra, J.; Jeger, M.J.; van den Bosch, F. Epidemic dynamics and patterns of plant diseases. Phytopathology 2001, 91, 1001–1010. [Google Scholar] [CrossRef] [Green Version]
- Van den Bosch, F.; McRoberts, N.; van den Berg, F.; Madden, L.V. The basic reproduction number of plant pathogens: Matrix approaches to complex dynamics. Phytopathology 2008, 98, 239–249. [Google Scholar] [CrossRef] [Green Version]
- Van den Bosch, F.; Jeger, M.J. The basic reproduction number of vector-borne plant virus epidemics. Virus Res. 2017, 241, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Wesley, C.L.; Allen, L.J.S. The basic reproduction number in epidemic models with periodic demographics. J. Biol. Dynam. 2009, 3, 116–129. [Google Scholar] [CrossRef]
- Cunniffe, N.J.; Gilligan, C.A. Invasion, persistence and control in epidemic models for plant pathogens: The effect of host demography. J. R. Soc. Interface 2010, 7, 439–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, R.; Zhao, H.; Tang, S. Global dynamic analysis of a vector-borne plant disease model. Adv. Diff. Equ. 2014, 59, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Yang, J. Global stability of an SEI model for plant diseases. Math. Slovaca 2016, 66, 305–311. [Google Scholar] [CrossRef]
- Jeger, M.J.; van den Bosch, F.; Madden, L.V. Modelling virus- and host-limitation in vectored plant disease epidemics. Virus Res. 2011, 159, 215–222. [Google Scholar] [CrossRef]
- Bremermann, H.J.; Thieme, H.R. A competitive exclusion principle for pathogen virulence. J. Math. Biol. 1989, 27, 179–190. [Google Scholar] [CrossRef]
- Mylius, S.D.; Diekmann, O. On evolutionarily stable life histories, optimization and the need to be specific about density dependence. OIKOS 1995, 74, 218–224. [Google Scholar] [CrossRef] [Green Version]
- Metz, J.A.J.; Mylius, S.D.; Diekmann, O. When does evolution optimize? Evol. Ecol. Res. 2008, 10, 629–654. [Google Scholar]
- Brauer, F.; Castillo-Chavez, C.; Mubayi, A.; Towers, S. Some models for epidemics of vector-transmitted diseases. Infect. Dis. Model. 2016, 1, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Brauer, F. A final size relation for epidemic models of vector transmitted diseases. Infect. Dis. Model. 2017, 2, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.A.; Seal, D.R.; Zhang, S.; Liburd, O.E.; Srinivasan, R.; Evans, E. Distribution pattern of thrips (Thysanoptera: Thripidae) and tomato chlorotic spot virus in south Florida tomato fields. Environ. Entomol. 2020, 49, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Cieniewicz, E.; Flasco, M.; Brunelli, M.; Onmuwelo, M.; Wise, A.; Fuchs, M.F. Differential spread of grapevine red blotch virus in California and New York vineyards. Phytobiomes J. 2019, 3, 203–211. [Google Scholar] [CrossRef] [Green Version]
- Stilwell, A.R.; Rundquist, D.C.; Marx, D.B.; Hein, G.L. Differential gradients of wheat streak mosaic virus into winter wheat from a central mite-virus source. Plant Dis. 2019, 103, 338–344. [Google Scholar] [CrossRef] [Green Version]
- Legg, J.P.; Ogwal, S. Changes in the incidence of African cassava mosaic virus disease and the abundance of its whitefly vector along south-north transects in Uganda. J. Appl. Ent. 1998, 122, 169–178. [Google Scholar] [CrossRef]
- Picard, C.; Soubeyrand, S.; Jacquot, E.; Thébaud, G. Analyzing the influence of landscape aggregation on disease spread to improve management strategies. Phytopathology 2019, 109, 1198–1207. [Google Scholar] [CrossRef]
- Moore, S.M.; Manore, C.A.; Bokil, V.A.; Borer, E.T.; Hosseini, P.R. Spatiotemporal model of barley and cereal yellow dwarf virus transmission dynamics with seasonality and plant competition. Bull. Math. Biol. 2011, 73, 2707–2730. [Google Scholar] [CrossRef] [Green Version]
- Jablońska-Sabuka, M.; Kalaria, R.; Kauranne, T. A dynamical model for epidemic outbursts by begomovirus population clusters. Ecol. Model. 2015, 297, 60–68. [Google Scholar] [CrossRef]
- Kho, J.-W.; Kim, K.H.; Lee, D.-H. Comparing the plant virus spread patterns of non-persistently transmitted virus and persistently transmitted virus using an individual-based model. J. Asia-Pac. Entomol. 2020, 23, 371–379. [Google Scholar] [CrossRef]
- Jeger, M.J.; Ollenu, L.A.; Hagen, L.S.; Jacquemond, M. Cocoa. In Virus and Virus-Like Diseases of Major Crops in Developing Countries; Loebenstein, G., Thottappilly, G., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; pp. 519–542. [Google Scholar]
- Trebicki, P. Climate change and plant virus epidemiology. Virus Res. 2020, 286, 198059. [Google Scholar] [CrossRef]
- Juroszek, P.; Racca, P.; Link, S.; Farhumand, J.; Kleinhenz, B. Overview on the review articles published during the past 30 years relating to the potential climate change effects on plant pathogens and crop disease risks. Plant Pathol. 2020, 69, 179–193. [Google Scholar] [CrossRef]
- Szczepaniec, A.; Finke, D. Plant-vector-pathogen interactions in the context of drought stress. Front. Ecol. Evol. 2019, 7, 262. [Google Scholar] [CrossRef] [Green Version]
- Van Munster, M. Impact of abiotic stresses on plant virus transmission by aphids. Viruses 2020, 12, 216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, T.S.; Bosque-Pérez, N.A.; Foote, N.E.; Magney, T.; Eigenbrode, S.D. Environmentally dependent host-pathogen and vector-pathogen interactions in the Barley yellow dwarf virus pathosystem. J. Appl. Ecol. 2015, 52, 1392–1401. [Google Scholar] [CrossRef]
- González, R.; Butković, A.; Escaray, F.J.; Martínez-Latorre, J.; Melero, I.; Pérez-Parets, E.; Gómez-Cadenas, A.; Carrasco, P.; Elena, S.F. Plant Virus Evolution under Strong Drought Conditions Results in a Transition from Parasitism to Mutualism. Available online: https://assets.researchsquare.com/files/rs-78584/v1/Nat.Microbiol.v1.SFE200916.pdf (accessed on 24 November 2020).
