The Search for Quorum Sensing in Botrytis cinerea: Regulatory Activity of Its Extracts on Its Development
Abstract
:1. Introduction
2. Results
2.1. Extracts Preparation and Liquid Chromatography/Mass Spectrometry Analysis
2.2. Effect of Extracts on B. cinerea
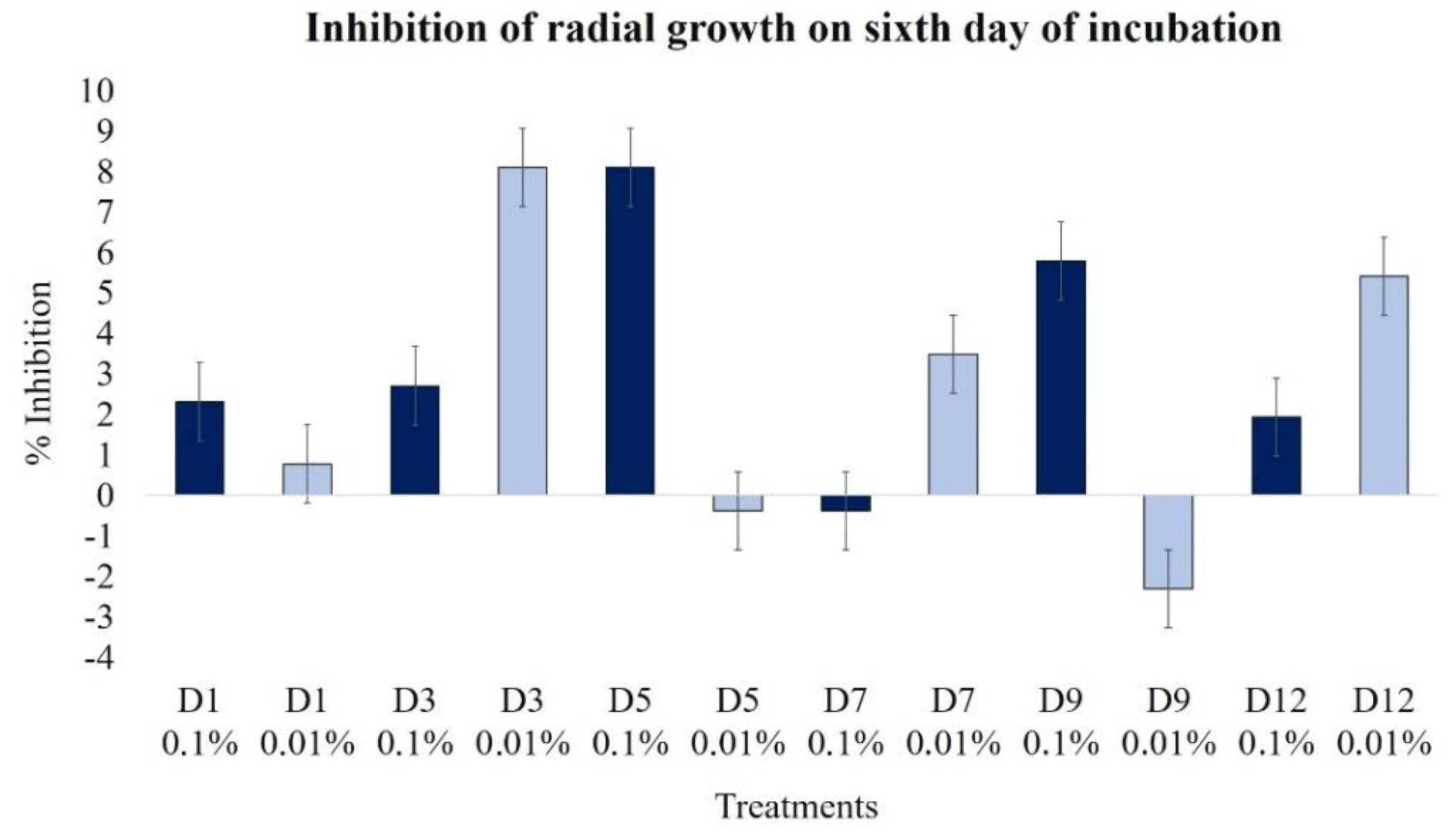
2.2.1. Effect on Radial Growth
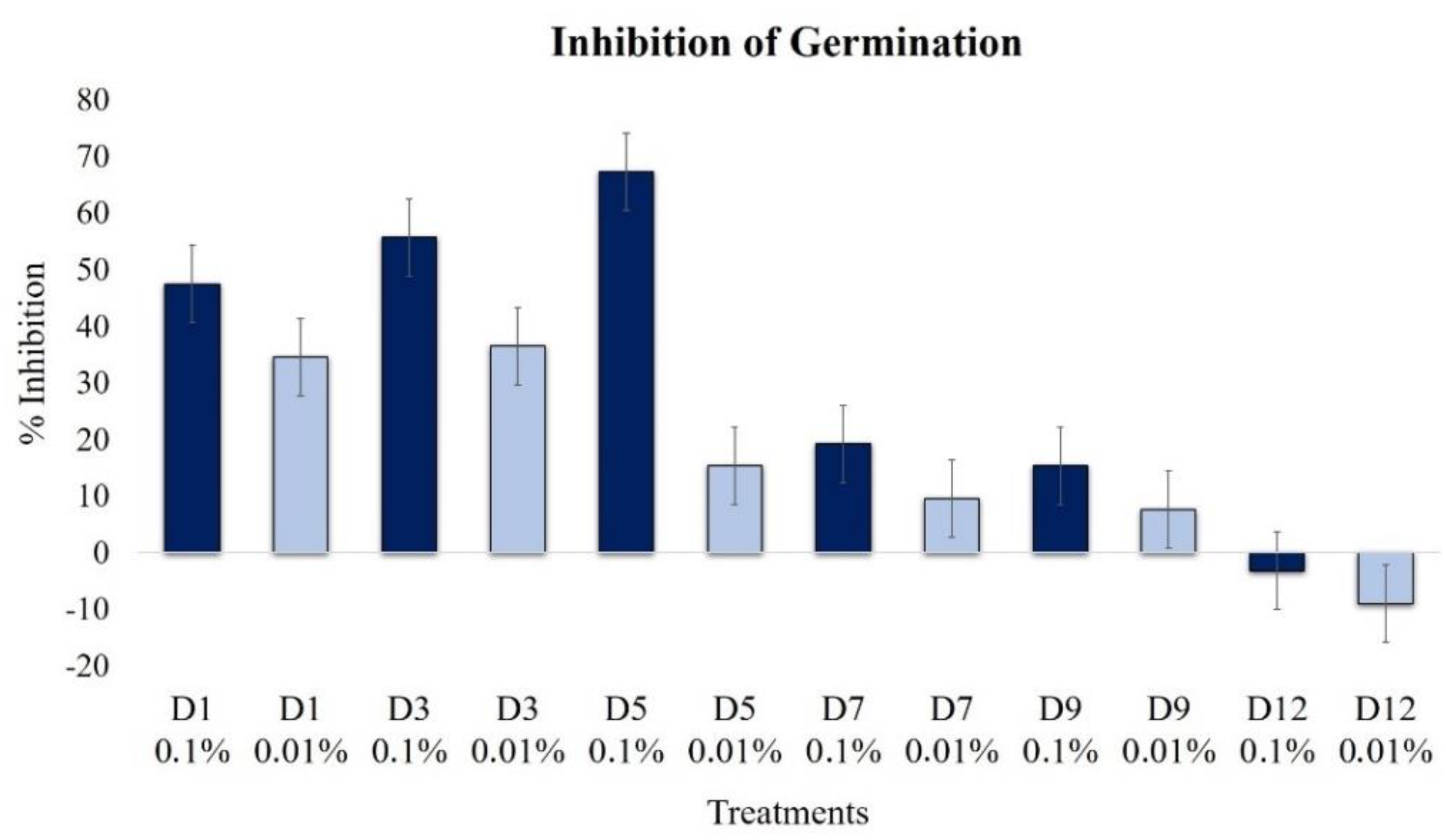
2.2.2. Effect on Conidia Germination
2.2.3. Effect on Elongation of Germ Tubes
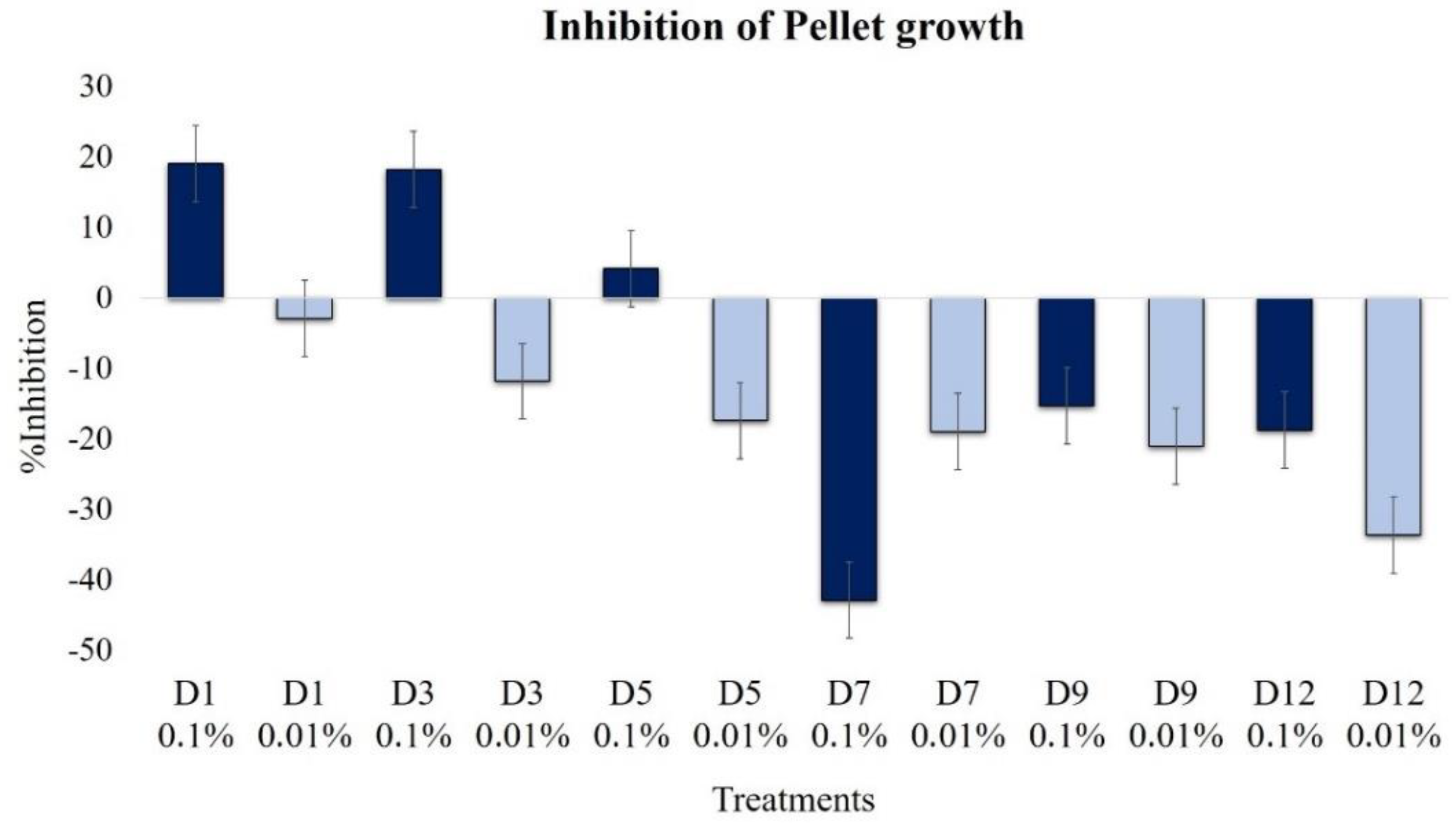
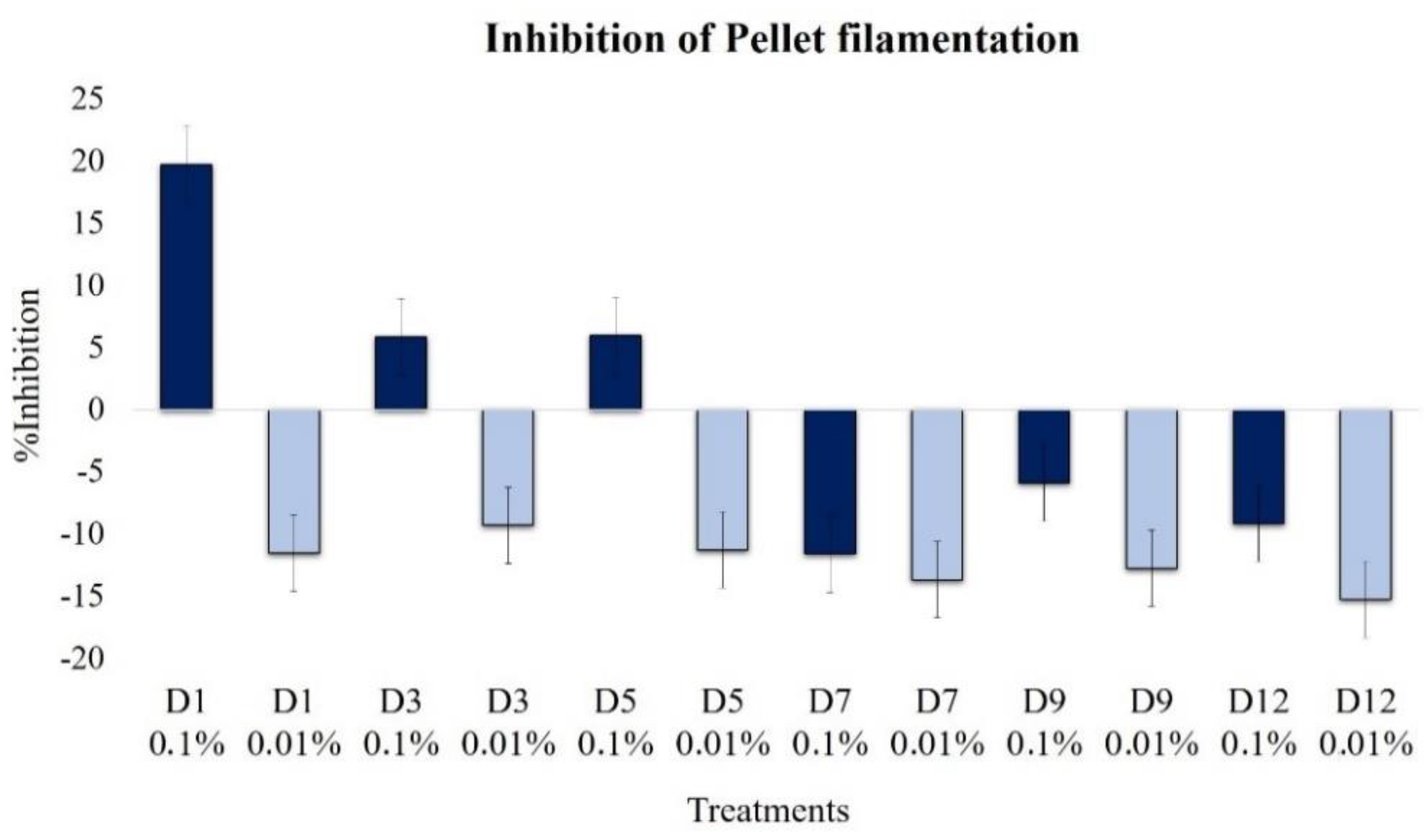
2.2.4. Effect on Pellet Formation and Filamentation
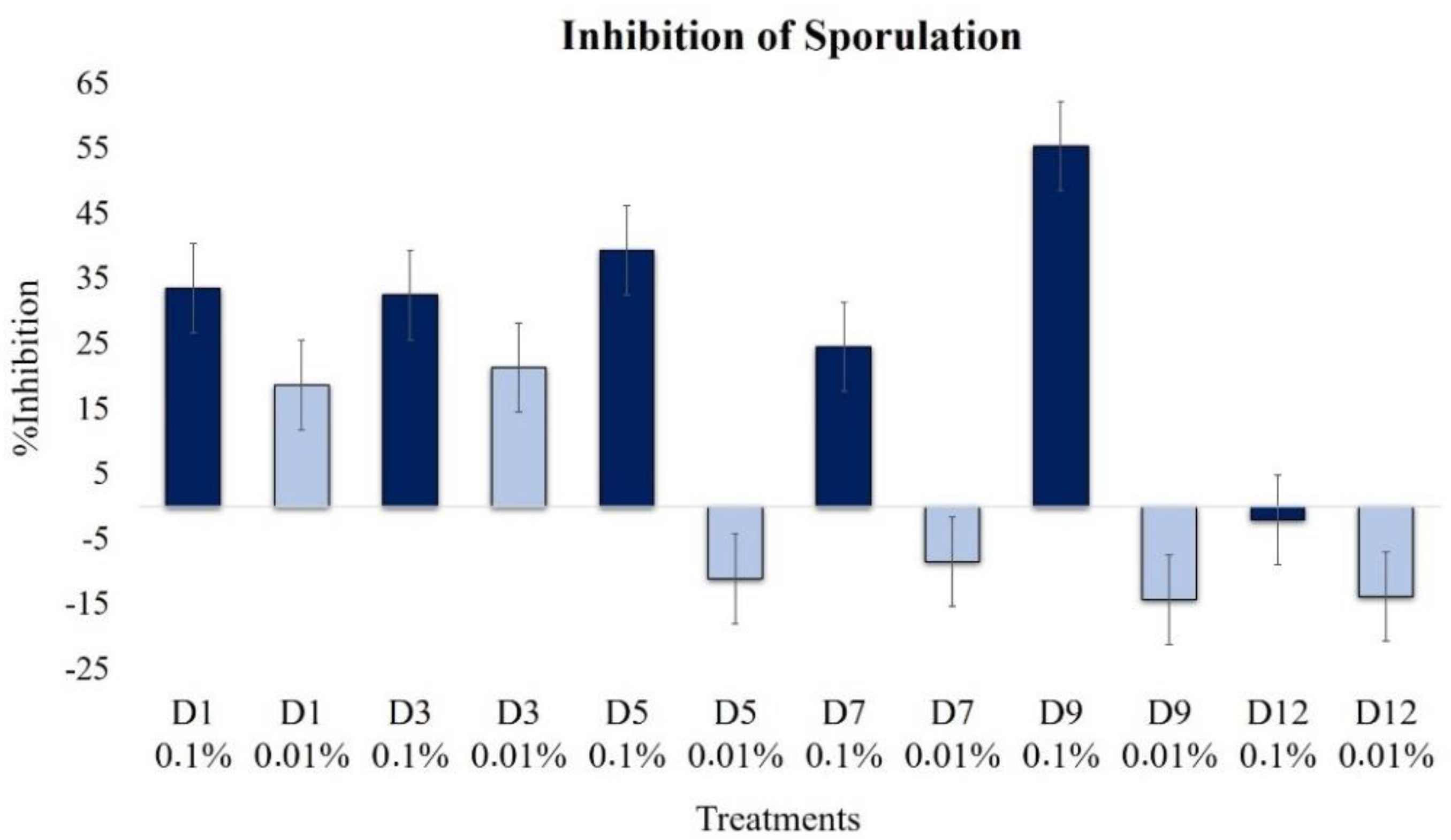
2.2.5. Effect on Sporulation
2.2.6. Effect on Biofilm Formation
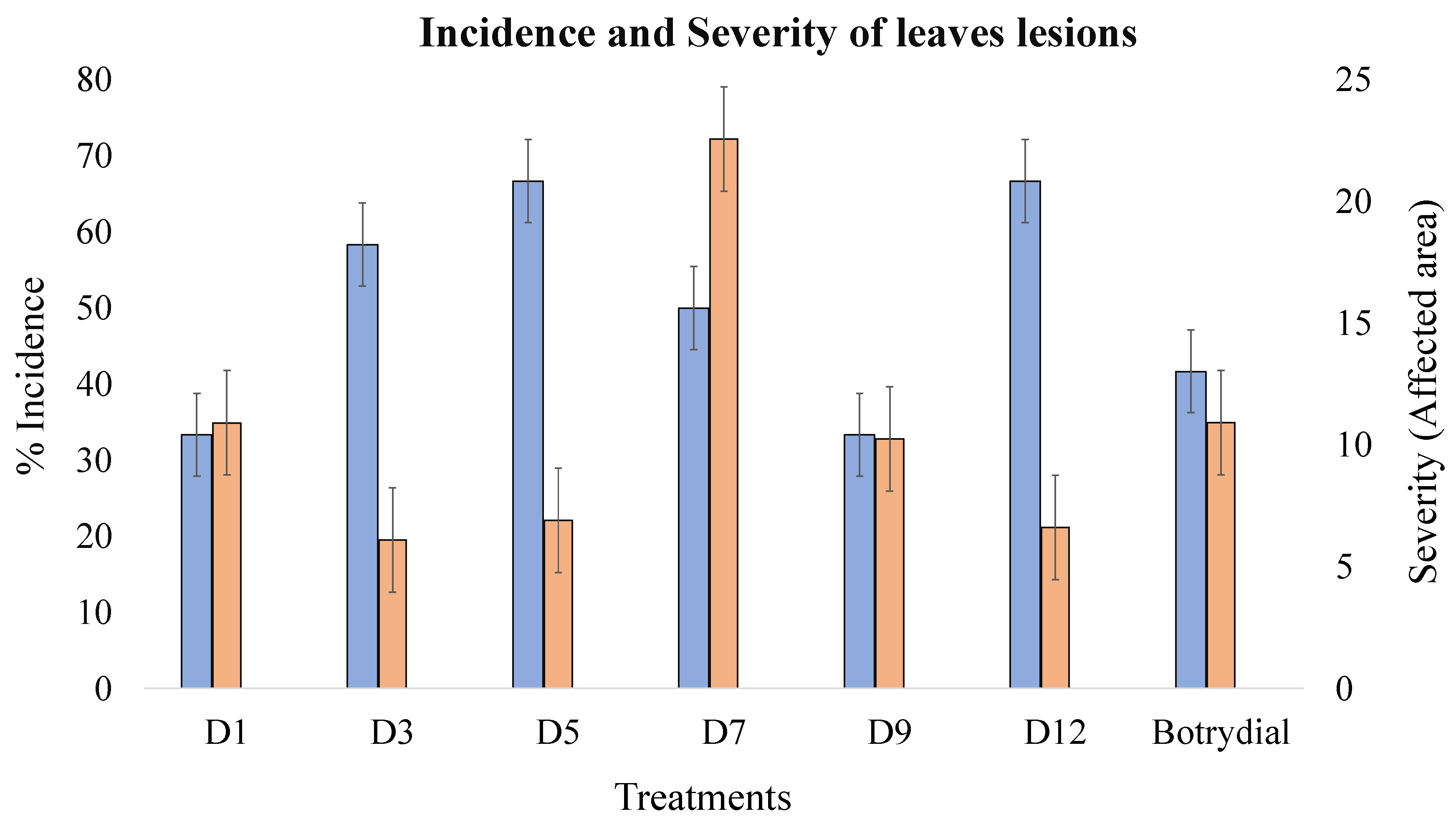
2.3. Extract Phytotoxicity
3. Discussion
4. Materials and Methods
4.1. Equipment and Software
4.2. Fungal Strain
4.3. Molecular Identification
4.4. Obtention and Analysis of B. cinerea Extracts
4.5. Effect of Extracts on B. cinerea Growth
4.5.1. Effect on Radial Growth
4.5.2. Effect on Germination
4.5.3. Effect on Germ Tube Development
4.5.4. Effect on pellet formation
4.5.5. Effect on Biofilm Formation in Submerged Cultures
4.5.6. Effect on Sporulation
4.6. Phytotoxic Effects of the Extracts of B. cinerea
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boddy, L. Pathogens of Autotrophs. In The Fungi, 3rd ed.; Academic Press: Cardiff, UK, 2015; pp. 245–292. ISBN 9780123820341. [Google Scholar]
- Elad, Y.; Pertot, I.; Cotes Prado, A.M.; Stewart, A. Plant Hosts of Botrytis spp. In Botrytis—The Fungus, the Pathogen and Its Management in Agricultural Systems; Springer International Publishing: Cham, Switzerland, 2016; pp. 413–486. [Google Scholar]
