Exogenous Salicylic Acid Modulates the Response to Combined Salinity-Temperature Stress in Pepper Plants (Capsicum annuum L. var. Tamarin)
Abstract
:1. Introduction
2. Results
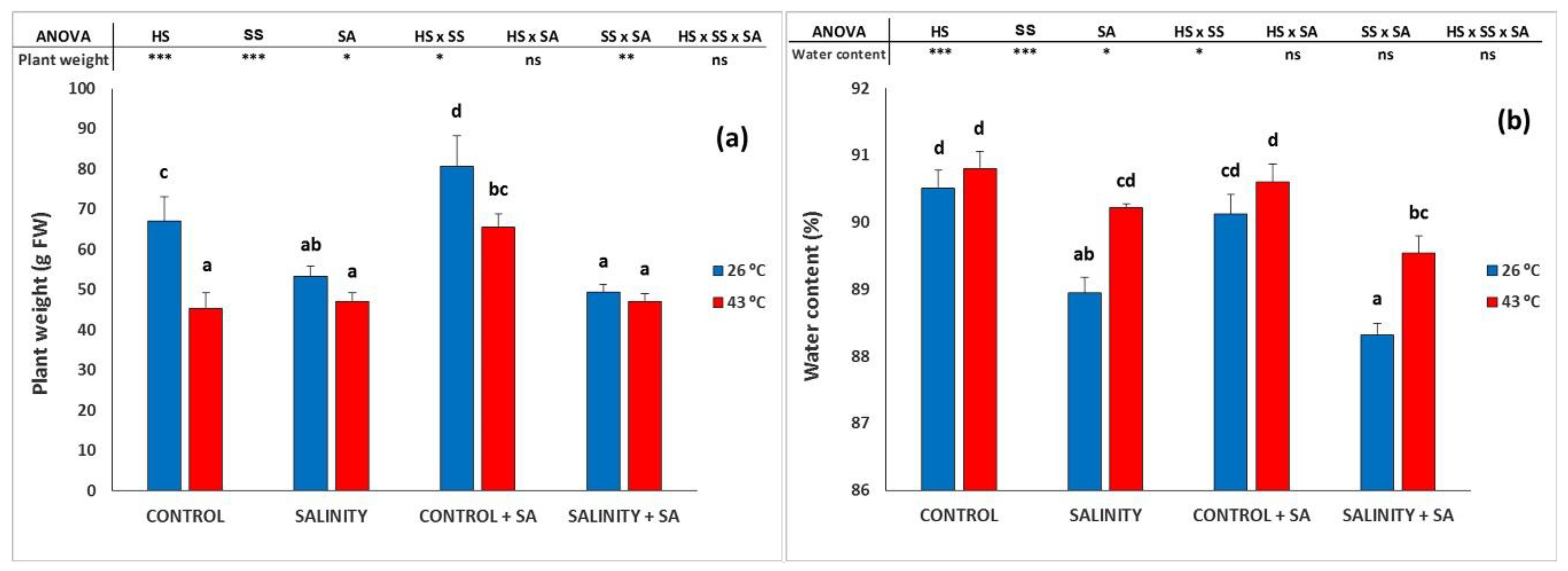
2.1. Plant Growth
2.2. Ion Concentrations
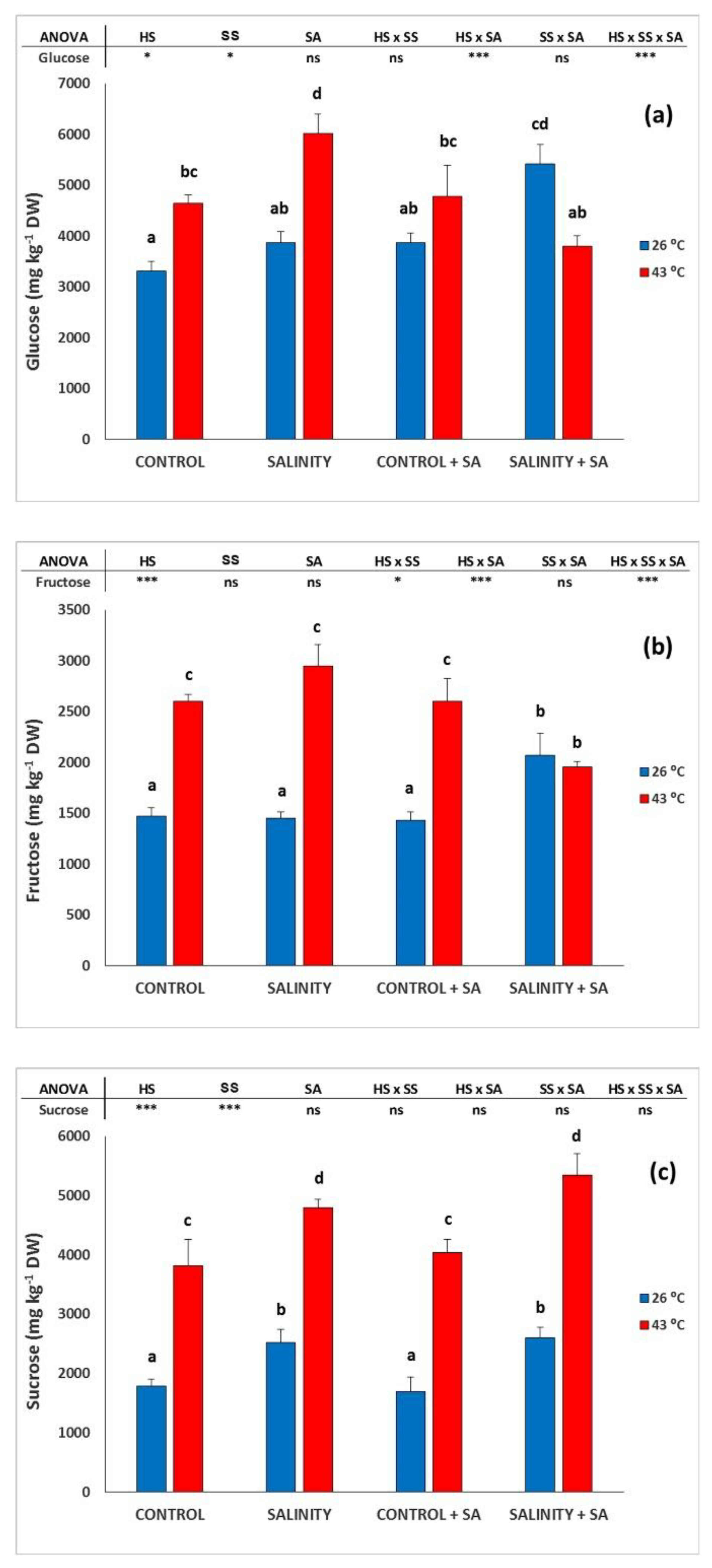
2.3. Total Soluble Sugars
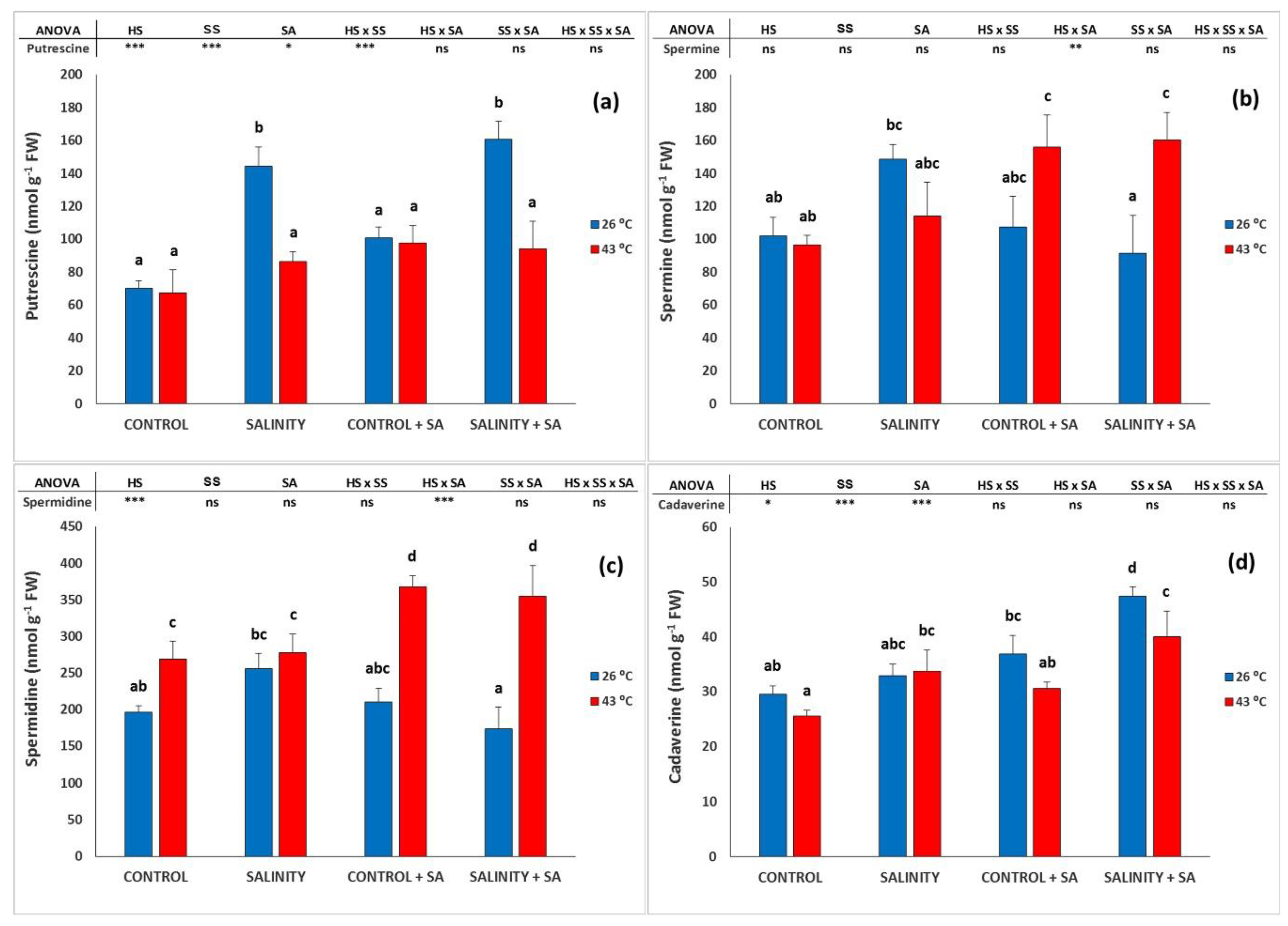
2.4. Polyamines Analysis
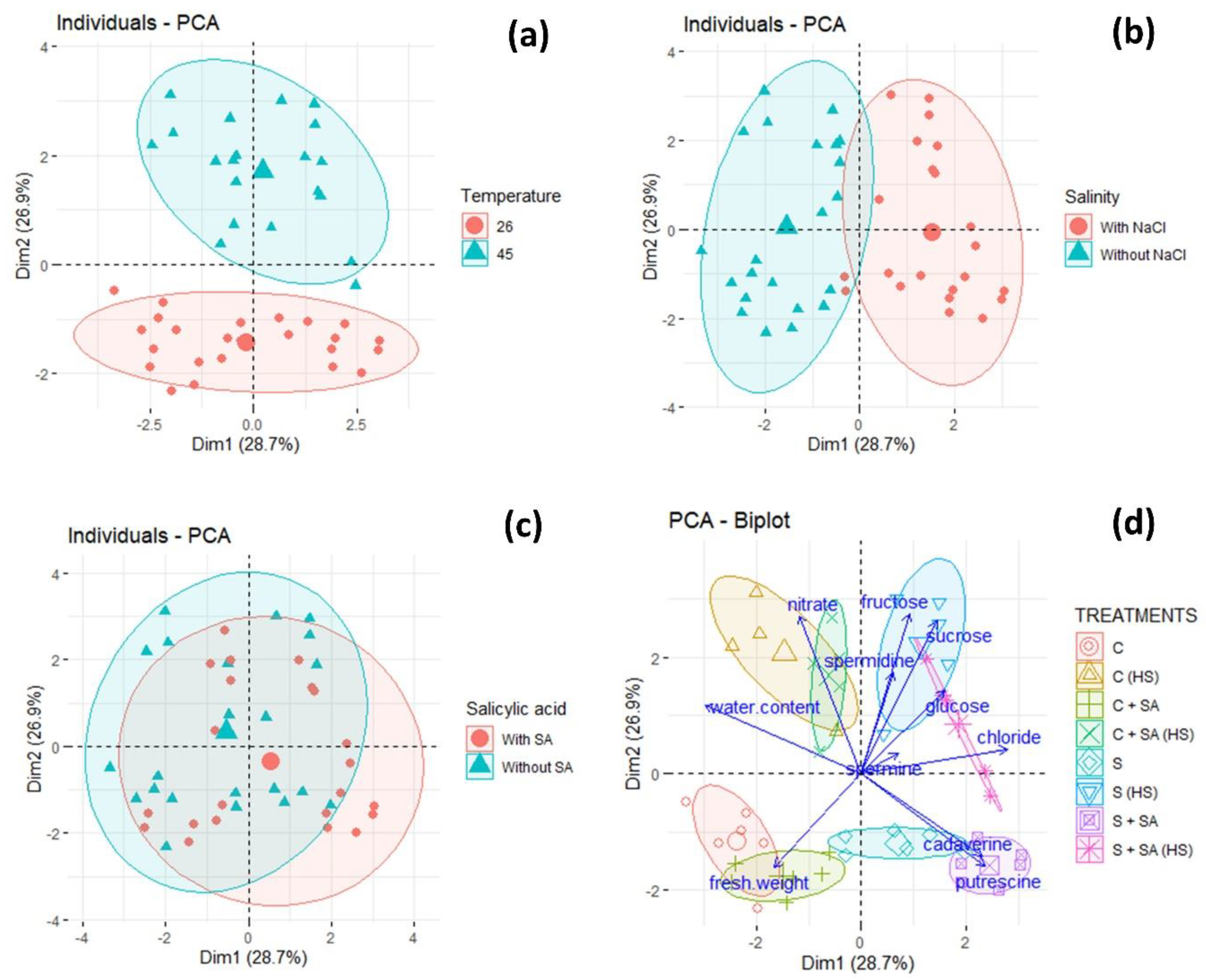
2.5. Principal Component Analysis (PCA)
3. Discussion
4. Materials and Methods
4.1. Growth Conditions and Treatments Applied
4.2. Plant Growth
4.3. Ion Concentrations
4.4. Total Soluble Sugars
4.5. Polyamines Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pandey, P.; Irulappan, V.; Bagavathiannan, M.V.; Senthil-Kumar, M. Impact of Combined Abiotic and Biotic Stresses on Plant Growth and Avenues for Crop Improvement by Exploiting Physio-morphological Traits. Front. Plant Sci. 2017, 8, 537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, N.J.; Lilley, C.J.; Urwin, P.E. Identification of Genes Involved in the Response of Arabidopsis to Simultaneous Biotic and Abiotic Stresses. Plant Physiol. 2013, 162, 2028–2041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittler, R.; Blumwald, E. Genetic Engineering for Modern Agriculture: Challenges and Perspectives. Annu. Rev. Plant Biol. 2010, 61, 443–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francini, A.; Sebastiani, L. Abiotic Stress Effects on Performance of Horticultural Crops. Horticulturae 2019, 5, 67. [Google Scholar] [CrossRef] [Green Version]
- E Hansen, J.; Sato, M.; Ruedy, R. Perception of climate change. Proc. Natl. Acad. Sci. USA 2012, 109, E2415–E2423. [Google Scholar] [CrossRef] [Green Version]