- Montes, N.; Pagán, I. Light intensity modulates the efficiency of virus seed transmission through modifications of plant tolerance. Plants 2019, 8, 304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chappell, T.M.; Codod, C.B.; Williams, B.W.; Kemerait, R.C.; Culbreath, A.K.; Kennedy, G.G. Adding epidemiologically important meteorological data to Peanut Rx the risk assessment framework for spotted wilt of peanut. Phytopathology 2020, 110, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Chappell, T.M.; Ward, R.V.; DePolt, K.T.; Roberts, P.M.; Greene, J.K.; Kennedy, G.G. Cotton thrips infestation predictor: A practical tool for predicting tobacco thrips (Frankliniella fusca) infestation of cotton seedlings in the south-eastern United States. Pest Manag. Sci. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Zaffaroni, M.; Cunniffe, N.J.; Bevacqua, D. An ecophysiological model of plant-aphid interactions: The role of nutrient and water availability. J. R. Soc. Interface 2020, 17, 20200356. [Google Scholar] [CrossRef]
- Pell, B.; Kendig, A.E.; Borer, E.T.; Kuang, Y. Modeling nutrient and disease dynamics in a plant-pathogen system. Math. Biosci. Eng. 2018, 16, 234–264. [Google Scholar] [CrossRef]
- Ssamula, A.; Okiror, A.; Avrahami-Moyal, L.; Tam, Y.; Gaba, V.; Gibson, R.W.; Gal-On, A.; Mukasa, S.B.; Wasswa, P. Factors influencing reversion from virus infection in sweet potato. Ann. Appl. Biol. 2020, 17, 109–121. [Google Scholar] [CrossRef]
- Hančinský, R.; Mihálik, D.; Mrkvová, M.; Candresse, T.; Glasa, M. Plant Viruses Infecting Solanaceae Family Members in the Cultivated and Wild Environments: A Review. Plants 2020, 9, 667. [Google Scholar] [CrossRef] [PubMed]
- Holt, J.; Pavis, C.; Marquier, M.; Chancellor, T.C.B.; Urbino, C.; Boissot, N. Insect-screened cultivation to reduce the invasion of tomato crops by Bemisia tabaci: Modelling the impact on virus disease and vector. Agric. For. Entomol. 2008, 10, 61–67. [Google Scholar] [CrossRef]
- Viteri, D.; Gordillo, L.F. Modelling and control of non-persistent plant virus transmission for annual production cycles. Eur. J. Plant Pathol. 2009, 125, 435–444. [Google Scholar] [CrossRef]
- Chan, M.-S.; Jeger, M.J. An analytical model of plant virus disease dynamics with roguing and replanting. J. Appl. Ecol. 1994, 31, 413–427. [Google Scholar] [CrossRef]
- Gao, S.; Xia, L.; Liu, Y.; Xie, D. A plant virus disease model with periodic environment and pulse roguing. Stud. Appl. Math. 2015, 136, 357–381. [Google Scholar] [CrossRef]
- Smith, M.C.; Holt, J.; Kenyon, L.; Foot, C. Quantitative epidemiology of banana bunchy top virus disease and its control. Plant Pathol. 1998, 47, 177–187. [Google Scholar] [CrossRef]
- Holt, J.; Jeger, M.J.; Thresh, J.M.; Otim-Nape, G.W. An epidemiological model incorporating vector population dynamics applied to African cassava mosaic virus disease. J. Appl. Ecol. 1997, 34, 793–806. [Google Scholar] [CrossRef]
- Bokil, V.A.; Allen, L.J.S.; Jeger, M.J.; Lenhart, S. Optimal control of a vectored plant disease model for a crop with continuous replanting. J. Biol. Dynam. 2019, 13, 325–353. [Google Scholar] [CrossRef]
- Jeger, M.J.; van den Bosch, F.; Dutmer, M.Y. Modelling plant virus epidemics in a plantation-nursery system. Math. Med. Biol. J. IMA 2002, 19, 75–94. [Google Scholar] [CrossRef]
- Holt, J.; Chancellor, T.C.B. Simulation modelling of the spread of rice tungro virus disease: The potential for management by roguing. J. Appl. Ecol. 1996, 33, 927–936. [Google Scholar] [CrossRef]
- Karasev, A.V.; Gray, S.M. Continuous and emerging challenges of Potato virus Y in potato. Annu. Rev. Phytopathol. 2013, 51, 571–586. [Google Scholar] [CrossRef]
- Zhang, K.; Lu, H.; Wan, C.; Tang, D.; Zhao, Y.; Luo, K.; Li, S.; Wang, J. The spread and transmission of sweet potato virus disease (SPVD) and its effect on the gene expression profile in sweet potato. Plants 2020, 9, 492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redinbaugh, M.G.; Molineros, J.E.; Vacha, J.; Berry, S.A.; Hammond, R.B.; Madden, L.V.; Dorrance, A.E. Bean pod mottle virus spread in insect-feeding-resistant soybean. Plant Dis. 2010, 94, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Sundaraj, S.; Srinivasan, R.; Culbreath, A.K.; Riley, D.G.; Pappu, H.R. Host plant resistance against Tomato spotted wilt virus in peanut (Arachis hypogaea) and its impact on susceptibility to the virus, virus population genetics, and vector feeding behaviour and survival. Phytopathology 2014, 104, 202–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsharkawy, M.M.; Hanbal, S.E.; Sidaros, S.A.; Elsawy, M.M.; Hamed, A.H. Plant growth promoting fungi stimulate growth and confer protection against Bean yellow mosaic virus in faba bean. J. Virol. Sci. 2019, 6, 55–66. [Google Scholar]
- Aseel, D.G.; Younes, M.; Rashad, Y.M.; Hammad, S.M. Arbuscular mycorrhizal fungi trigger transcriptional expression of flavonoid and chlorogenic acid biosynthetic pathways genes in tomato against tomato mosaic virus. Sci. Rep. 2019, 9, 9692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Chen, L.; Liu, M.; García-Guzmán, G.; Gilbert, G.S.; Zhou, S. Dilution effect of plant diversity on infectious diseases: Latitudinal trend and biological context dependence. Oikos 2020, 129, 457–465. [Google Scholar] [CrossRef] [Green Version]
- González, R.; Butković, A.; Elena, S.F. Role of host genetic diversity for susceptibility-to-infection in the evolution of virulence of a plant virus. Virus Evol. 2019, 5, vez024. [Google Scholar]
- Wilhoit, L.R. Modelling the population dynamics of different aphid genotypes in plant variety mixtures. Ecol. Model. 1991, 55, 257–283. [Google Scholar] [CrossRef]
- Holt, J.; Chancellor, T.C.B. Modelling the spatio-temporal deployment of resistant varieties to reduce the incidence of rice tungro disease in a dynamic cropping system. Plant Pathol. 1999, 48, 453–461. [Google Scholar] [CrossRef]
- Allen-Perkins, A.; Estrada, E. Mathematical modeling for sustainable aphid control in agriculture via intercropping. Proc. R. Soc. A 2019, 475, a20190136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demon, I.; Jeger, M.J.; van den Bosch, F. The effect of crop and pest management practices on the evolution within Bemisia tabaci. In 2nd European Whitefly Symposium [EWS11] Abstract Compendium; Institute For Adriatic Crops and Karst Reclamation Put Duilova, Split: Cavtat, Croatia, 2004; p. 43. [Google Scholar]
- Jeger, M.J. Bottlenecks in IPM. Crop Prot. 2000, 19, 787–792. [Google Scholar] [CrossRef]
- Reitz, S.R.; Gao, Y.; Kirk, W.D.J.; Hoddle, M.S.; Leiss, K.A.; Funderburk, J.E. Invasion biology, ecology, and management of Western Flower Thrips. Annu. Rev. Entomol. 2020, 65, 17–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batuman, O.; Turini, T.A.; LeStrange, M.; Stoddard, S.; Miyao, G.; Aegerter, B.J.; Chen, L.-F.; McRoberts, N.; Ullman, D.E.; Gilbertson, R.L. Development of an IPM strategy for thrips and Tomato spotted wilt virus in processing tomatoes in the Central Valley of California. Pathogens 2020, 9, 636. [Google Scholar] [CrossRef]