- Romanazzi, G.; Smilanick, J.L.; Feliziani, E.; Droby, S. Integrated management of postharvest gray mold on fruit crops. Postharvest Biol. Technol. 2016, 113, 69–76. [Google Scholar] [CrossRef]
- Barriuso, J.; Hogan, D.A.; Keshavarz, T.; Martínez, M.J. Role of quorum sensing and chemical communication in fungal biotechnology and pathogenesis. FEMS Microbiol. Rev. 2018, 42, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, P.; Casadevall, A. Quorum sensing in fungi—A review. Med. Mycol. 2012, 50, 337–345. [Google Scholar] [CrossRef] [Green Version]
- Harding, M.W.; Marques, L.L.R.; Howard, R.J.; Olson, M.E. Can filamentous fungi form biofilms? Trends Microbiol. 2009, 17, 475–480. [Google Scholar] [CrossRef]
- Sharma, R.; Jangid, K. Fungal quorum sensing inhibitors. In Quorum Sensing vs. Quorum Quenching: A Battle with No End in Sight; Springer: New Delhi, India, 2015; pp. 237–257. ISBN 9788132219828. [Google Scholar]
- Padder, S.A.; Prasad, R.; Shah, A.H. Quorum sensing: A less known mode of communication among fungi. Microbiol. Res. 2018, 210, 51–58. [Google Scholar] [CrossRef]
- Johansen, P.; Jespersen, L. Impact of quorum sensing on the quality of fermented foods. Curr. Opin. Food Sci. 2017, 13, 16–25. [Google Scholar] [CrossRef]
- Majumdar, S.; Mondal, S. Perspectives on quorum sensing in fungi. Int. J. Mod. Biol. Med. 2015, 6, 170–180. [Google Scholar]
- Rosero-Hernández, E.D.; Moraga, J.; Collado, I.G.; Echeverri, F.; Rosero-Hernández, E.D.; Moraga, J.; Collado, I.G.; Echeverri, F. Natural Compounds That Modulate the Development of the Fungus Botrytis cinerea and Protect Solanum lycopersicum. Plants 2019, 8, 111. [Google Scholar] [CrossRef] [Green Version]
