Physiological Responses to the Foliar Application of Synthetic Resistance Elicitors in Cape Gooseberry Seedlings Infected with Fusarium oxysporum f. sp. physali
Abstract
:1. Introduction
2. Results
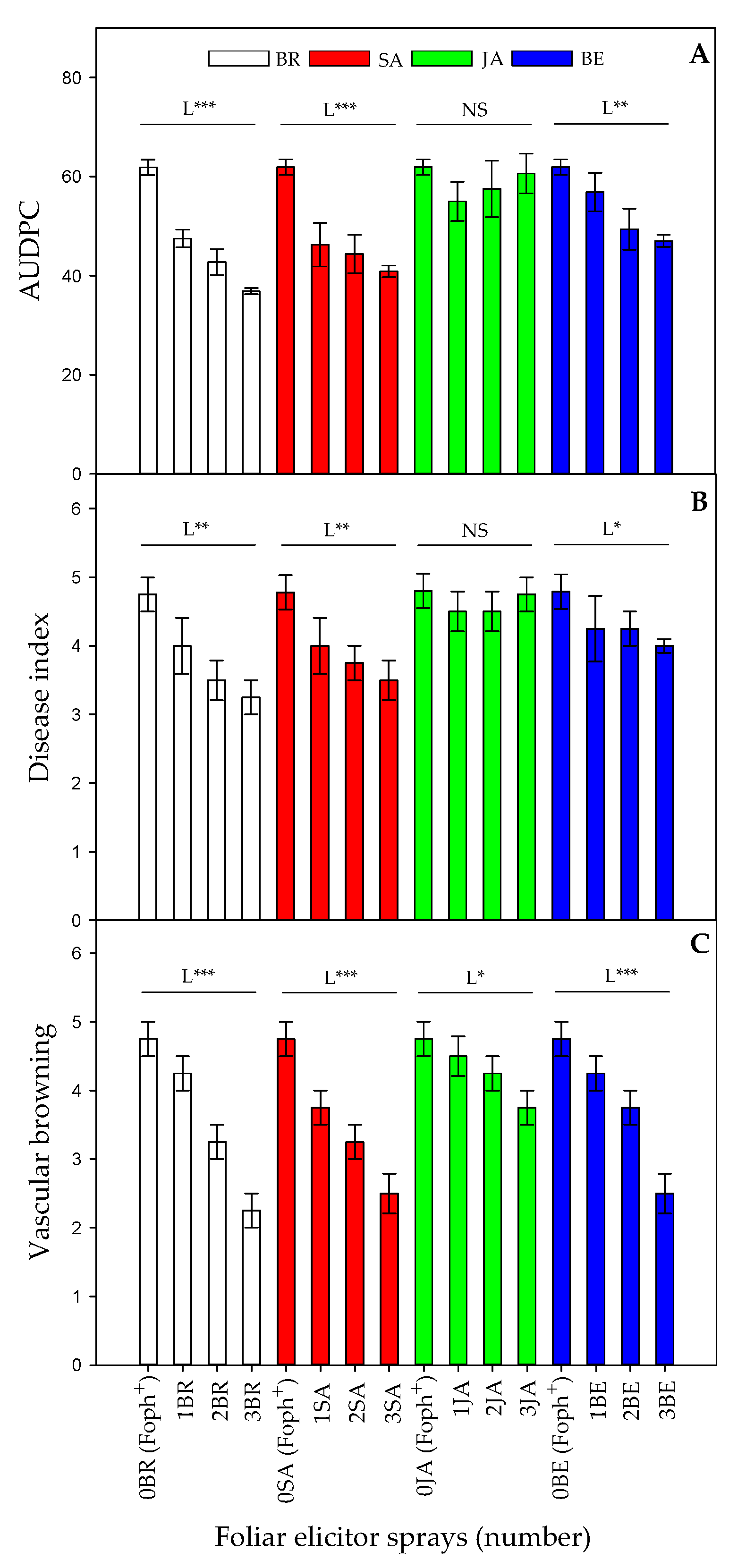
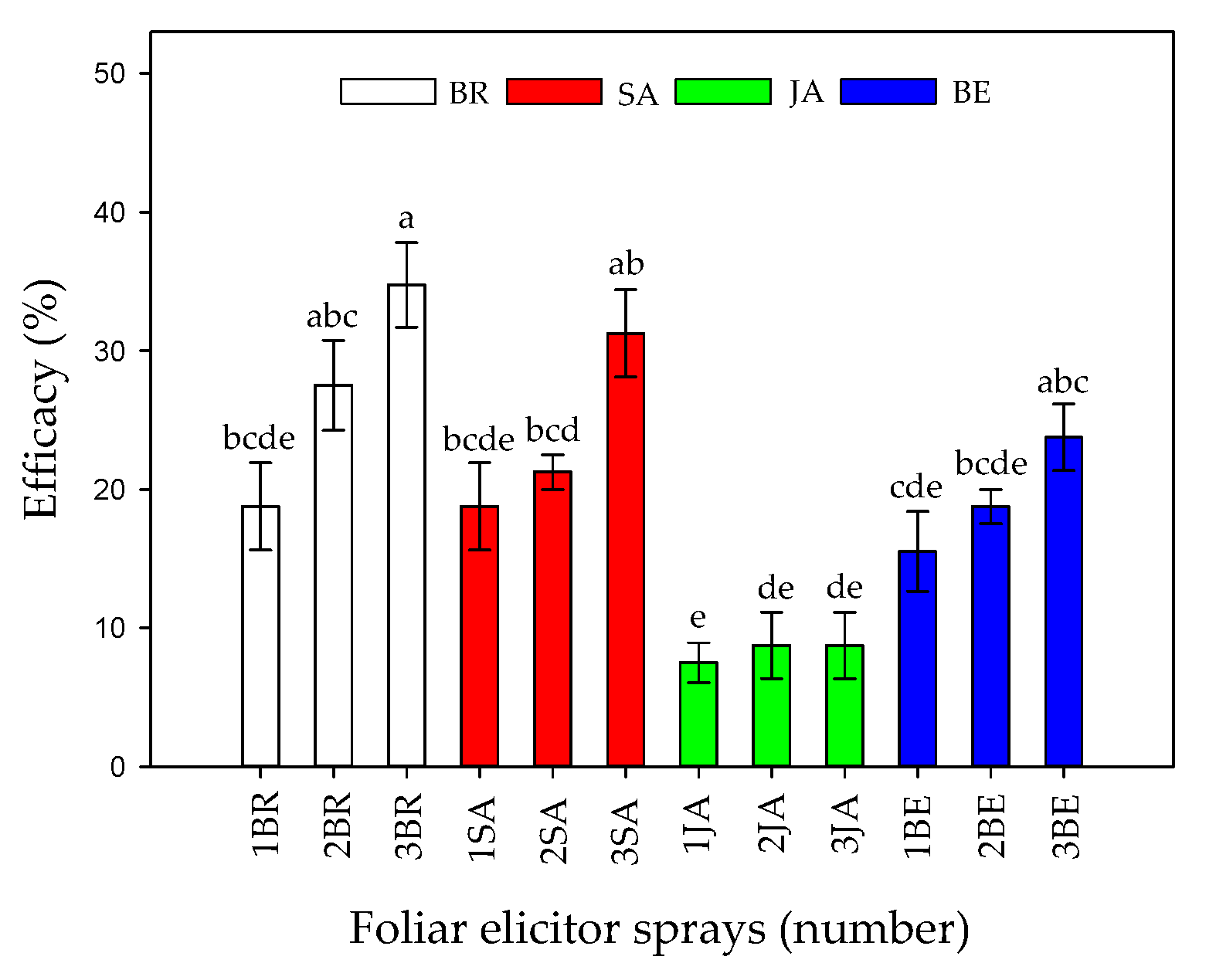
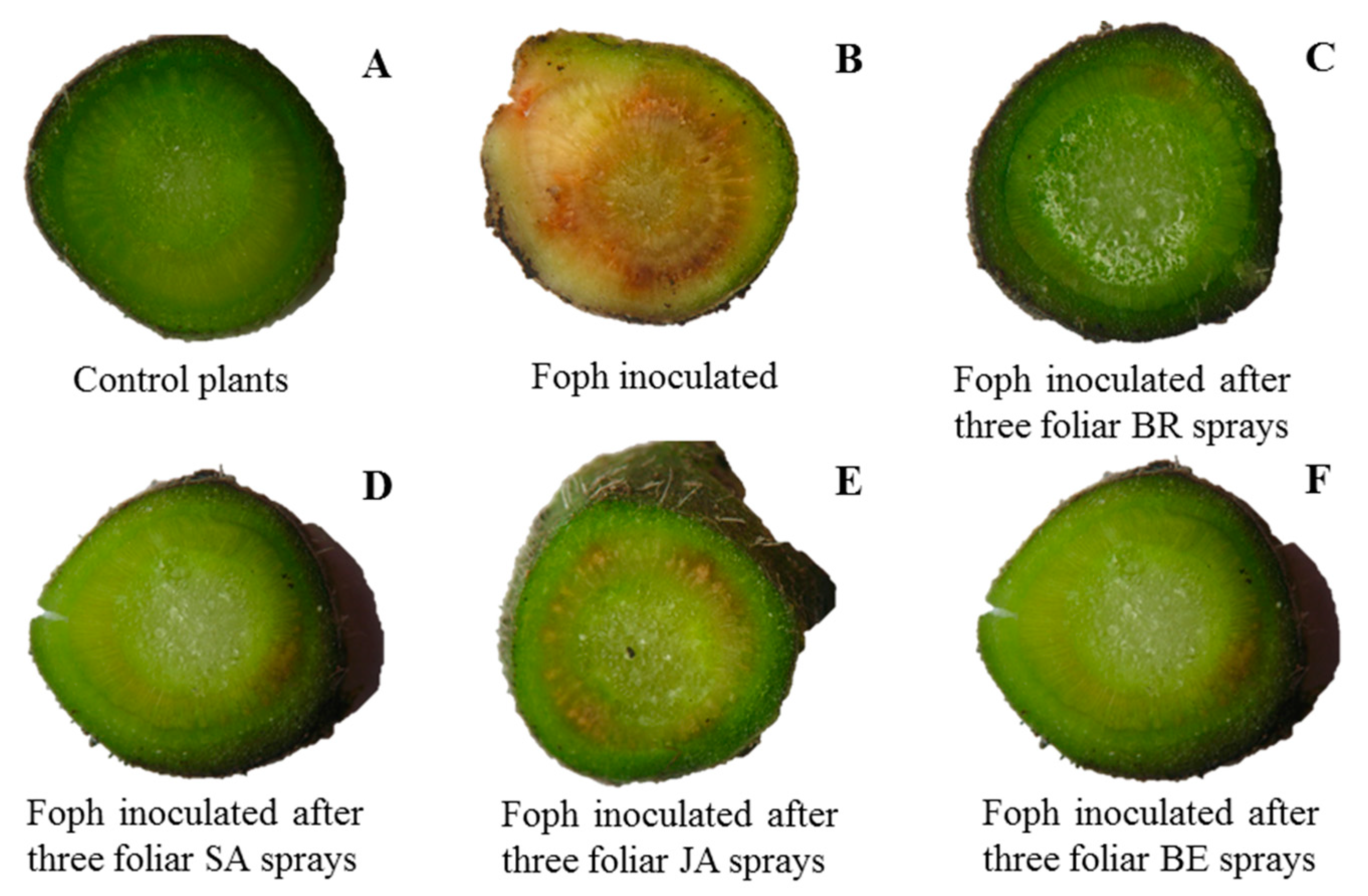
2.1. Vascular Wilt Severity Expressed as AUDPC, Disease Index, and Vascular Browning
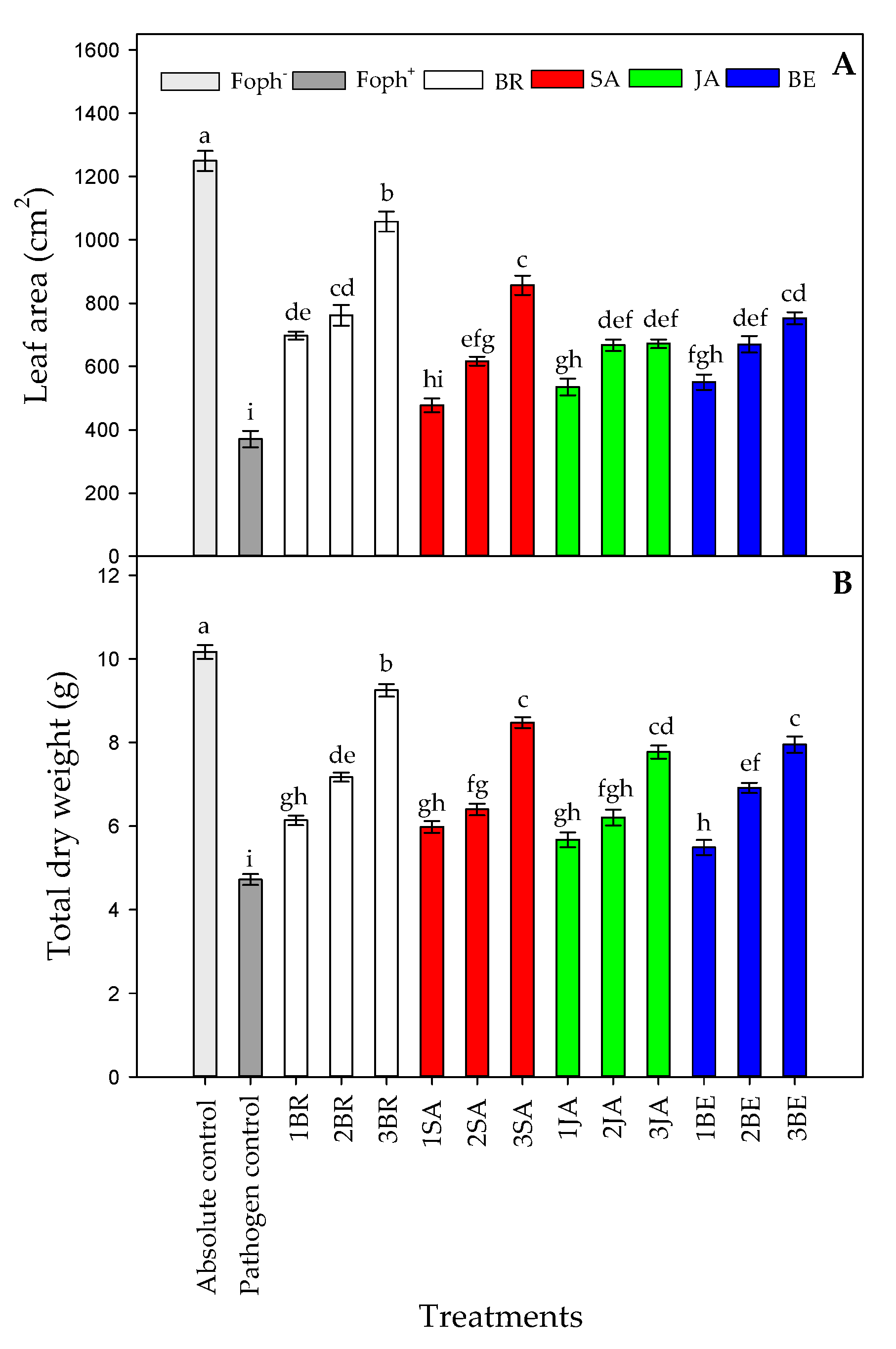
2.2. Plant Growth Variables
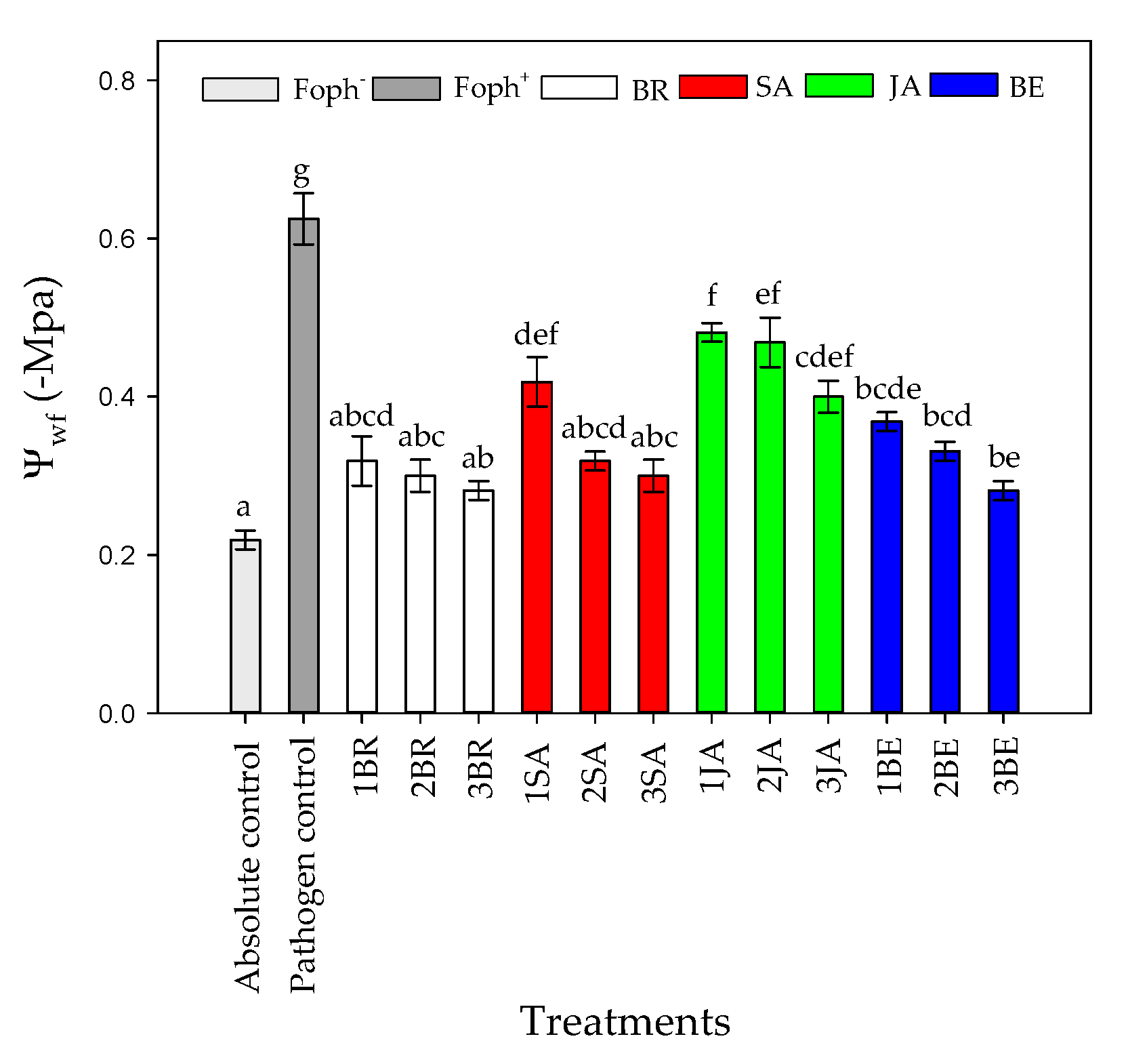
2.3. Stomatal Conductance (gs), Leaf Temperature (TL) and Leaf Water Potential (Ψwf)
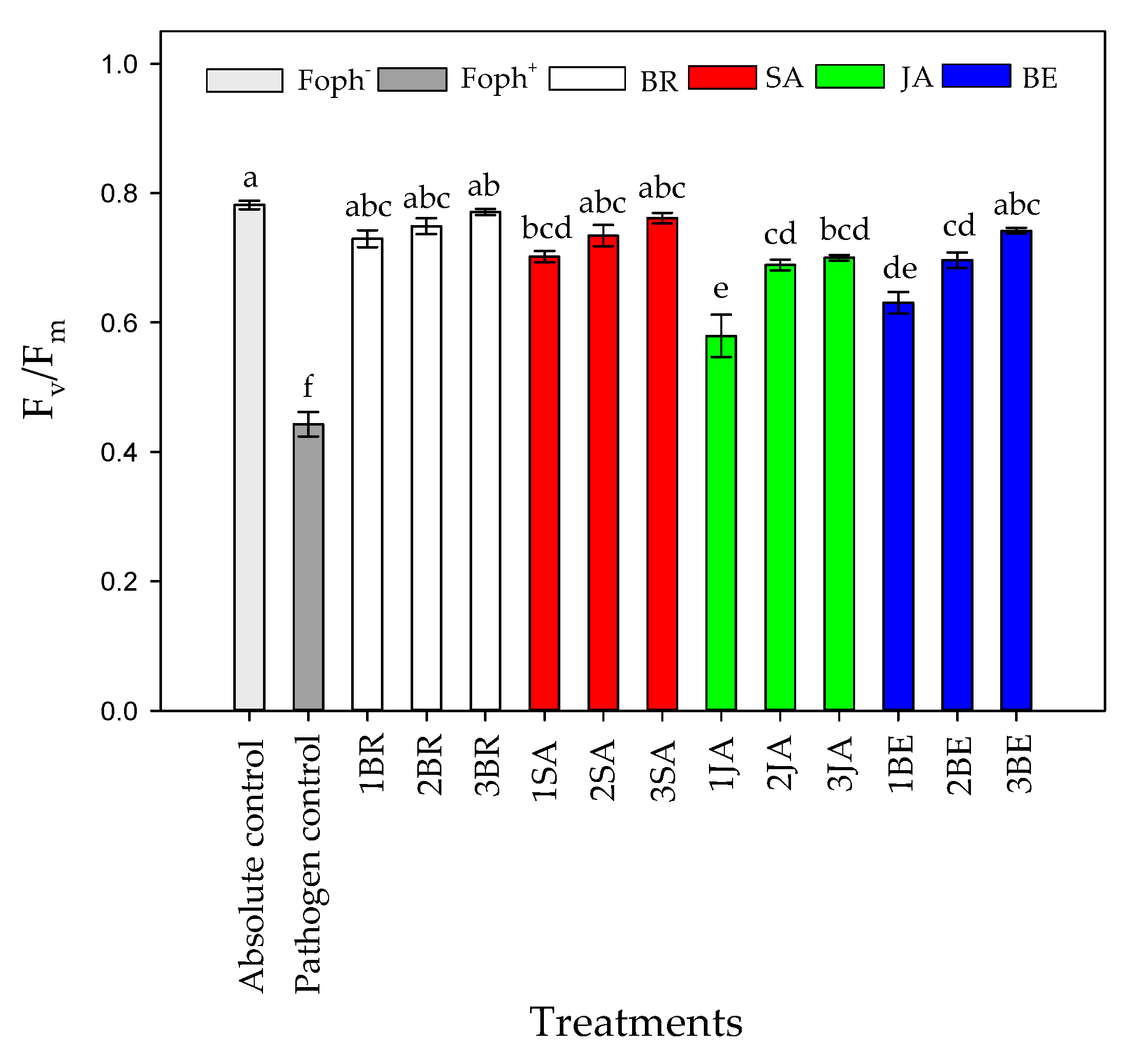
2.4. Parameters of Maximum Photochemical Efficiency of PSII (Fv/Fm) and Rapid Light-Response Curves
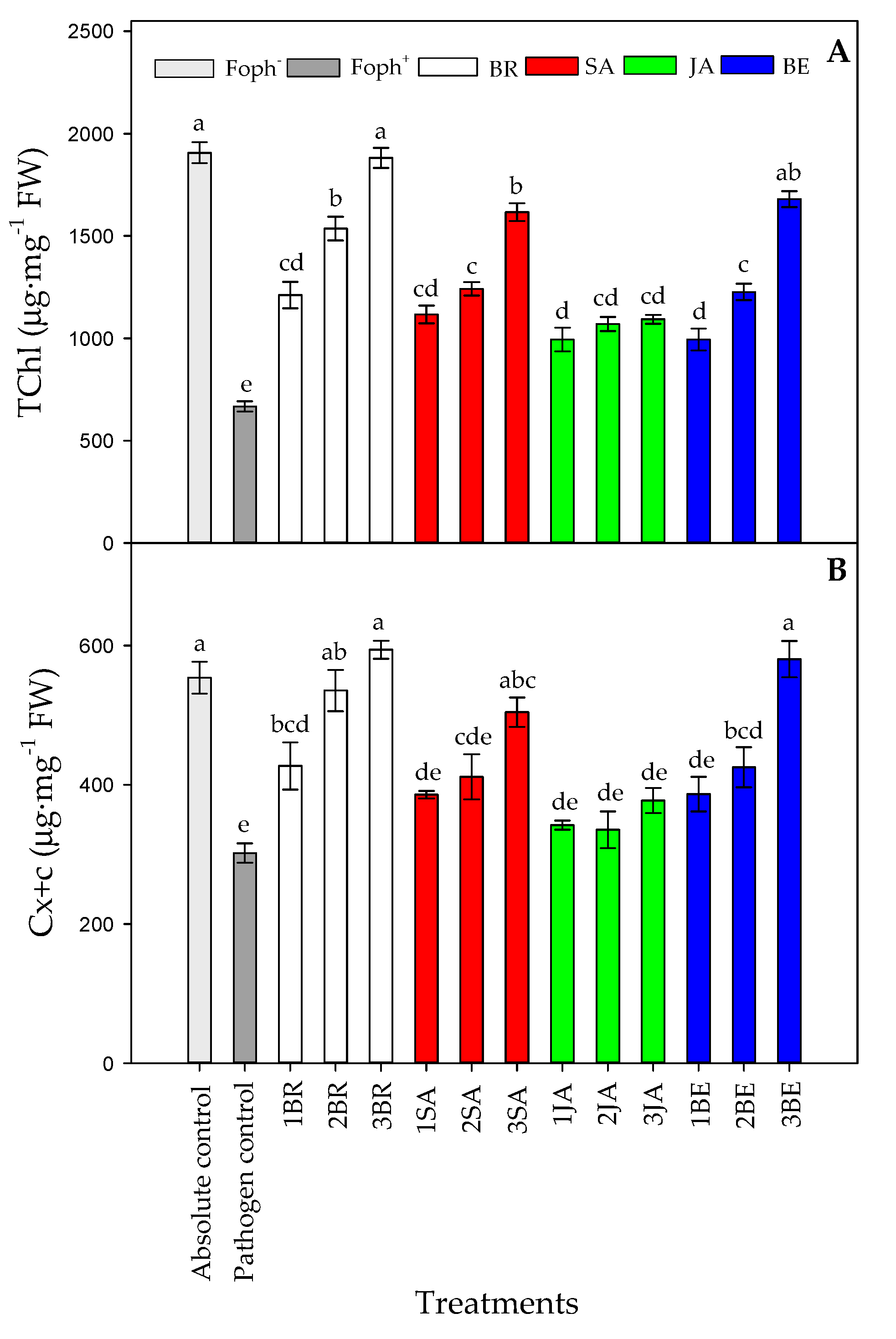
2.5. Leaf Photosynthetic Pigments
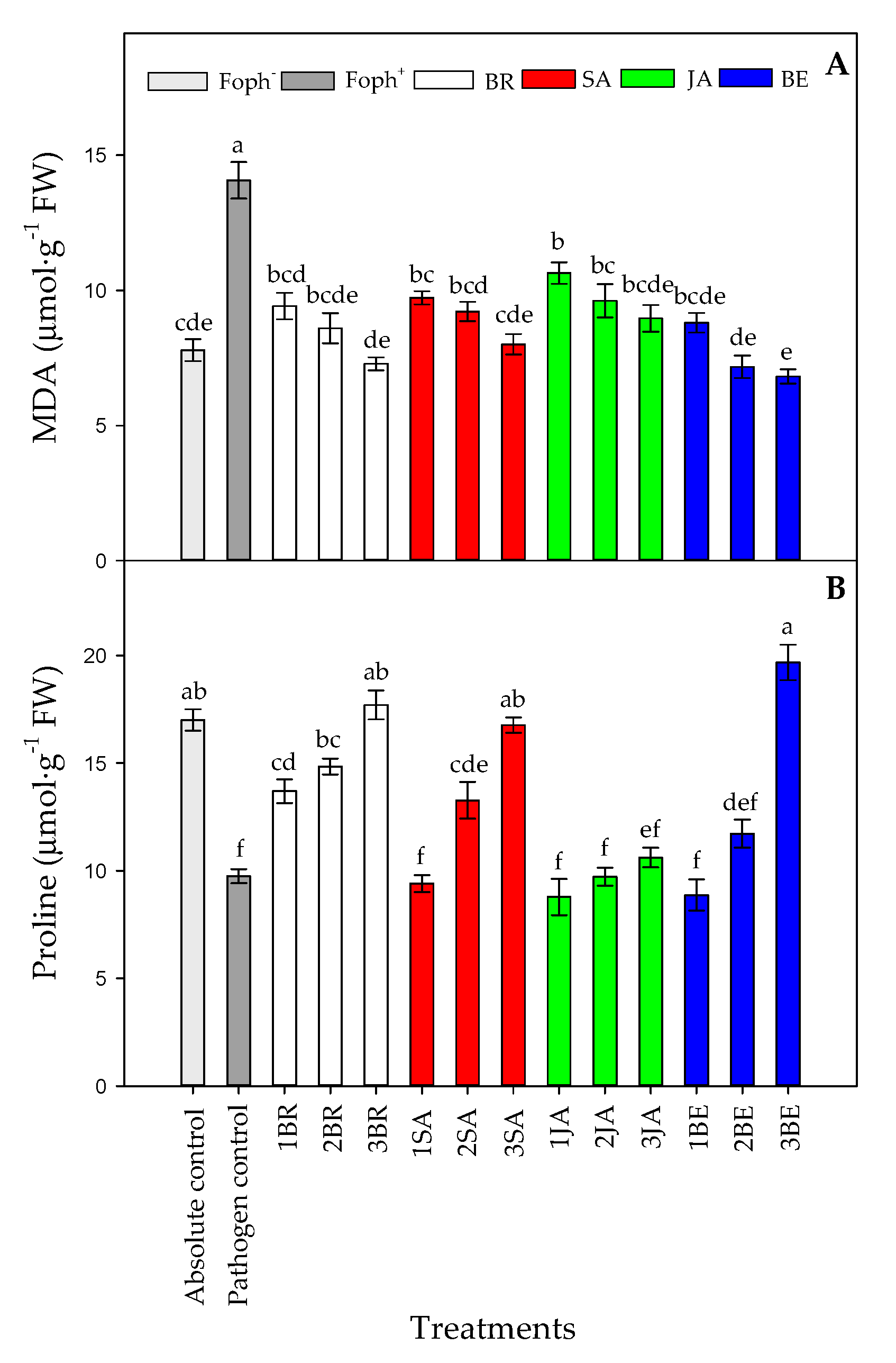
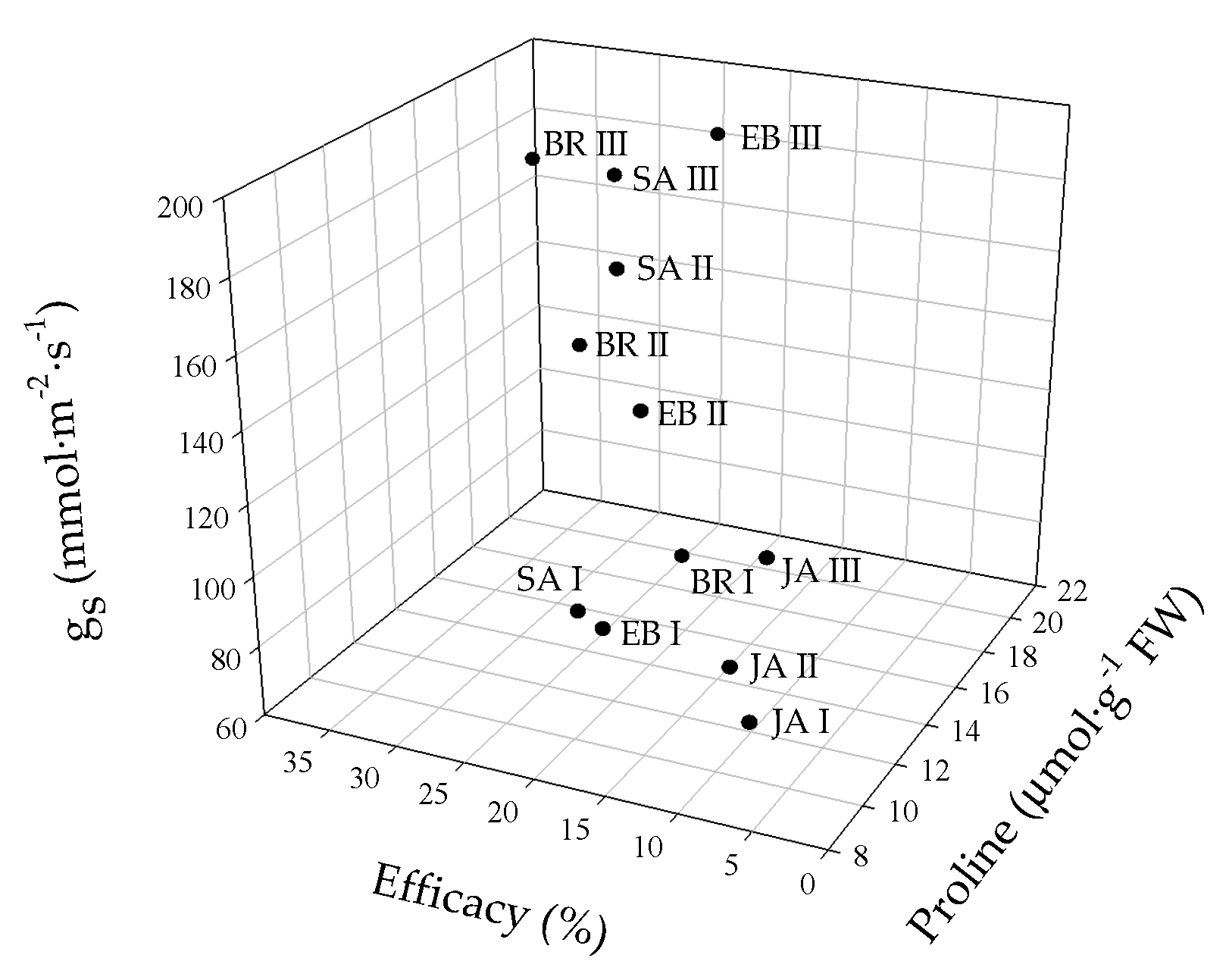
2.6. MDA Production and Leaf Proline Content
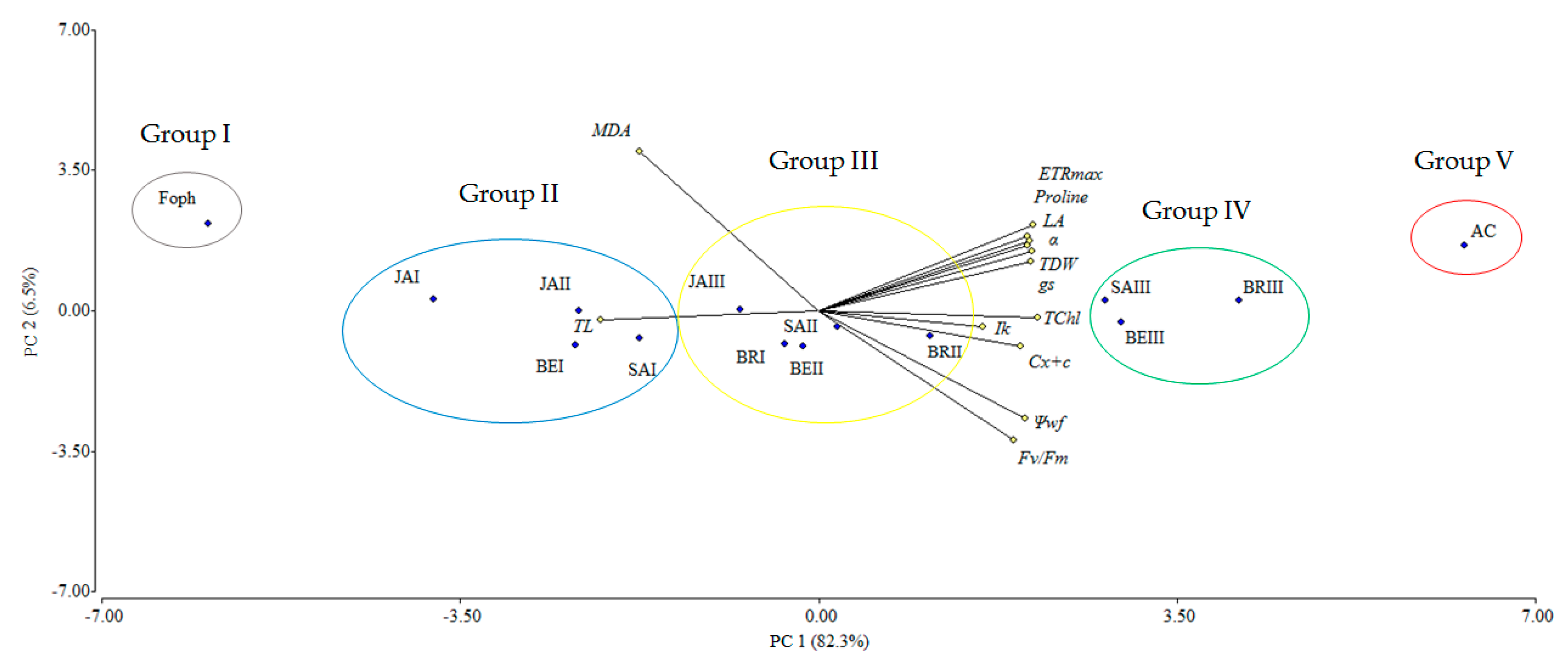
2.7. Biplot Analysis of Physiological and Biochemical Responses to Foph Management with Synthetic Elicitors
3. Discussion
4. Materials and Methods
4.1. General Growth Conditions
4.2. Plant Inoculation and Resistance Elicitors Application
4.3. Analysis of Vascular Wilt Development
4.4. Physiological and Biochemical Variables
4.4.1. Stomatal Conductance and Leaf Water Status
4.4.2. Fv/Fm Ratio and Rapid Light-Response Curves
4.4.3. Growth Parameters
4.4.4. Leaf Photosynthetic Pigments
4.4.5. Lipid Peroxidation (malondialdehyde-MDA)
4.4.6. Proline Concentration
4.5. Experimental Design and Data Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fischer, G.; Almanza-Merchán, P.J.; Miranda, D. Importancia y cultivo de la uchuva (Physalis peruviana L.). Rev. Bras. Frutic. 2014, 36, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Puente, L.A.; Pinto-Muñoz, C.A.; Castro, E.S.; Cortés, M. Physalis peruviana Linnaeus, the multiple properties of a highly functional fruit: A review. Food Res. Int. 2011, 44, 1733–1740. [Google Scholar] [CrossRef]
- Ramadan, M.F. Bioactive phytochemicals, nutritional value, and functional properties of cape gooseberry (Physalis peruviana): An overview. Food Res. Int. 2011, 44, 1830–1836. [Google Scholar] [CrossRef]
- Erkaya, T.; Dağdemir, E.; Şengül, M. Influence of Cape gooseberry (Physalis peruviana L.) addition on the chemical and sensory characteristics and mineral concentrations of ice cream. Food Res. Int. 2012, 45, 331–335. [Google Scholar] [CrossRef]