- Fargette, D.; Vié, K. Simulation of the effects of host resistance, reversion, and cutting selection on incidence of African cassava mosaic virus and yield losses in cassava. Phytopathology 1995, 85, 370–375. [Google Scholar] [CrossRef]
- Seal, S.E.; van den Bosch, F.; Jeger, M.J. Factors influencing begomovirus evolution and their increasing global significance: Implications for sustainable control. Crit. Rev. Plant Sci. 2006, 25, 23–46. [Google Scholar] [CrossRef]
- Thomas-Sharma, S.; Andrade-Piedra, J.; Carvajal Yepes, M.; Hernandez Nopsa, J.F.; Jeger, M.J.; Jones, R.A.C.; Kromann, P.; Legg, J.P.; Yuen, Y.; Forbes, G.A.; et al. A risk assessment framework for seed degeneration: Informing an integrated seed health strategy for vegetatively-propagated crops. Phytopathology 2017, 107, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Jones, R.A.C. Plant and insect viruses in managed and natural environments: Novel and neglected transmission pathways. Adv. Virus Res. 2018, 101, 149–187. [Google Scholar] [PubMed]
- Ng, J.C.K.; Zhou, J.S. Insect vector-plant virus interactions associated with non-circulative, semi-persistent transmission: Current perspectives and future challenges. Curr. Opin. Virol. 2015, 15, 48–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stafford, C.A.; Walker, G.P.; Ullman, D.E. Vector feeding and virus transmission. Comm. Integr. Biol. 2011, 5, 43–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stafford, C.A.; Walker, G.P.; Ullman, D.E. Infection with a plant virus modifies vector feeding behaviour. Proc. Natl. Acad. Sci. USA 2011, 108, 9350–9355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, S.M.; Banerjee, N. Mechanisms of arthropod transmission of plant and animal viruses. Microbiol. Mol. Biol. Rev. 1999, 63, 128–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitfield, A.E.; Falk, B.W.; Rotenberg, D. Insect vector-mediated transmission of plant viruses. Virology 2015, 479–480, 278–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogenhout, S.A.; Ammar, E.D.; Whitfield, A.E.; Redinbaugh, M.G. Insect vector interactions with persistently transmitted viruses. Annu. Rev. Phytopathol. 2008, 46, 327–359. [Google Scholar] [CrossRef] [Green Version]
- Jia, D.; Chen, Q.; Mao, Q.; Zhang, X.; Wu, W.; Chen, H.; Yu, X.; Wang, Z.; Wei, T. Vector mediated transmission of persistently transmitted plant viruses. Curr. Opin. Virol. 2018, 18, 127–132. [Google Scholar] [CrossRef]
- Zhou, J.C.; Drucker, M.; Ng, J.C.K. Direct and indirect influences of virus-insect vector-plant interactions on non-circulative semi-persistent virus transmission. Curr. Opin. Virol. 2018, 33, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Brault, V.; Uzest, M.; Monzion, B.; Jacquot, E.; Blanc, E. Aphids as transport devices for plant viruses. C. R. Biol. 2010, 333, 524–538. [Google Scholar] [CrossRef]
- Liu, W.; Hajano, J.-U.-D.; Wang, X. New insights on the transmission mechanism of tenuiviruses by their vector insects. Curr. Opin. Virol. 2018, 33, 13–17. [Google Scholar] [CrossRef]
- Verbeek, M.; van Bekkum, P.J.; Dullemans, A.M.; van der Vlugt, R.A.A. Torradoviruses are transmitted in a semi-persistent and stylet-borne manner by three whitefly vectors. Virus Res. 2014, 186, 55–60. [Google Scholar] [CrossRef]
- Bragard, C.; Caciagli, P.; Lemaire, O.; Lopez-Moya, J.J.; MacFarlane, S.; Peters, D.; Susi, P.; Torrance, L. Status and prospects of plant virus control through interference with vector transmission. Annu. Rev. Phytopathol. 2013, 51, 177–201. [Google Scholar] [CrossRef]
- King, A.M.Q.; Adams, M.J.; Carstens, E.B.; Lefkowitz, E.J. Tenuivirus. In Virus Taxonomy, Ninth Report of the International Committee on Taxonomy of Viruses; ICTV: Leiden, The Netherlands, 2012; pp. 771–776. [Google Scholar]
- Gadhave, K.R.; Gautam, S.; Dutta, B.; Coolong, T.; Adkins, S.; Srinivasan, R. Low frequency of horizontal and vertical transmission of cucurbit leaf crumple virus in whitefly Bemisia tabaci Gennardius. Phytopathology 2020, 110, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Jeger, M.J.; Madden, L.V.; van den Bosch, F. The effect of transmission route on plant virus epidemic development and disease control. J. Theor. Biol. 2009, 258, 198–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsar, C.S.; Hunter, W.B.; Sinisterra, X.H. Phytoreovirus-like sequences isolated from salivary glands of the glassy-winged sharpshooter Homalodisca vitripennis (Hemiptera: Cicadellidae). Fla. Entomol. 2007, 90, 196–203. [Google Scholar] [CrossRef]
- Wan, J.; Cabanillas, D.G.; Zheng, H.; Laliberté, J.-F. Turnip mosaic virus moves systemically through both phloem and xylem as membrane associated complexes. Plant Physiol. 2015, 167, 1374–1388. [Google Scholar] [CrossRef] [Green Version]
- Pompon, J.; Quiring, D.; Goyer, C.; Giordanengo, P.; Pelletier, Y. A phloem-sap feeder mixes phloem and xylem sap to regulate osmotic potential. J. Insect Physiol. 2011, 57, 1317–1322. [Google Scholar] [CrossRef]
- Stafford, C.A.; Walker, G.P. Characterization and correlation of DC electrical penetration graph waveforms with feeding behaviour of beet leafhopper, Circulifer tenullus. Entomol. Exp. Appl. 2009, 130, 113–129. [Google Scholar] [CrossRef]
- Jeger, M.J.; van den Bosch, F.; McRoberts, N. Modelling transmission characteristics and epidemic development of the tospovirus—Thrip interaction. Arthropod-Plant Interact. 2015, 9, 107–120. [Google Scholar] [CrossRef]
- Wei, J.; Jia, D.; Mao, Q.; Zhang, X.; Chen, Q.; Wu, W.; Chen, H.; Wei, T. Complex interactions between insect-borne rice viruses and their vectors. Curr. Opin. Virol. 2018, 33, 18–23. [Google Scholar] [CrossRef]
- Jeger, M.J.; van den Bosch, F.; Madden, L.V.; Holt, J. A model for analysing plant-virus transmission characteristics and epidemic development. Math. Med. Biol. J. IMA 1998, 15, 1–18. [Google Scholar] [CrossRef]
- Madden, L.V.; Jeger, M.J.; van den Bosch, F. A theoretical assessment of the effects of vector-virus transmission mechanism on plant virus disease epidemics. Phytopathology 2000, 90, 576–594. [Google Scholar] [CrossRef] [Green Version]
- Guitiérrez, S.; Michalakis, Y.; Blanc, S. Virus population bottlenecks during within-host progression and host-to-host transmission. Curr. Opin. Virol. 2012, 2, 546–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moury, B.; Fabre, F.; Senoussi, R. Estimation of the number of virus particles transmitted by an insect vector. Proc. Natl. Acad. Sci. USA 2007, 104, 17891–17896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.-S.; Holt, J.; Colvin, J. A general model of plant-virus disease infection incorporating vector aggregation. Plant Pathol. 2000, 49, 435–444. [Google Scholar] [CrossRef]
