Foliar Application of Polyamines Modulates Winter Oilseed Rape Responses to Increasing Cold
Abstract
:1. Introduction
2. Results
2.1. Survival
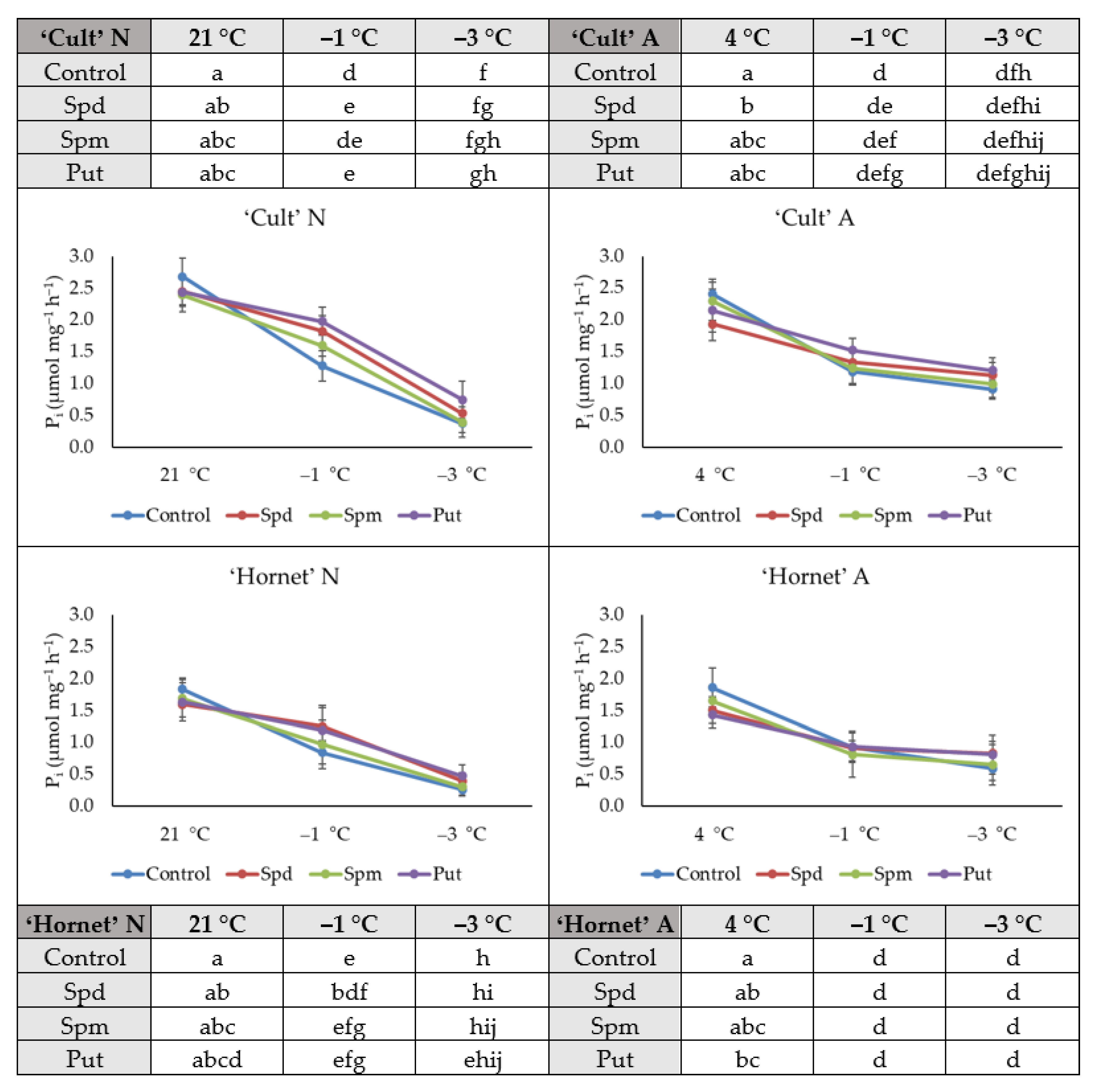
2.2. H+-ATPase Activity
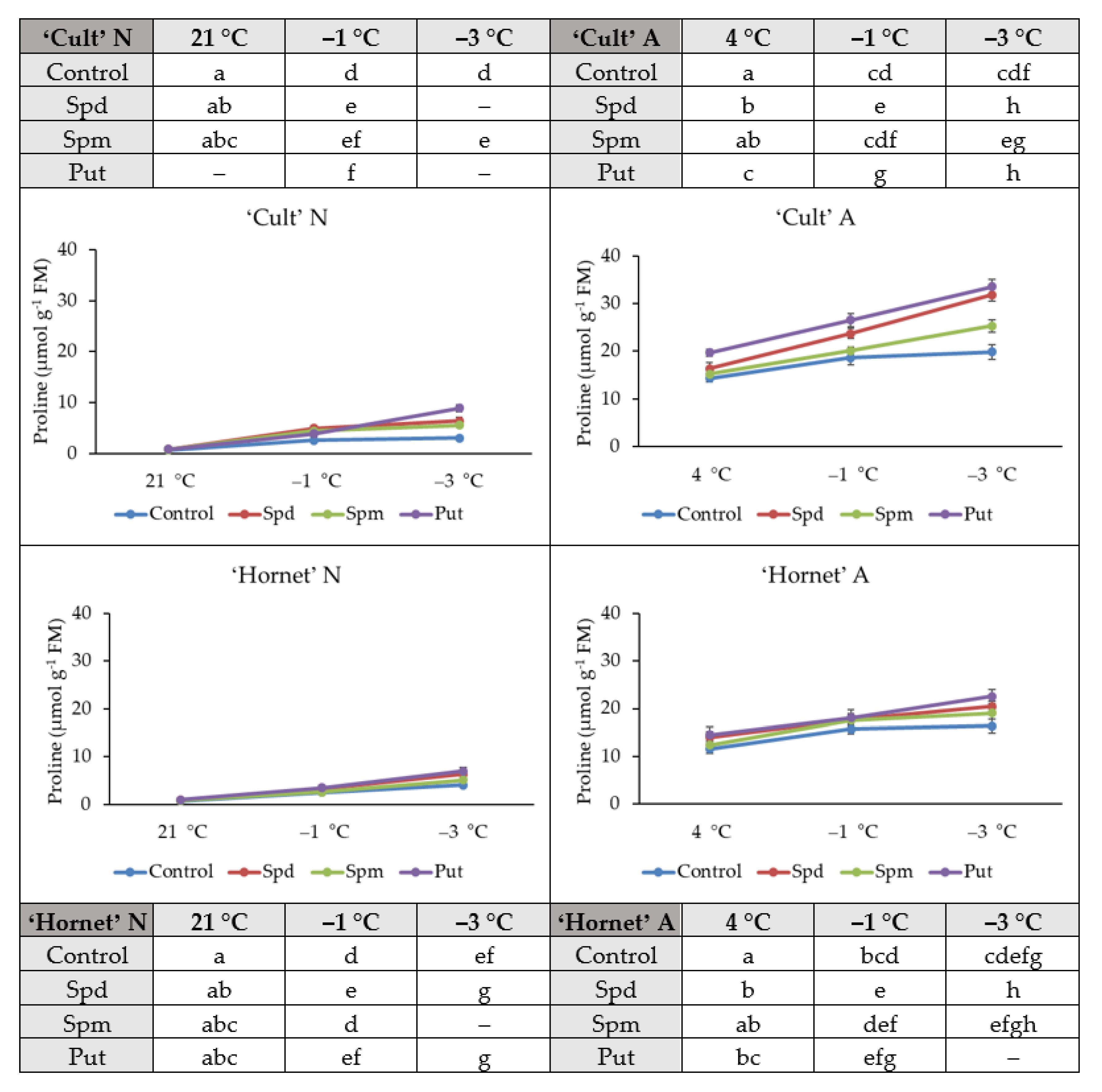
2.3. Proline Accumulation