- Olivares-Tenorio, M.L.; Verkerk, R.; van Boekel, M.A.; Dekker, M. Thermal stability of phytochemicals, HMF and antioxidant activity in cape gooseberry (Physalis peruviana L.). J. Funct. Foods 2017, 32, 46–57. [Google Scholar] [CrossRef]
- Agronet. Available online: http://www.agronet.gov.co/estadistica/Paginas/default.aspx (accessed on 1 November 2019).
- Osorio-Guarín, J.A.; Enciso-Rodríguez, F.E.; González, C.; Fernández-Pozo, N.; Mueller, L.A.; Barrero, L. Association analysis for disease resistance to Fusarium oxysporum in cape gooseberry (Physalis peruviana L). BMC Genom. 2016, 17, 248. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Velandia, C.A.; Izquierdo-García, L.F.; Ongena, M.; Kloepper, J.W.; Cotes, A.M. Soil sterilization, pathogen and antagonist concentration affect biological control of Fusarium wilt of cape gooseberry by Bacillus velezensis Bs006. Plant Soil 2018, 435, 39–55. [Google Scholar] [CrossRef] [Green Version]
- Simbaqueba, J.; Catanzariti, A.; Gonzalez, C.; Jones, D. Evidence for horizontal gene transfer and separation of effector recognition form effector function revealed by analysis of effector genes shared between cape-gooseberry and tomate-infecting formae speciales of Fusarium oxysporum. Mol. Plant Pathol. 2018, 9, 2302–2318. [Google Scholar] [CrossRef] [Green Version]
- Urrea, R.; Cabezas, L.; Sierra, R.; Cárdenas, M.; Restrepo, S.; Jiménez, P. Selection of antagonistic bacteria isolated from the Physalis peruviana rhizosphere against Fusarium oxysporum. J. Appl. Microbiol. 2011, 111, 707–716. [Google Scholar] [CrossRef]
- McGovern, R.J. Management of tomato diseases caused by Fusarium oxysporum. Crop. Prot. 2015, 73, 78–92. [Google Scholar] [CrossRef]
- Tamm, L.; Thürig, B.; Fliessbach, A.; Goltlieb, A.E.; Karavani, S.; Cohen, Y. Elicitors and soil management to induce resistance against fungal plant diseases. Njas-Wagen. J. Life Sc. 2011, 58, 131–137. [Google Scholar] [CrossRef]
- Thakur, M.; Sohal, B.S. Role of elicitors in inducing resistance in plants against pathogen infection: A review. ISRN Biochem. 2013, 1, 762412. [Google Scholar] [CrossRef] [Green Version]
- Mejía-Teniente, L.; Torres-Pacheco, I.; González-Chavira, M.M.; Ocampo-Velazquez, R.V.; Herrera-Ruiz, G.; Chapa-Oliver, A.M.; Guevara-González, R.G. Use of elicitors as an approach for sustainable agricultura. Afr. J. Biotechnol. 2010, 9, 9155–9162. [Google Scholar]