- Makkouk, K.M.; Bos, L.; Azzam, O.I.; Katul, L.; Rizkallah, A. Broad bean stain virus: Detectability with ELISA in faba bean, occurrence in leaves and seed in West Asia and North Africa, and possible wild hosts. Neth. J. Plant Path. 1987, 93, 97–106. [Google Scholar] [CrossRef]
- Nunes, M.A.; de Oliveira, C.A.L.; de Oliveira, M.L.; Kitajima, E.W.; Hilf, M.E.; Gottwald, T.R.; Freitas-Astúa, J. Transmission of Citrus leprosis virus C by Brevipalpus phoenicis (Geijskes) to alternative host plants found in citrus orchards. Plant Dis. 2012, 96, 968–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lockhart, B.E.; Menke, J.; Dahal, G.; Olszewski, N.E. Characterization and genomic analysis of tobacco vein clearing virus, a plant pararetrovirus that is transmitted vertically and related to sequences integrated in the host genome. J. Gen. Virol. 2000, 81, 1579–1585. [Google Scholar] [CrossRef] [Green Version]
- Iskra-Caruana, M.-L.; Baurens, F.-C.; Gayral, P.; Chabannes, M. A four-partner plant–virus Interaction: Enemies can also come from within. MPMI 2010, 23, 1394–1402. [Google Scholar] [CrossRef] [Green Version]
- de Assis Filho, F.M.; Sherwood, L. Evaluation of seed transmission of Turnip yellow mosaic virus and Tobacco mosaic virus in Arabidopsis thaliana. Phytopathology 2000, 90, 1233–1238. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Kobayashi, M. Seed transmission of Cucumber mosaic virus in pepper. J. Virol. Meth. 2010, 263, 234–237. [Google Scholar] [CrossRef]
- Cobos, A.; Montes, N.; López-Herranz, M.; Gil-Valle, M.; Pagán, I. Within-host multiplication and speed of colonization as infection traits associated with plant virus vertical transmission. J. Virol. 2019, 93, e01078-19. [Google Scholar] [CrossRef] [Green Version]
- Johansen, E.; Edwards, M.C.; Hampton, R.O. Seed transmission of viruses: Current Perspectives. Annu. Rev. Phytopathol. 1994, 32, 363–386. [Google Scholar] [CrossRef]
- Maule, A.J.; Wang, D. Seed transmission of plant viruses: A lesson in biological complexity. Trends Microbiol. 1996, 4, 153–158. [Google Scholar] [CrossRef]
- Rebenstorf, K.; Candresse, T.; Dulucq, M.J.; Büttner, C.; Obermeier, C. Host species-dependent population structure of a pollen-borne plant virus, Cherry leaf roll virus. J. Virol. 2006, 80, 2453–2462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simmons, H.E.; Dunham, J.P.; Zinn, K.E.; Munkvold, G.P.; Holmes, E.C.; Stephenson, A.C. Zucchini yellow mosaic virus (ZYMV, Potyvirus): Vertical transmission, seed infection and cryptic infections. Virus Res. 2013, 176, 259–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delfosse, P.; Reddy, A.S.; Legrève, A.; Devi, P.S.; ThirumalaDevi, K.; Maraite, H.; Reddy, D.V.S. Indian peanut clump virus (IPCV) infection on wheat and barley: Symptoms, yield loss and transmission through seed. Plant Pathol. 1999, 48, 273–282. [Google Scholar] [CrossRef]
- Quainoo, A.K.; Wetten, A.C.; Allainguillaume, J. Transmission of cocoa swollen shoot virus by seeds. J. Virol. Meth. 2008, 150, 45–49. [Google Scholar] [CrossRef]
- Aramburu, J.; Galipienso, L.; Aparicio, F.; Soler, S.; López, C. Mode of transmission of Parieta mottle virus. J. Plant Pathol. 2010, 92, 679–684. [Google Scholar]
- Liu, H.W.; Luo, L.X.; Li, J.Q.; Liu, P.F.; Chen, X.Y.; Hao, J.J. Pollen and seed transmission of Cucumber green mottle mosaic virus in cucumber. Plant Pathol. 2014, 63, 72–77. [Google Scholar] [CrossRef]
- Lapidot, M.; Guenoune-Gelbart, D.; Leibman, D.; Holdengreber, V.; Davidovitz, M.; Machbash, Z.; Klieman-Shoval, S.; Cohen, S.; Gal-On, A. Pelargonium zonate spot virus is transmitted vertically via seed and pollen in tomato. Phytopathology 2010, 100, 798–804. [Google Scholar] [CrossRef] [Green Version]
- Lipsitch, M.; Siller, S.; Nowak, M.A. The evolution of virulence in pathogens with vertical and horizontal transmission. Evolution 1996, 50, 1729–1740. [Google Scholar] [CrossRef]
- Faeth, S.H.; Hadeler, K.P.; Thieme, H.R. An apparent paradox of horizontal and vertical disease transmission. J. Biol. Dyn. 2007, 1, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Van den Bosch, F.; Fraaije, B.A.; van den Berg, F.; Shaw, M.W. Evolutionary bi-stability in pathogen transmission mode. Proc. R. Soc. B 2010, 277, 1735–1742. [Google Scholar] [CrossRef] [Green Version]
- Blanc, S. Virus transmission—Getting out and in. Plant Cell Monogr. 2007, 7, 28. [Google Scholar]
- Sacristán, S.; García-Arenal, F. The evolution of virulence and pathogenicity in plant pathogen populations. Mol. Plant Pathol. 2008, 9, 369–384. [Google Scholar] [CrossRef]
- Pagán, I.; Montes, N.; Milgroom, M.G.; García-Arenal, F. Vertical transmission selects for reduced virulence in a plant virus and for increased resistance in the host. PLoS Pathog. 2014, 10, e1004293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Froissart, R.; Doumayrou, J.; Vuillaume, F.; Alizon, S.; Michalakis, Y. The virulence-transmission trade-off in vector-borne plant−viruses: A review of (non-)existing studies. Philos. Trans. R. Soc. B 2010, 365, 1907–1918. [Google Scholar] [CrossRef] [Green Version]
- Lafforgue, G.; Sardanyés, J.; Elena, S.F. Differences in accumulation and virulence determine the outcome of competition during Tobacco etch virus coinfection. PLoS ONE 2011, 6, e17917. [Google Scholar] [CrossRef] [Green Version]
- Lowery, T.; Vickers, P.M.; Bittner, L.A.; Stobbs, L.W.; Foottit, R.G. Aphid transmission of the Ontario Isolate of Plum pox virus. J. Econ. Entomol. 2015, 108, 2168–2173. [Google Scholar] [CrossRef] [Green Version]
- Ogada, P.A.; Moualeu, D.P.; Poshling, H.-M. Predictive models for Tomato spotted wilt virus spread dynamics, considering Frankliniella occidentalis specific life processes as influenced by the virus. PLoS ONE 2016, 11, e0154533. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Liu, C.; Deng, W.-H.; Yao, D.-M.; Pan, L.-L.; Li, Y.-Q.; Liu, Y.Q.; Liang, Y.; Zhou, X.-P.; Wang, X.-W. Plant begomoviruses subvert ubiquitination to suppress plant defenses against insect vectors. PLoS Pathog. 2019, 152, e1007607. [Google Scholar] [CrossRef] [Green Version]
- Sisterson, M.S. Transmission of insect-vectored pathogens: Effects of vector fitness as a function of infectivity status. Environ. Entomol. 2009, 38, 345–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeger, M.J. Improved understanding of dispersal in crop pest and disease management: Current status and future directions. Agric. For. Meteorol. 1999, 97, 331–349. [Google Scholar] [CrossRef]
- Pleydell, D.R.J.; Soubeyrand, S.; Dallot, S.; Labonne, G.; Chadoeuf, J.; Jacquot, E.; Thébaud, G. Estimation of the dispersal distances of an aphid-borne virus in a patchy landscape. PLoS Comput. Biol. 2018, 14, e1006085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schröder, M.L.; Glinwood, R.; Ignell, R.; Krüger, K. The role of visual and olfactory plant cues in aphid behaviour and the development of non-persistent virus management strategies. Arthropod-Plant Interact. 2017, 11, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Holt, J.; Colvin, J.; Muniyappa, V. Identifying control strategies for Tomato leaf curl virus disease using an epidemiological model. J. Appl. Ecol. 1999, 36, 625–633. [Google Scholar] [CrossRef]
