3. Discussion
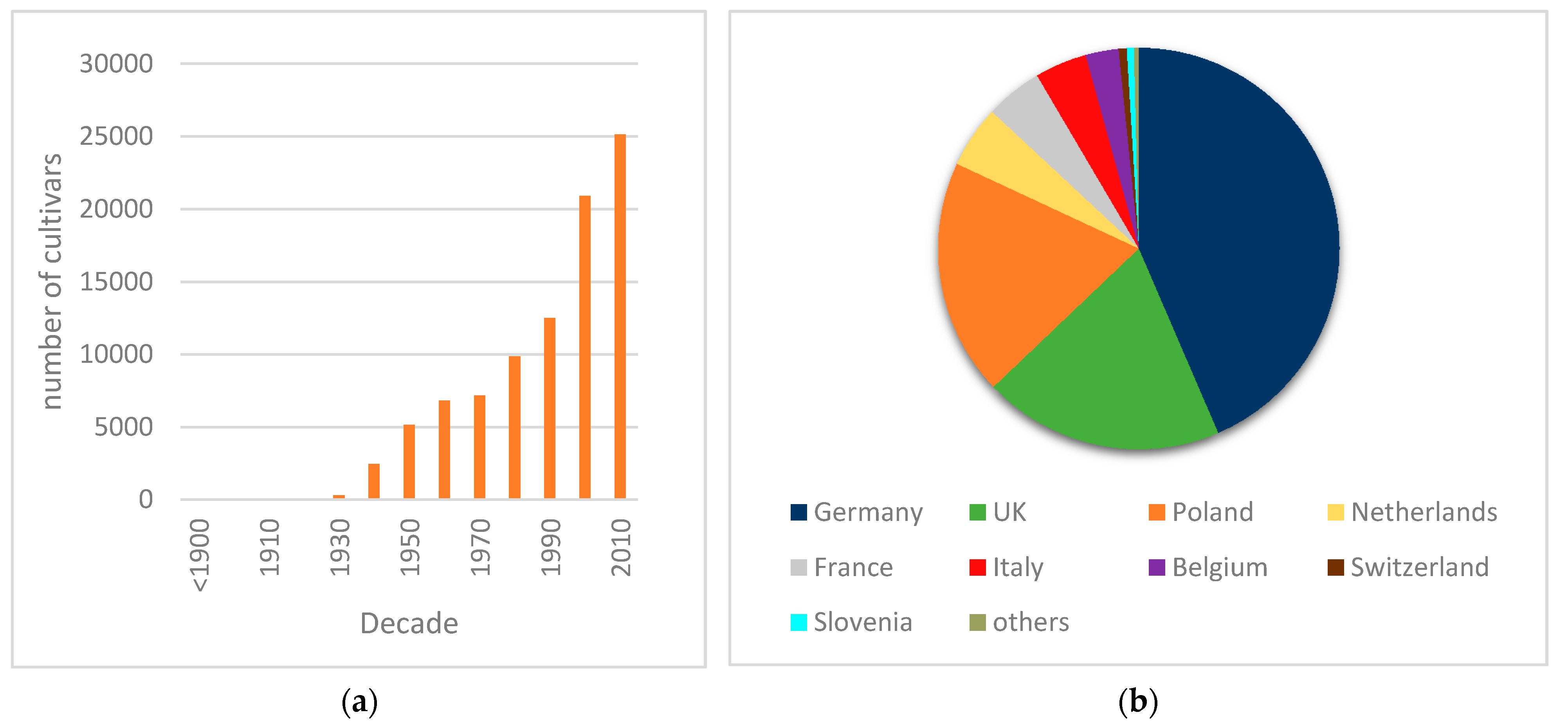
Intercontinental spread of plant diseases has played major roles in agriculture and society, among which rusts are of greatest importance, including examples such as wheat stem rust [
29], myrtle/eucalyptus rust [
30] and coffee leaf rust [
31]. Due to their biological properties, rusts are prone to natural and human-driven dissemination, either at short or long distances [
32]. In Europe, Portugal (including the foremost position of the Atlantic archipelagos of Azores and Madeira) is placed in a critical position concerning the entry of pathogens (including several rust pathogens) from the Americas or Western Africa, through international trade or natural dissemination [
33,
34]. After spreading through all continents, daylily rust reached Europe, being detected in several locations in Portugal [