- Bektas, Y.; Eulgem, T. Synthetic plant defense elicitors. Front. Plant Sci. 2015, 5, 804. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, G.H.C.; Dantas, R.L.; de Sousa, A.S.B.; Soares, L.G.; de Sá Melo, R.; da Silva, R.S.; Lima, R.P.; Mendonça, R.M.N.; Beaudry, R.M.; Silva, S.M. Impact of cassava starch-alginate based coatings added with ascorbic acid and elicitor on quality and sensory attributes during pineapple storage. Afr. J. Agric. Res. 2017, 12, 664–673. [Google Scholar]
- Mandal, S.; Mallick, N.; Mitra, A. Salicylic acid-induced resistance to Fusarium oxysporum f. sp. lycopersici in tomato. Plant Physiol. Bioch. 2009, 47, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.F.; Wu, J.; Wang, L.F.; Blair, M.W.; Wang, X.M.; De Ge, W.; Zhu, Z.D.; Wang, S.M. Salicylic acid enhances resistance to Fusarium oxysporum f. sp. phaseoli in common beans (Phaseolus vulgaris L.). J. Plant Growth Regul. 2014, 33, 470–476. [Google Scholar] [CrossRef]
- Jaiti, F.; Verdeil, J.L.; El Hadrami, I. Effect of jasmonic acid on the induction of polyphenoloxidase and peroxidase activities in relation to date palm resistance against Fusarium oxysporum f. sp. albedinis. Physiol. Mol. Plant Path. 2009, 74, 84–90. [Google Scholar] [CrossRef]
- Zhu, F.; Xi, D.H.; Yuan, S.; Xu, F.; Zhang, D.W.; Lin, H.H. Salicylic acid and jasmonic acid are essential for systemic resistance against tobacco mosaic virus in Nicotiana benthamiana. Mol. Plant Microbe Interact. 2014, 27, 567–577. [Google Scholar] [CrossRef] [Green Version]
- Ding, J.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Effects of root and foliar applications of 24-epibrassinolide on Fusarium wilt and antioxidant metabolism in cucumber roots. HortScience 2009, 44, 1340–1345. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.W.; Deng, X.G.; Fu, F.Q.; Lin, H.H. Induction of plant virus defense response by brassinosteroids and brassinosteroid signaling in Arabidopsis thaliana. Planta 2014, 241, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Vásquez, A.; Salinas, P.; Holuigue, L. Salicylic acid and reactive oxygen species interplay in the transcriptional control of defense genes expression. Front. Plant Sci. 2015, 6, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinod, K.; Sabah, A. Plant Defense against Pathogens: The Role of Salicylic Acid. Res. J. Biotechnol. 2018, 13, 97–103. [Google Scholar]
- Le Thanh, T.; Thumanu, K.; Wongkaew, S.; Boonkerd, N.; Teaumroong, N.; Phansak, P.; Buensanteai, N. Salicylic acid-induced accumulation of biochemical components associated with resistance against Xanthomonas oryzae pv. oryzae in rice. J. Plant Interact. 2017, 12, 108–120. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Shi, X.; Li, B.; Zhang, Q.; Liang, W.; Wang, C. Salicylic acid confers enhanced resistance to Glomerella leaf spot in apple. Plant Physiol. Bioch. 2016, 106, 64–72. [Google Scholar] [CrossRef]