- Ciais, P.; Reichstein, M.; Viovy, N.; Granier, A.; Ogée, J.; Allard, V.; Aubinet, M.; Buchmann, N.; Bernhofer, C.; Carrara, A.; et al. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 2005, 437, 529–533. [Google Scholar] [CrossRef]
- Bastos, A.; Gouveia, C.; Trigo, R.M.; Running, S.W. Comparing the impacts of 2003 and 2010 heatwaves in NPP over Europe. Biogeosci. Discuss. 2013, 10, 15879–15911. [Google Scholar] [CrossRef]
- Ding, P.; Ding, Y. Stories of Salicylic Acid: A Plant Defense Hormone. Trends Plant Sci. 2020, 25, 549–565. [Google Scholar] [CrossRef]
- Rekhter, D.; Lüdke, D.; Ding, Y.; Feussner, K.; Zienkiewicz, K.; Lipka, V.; Wiermer, M.; Zhang, Y.; Petit-Houdenot, Y. Isochorismate-derived biosynthesis of the plant stress hormone salicylic acid. Science 2019, 365, 498–502. [Google Scholar] [CrossRef]
- Shaar-Moshe, L.; Blumwald, E.; Peleg, Z. Unique Physiological and Transcriptional Shifts under Combinations of Salinity, Drought, and Heat. Plant Physiol. 2017, 174, 421–434. [Google Scholar] [CrossRef] [Green Version]
- Torun, H. Time-course analysis of salicylic acid effects on ROS regulation and antioxidant defense in roots of hulled and hulless barley under combined stress of drought, heat and salinity. Physiol. Plant. 2018, 165, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wang, Y.; Li, X.; Zhang, Y. Biosynthesis and Regulation of Salicylic Acid and N-Hydroxypipecolic Acid in Plant Immunity. Mol. Plant 2020, 13, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Janda, T.; Szalai, G.; Pál, M. Salicylic Acid Signalling in Plants. Int. J. Mol. Sci. 2020, 21, 2655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Amor, F.M.; Cuadra-Crespo, P. Plant growth-promoting bacteria as a tool to improve salinity tolerance in sweet pepper. Funct. Plant Biol. 2012, 39, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Rameshwaran, P.; Tepe, A.; Yazar, A.; Ragab, R. Effects of drip-irrigation regimes with saline water on pepper productivity and soil salinity under greenhouse conditions. Sci. Hortic. 2016, 199, 114–123. [Google Scholar] [CrossRef] [Green Version]