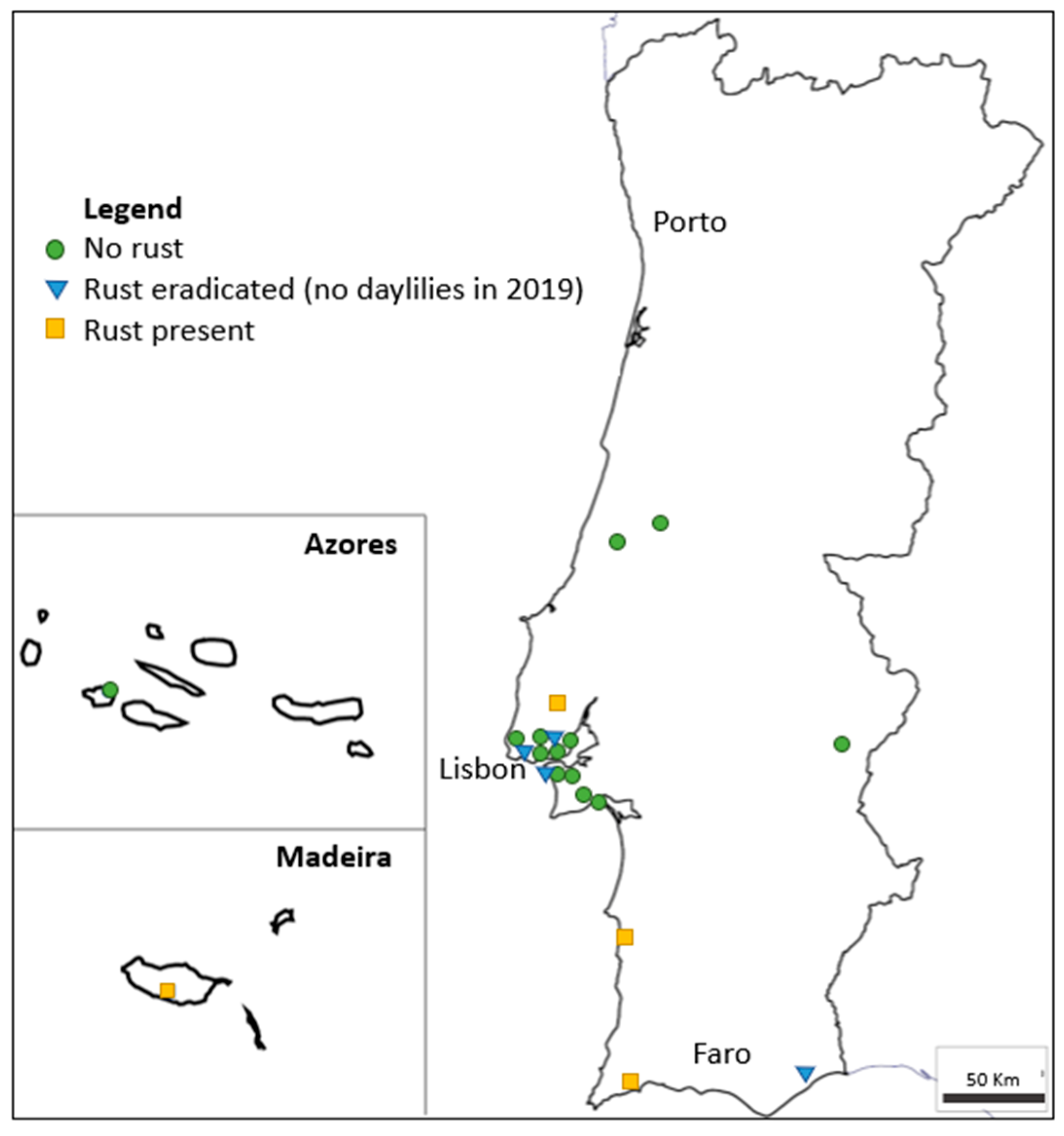
15] in 2015. With the goal to prevent further dissemination of the disease in Portugal, some of the initial foci were destroyed. Disease prevalence has thus decreased from 67% in 2015/16 to 31% in 2019, although new occurrences were detected, suggesting some inoculum dissemination or maintenance of routes of introduction of the pathogen. There is no breeding activity of daylilies in Portugal, nor is there any known activity of propagule multiplication, unlike as is the case with several other ornamentals. In countries with cooler winters, such as most European countries, the disease cycle is naturally broken by the loss of daylily foliage, which prevents urediniospore survival. However, climate changes and global warming may contribute to rust dissemination by reducing such wintery periods.