- Witzany, G. Introduction: Keylevels of biocommunication in fungi. In Biocommunication of Fungi; Witzany, G., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–18. ISBN 9789400742642. [Google Scholar]
- Verheecke, C.; Choque, E.; Mathieu, F. Application of fungal metabolites against mycotoxins production. In Fungal Metabolites; Springer International Publishing: Cham, Switzerland, 2016; pp. 701–737. ISBN 978-3-319-19456-1. [Google Scholar]
- Tseng, C.C.; Fink, G.R. Quorum Sensing in Fungi. In Chemical Communication among Bacteria; Winans, S.C., Bassler, B.L., Eds.; American Society of Microbiology: Washington, DC, USA, 2008; pp. 443–451. [Google Scholar]
- Yannai, S. Dictionary of Food Compounds with CD-ROM; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Nickerson, K.W.; Atkin, A.L.; Hornby, J.M. Quorum sensing in dimorphic fungi: Farnesol and beyond. Appl. Environ. Microbiol. 2006, 72, 3805–3813. [Google Scholar] [CrossRef] [Green Version]
- Collado, I.G.; Sánchez, A.J.M.; Hanson, J.R. Fungal terpene metabolites: Biosynthetic relationships and the control of the phytopathogenic fungus Botrytis cinerea. Nat. Prod. Rep. 2007, 24, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Akutsu, K. Virulence factors of Botrytis cinerea. J. Gen. Plant Pathol. 2014, 80, 15–23. [Google Scholar] [CrossRef]
- Durán-Patrón, R.; Cantoral, J.M.; Hernández-Galán, R.; Hanson, J.R.; Collado, I.G. The biodegradation of the phytotoxic metabolite botrydial by its parent organism, Botrytis cinerea. J. Chem. Res. 2004, 2004, 441–443. [Google Scholar] [CrossRef]