- Belkhadir, Y.; Jaillais, Y.; Epple, P.; Balsemão-Pires, E.; Dangl, J.L.; Chory, J.L. Brassinosteroids modulate the efficiency of plant immune responses to microbe-associated molecular patterns. Proceeding Natl. Acad. Sci. USA 2012, 109, 297–302. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.Y.; Bai, M.Y.; Oh, E.; Zhu, J.Y. Brassinosteroid signaling network and regulation of photomorphogenesis. Annu. Rev. Genet. 2012, 46, 701–724. [Google Scholar] [CrossRef]
- Nawaz, F.; Naeem, M.; Zulfiqar, B.; Akram, A.; Ashraf, M.Y.; Raheel, M.; Shabbir, R.N.; Hussain, R.A.; Anwar, I.; Aurangzaib, M. Understanding brassinosteroid-regulated mechanisms to improve stress tolerance in plants: A critical review. Environ. Sci. Pollut. R. 2017, 24, 15959–15975. [Google Scholar] [CrossRef]
- Furio, R.N.; Albornoz, P.L.; Coll, Y.; Zamora, G.M.; Salazar, S.M.; Martos, G.G.; Ricci, J.D. Effect of natural and synthetic Brassinosteroids on strawberry immune response against Colletotrichum acutatum. Eur. J. Plant Pathol. 2018, 153, 167–181. [Google Scholar] [CrossRef]
- Canales, E.; Coll, Y.; Hernández, I.; Portieles, R.; García, M.R.; López, Y.; Aranguren, M.; Alonso, E.; Delgado, R.; Luis, M.; et al. ‘Candidatus Liberibacter asiaticus’, causal agent of citrus Huanglongbing, is reduced by treatment with Brassinosteroids. Plos ONE 2016, 11, e0146223. [Google Scholar] [CrossRef]
- Liu, S.; Vargas, J.; Merewitz, E. Jasmonic and salicylic acid effects on bacterial etiolation and decline disease of creeping bentgrass. Crop. Prot. 2018, 109, 9–16. [Google Scholar] [CrossRef]
- Conrath, U.; Beckers, G.J.; Flors, V.; García-Agustín, P.; Jakab, G.; Mauch, F.; Newman, M.A.; Pieterse, C.M.J.; Poinssot, B.; Pozo, M.J.; et al. Priming: Getting ready for battle. Mol. Plant Microbe. Interact. 2006, 19, 1062–1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehari, Z.H.; Elad, Y.; Rav-David, D.; Graber, E.R.; Harel, Y.M. Induced systemic resistance in tomato (Solanum lycopersicum) against Botrytis cinerea by biochar amendment involves jasmonic acid signaling. Plant Soil 2015, 395, 31–44. [Google Scholar] [CrossRef]
- Ávila, A.C.; Ochoa, J.; Proaño, K.; Martínez, M.C. Jasmonic acid and nitric oxide protects naranjilla (Solanum quitoense) against infection by Fusarium oxysporum f. sp. quitoense by eliciting plant defense responses. Physiol. Mol. Plant Pathol. 2019, 106, 129–136. [Google Scholar]