The morphological characteristics of urediniospores and teliospores present in Portugal agreed with the descriptions made by Hernández et al. [
18] and von Thümen [
8]. The average size of urediniospores among isolates was different. While the urediniospores of isolates Ph02 and Ph06 agreed with the description made by Hernández [
18] and von Thümen [
8], the average urediniospores size of Ph14 and Ph16 isolates was slightly larger, matching that of urediniospores associated with the first report of
P. hemerocallidis in South Africa (25.5 × 22 µm; [
12]).
The dimensions recorded for the nonseptate and 1-septate teliospores of the Ph02 isolate in accordance with the description made by Thümen [
8], Hernández et al. [
18], Inokuti et al. [
35] and Silva et al. [
15]. However, the teliospores of isolate Ph14 had a smaller average size than teliospores found in other geographic regions. The minimum and maximum pedicel length recorded for the two isolates approximate the measurements made by Inokuti et al. [
35] and von Thümen [
8]. Most teliospores were single celled, similarly to the report by Hernandez et al. [
18] for pathogens occurring in North America, contrasting with Asian specimens [
18] but also with reports from South America [
35,
36] where only septate teliospores were found.
The nucleotide sequences of the isolates Ph06, Ph14 and Ph16 did not differ from those of the
P. hemerocallidis isolates from the USA and Costa Rica. The sequence corresponding to Ph02 isolate was more closely related to the isolate from Mexico. Yet, these differences are based on a very limited number of informative nucleotides. Still, it is worth noting that Asian isolates were clustered together in this analysis, while Mexican isolates were the most diverse. Clearly, a larger set of isolates is needed for this type of study. It is tempting to speculate that more informative markers should be identified and used, although this should be addressed cautiously (the rDNA-ITS region is the universal DNA barcode marker for fungi [
37]). One of the objectives of such analyses is to relate genetic diversity to putative physiological races, and this should be considering when choosing appropriate markers. Considering the narrow representativity of the isolates in this analysis and the low levels of diversity revealed, it is still possible to suggest that daylily rust may have entered Portugal in more than one event. Considering morphologic and genetic data, this introduction may have occurred from North or Central America, although the absence of sequences corresponding to isolates from South America, South Africa and Central and Southern Asia does not enable a more thorough analysis.
Considering all measurements made to the size of the
P. hemerocallidis genome, it is possible to state that the species has a genome with an estimated size of 318 Mbp, matching the single value previously obtained for this species [
17], but matching also the average genome size of the order Pucciniales (335 Mbp), one of the largest among fungi [
38]. Tavares et al. [
39] noted that rust species with uredinial/telial Poaceae hosts have smaller genomes that those infecting Fabaceae. The present genome size value for
P. hemerocallidis is similar that of
P. allii (352 Mpb) and of
Uromyces transversalis (377 Mbp), suggesting that rusts with Asparagales hosts (Xanthorrhoeaceae, Amaryllidaceae and Iridaceae, respectively for those three rust species) have larger genomes that those with Poaceae hosts. Large genomes may be due to activity of transposable elements, chromosome transfer and polyploidization [
40], which may be important sources of diversity and variation in genome size, particularly in the absence of sexual reproduction [
41]. Genome size analyses of closely related taxa may add information to the events that led to the speciation of
P. hemerocallidis [
26], but they may also be useful for the discrimination of intra-specific diversity of the fungus. To this end, analyses of additional isolates is needed.