- Garzo, E.I.; Duque, M.; Fereres, A. Transmission efficiency of different non-persistent viruses infecting melon by four aphid species. Span. J. Agric. Res. 2004, 2, 369–376. [Google Scholar] [CrossRef]
- Katis, N.I.; Tsitsipis, J.A.; Lykouressis, D.P.; Papapanayotou, A.; Margaritopoulos, J.T.; Kokinis, G.M.; Perdikis, D.C.; Manoussopoulos, I.N. Transmission of Zucchini yellow mosaic virus by colonizing and non-colonizing aphids in Greece and new aphid species vectors of the virus. J. Phytopathol. 2006, 154, 293–302. [Google Scholar] [CrossRef]
- Zogli, P.; Pingault, L.; Grover, S.; Louis, J. Ento(o)mics: The intersection of ‘omic’ approaches to decipher plant defense against sap-sucking insect pests. Curr. Opin. Plant Biol. 2020, 56, 153–161. [Google Scholar] [CrossRef]
- Lu, S.; Chen, M.; Li, J.; Shi, Y.; Gu, Q.; Yan, F. Changes in Bemisia tabaci feeding behaviors caused directly and indirectly by cucurbit chlorotic yellows virus. Virol. J. 2019, 16, 106. [Google Scholar] [CrossRef] [Green Version]
- Nakazawa, T.; Yamanaka, T.; Urano, S. Model analysis for plant disease dynamics co-mediated by herbivory and herbivore-borne phytopathogens. Biol. Lett. 2012, 8, 685–688. [Google Scholar] [CrossRef]
- Grilli, M.P.; Holt, J. Vector feeding period variability in epidemiological models of persistent plant viruses. Ecol. Model. 2000, 126, 49–57. [Google Scholar] [CrossRef]
- Leybourne, D.J.; Valentine, T.A.; Bos, J.I.B.; Karley, A.J. A fitness cost resulting from Hamiltonella defensa infection is associated with altered probing and feeding behaviour in Rhopalosiphum padi. J. Exp. Biol. 2020, 223, jeb207936. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, P.J.; Eigenbrode, S.D.; Clark, R.E.; Basu, S.; Crowder, D.W. Plant-mediated interactions between a vector and a non-vector herbivore promote the spread of a plant virus. Proc. R. Soc. B 2019, 286, 20191383. [Google Scholar] [CrossRef] [PubMed]
- Carr, J.P.; Murphy, A.M.; Tungadi, T.; Yoon, J.-Y. Plant defense signals: Players and pawns in plant-virus-vector interactions. Plant Sci. 2019, 279, 87–95. [Google Scholar] [CrossRef]
- Hodge, S.; Hardie, J.; Powell, G. Parasitoids aid dispersal of a nonpersistently transmitted plant virus by disturbing the aphid vector. Agric. For. Entomol. 2011, 13, 83–88. [Google Scholar] [CrossRef]
- Jeger, M.J.; Chen, Z.; Powell, G.; Hodge, S.; van den Bosch, F. Interactions in a host plant-virus–vector–parasitoid system: Modelling the consequences for virus transmission and disease dynamics. Virus Res. 2011, 159, 183–193. [Google Scholar] [CrossRef]
- Jeger, M.J.; Chen, Z.; Cunningham, E.; Martin, G.; Powell, G. Population biology and epidemiology of plant virus epidemics: From tripartite to tritrophic interactions. Eur. J. Plant Pathol. 2012, 133, 3–23. [Google Scholar] [CrossRef]
- Dáder, B.; Moreno, A.; Viñuela, E.; Fereres, A. Spatio-temporal dynamics of viruses are differentially affected by parasitoids depending on the mode of transmission. Viruses 2012, 4, 3069–3089. [Google Scholar] [CrossRef] [Green Version]
- Keough, S.; Han, J.; Shuman, T.; Wise, K.; Nachappa, P. Effects of Soybean Vein Necrosis Virus on life history and host preference of its vector, Neohydatothrips variabilis, and evaluation of vector status of Frankliniella tritici and Frankliniella fusca. J. Econ. Entomol. 2016, 109, 1979–1987. [Google Scholar] [CrossRef]
- Shalileh, S.; Ogada, P.A.; Moualeu, D.P.; Poehling, H.-M. Manipulation of Frankliniella occidentalis (Thysanoptera: Thripidae) by Tomato Spotted Wilt Virus (Tospovirus) via the host plant nutrients to enhance its transmission and spread. Environ. Entomol. 2016, 45, 1235–1242. [Google Scholar] [CrossRef] [Green Version]
- Tomitaka, Y.; Abe, H.; Sakurai, T.; Tsuda, S. Preference of the vector thrips Frankliniella occidentalis for plants infected with thrips-non-transmissible Tomato spotted wilt virus. J. Appl. Entomol. 2015, 139, 250–259. [Google Scholar] [CrossRef]
- Chen, W.-T.; Tseng, C.-H.; Tsai, C.-W. Effect of watermelon silver mottle virus on the life history and feeding preference of Thrips palmi. PLoS ONE 2014, 9, e102021. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Srinivasan, R.; Riley, D.G.; Culbreath, A.K. Direct and indirect effects of a thrips-transmitted Tospovirus on the preference and fitness of its vector, Frankliniella fusca. Entomol. Exp. Appl. 2012, 145, 260–271. [Google Scholar] [CrossRef]
- Fereres, A.; Fernanda, G.V.M.; Peñaflor, M.F.G.V.; Carla, F.; Favaro, C.F.; Azevedo, K.E.X.; Landi, C.H.; Maluta, N.K.P.; Bento, J.M.S.; Lopes, J.R.S. Tomato infection by whitefly-transmitted circulative and non-circulative viruses induce contrasting changes in plant volatiles and vector behaviour. Viruses 2016, 8, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legarrea, S.; Barman, A.; Marchant, W.; Diffie, S.; Srinivasan, R. Temporal effects of a begomovirus infection and host plant resistance on the preference and development of an insect vector, Bemisia tabaci, and implications for epidemics. PLoS ONE 2015, 10, e0142114. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.; Jiao, X.; Xie, W.; Wang, S.; Wu, Q.; Shi, X.; Chen, G.; Su, Q.; Yang, X.; Pan, H.; et al. Tomato yellow leaf curl virus alters the host preferences of its vector Bemisia tabaci. Sci. Rep. 2013, 3, 2876. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, R.C.; Peñaflor, M.F.; Sanches, P.A.; Nardi, C.; Bento, J.M. The effects of Gibberella zeae, Barley Yellow Dwarf Virus, and co-infection on Rhopalosiphum padi olfactory preference and performance. Phytoparasitica 2016, 44, 47–54. [Google Scholar] [CrossRef]
- Ghosh, A.; Das, A.; Vijayanandraj, S.; Mandal, B. Cardamom bushy dwarf virus infection in large cardamom alters plant selection preference, life stages, and fecundity of aphid vector, Micromyzus kalimpongensis (Hemiptera: Aphididae). Environ. Entomol. 2016, 45, 178–184. [Google Scholar] [CrossRef]
- Wu, Y.; Davis, T.S.; Eigenbrode, S.D. Aphid behavioral responses to virus-infected plants are similar despite divergent fitness effects. Entomol. Exp. Appl. 2014, 153, 246–255. [Google Scholar] [CrossRef]
- Mauck, K.E.; De Moraes, C.M.; Mescher, M.C. Evidence of local adaptation in plant virus effects on host–vector interactions. Integr. Comp. Biol. 2014, 54, 193–209. [Google Scholar] [CrossRef] [Green Version]