- Dietrich, R.; Ploss, K.; Heil, M. Growth responses and fitness costs after induction of pathogen resistance depend on environmental conditions. Plant Cell Environ. 2005, 28, 211–222. [Google Scholar] [CrossRef]
- Parkinson, L.E.; Crew, K.S.; Thomas, J.E.; Dann, E.K. Efficacy of acibenzolar-S-methyl (Bion®) treatment of Australian commercial passionfruit, Passiflora edulis f. sp. flavicarpa, on resistance to Passionfruit woodiness virus (PWV) and activities of chitinase & β-1, 3-glucanase. Australas. Plant Path. 2015, 44, 311–318. [Google Scholar]
- Akila, R.; Rajendran, L.; Harish, S.; Saveetha, K.; Raguchander, T.; Samiyappan, R. Combined application of botanical formulations and biocontrol agents for the management of Fusarium oxysporum f. sp. cubense (Foc) causing Fusarium wilt in banana. Biol. Control. 2011, 57, 175–183. [Google Scholar] [CrossRef]
- Gurjar, M.S.; Ali, S.; Akhtar, M.; Singh, K.S. Efficacy of plant extracts in plant disease management. Agric. Sci. 2012, 3, 425–433. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Zhu, S.; Wu, W.; Zhang, C.; Peng, Y.; Wang, Q.; Shi, J. Exogenous nitric oxide induces disease resistance against Monilinia fructicola through activating the phenylpropanoid pathway in peach fruit. J. Sci. Food Agr. 2017, 97, 3030–3038. [Google Scholar] [CrossRef]
- Jayaraman, J.; Norrie, J.; Punja, Z.K. Commercial extract from the brown seaweed Ascophyllum nodosum reduces fungal diseases in greenhouse cucumber. J. Appl. Phycol. 2011, 23, 353–361. [Google Scholar] [CrossRef]
- Shifa, H.; Gopalakrishnan, C.; Velazhahan, R. Management of late leaf spot (Phaeoisariopsis personata) and root rot (Macrophomina phaseolina) diseases of groundnut (Arachis hypogaea L.) with plant growth-promoting rhizobacteria, systemic acquired resistance inducers and plant extracts. Phytoparasitica 2018, 46, 19–30. [Google Scholar] [CrossRef]
- Pandey, P.; Ramegowda, V.; Senthil-Kumar, M. Shared and unique responses of plants to multiple individual stresses and stress combinations: Physiological and molecular mechanisms. Front. Plant Sci. 2015, 6, 723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irulappan, V.; Senthil-Kumar, M. Morpho-Physiological Traits and Molecular Intricacies Associated with Tolerance to Combined Drought and Pathogen Stress in Plants. In Biotechnologies of Crop. Improvement; Gosal, S.S., Wani, S.H., Eds.; Springer: Cham, Switzerland, 2018; Volume 3, pp. 59–74. [Google Scholar]