In the present study we have analyzed the rust response of 17 daylily cultivars, seven of which of European origin. Three cultivars were classified as moderately resistant, two as moderately susceptible and the remaining as susceptible. Only three of these cultivars had been previously analyzed for rust response. The cultivar ‘Roswitha’ was classified as susceptible to rust in our study, not corresponding to the resistance recorded in previous studies [
27]. The cultivar ‘Stella d’Oro’ showed to be moderately resistant, corroborating previous results reporting this cultivar as resistant or moderately resistant [
20,
21,
24,
42]. A similar result was found for ‘Bitsy’ [
43]. None of the plants analyzed in the present study was considered as resistant, mostly because of late sporulation over necrotic areas, leading to considering them as moderately resistant. Late sporulation, sometimes as late as 30 days after inoculation, may not be recorded in common resistance trials. While immune response is highly desirable as a resistance trait for daylily breeders, other types of hypersensitive response are common in incompatible interactions, often originating macroscopic necroses [
21]. Such resistance responses based on macroscopic necroses are less desirable as the ornamental value of the plants depreciate, although the disease cycle is broken. Late sporulation in moderately resistant cultivars does not totally impair inoculum dissemination. Nevertheless, moderate resistance may still be a desirable trait in the absence of higher levels of resistance. Moderately susceptible plants show reduced levels of sporulation and longer latent periods than fully susceptible plants (
Table 5) and such quantitative responses may also be used in plant protection schemes to reduce disease while minimizing fungicide application. Disease rating scales in daylily rust must therefore be able to accommodate both qualitative and quantitative variations in disease parameters, as well as to consider the time course of infection. Results from this work, along with those from other authors (e.g., [
1,
21]), depict necrotic areas as typical of intermediate responses of plants to rust: neither fully susceptible nor fully resistant (immune) responses develop necroses, while moderately susceptible and especially moderately resistant interactions do. A detailed and quantified discrimination of necrotic leaf areas along time, by comparison of healthy areas as well as of diseased (chloroses, pustules and senescence), was shown in this work to be useful to evaluate daylily cultivars. Ideally, disease rating scales should be both scientifically accurate and practical to use in large scale studies. The present image analysis-based study has the potential to be adapted to field surveys using images obtained by robotics or air-driven automated systems.
Among the seven European daylily cultivars analyzed in this study, one was considered as moderately resistant (‘Cherry Tiger’) and two as moderately susceptible (‘German Highlight’ and ‘Romantic Rose’), whereas the other four cultivars shown susceptible to rust. Rust resistance became an important trait for breeders when the disease reached North America, leading to several disease resistance screening studies.
Sources of resistance in European cultivars are however still largely unknown. Interestingly, no clear resistance sources have been reported in wild germplasm, an area that certainly deserves further deepening.
5. Conclusions
After spreading from Asia to all other continents in the 21st century, daylily rust has entered Europe through its Southwestern point. Four years after being detected in multiple locations in mainland Portugal and Madeira, this study showed that although the prevalence of the disease decreased since 2016, new occurrences were identified and showed a high incidence and severity.
Morphologic, genetic and genomic analyses show that the pathogens present in Portugal exhibit narrow diversity, suggesting that the pathogen may have been introduced in Europe from North or Central America. Results obtained in this study indicate a need for a global study comparing Puccinia hemerocallidis isolates, mainly aiming at the identification of physiological races.
Detailed analysis of the daily evolution of diverse symptomatological parameters has enabled providing qualitative and quantitative data for accurate classification of cultivars. Additionally, the phenomenon of late sporulation over necrotic tissues was highlighted, suggesting that cultivars classified as resistant may be better characterized as moderately resistant.
Experience from the American continent shows that, once introduced, daylily rust is unlikely to be eradicated. Either naturally or human-driven, daylily rust has potential to reach other parts of Europe and compromise breeding and nursery activities of the ornamental horticulture sector. This study emphasizes the fact that rust response in European cultivars is largely unknown, although resistant germplasm is available and commonly used in breeding programs. Achievement of knowledge of the resistance levels of European daylily cultivars, including also wild genotypes, is required to maintain daylilies as disease-free prime-choice garden plants. It is also urgent that plant breeders, especially those who only breed Hemerocallis spp., take awareness of the resistance level of the existing varieties to avoid future economic losses.