- Erickson, A.N.; Markhart, A.H. Flower developmental stage and organ sensitivity of bell pepper (Capsicum annuum L.) to elevated temperature. Plant Cell Environ. 2002, 25, 123–130. [Google Scholar] [CrossRef]
- Guo, M.; Yin, Y.-X.; Ji, J.-J.; Ma, B.-P.; Lu, M.-H.; Gong, Z.-H. Cloning and expression analysis of heat-shock transcription factor gene CaHsfA2 from pepper (Capsicum annuum L.). Genet. Mol. Res. 2014, 13, 1865–1875. [Google Scholar] [CrossRef]
- Kafizadeh, N.; Carapetian, J.; Manouchehri Kalantari, K. Effects of Heat Stress on Pollen Viability and Pollen Tube Growth in Pepper. Res. J. Biol. Sci. 2008, 3, 1159–1162. [Google Scholar]
- Li, T.; Xu, X.; Li, Y.; Wang, H.; Li, Z.; Li, Z. Comparative transcriptome analysis reveals differential transcription in heat-susceptible and heat-tolerant pepper (Capsicum annum L.) cultivars under heat stress. J. Plant Biol. 2015, 58, 411–424. [Google Scholar] [CrossRef]
- Vicente, M.R.-S.; Plasencia, J. Salicylic acid beyond defence: Its role in plant growth and development. J. Exp. Bot. 2011, 62, 3321–3338. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.I.R.; Iqbal, N.; Masood, A.; Per, T.S.; A Khan, N. Salicylic acid alleviates adverse effects of heat stress on photosynthesis through changes in proline production and ethylene formation. Plant Signal. Behav. 2013, 8, e26374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horváth, E.; Szalai, G.; Janda, T. Induction of Abiotic Stress Tolerance by Salicylic Acid Signaling. J. Plant Growth Regul. 2007, 26, 290–300. [Google Scholar] [CrossRef]