- Wosula, E.N.; Davis, J.A.; Clark, C.A. Stylet Penetration behaviors of Myzus persicae (Hemiptera: Aphididae) on four Ipomoea spp. infected or noninfected with sweet potato potyviruses. J. Econ. Entomol. 2014, 107, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Fariña, A.E.; Rezende, J.A.M.; Wintermantel, W.M. Expanding knowledge of the host range of tomato chlorosis virus and host plant preference of Bemisia tabaci MEAM1. Plant Dis. 2019, 103, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Lagos-Kutz, D.; Pawlowski, M.L.; Haudenshield, J.; Han, J.; Domier, L.L.; Hartman, G.L. Evaluation of soybean for resistance to Neohyadatothrips variabilis (Thysanoptera: Thripidae) noninfected and infected with soybean vein necrosis virus. J. Econ. Entomol. 2020, 113, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Bosque-Pérez, N.A.; Eigenbrode, S.D. The influence of virus-induced changes in plants on aphid vectors: Insights from luteovirus pathosystems. Virus Res. 2011, 159, 201–205. [Google Scholar] [CrossRef]
- Zeilinger, A.R.; Daugherty, M.P. Vector preference and host defense against infection interact to determine disease dynamics. Oikos 2014, 123, 613–622. [Google Scholar] [CrossRef]
- Penaflor, M.F.; Mauck, K.E.; Alves, K.J.; de Moraes, C.M.; Mescher, M.C. Effects of single and mixed infections of Bean pod mottle virus and Soybean mosaic virus on host-plant chemistry and host-vector interactions. Funct. Ecol. 2016, 30, 1648–1659. [Google Scholar] [CrossRef]
- Carr, J.P.; Tungadi, T.; Donnelly, R.; Bravo-Cazar, A.; Rhee, S.-J.; Watt, L.G.; Mutuku, J.M.; Wamonje, F.O.; Murphy, A.M.; Arinaitwe, W.; et al. Modeling and manipulation of aphid-mediated spread of non-persistently transmitted viruses. Virus Res. 2020, 277, 197845. [Google Scholar] [CrossRef]
- Mauck, K.E.; Smyers, E.; De Moraes, C.M.; Mescher, M.C. Virus infection influences host plant interactions with non-vector herbivores and predators. Funct. Ecol. 2015, 29, 662–673. [Google Scholar] [CrossRef] [Green Version]
- Ingwell, L.L.; Eigenbrode, S.D.; Bosque-Pérez, N.A. Plant viruses alter insect behavior to enhance their spread. Sci. Rep. 2012, 2, 578. [Google Scholar] [CrossRef] [Green Version]
- Mauck, K.; Bosque-Pérez, N.A.; Eigenbrode, S.D.; De Moraes, C.M.; Mescher, M.C. Transmission mechanisms shape pathogen effects on host–vector interactions: Evidence from plant viruses. Funct. Ecol. 2012, 26, 1162–1175. [Google Scholar] [CrossRef]
- Carmo-Souza, M.; Moreno, A.; Garzo, E.; Fereres, A. A non-persistently transmitted-virus induces a push-pull strategy in its aphid vector to optimize transmission and spread. Virus Res. 2014, 186, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Rajabaskar, D.; Bosque-Pérez, N.A.; Eigenbrode, S.D. Preference for a virus vector for infected plants is reversed after virus acquisition. Virus Res. 2014, 186, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Mauck, K.E.; Chesnais, Q.; Shapiro, L.R. Evolutionary determinants of host and vector manipulation by plant viruses. Adv. Virus Res. 2018, 101, 189–250. [Google Scholar] [PubMed]
- Mauck, K.E.; Chesnais, Q. A synthesis of virus-vector associations reveals important deficiencies in studies on host and vector manipulation by plant viruses. Virus Res. 2020, 285, 197957. [Google Scholar] [CrossRef] [PubMed]
- Roosien, B.K.; Gomulkiewicz, R.; Ingwell, L.L.; Bosque-Pérez, N.A.; Rajabaskar, D.; Eigenbrode, S.D. Conditional vector preference aids the spread of plant pathogens: Results from a model. Environ. Entomol. 2013, 42, 1299–1308. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.K.; Peace, A.; Power, A.G.; Bosque-Pérez, N.A. Vector population growth and condition-dependent movement drive the spread of plant pathogens. Ecology 2017, 98, 2145–2157. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, G.; Zhang, Y.J.; Xie, W.; Wu, Q.J.; Wang, S.L. Virus-infected plants altered the host selection of Encarsia formosa, a parasite of whiteflies. Front. Physiol. 2017, 8, 937. [Google Scholar] [CrossRef] [Green Version]
- Daimei, G.; Raina, H.S.; Devi, P.P.; Saurav, G.K.; Renukadevi, P.; Malathi, V.G.; Senthilraja, C.; Mandel, B.; Rajagopal, R. Influence of Groundnut bud necrosis on the life history traits and feeding preferences of its vector, Thrips palmi. Phytopathology 2017, 107, 1440–1445. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, D.; McAuslane, H.J.; Adkins, S.T.; Smith, H.A.; Dufault, N.; Colee, J.; Webb, S.E. Host-mediated effects of semipersistently transmitted Squash vein yellowing virus on Sweetpotato whitefly (Hemiptera: Aleyrodidae) behaviour and fitness. J. Econ. Entomol. 2017, 110, 1433–1441. [Google Scholar] [CrossRef]
- Tungadi, T.; Groen, S.C.; Murphy, A.M.; Pate, A.E.; Iqbal, J.; Bruce, T.J.A.; Cunniffe, N.J.; Carr, J.P. Cucumber mosaic virus and its 2b protein alter emission of host volatile organic compounds but not aphid vector settling in tobacco. Virol. J. 2017, 14, 91. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Su, Q.; Shi, X.B.; Liu, X.; Peng, Z.K.; Zheng, H.X.; Xie, W.; Xu, B.Y.; Wang, S.L.; Wu, Q.J.; et al. Odor, not performance dictates Bemisia tabaci’s selection between healthy and virus infected plants. Front. Physiol. 2017, 8, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, C.M.; Gedling, C.R.; Wiebe, K.F.; Cassone, B.J. A sweet story: Bean pod mottle virus transmission dynamics by Mexican bean beetles (Epilachna varivestis). Genome Biol. Evol. 2017, 9, 714–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, T.S.; Wu, Y.; Eigenbrode, S.D. The effects of bean leafroll virus on life history traits and host selection behaviour of specialized pea aphid (Acyrthosiphon pisum, Hemiptera: Aphididae) genotypes. Environ. Entomol. 2017, 46, 68–74. [Google Scholar] [PubMed]
- Chesnais, Q.; Couty, A.; Uzest, M.; Brault, V.; Ameline, A. Plant infection by two different viruses induce contrasting changes of vector fitness and behavior. Insect Sci. 2019, 26, 86–96. [Google Scholar] [CrossRef] [Green Version]
- Fox, A. Reconsidering causal association in plant virology. Plant Pathol. 2020, 69, 956–961. [Google Scholar] [CrossRef]
- Vautrin, E.; Vavre, F. Interactions between vertically transmitted symbionts. Trends Microbiol. 2009, 17, 95–99. [Google Scholar] [CrossRef]
- Wu, L.; Zu, X.; Wang, S.; Chen, Y. Sugarcane mosaic virus—Long history but still a threat to industry. Crop Prot. 2012, 42, 74–78. [Google Scholar] [CrossRef]
- Syller, J. Biological and molecular events associated with simultaneous transmission of plant viruses by invertebrate and fungal vectors. Mol. Plant Pathol. 2014, 15, 417–426. [Google Scholar] [CrossRef]
- Syller, J.; Grupa, A. Antagonistic within-host interactions between plant viruses: Molecular basis and impact on viral and host fitness. Mol. Plant Pathol. 2016, 17, 769–782. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.-S.; Holt, J. Mathematical models of cross protection in the epidemiology of plant-virus diseases. Phytopathology 2001, 91, 924–934. [Google Scholar] [CrossRef] [Green Version]