- Bautista Baños, S. Postharvest Environmental Factors Affecting Infection of Kiwifruit by Botrytis cinerea. Ph.D. Thesis, Massey University, Palmerston North, New Zealand, 1995. [Google Scholar]
- Nassr, S.; Barakat, R. Effect of Factors on Conidium Germination of Botrytis cinerea in vitro. Int. J. Plant Soil Sci. 2013, 2, 41–54. [Google Scholar] [CrossRef]
- Sharma, R.; Jangid, K. Role of Quorum Sensing in fungal morphogenesis and pathogenesis. In Fungal Metabolites; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–28. ISBN 978-3-319-19456-1. [Google Scholar]
- Schügerl, K.; Wittler, R.; Lorenz, T. The use of molds in pellet form. Trends Biotechnol. 1983, 1, 120–123. [Google Scholar] [CrossRef]
- Alem, M.A.S.; Oteef, M.D.Y.; Flowers, T.H.; Douglas, L.J. Production of tyrosol by Candida albicans biofilms and its role in Quorum Sensing and biofilm development. Eukaryot. Cell 2006, 5, 1770–1779. [Google Scholar] [CrossRef] [Green Version]
- Krzyczkowska, J.; Phan-Thi, H.; Waché, Y. Lactone Formation in Yeast and Fungi. In Fungal Metabolites; Springer International Publishing: Cham, Switzerland, 2017; pp. 461–498. [Google Scholar]
- Pinedo, C.; Moraga, J.; Barua, J.; Gonzalez-Rodriguez, V.E.; Aleu, J.; Duran-Patron, R.; Macias-Sanchez, A.J.; Hanson, J.R.; Viaud, M.; Hernandez-Galan, R.; et al. Chemically induced cryptic sesquiterpenoids and expression of sesquiterpene cyclases in Botrytis cinerea revealed new sporogenic (+)-4-epieremophil-9-en-11-ols. ACS Chem. Biol. 2016, 11, 1391–1400. [Google Scholar] [CrossRef]