- Pancheva, T.; Popova, L.; Uzunova, A. Effects of salicylic acid on growth and photosynthesis in barley plants. J. Plant Physiol. 1996, 149, 57–63. [Google Scholar] [CrossRef]
- Gunes, A.; Inal, A.; Alpaslan, M.; Eraslan, F.; Bagci, E.G.; Cicek, N. Salicylic acid induced changes on some physiological parameters symptomatic for oxidative stress and mineral nutrition in maize (Zea mays L.) grown under salinity. J. Plant Physiol. 2007, 164, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Hayat, Q.; Hayat, S.; Irfan, M.; Ahmad, A. Effect of exogenous salicylic acid under changing environment: A review. Environ. Exp. Bot. 2010, 68, 14–25. [Google Scholar] [CrossRef]
- Barba-Espín, G.; Clemente-Moreno, M.J.; Álvarez, S.; García-Legaz, M.F.; Hernández, J.A.; Diaz-Vivancos, P. Salicylic acid negatively affects the response to salt stress in pea plants. Plant Biol. 2011, 13, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Borsani, O.; Valpuesta, V.; Botella, J.R. Evidence for a Role of Salicylic Acid in the Oxidative Damage Generated by NaCl and Osmotic Stress in Arabidopsis Seedlings. Plant Physiol. 2001, 126, 1024–1030. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Coronado, M.A.; Trejo-López, C.; Larqué-Saavedra, A. Effects of salicylic acid on the growth of roots and shoots in soybean. Plant Physiol. Biochem. 1998, 36, 563–565. [Google Scholar] [CrossRef]
- Nazar, R.; Iqbal, N.; Syeed, S.; Khan, N.A. Salicylic acid alleviates decreases in photosynthesis under salt stress by enhancing nitrogen and sulfur assimilation and antioxidant metabolism differentially in two mungbean cultivars. J. Plant Physiol. 2011, 168, 807–815. [Google Scholar] [CrossRef]