- Mascia, T.; Gallitelli, D. Synergies and antagonisms in virus interactions. Plant Sci. 2016, 252, 176–192. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-M.; Jeffries, P.; Pautasso, M.; Jeger, M.J. Combined use of biocontrol agents to manage plant diseases in theory and practice: A review. Phytopathology 2011, 101, 1024–1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tahmasebi, A.; Zarghani, H.H.; Afsharifar, A.R.; Dizadji, A. An appropriate method to determine the interaction type of Cucumber mosaic virus (CMV) and Bean yellow mosaic virus (BYMV). Iran Agric. Res. 2019, 38, 47–56. [Google Scholar]
- Zhang, X.-S.; Holt, J.; Colvin, J. Mathematical models of host plant infection by helper-dependent virus complexes: Why are helper viruses always avirulent? Phytopathology 2000, 90, 85–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrol, J.E.; Smith, D.M.; Gray, S.M. Preferential acquisition and inoculation of PVYNTN over PVYO in potato by the green peach aphid Myzus persicae (Sulzer). J. Gen. Virol. 2016, 97, 797–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivasan, R.; Hall, D.G.; Cervantes, F.A.; Alvarez, J.M.; Whitworth, J.L. Strain specificity and simultaneous transmission of closely related strains of a Potyvirus by Myzus persicae. J. Econ. Entomol. 2012, 105, 783–791. [Google Scholar] [CrossRef]
- Mondal, S.; Wenninger, E.J.; Hutchinson, P.J.S.; Whitworth, J.L.; Shrestha, D.; Eigenbrode, S.D.; Bosque-Perez, N.A. Comparison of transmission efficiency of various isolates of Potato virus Y among three aphid species. Entomol. Exp. Appl. 2016, 158, 258–268. [Google Scholar] [CrossRef]
- Lacroix, C.; Seabloom, E.W.; Borer, E.T. Environmental nutrient supply alters prevalence and weakens competitive interactions among coinfecting viruses. New Phytol. 2014, 204, 424–433. [Google Scholar] [CrossRef]
- Salvaudon, L.; De Moraes, C.M.; Mescher, M.C. Outcomes of co-infection by two potyviruses: Implications for the evolution of manipulative strategies. Proc. R. Soc. B 2013, 280, 20122959. [Google Scholar] [CrossRef] [Green Version]
- Beltran-Beltran, A.K.; Santillán-Galicia, M.T.; Guzmán-Franco, A.W.; Teliz-Ortiz, D.; Gutiérrez-Espinoza, M.A.; Romero-Rosales, F.; Robles-García, P.L. Incidence of Citrus leprosis virus C and Orchid fleck dichorhavirus citrus strain in mites of the genus Brevipalpus in Mexico. J. Econ. Entomol. 2020, 113, 1576–1581. [Google Scholar] [CrossRef]
- Hamelin, F.M.; Allen, L.J.S.; Bokil, V.A.; Gross, L.J.; Hilker, F.M.; Jeger, M.J.; Manore, C.A.; Power, A.G.; Rúa, M.A.; Cunniffe, N.J. Co-infections by non-interacting pathogens are not independent: Deriving new tests of interaction. PLoS Biol. 2019, 17, e3000551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naidu, R.A.; Maree, H.J.; Burger, J.T. Grapevine leafroll disease and associated viruses: A unique pathosystem. Annu. Rev. Phytopathol. 2015, 53, 613–634. [Google Scholar] [CrossRef] [PubMed]
- Untiveros, M.; Fuentes, S.; Salazar, L.F. Synergistic interaction of Sweet potato chlorotic stunt virus (Crinivirus) with carla-, cucumo-, ipomo-, and potyviruses infecting sweet potato. Plant Dis. 2007, 91, 669–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kendig, A.E.; Borer, E.T.; Mitchell, C.E.; Power, A.G.; Seabloom, E.W. Characteristics and drivers of plant virus community spatial patterns in US west coast grasslands. Oikos 2017, 126, 1281–1290. [Google Scholar] [CrossRef]
- McLeish, M.J.; Sacristán, S.; Fraile, A.; García-Arenal, F. Co-infection organises epidemiological networks of viruses and hosts and reveals hubs of transmission. Phytopathology 2019, 109, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Alcalá-Briseño, R.I.; Casarrubias-Castillo, K.; López-Ley, D.; Garrett, K.A.; Silva-Rosales, L. Network analysis of the papaya orchard virome from two agroecological regions of Chiapas, Mexico. mSystems 2020, 5, e00423-19. [Google Scholar]
- Jeger, M.J.; Pautasso, M.; Holdenrieder, O.; Shaw, M.W. Modelling disease spread and control in networks: Implications for plant sciences. New Phytol. 2007, 174, 179–197. [Google Scholar] [CrossRef]
- Moslonka-Lefebre, M.; Finley, A.; Dorigatti, I.; Dehnen-Schmutz, K.; Harwood, T.; Jeger, M.J.; Xu, X.; Holdenrieder, O.; Pautasso, M. Networks in plant epidemiology: From genes to landscapes, countries, and continents. Phytopathology 2011, 101, 392–403. [Google Scholar] [CrossRef] [Green Version]
- Lightle, D.; Lee, J. Raspberry viruses affect the behavior and performance of Amphorophora agathonica in single and mixed infections. Entomol. Exp. Appl. 2014, 151, 57–64. [Google Scholar] [CrossRef]
- Gautam, S.; Gadhave, K.R.; Buck, J.W.; Dutta, B.; Coolong, T.; Adkins, S.; Srinivasan, R. Virus-virus interactions in a plant host and in a hemipteran vector: Implications for vector fitness and virus epidemics. Virus Res. 2020, 286, 198069. [Google Scholar] [CrossRef]
- Wang, H.; Xu, D.; Pu, L.; Zhou, G. Southern rice black-streaked dwarf virus alters insect vectors’ host orientation preferences to enhance spread and increase Rice ragged stunt virus co-infection. Phytopathology 2014, 104, 196–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nee, S. Mutualism, parasitism and competition in the evolution of coviruses. Philos. Trans. R. Soc. Lond. B 2000, 355, 1607–1613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucía-Sanz, A.; Aguirre, J.; Manrubia, S. Theoretical approaches to disclosing the emergence and adaptive advantages of multipartite viruses. Curr. Opin. Virol. 2018, 33, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Gallet, R.; Fabre, F.; Thébaud, G.; Sofonea, M.T.; Sicard, A.; Blanc, S.; Michalakis, Y. Small bottleneck size in a highly multipartite virus during a complete infection cycle. J. Virol. 2018, 92, e00139-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leeks, A.; Segredo-Otero, E.A.; Sanjuán, R.; West, S.A. Beneficial coinfection can promote within-host viral diversity. Virus Evol. 2018, 4, vey028. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, S.; Zwart, M.P. Population bottlenecks in multicomponent viruses: First forays into the uncharted territory of genome-formula drift. Curr. Opin. Virol. 2018, 33, 184–190. [Google Scholar] [CrossRef] [Green Version]
- Sicard, A.; Pirolles, E.; Gallet, R.; Vernerey, M.-S.; Yvon, M.; Urbino, C.; Peterschmitt, M.; Gutierrez, S.; Michalakis, Y.; Blanc, S. A multicellular way of life for a multipartite virus. eLife 2019, 8, e43599. [Google Scholar] [CrossRef]
- Lucía-Sanz, A.; Manrubia, S. Multipartite viruses: Adaptive trick or evolutionary treat? Syst. Biol. Appl. 2017, 3, 34. [Google Scholar] [CrossRef]
- Gilmer, D.; Ratti, C.; Michel, F. Long-distance movement of helical multipartite phytoviruses: Keep connected or die? Curr. Opin. Virol. 2018, 33, 120–128. [Google Scholar] [CrossRef]
- Sofonea, M.T.; Alizon, S.; Michelakis, Y. From within-host interactions to epidemiological competition: A general model for multiple infections. Philos. Trans. R. Soc. B 2015, 370, 2014303. [Google Scholar] [CrossRef] [Green Version]