- Suárez, I.; da Silva Lima, G.; Conti, R.; Pinedo, C.; Moraga, J.; Barúa, J.; de Oliveira, A.L.L.; Aleu, J.; Durán-Patrón, R.; Macías-Sánchez, A.J.; et al. Structural and biosynthetic studies on eremophilenols related to the phytoalexin capsidiol, produced by Botrytis cinerea. Phytochemistry 2018, 154, 10–18. [Google Scholar] [CrossRef]
- Malmierca, M.G.; Izquierdo-Bueno, I.; Mccormick, S.P.; Cardoza, R.E.; Alexander, N.J.; Moraga, J.; Gomes, E.V.; Proctor, R.H.; Collado, I.G.; Monte, E.; et al. Botrydial and botcinins produced by Botrytis cinerea regulate the expression of Trichoderma arundinaceum genes involved in trichothecene biosynthesis. Mol. Plant Pathol. 2016, 17, 1017–1031. [Google Scholar] [CrossRef]
- Bacon, C.W.; Hinton, D.M.; Mitchell, T.R. Is Quorum Signaling by Mycotoxins a New Risk-Mitigating Strategy for Bacterial Biocontrol of Fusarium verticillioides and Other Endophytic Fungal Species? J. Agric. Food Chem. 2017, 65, 7071–7080. [Google Scholar] [CrossRef]
- Venkatesh, N.; Keller, N.P. Mycotoxins in Conversation with Bacteria and Fungi. Front. Microbiol. 2019, 10, 403. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Shao, S.; Zheng, C.; Sun, Z.; Shi, J.; Yu, J.; Qi, Z.; Shi, K. Induction of systemic resistance in tomato against Botrytis cinerea by N-decanoyl-homoserine lactone via jasmonic acid signaling. Planta 2018, 247, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, O.; Bélanger, A.; Khanizadeh, S. Variable inhibitory activities of essential oils of three Monarda species on the growth of Botrytis cinerea. Can. J. Plant Sci. 2013, 93, 987–995. [Google Scholar] [CrossRef]