- Silva, E.N.; Vieira, S.A.; Ribeiro, R.V.; Ponte, L.F.A.; Ferreira-Silva, S.L.; Silveira, J.A.G. Contrasting Physiological Responses of Jatropha curcas Plants to Single and Combined Stresses of Salinity and Heat. J. Plant Growth Regul. 2013, 32, 159–169. [Google Scholar] [CrossRef]
- Rivero, R.M.; Mestre, T.C.; Mittler, R.; Rubio, F.; Garcia-Sanchez, F.; Martinez, V. The combined effect of salinity and heat reveals a specific physiological, biochemical and molecular response in tomato plants. Plant Cell Environ. 2013, 37, 1059–1073. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Li, S.; Liang, Y.; Zhao, H.; Hou, L.; Shi, Y.; Ahammed, G.J. Effects of Exogenous Spermidine and Elevated CO2 on Physiological and Biochemical Changes in Tomato Plants Under Iso-osmotic Salt Stress. J. Plant Growth Regul. 2018, 37, 1222–1234. [Google Scholar] [CrossRef]
- Ruan, Y.-L. Sucrose Metabolism: Gateway to Diverse Carbon Use and Sugar Signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef]
- Elwan, M.; El-Hamahmy, M. Improved productivity and quality associated with salicylic acid application in greenhouse pepper. Sci. Hortic. 2009, 122, 521–526. [Google Scholar] [CrossRef]
- Zhou, R.; Yu, X.; Kjær, K.H.; Rosenqvist, E.; Ottosen, C.-O.; Wu, Z. Screening and validation of tomato genotypes under heat stress using Fv/Fm to reveal the physiological mechanism of heat tolerance. Environ. Exp. Bot. 2015, 118, 1–11. [Google Scholar] [CrossRef]
- Teskey, R.; Wertin, T.; Bauweraerts, I.; Ameye, M.; McGuire, M.A.; Steppe, K. Responses of tree species to heat waves and extreme heat events. Plant Cell Environ. 2015, 38, 1699–1712. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, J.G. Osmotic adjustment and plant adaptation to environmental changes related to drought and salinity. Environ. Rev. 2010, 18, 309–319. [Google Scholar] [CrossRef]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef]