- Berger, S.; Sinha, A.K.; Roitsch, T. Plant physiology meets phytopathology: Plant primary metabolism and plant–pathogen interactions. J. Exp. Bot. 2007, 58, 4019–4026. [Google Scholar] [CrossRef] [PubMed]
- Qamar, A.; Mysore, K.S.; Senthil-Kumar, M. Role of proline and pyrroline-5-carboxylate metabolism in plant defense against invading pathogens. Front. Plant Sci. 2015, 6, 503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yusuf, M.; Hayat, S.; Alyemeni, M.N.; Fariduddin, Q.; Ahmad, A. Salicylic Acid: Physiological Roles in Plants. In Salicylic Acid; Hayat, S., Ahmad, A., Alyemeni, M., Eds.; Springer: Dordrecht, Netherlands, 2013; pp. 15–30. [Google Scholar]
- Sirhindi, G.; Kumar, M.; Kumar, S.; Bhardwaj, R. Brassinosteroids: Physiology and Stress Management in Plants. In Abiotic stress response in plants; Tuteja, N., Gill, S.S., Eds.; Wiley: Weinheim, Germany, 2016; pp. 279–314. [Google Scholar]
- Huang, H.; Liu, B.; Liu, L.; Song, S. Jasmonate action in plant growth and development. J. Exp. Bot. 2017, 68, 1349–1359. [Google Scholar] [CrossRef] [Green Version]
- Radwan, D.E.M.; Soltan, D.M. The negative effects of clethodim in photosynthesis and gas-exchange status of maize plants are ameliorated by salicylic acid pretreatment. Photosynthetica 2012, 50, 171–179. [Google Scholar] [CrossRef]
- Mahmood, M.; Bidabadi, S.S.; Ghobadi, C.; Gray, D.J. Effect of methyl jasmonate treatments on alleviation of polyethylene glycol -mediated water stress in banana (Musa acuminata cv. ‘Berangan’, AAA) shoot tip cultures. Plant Growth Regul. 2012, 68, 161–169. [Google Scholar] [CrossRef]
- Qiu, Z.; Guo, J.; Zhu, A.; Zhang, L.; Zhang, M. Exogenous jasmonic acid can enhance tolerance of wheat seedlings to salt stress. Ecotoxicol. Environ. Saf. 2014, 104, 202–208. [Google Scholar] [CrossRef]
- Fariduddin, Q.; Yusuf, M.; Ahmad, I.; Ahmad, A. Brassinosteroids and their role in response of plants to abiotic stresses. Biol. Plant. 2014, 58, 9–17. [Google Scholar] [CrossRef]
- Anwar, A.; Liu, Y.; Dong, R.; Bai, L.; Yu, X.; Li, Y. The physiological and molecular mechanism of brassinosteroid in response to stress: A review. Biol. Res. 2018, 51, 46. [Google Scholar] [CrossRef] [Green Version]
- Rongai, D.; Pulcini, P.; Pesce, B.; Milano, F. Antifungal activity of some botanical extracts on Fusarium oxysporum. Open Life Sci. 2015, 10, 409–416. [Google Scholar] [CrossRef] [Green Version]
- NC State University Home Page. Available online: https://projects.ncsu.edu/cals/course/pp728/Fusarium/Fusarium_oxysporum.htm (accessed on 1 November 2019).