- Mukaremera, L.; Lee, K.K.; Mora-Montes, H.M.; Gow, N.A.R. Candida albicans Yeast, Pseudohyphal, and Hyphal Morphogenesis Differentially Affects Immune Recognition. Front. Immunol. 2017, 8, 629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moraga, J.; Pinedo, C.; Durán-Patrón, R.; Collado, I.G.; Hernández-Galán, R. Botrylactone: New interest in an old molecule—Review of its absolute configuration and related compounds. Tetrahedron 2011, 67, 417–420. [Google Scholar] [CrossRef] [Green Version]
- Olmedo, G.M.; Cerioni, L.; González, M.M.; Cabrerizo, F.M.; Rapisarda, V.A.; Volentini, S.I. Antifungal activity of β-carbolines on Penicillium digitatum and Botrytis cinerea. Food Microbiol. 2017, 62, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Kaliňák, M.; Šimkovič, M.; Žemla, P.; Maťaťa, M.; Molnár, T.; Liptaj, T.; Varečka, Ľ.; Hudecová, D. Changes in metabolome and in enzyme activities during germination of Trichoderma atroviride conidia. FEMS Microbiol. Lett. 2014, 357, 201–207. [Google Scholar]
- Sharifi, R.; Ryu, C.M. Are bacterial volatile compounds poisonous odors to a fungal pathogen Botrytis cinerea, alarm signals to arabidopsis seedlings for eliciting induced resistance, or both? Front. Microbiol. 2016, 7, 196. [Google Scholar] [CrossRef]
- Małolepsza, U.; Urbanek, H. The oxidants and antioxidant enzymes in tomato leaves treated with O-Hydroxyethylorutin and infected with Botrytis cinerea. Eur. J. Plant Pathol. 2000, 106, 657–665. [Google Scholar] [CrossRef]
- Rebordinos, L.; Cantoral, J.M.; Prieto, M.V.; Hanson, J.R.; Collado, I.G. The phytotoxic activity of some metabolites of Botrytis cinerea. Phytochemistry 1996, 42, 383–387. [Google Scholar] [CrossRef]