- Mostafaei, E.; Zehtab-Salmasi, S.; Salehi-Lisar, Y.; Ghassemi-Golezani, K. Changes in photosynthetic pigments, osmolytes and antioxidants of Indian mustard by drought and exogenous polyamines. Acta Biol. Hung. 2018, 69, 313–324. [Google Scholar] [CrossRef] [Green Version]
- Piñero, M.C.; Otálora, G.; Porras, M.E.; Sánchez-Guerrero, M.C.; Lorenzo, P.; Medrano, E.; Del Amor, F.M. The Form in Which Nitrogen Is Supplied Affects the Polyamines, Amino Acids, and Mineral Composition of Sweet Pepper Fruit under an Elevated CO2Concentration. J. Agric. Food Chem. 2017, 65, 711–717. [Google Scholar] [CrossRef]
- Piñero, M.C.; Porras, M.E.; López-Marín, J.; Sánchez-Guerrero, M.C.; Medrano, E.; Lorenzo, P.; Del Amor, F.M. Differential Nitrogen Nutrition Modifies Polyamines and the Amino-Acid Profile of Sweet Pepper Under Salinity Stress. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Tajti, J.; Hamow, K.Á.; Majláth, I.; Gierczik, K.; Németh, E.; Janda, T.; Pál, M. Polyamine-Induced Hormonal Changes in eds5 and sid2 Mutant Arabidopsis Plants. Int. J. Mol. Sci. 2019, 20, 5746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collado-González, J.; Piñero, M.C.; Otálora, G.; López-Marín, J.; Del Amor, F.M. Exogenous spermidine modifies nutritional and bioactive constituents of cauliflower (Brassica oleracea var. botrytis L.) florets under heat stress. Sci. Hortic. 2021, 277, 109818. [Google Scholar] [CrossRef]
- Gupta, K.; Dey, A.; Gupta, B. Plant polyamines in abiotic stress responses. Acta Physiol. Plant. 2013, 35, 2015–2036. [Google Scholar] [CrossRef]
- Tanou, G.; Ziogas, V.; Belghazi, M.; Christou, A.; Filippou, P.; Job, D.; Fotopoulos, V.; Molassiotis, A. Polyamines reprogram oxidative and nitrosative status and the proteome of citrus plants exposed to salinity stress. Plant Cell Environ. 2013, 37, 864–885. [Google Scholar] [CrossRef]