- Ignjatov, M.; Milosevic, D.; Nikolic, Z.; Gvozdanovic-Varga, J.; Jovicic, D.; Zdjelar, G. Fusarium oxysporum as causal agent of tomato wilt and fruit rot. Pestic. Phytomed. 2015, 27, 25–31. [Google Scholar] [CrossRef]
- Chakraborty, N.; Ghosh, S.; Chandra, S.; Sengupta, S.; Acharya, K. Abiotic elicitors mediated elicitation of innate immunity in tomato: An ex vivo comparison. Physiol. Mol. Biol. Plants 2016, 22, 307–320. [Google Scholar] [CrossRef] [Green Version]
- Jendoubi, W.; Harbaoui, K.; Hamada, W. Salicylic acid-induced resistance against Fusarium oxysporumf.s.pradicis lycopercisi in hydroponic grown tomato plants. J. New Sci. 2015, 21, 985–995. [Google Scholar]
- Ramírez-Godoy, A.; Vera-Hoyos, A.P.; Jiménez-Beltrán, N.; Restrepo-Diaz, H. Application of Foliar Synthetic Elicitors for the Management of Diaphorina citri (Hemiptera: Liviidae) Populations in Tahiti Lime (Citrus latifolia Tanaka). HortScience 2018, 53, 1012–1020. [Google Scholar] [CrossRef] [Green Version]
- Bibi, N.; Fan, K.; Dawood, M.; Nawaz, G.; Yuan, S.; Xuede, W. Exogenous application of epibrassinolide attenuated Verticillium wilt in upland cotton by modulating the carbohydrates metabolism, plasma membrane ATPases and intracellular osmolytes. Plant Growth Regul. 2013, 73, 155–164. [Google Scholar] [CrossRef]
- Tosun, N.; Türküsay, H.; Aktaş, L.; Yavaşoğlu, N.Ü. Effects of salicylic acid, harpin and phosphorus acid in control of late blight (Phytophthora infestans Mont. De Barry) disease and some physiological parameters of tomato. Turk. Phytopathol. Soc. 2006, 32, 1–10. [Google Scholar]
- Parvu, M.; Vlase, L.; Fodorpataki, L.; Parvu, O.; Rosca-Casian, O.; Bartha, C.; Barbu-Tudoran, L.; Parvu, A.E. Chemical composition of celandine (Chelidonium majus L.) extract and its effects on Botrytis tulipae (Lib.) Lind fungus and the tulip. Not. Bot. Horti Agrobot. Cluj Napoca 2013, 41, 414–426. [Google Scholar] [CrossRef] [Green Version]
- Bibi, N.; Ahmed, I.M.; Fan, K.; Dawood, M.; Li, F.; Yuan, S.; Wang, X. Role of brassinosteroids in alleviating toxin-induced stress of Verticillium dahliae on cotton callus growth. Environ. Sci. Pollut. R. 2017, 24, 12281–12292. [Google Scholar] [CrossRef]
- Kaur, G.; Asthir, B. Proline: A key player in plant abiotic stress tolerance. Biol. Plant. 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Khoubnasabjafari, M.; Ansarin, K.; Jouyban, A. Reliability of malondialdehyde as a biomarker of oxidative stress in psychological disorders. BioImpacts 2015, 5, 123–127. [Google Scholar] [PubMed]
- Taïbi, K.; Taïbi, F.; Abderrahim, L.A.; Ennajah, A.; Belkhodja, M.; Mulet, J.M. Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. S. Afr. J. Bot. 2016, 105, 306–312. [Google Scholar] [CrossRef]
- Zehra, A.; Meena, M.; Dubey, M.K.; Aamir, M.; Upadhyay, R.S. Activation of defense response in tomato against Fusarium wilt disease triggered by Trichoderma harzianum supplemented with exogenous chemical inducers (SA and MeJA). Braz. J. Bot. 2017, 40, 651–664. [Google Scholar] [CrossRef]
- Radwan, D.E.M.; Fayez, K.A.; Mahmoud, S.Y.; Lu, G. Modifications of antioxidant activity and protein composition of bean leaf due to Bean yellow mosaic virus infection and salicylic acid treatments. Acta Physiol. Plant 2010, 32, 891–904. [Google Scholar] [CrossRef]
- Naz, R.; Nosheen, A.; Yasmin, H.; Bano, A.; Keyani, R. Botanical-chemical formulations enhanced yield and protection against Bipolaris sorokiniana in wheat by inducing the expression of pathogenesis-related proteins. PLoS ONE 2018, 13, e0196194. [Google Scholar] [CrossRef] [Green Version]
- Lima, J.V.; Lobato, A.K.S. Brassinosteroids improve photosystem II efficiency, gas exchange, antioxidant enzymes and growth of cowpea plants exposed to water deficit. Physiol. Mol. Biol. Plants 2017, 23, 59–72. [Google Scholar] [CrossRef]
- Ali, S.S.; Kumar, G.S.; Khan, M.; Doohan, F.M. Brassinosteroid enhances resistance to fusarium diseases of barley. Phytopathology 2013, 103, 1260–1267. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Z.; Wang, L.; Zhou, Q. Effects of bisphenol A on growth, photosynthesis and chlorophyll fluorescence in above-ground organs of soybean seedlings. Chemosphere 2013, 90, 1274–1280. [Google Scholar] [CrossRef]
- Li, J.; Yang, P.; Gan, Y.; Yu, J.; Xie, J. Brassinosteroid alleviates chilling-induced oxidative stress in pepper by enhancing antioxidation systems and maintenance of photosystem II. Acta Physiol. Plant 2015, 37, 222. [Google Scholar] [CrossRef]
- Yadav, S.; Hayat, S.; Wani, A.S.; Irfan, M.; Ahmad, A. Comparison of the Influence of 28-Homobrassinolide and 24-Epibrassinolide on Nitrate Reductase Activity, Proline Content, and Antioxidative Enzymes of Tomato. Int. J. Veg. Sci. 2012, 18, 161–170. [Google Scholar] [CrossRef]
- Smith, J.L.; De Moraes, C.M.; Mescher, M.C. Jasmonate-and salicylate-mediated plant defense responses to insect herbivores, pathogens and parasitic plants. Pest Manag. Sci. 2009, 65, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.M.; Zhu, S.; Kachroo, P.; Kachroo, A. Signal regulators of systemic acquired resistance. Front. Plant Sci. 2015, 6, 228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, W.G.; Miller, G.; Wallace, I.; Harper, J.; Mittler, R.; Gilroy, S. Orchestrating rapid long-distance signaling in plants with Ca2+, ROS and electrical signals. Plant J. 2017, 90, 698–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khoshbakht, D.; Asgharei, M.R. Influence of foliar-applied salicylic acid on growth, gas-exchange characteristics, and chlorophyll fluorescence in citrus under saline conditions. Photosynthetica 2015, 53, 410–418. [Google Scholar] [CrossRef]
- Razmi, N.; Ebadi, A.; Daneshian, J.; Jahanbakhsh, S. Salicylic acid induced changes on antioxidant capacity, pigments and grain yield of soybean genotypes in water deficit condition. J. Plant Interact. 2017, 12, 457–464. [Google Scholar] [CrossRef]
- Nazar, R.; Umar, S.; Khan, N.A.; Sareer, O. Salicylic acid supplementation improves photosynthesis and growth in mustard through changes in proline accumulation and ethylene formation under drought stress. S. Afr. J. Bot. 2015, 98, 84–94. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H. Interactive effect of salicylic acid on some physiological features and antioxidant enzymes activity in ginger (Zingiber officinale Roscoe). Molecules 2013, 18, 5965–5979. [Google Scholar] [CrossRef]
- Fodor, J.; Gullner, G.; Adam, A.L.; Barna, B.; Komives, T.; Király, Z. Local and systemic responses of antioxidants to tobacco mosaic virus infection and to salicylic acid in tobacco (role in systemic acquired resistance). Plant Physiol. 1997, 114, 1443–1451. [Google Scholar] [CrossRef] [Green Version]
- Király, Z.; Barna, B.; Kecskés, A.; Fodor, J. Down-regulation of antioxidative capacity in a transgenic tobacco which fails to develop acquired resistance to necrotization caused by TMV. Free Radic. Res. 2002, 36, 981–991. [Google Scholar] [CrossRef]
- Preciado-Rangel, P.; Reyes-Pérez, J.J.; Ramírez-Rodríguez, S.C.; Salas-Pérez, L.; Fortis-Hernández, M.; Murillo-Amador, B.; Troyo-Diéguez, E. Foliar Aspersion of Salicylic Acid Improves Phenolic and Flavonoid Compounds, and Also the Fruit Yield in Cucumber (Cucumis sativus L.). Plants 2019, 8, 44. [Google Scholar] [CrossRef] [Green Version]