| Time (day) | Name of Extract | Weight (mg) |
|---|---|---|
| 1 | D1 | 111.5 |
| 3 | D3 | 98.9 |
| 5 | D5 | 282.9 |
| 7 | D7 | 278.5 |
| 9 | D9 | 393.9 |
| 12 | D12 | 219.6 |
| Type of Compound | Compound | Fermentation Time (days) | |||||
|---|---|---|---|---|---|---|---|
| 1 | 3 | 5 | 7 | 9 | 12 | ||
| Terpenes | Botrydial | - | + | + | + | - | - |
| Botrydiol | + | - | - | - | - | - | |
| Dihydrobotrydial | - | + | + | - | - | - | |
| Botryendial | - | - | - | + | + | + | |
| Eremophil-9-ene-1α,11-diol | - | - | - | - | + | + | |
| Polyketides | Botcinin D | - | - | + | + | + | + |
| QS Molecules | 1-Phenylethanol | - | - | + | + | + | - |
| 3-Phenyl-1-propanol | - | - | + | + | + | + | |
| Farnesol | - | - | - | - | - | - | |
| Tyrosol | - | - | - | - | - | - | |
| Evaluated Parameter | Percentage of Inhibition (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D1 | D3 | D5 | D7 | D9 | D12 | |||||||
| 0.1% | 0.01% | 0.1% | 0.01% | 0.1% | 0.01% | 0.1% | 0.01% | 0.1% | 0.01% | 0.1% | 0.01% | |
| Radial growth (day 6) | 2.3 | 0.8 | 2.7 | 8.1 | 8.1 | −0.4 | −0.4 | 3.5 | 5.8 | −2.3 | 1.9 | 5.4 |
| Conidia Germination | 47.4 | 34.6 | 55.8 | 36.5 | 67.3 | 15.4 | 19.2 | 9.6 | 15.4 | 7.7 | 3.2 | 9.0 |
| Germ tube growth | 0.8 | 68.7 | 44.8 | 93.7 | 51.9 | −20.2 | 40.4 | −22.5 | 34.3 | −14.2 | 3.8 | −5.1 |
| Pellet growth | 19.1 | −2.9 | 18.2 | −11.9 | 4.1 | 17.4 | −42.9 | −19.0 | −15.3 | −21.1 | −18.8 | −33.7 |
| Pellet hairy region | 19.7 | −11.6 | 5.8 | −9.3 | −6.0 | −11.3 | 11.4 | −13.7 | −5.9 | −12.8 | −9.2 | −15.3 |
| Sporulation | 33.5 | 18.6 | 32.4 | 21.3 | 39.4 | −11.2 | 24.5 | −8.5 | 55.3 | −14.4 | −2.1 | −13.8 |