- Hilker, F.M.; Allen, L.J.; Bokil, V.A.; Briggs, C.J.; Feng, Z.; Garrett, K.A.; Gross, L.J.; Hamelin, F.M.; Jeger, M.J.; Manore, C.A.; et al. Modeling virus coinfection to inform management of maize lethal necrosis in Kenya. Phytopathology 2017, 107, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Marchetto, K.M.; Power, A.G. Coinfection timing drives host population dynamics through changes in virulence. Am. Nat. 2018, 191, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Valdano, E.; Manrubia, S.; Gómez, S.; Arenas, A. Endemicity and prevalence of multipartite viruses under heterogeneous between-host transmission. PLoS Comput. Biol. 2019, 15, e1006876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, L.J.S.; Bokil, V.A.; Cunniffe, N.J.; Hamelin, F.M.; Hilker, F.M.; Jeger, M.J. Modelling vector transmission and epidemiology of co-infecting plant viruses. Viruses 2019, 11, 1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno-Delafuente, A.; Vinuela, E.; Fereres, A.; Medina, P.; Trebicki, P. Simultaneous increase in CO(2) and temperature alters wheat growth and aphid performance differently depending on virus infection. Insects 2020, 11, 459. [Google Scholar] [CrossRef] [PubMed]
- Thackray, D.J.; Diggle, A.J.; Jones, R.A.C. BYDV PREDICTOR: A simulation model to predict aphid arrival, epidemics of Barley yellow dwarf virus and yield losses in wheat crops in a Mediterranean-type environment. Plant Pathol. 2009, 58, 186–202. [Google Scholar] [CrossRef]
- van den Bosch, F.; Akudibilah, G.; Seal, S.E.; Jeger, M.J. Host resistance and the evolutionary response of plant viruses. J. Appl. Ecol. 2006, 43, 506–516. [Google Scholar] [CrossRef]
- Rousseau, E.; Bonneault, M.; Fabre, F.; Moury, B.; Mailleret, L.; Grognard, F. Virus epidemics, plant-controlled population bottlenecks and the durability of plant resistance. Philos. Trans. R. Soc. B 2019, 374, 20180263. [Google Scholar] [CrossRef] [Green Version]
- Van den Bosch, F.; Jeger, M.J.; Gilligan, C.A. Disease control and its selection for damaging plant virus strains in vegetatively propagated staple food crops: A theoretical assessment. Proc. Biol. Sci. 2007, 274, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Hamelin, F.M.; Allen, L.J.; Prendeville, H.R.; Hajimorad, M.R.; Jeger, M.J. The evolution of plant virus transmission pathways. J. Theor. Biol. 2016, 396, 75–89. [Google Scholar] [CrossRef] [Green Version]
- Fabre, F.; Montarry, J.; Coville, J.; Senoussi, R.; Simon, V.; Moury, B. Modelling the evolutionary dynamics of viruses within their hosts: A case study using high-throughput sequencing. PLoS Pathog. 2012, 8, e1002654. [Google Scholar] [CrossRef] [PubMed]
- Mauck, K.E.; Kenney, J.; Chesnais, Q. Progress and challenges in identifying molecular mechanisms underlying host and vector manipulation by plant viruses. Curr. Opin. Insect Sci. 2019, 33, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Chamchod, F.; Britton, N.F. Analysis of a vector-bias model on malaria transmission. Bull. Math. Biol. 2011, 73, 639–657. [Google Scholar] [CrossRef] [PubMed]





| Virus | Host | Vector | A | B | Additional Comments | Ref. |
|---|---|---|---|---|---|---|
| Soybean vein necrosis virus | Soybean | Neohydatothrips variabilis | ? | Y | Viruliferous vectors produced more offspring but excessive accumulation led to lower viability | [187] |
| Tomato spotted wilt virus | Pepper | Frankliniella occidentalis | Y | Y | Exposure to TSWV as larvae gave shorter developmental times | [188] |
| Tomato spotted wilt virus | Arabidpsis thaliana | Frankliniella occidentalis/Thrips tabaci | ? | ? | Plants infected with a non-transmissible thrips strain were preferred over uninfected plants. Transmissibility by thrips of TSWV was unrelated to vector preference | [189] |
| Watermelon silver mottle virus (P) | Watermelon | Thrips palmi | Y | N | T palmi also preferred feeding on thrips -damaged plants to healthy plants. Mixed effect on thrips performance parameters | [190] |
| Tomato spotted wilt virus (P) | Peanut | Frankliniella fusca | ? | ? | Preference refers to “speed of feeding” of non-viruliferous compared with viruliferous F fusca | [191] |
| Tomato chlorosis virus (SP)/Tomato severe rugose virus (P) | Tomato | Bemisia tabaci | ? | ? | ToSRV whiteflies preferred volatiles from non-infected plants; non-viruliferous whiteflies avoided volatiles from ToCV infected plants | [192] |
| Tomato yellow leaf curl virus (P) | Tomato | Bemisia tabaci | Y | Y | Preferences were only prominent on a susceptible rather than resistant genotype. Developmental time was only reduced on TYLCV-infected plants | [193] |
| Tomato yellow leaf curl virus (P) | Tomato | Bemisia tabaci | Y/N | N | Virus-free Q-type preferred TYLCV infected plants; virus-free B-type preferred healthy plants. TYLCV whiteflies (both Q and B) show no preference for TYLCV-infected or virus-free plants | [194] |
| Barley yellow dwarf virus | Wheat | Rhopalosiphum padi | Y | N | Non-viruliferous preference was not affected by plants co-infected with Gibberella zeae, which also supported greater population growth | [195] |
| Cardamon bushy dwarf virus | Cardamon | Micromyzus kalimpongensis | ? | Y | Aphids grown on CBDV plants had shortened nymphal periods and increased longevity and fecundity | [196] |
| Pea enation mosaic virus (P)/Bean leaf roll virus (P) | Pea | Acyrthrosiphum pisum | Y | ? | The two viruses differed in their distribution within the plant, but aphids did not discriminate between plants infected by the two viruses. There was earlier nymph production on both infected plants but divergent age specific effects depending on the virus | [197] |
| Cucumber mosaic virus (NP) | Squash/Pepper | Aphis gossypii | Y | ? | Isolates from squash induced in squash the type of preference behaviour previously found. An isolate from pepper on pepper was more neutral. Cross-host inoculations showed (mal)adaptive effects. | [198] |
| Sweet potato potyviruses | Sweet potato and Ipomea weeds | Myzus persicae | Y/N | ? | In sweet potato there was preference for virus-infected plants. In the Ipomea weeds, there was preference for noninfected plants | [199] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeger, M.J. The Epidemiology of Plant Virus Disease: Towards a New Synthesis. Plants 2020, 9, 1768. https://doi.org/10.3390/plants9121768
Jeger MJ. The Epidemiology of Plant Virus Disease: Towards a New Synthesis. Plants. 2020; 9(12):1768. https://doi.org/10.3390/plants9121768
Chicago/Turabian StyleJeger, Michael J. 2020. "The Epidemiology of Plant Virus Disease: Towards a New Synthesis" Plants 9, no. 12: 1768. https://doi.org/10.3390/plants9121768
APA StyleJeger, M. J. (2020). The Epidemiology of Plant Virus Disease: Towards a New Synthesis. Plants, 9(12), 1768. https://doi.org/10.3390/plants9121768