- Piñero, M.C.; Otálora, G.; Collado, J.; López-Marín, J.; Del Amor, F.M. Foliar application of putrescine before a short-term heat stress improves the quality of melon fruits (Cucumis melo L.). J. Sci. Food Agric. 2020. [Google Scholar] [CrossRef]
- Upadhyay, R.; Fatima, T.; Handa, A.K.; Mattoo, A.K. Polyamines and Their Biosynthesis/Catabolism Genes are Differentially Modulated in Response to Heat Versus Cold Stress in Tomato Leaves (Solanum lycopersicum L.). Cells 2020, 9, 1749. [Google Scholar] [CrossRef]
- Colmenero-Flores, J.M.; Rosales, M.A. Interaction between salt and heat stress: When two wrongs make a right. Plant Cell Environ. 2014, 37, 1042–1045. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 12 November 2020).

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otálora, G.; Piñero, M.C.; Collado-González, J.; López-Marín, J.; del Amor, F.M. Exogenous Salicylic Acid Modulates the Response to Combined Salinity-Temperature Stress in Pepper Plants (Capsicum annuum L. var. Tamarin). Plants 2020, 9, 1790. https://doi.org/10.3390/plants9121790
Otálora G, Piñero MC, Collado-González J, López-Marín J, del Amor FM. Exogenous Salicylic Acid Modulates the Response to Combined Salinity-Temperature Stress in Pepper Plants (Capsicum annuum L. var. Tamarin). Plants. 2020; 9(12):1790. https://doi.org/10.3390/plants9121790
Chicago/Turabian StyleOtálora, Ginés, María Carmen Piñero, Jacinta Collado-González, Josefa López-Marín, and Francisco M. del Amor. 2020. "Exogenous Salicylic Acid Modulates the Response to Combined Salinity-Temperature Stress in Pepper Plants (Capsicum annuum L. var. Tamarin)" Plants 9, no. 12: 1790. https://doi.org/10.3390/plants9121790