- Bhutia, D.D.; Zhimo, Y.; Kole, R.; Saha, J. Antifungal activity of plant extracts against Colletotrichum musae, the post-harvest anthracnose pathogen of banana cv. Martaman. Nutr. Food Sci. 2016, 46, 2–15. [Google Scholar] [CrossRef]
- Bulgari, R.; Cocetta, G.; Trivellini, A.; Vernieri, P.; Ferrante, A. Biostimulants and crop responses: A review. Biol. Agric. Hortic. 2015, 31, 1–17. [Google Scholar] [CrossRef]
- Massa, D.; Prisa, D.; Montoneri, E.; Battaglini, D.; Ginepro, M.; Negre, M.; Burchi, G. Application of municipal biowaste derived products in Hibiscus cultivation: Effect on leaf gaseous exchange activity, and plant biomass accumulation and quality. Sci. Horict. 2016, 205, 59–69. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaige, A.R.; Ayella, A.; Shuai, B. Methyl jasmonate and ethylene induce partial resistance in Medicago truncatula against the charcoal rot pathogen Macrophomina phaseolina. Physiol. Mol. Plant Pathol. 2010, 74, 412–418. [Google Scholar] [CrossRef]
- Kępczyńska, E.; Król, P. The phytohormone methyl jasmonate as an activator of induced resistance against the necrotroph Alternaria porri f. sp. solani in tomato plants. J. Plant Interact. 2012, 7, 307–315. [Google Scholar] [CrossRef]
- Jiang, L.; Jin, P.; Wang, L.; Yu, X.; Wang, H.; Zheng, Y. Methyl jasmonate primes defense responses against Botrytis cinerea and reduces disease development in harvested table grapes. Sci. Hortic. 2015, 192, 218–223. [Google Scholar] [CrossRef]
- Wang, K.; Liao, Y.; Kan, J.; Han, L.; Zheng, Y. Response of direct or priming defense against Botrytis cinerea to methyl jasmonate treatment at different concentrations in grape berries. Int. J. Food Microbiol. 2015, 194, 32–39. [Google Scholar] [CrossRef]
- Ralhan, A.; Schöttle, S.; Thurow, C.; Iven, T.; Feussner, I.; Polle, A.; Gatz, C. The vascular pathogen Verticillium longisporum requires a jasmonic acid-independent COI1 function in roots to elicit disease symptoms in Arabidopsis shoots. Plant Physiol. 2012, 159, 1192–1203. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.C.; Wong, C.L.; Muzzi, F.; Vlaardingerbroek, I.; Kidd, B.N.; Schenk, P.M. Root defense analysis against Fusarium oxysporum reveals new regulators to confer resistance. Sci. Rep. 2014, 4, 5584. [Google Scholar] [CrossRef] [Green Version]
- Lyons, R.; Stiller, J.; Powell, J.; Rusu, A.; Manners, J.M.; Kazan, K. Fusarium oxysporum triggers tissue-specific transcriptional reprogramming in Arabidopsis thaliana. PLoS ONE 2015, 10, e0121902. [Google Scholar] [CrossRef] [PubMed]
- Rahman, T.A.E.; Oirdi, M.E.; Gonzalez-Lamothe, R.; Bouarab, K. Necrotrophic pathogens use the salicylic acid signaling pathway to promote disease development in tomato. Mol. Plant Microbe Interact. 2012, 25, 1584–1593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwari, S.; Pandey, D.; Gaur, M.; Kumar, A. Effect of Methyl Jasmonate on Disease Severity and Expression of Plant Defensin Gene during Alternaria brassicae Infection in Arabidopsis. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 857–865. [Google Scholar] [CrossRef]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual, 1st ed.; Blackwell Publishing: Ames, IA, USA, 2006; p. 382. [Google Scholar]
- Hainaut, P.; Remacle, T.; Decamps, C.; Lambert, R.; Sadok, W. Higher forage yields under temperate drought explained by lower transpiration rates under increasing evaporative demand. Eur. J. Agron. 2016, 72, 91–98. [Google Scholar] [CrossRef]
- Park, D. Isolation of Fusarium oxysporum from soils. Trans. Br. Mycol. Soc. 1961, 44, 119–122. [Google Scholar] [CrossRef]
- Makandar, R.; Nalam, V.J.; Lee, H.; Trick, H.N.; Dong, Y.; Shah, J. Salicylic acid regulates basal resistance to Fusarium head blight in wheat. Mol. Plant Microbe Interact. 2012, 25, 431–439. [Google Scholar] [CrossRef] [Green Version]
- Thaler, J.S.; Owen, B.; Higgins, V.J. The role of the jasmonate response in plant susceptibility to diverse pathogens with a range of lifestyles. Plant Physiol. 2004, 135, 530–538. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Velandia, C. Interactions between Bacillus amyloliquefaciens Bs006, Fusarium oxysporun Map5 and Cape gooseberry (Physalis peruviana). Ph.D. Thesis, Universidad Nacional de Colombia, Bogotá, Colombia, 25 January 2018. [Google Scholar]
- Townsend, G.R.; Heuberger, J.W. Methods for estimating losses caused by diseases in fungicide experiments. Plant Dis. Report. 1943, 27, 340–343. [Google Scholar]
- Campbell, C.L.; Madden, L.V. Introduction to Plant Disease Epidemiology; Wiley-Interscience: New York, NY, USA, 1990; p. 532. [Google Scholar]
- Alves, D.P.; Tomaz, R.S.; Laurindo, B.S.; Laurindo, R.D.F.; Cruz, C.D.; Nick, C.; Silva, D.J.H.D. Artificial neural network for prediction of the area under the disease progress curve of tomato late blight. Sci. Agr. 2017, 74, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.Z.; Deng, X.P.; Xu, B.C.; Gao, Z.J.; Ding, W.L. Photosynthetic activity and efficiency of Bothriochloa ischaemum and Lespedeza davurica in mixtures across growth periods under water stress. Acta Physiol. Plant 2014, 36, 1033–1044. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]

| Treatments | Rapid Light-Response Curve Parameters | |||
|---|---|---|---|---|
| Elicitor | Spray number | α | ETRmax | Ik |
| µmol∙m−2∙s−1 | µmol∙m−2∙s−1 | |||
| 1 | ||||
| BR | 0 | 0.36 1 | 4.21 | 10.27 |
| 1 | 0.44 | 6.21 | 14.16 | |
| 2 | 0.59 | 7.09 | 11.85 | |
| 3 | 0.82 | 10.81 | 12.82 | |
| Significance 2 | L, Q *** | L *** | Q, C *** | |
| SA | 0 | 0.36 | 4.16 | 10.32 |
| 1 | 0.44 | 6.04 | 13.68 | |
| 2 | 0.57 | 7.47 | 13.07 | |
| 3 | 0.80 | 10.01 | 12.69 | |
| Significance | L *** | L *** | Q ** | |
| JA | 0 | 0.37 | 4.19 | 10.25 |
| 1 | 0.42 | 4.37 | 10.03 | |
| 2 | 0.55 | 5.92 | 10.79 | |
| 3 | 0.63 | 7.12 | 11.24 | |
| Significance | L *** | L *** | L * | |
| BE | 0 | 0.36 | 4.24 | 10.32 |
| 1 | 0.43 | 5.29 | 11.82 | |
| 2 | 0.59 | 7.05 | 11.99 | |
| 3 | 0.75 | 9.39 | 12.43 | |
| Significance | L *** | L *** | L *** | |
| CV 3 (%) | 5.7 | 5.83 | 6.89 | |
| Chemical Name of the Active Ingredient | Commercial Name (Manufacturer) |
|---|---|
| 2-Hydroxybenzoic acid | Salicylic acid (Panreac Applichem, Barcelona, Spain) |
| (±)-1α, 2β-3-Oxo-2-(cis-2 pentenyl) Cyclopentylacetic acid | (±)- Jasmonic acid (Sigma Aldrich, MO, US) |
| (25 R) – 3β. 5α – dihydroxy-spirostan-6-one | Biomex DI-31 (Minerales exclusivos SA, Bogotá, Colombia) |
| Echinacea, Tormentil and Aloe extracts, K and Mg salts | Loker® (Biolchim S. p. A., Medicina, Bologna, Italy) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chávez-Arias, C.C.; Gómez-Caro, S.; Restrepo-Díaz, H. Physiological Responses to the Foliar Application of Synthetic Resistance Elicitors in Cape Gooseberry Seedlings Infected with Fusarium oxysporum f. sp. physali. Plants 2020, 9, 176. https://doi.org/10.3390/plants9020176
Chávez-Arias CC, Gómez-Caro S, Restrepo-Díaz H. Physiological Responses to the Foliar Application of Synthetic Resistance Elicitors in Cape Gooseberry Seedlings Infected with Fusarium oxysporum f. sp. physali. Plants. 2020; 9(2):176. https://doi.org/10.3390/plants9020176
Chicago/Turabian StyleChávez-Arias, Cristhian C., Sandra Gómez-Caro, and Hermann Restrepo-Díaz. 2020. "Physiological Responses to the Foliar Application of Synthetic Resistance Elicitors in Cape Gooseberry Seedlings Infected with Fusarium oxysporum f. sp. physali" Plants 9, no. 2: 176. https://doi.org/10.3390/plants9020176