| Biofilm | −10.5 | −4.0 | −13.1 | −7.9 | −8.4 | −2.7 | −5.0 | −27.0 | −13.7 | −17.8 | −11.7 | −30.5 |
| Phytotoxicity (1%) | ||||||||||||
| Incidence | 20.1 | −39.8 | −60.0 | −19.9 | 20.1 | −60.0 | ||||||
| Severity | −81.1 | −3.9 | −12.0 | −218.1 | −131.3 | −31.7 | ||||||
| Treatment | Incidence (%) | Average Area of the Lesion (cm2) | Severity (%) (Lesion Area/Total Area) | Phytotoxicity |
|---|---|---|---|---|
| D1 | 33.3 | 0.42 | 10.9 | ** |
| D3 | 58.3 | 0.24 | 6.1 | * |
| D5 | 66.7 | 0.26 | 6.9 | * |
| D7 | 50 | 0.73 | 22.6 | *** |
| D9 | 33.3 | 0.53 | 10.3 | ** |
| D12 | 66.7 | 0.30 | 6.6 | * |
| Botrydial | 41.7 | 0.23 | 10.9 | ** |
| Methanol (control) | 0 | - | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosero-Hernández, E.D.; Echeverri, F.L. The Search for Quorum Sensing in Botrytis cinerea: Regulatory Activity of Its Extracts on Its Development. Plants 2020, 9, 168. https://doi.org/10.3390/plants9020168
Rosero-Hernández ED, Echeverri FL. The Search for Quorum Sensing in Botrytis cinerea: Regulatory Activity of Its Extracts on Its Development. Plants. 2020; 9(2):168. https://doi.org/10.3390/plants9020168
Chicago/Turabian StyleRosero-Hernández, Esteban D., and Fernando L. Echeverri. 2020. "The Search for Quorum Sensing in Botrytis cinerea: Regulatory Activity of Its Extracts on Its Development" Plants 9, no. 2: 168. https://doi.org/10.3390/plants9020168
APA StyleRosero-Hernández, E. D., & Echeverri, F. L. (2020). The Search for Quorum Sensing in Botrytis cinerea: Regulatory Activity of Its Extracts on Its Development. Plants, 9(2), 168. https://doi.org/10.3390/plants9020168