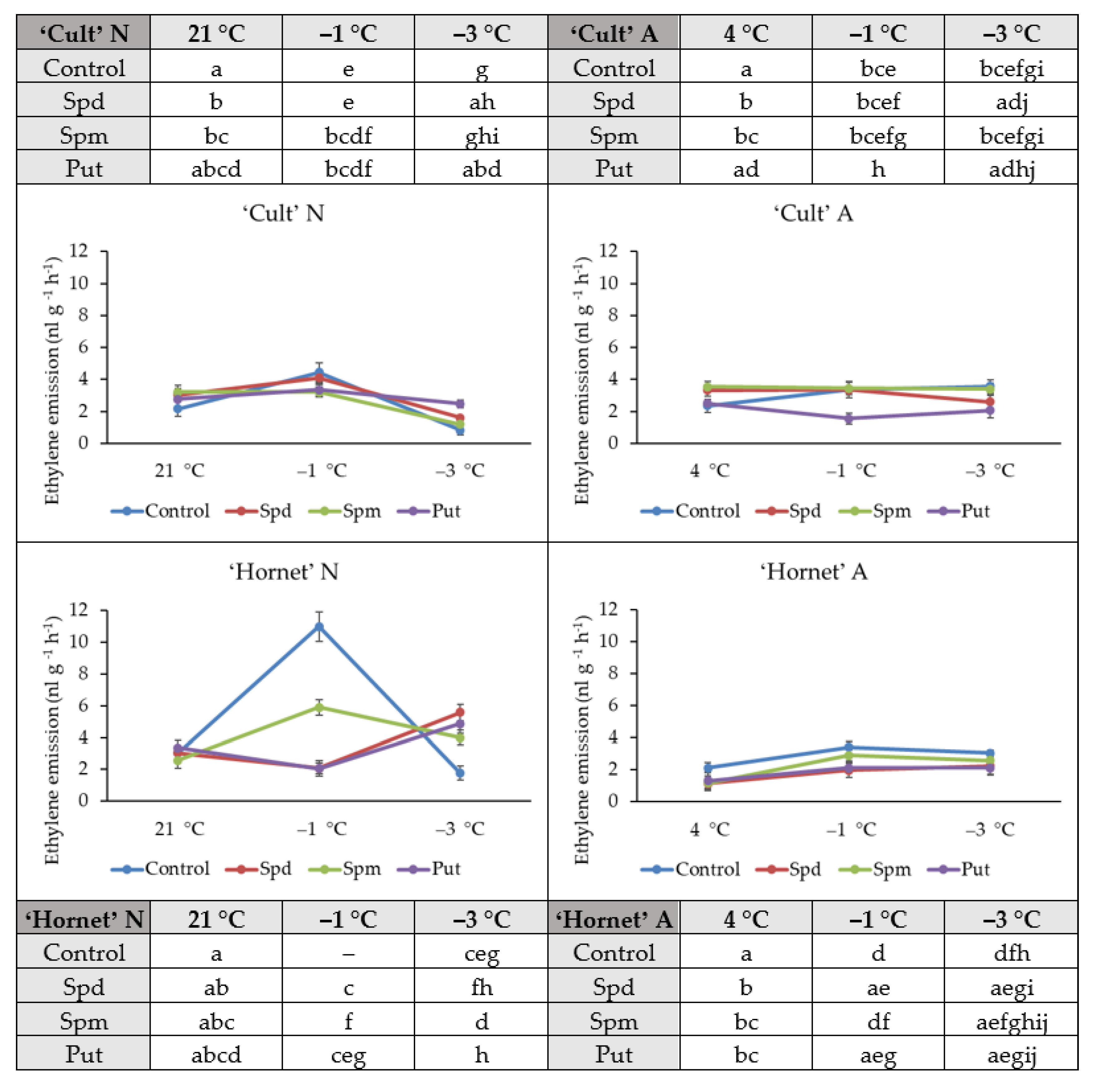
2.4. Ethylene Emissions
3. Discussion
4. Materials and Methods
4.1. Plant Material and Cultivation Conditions
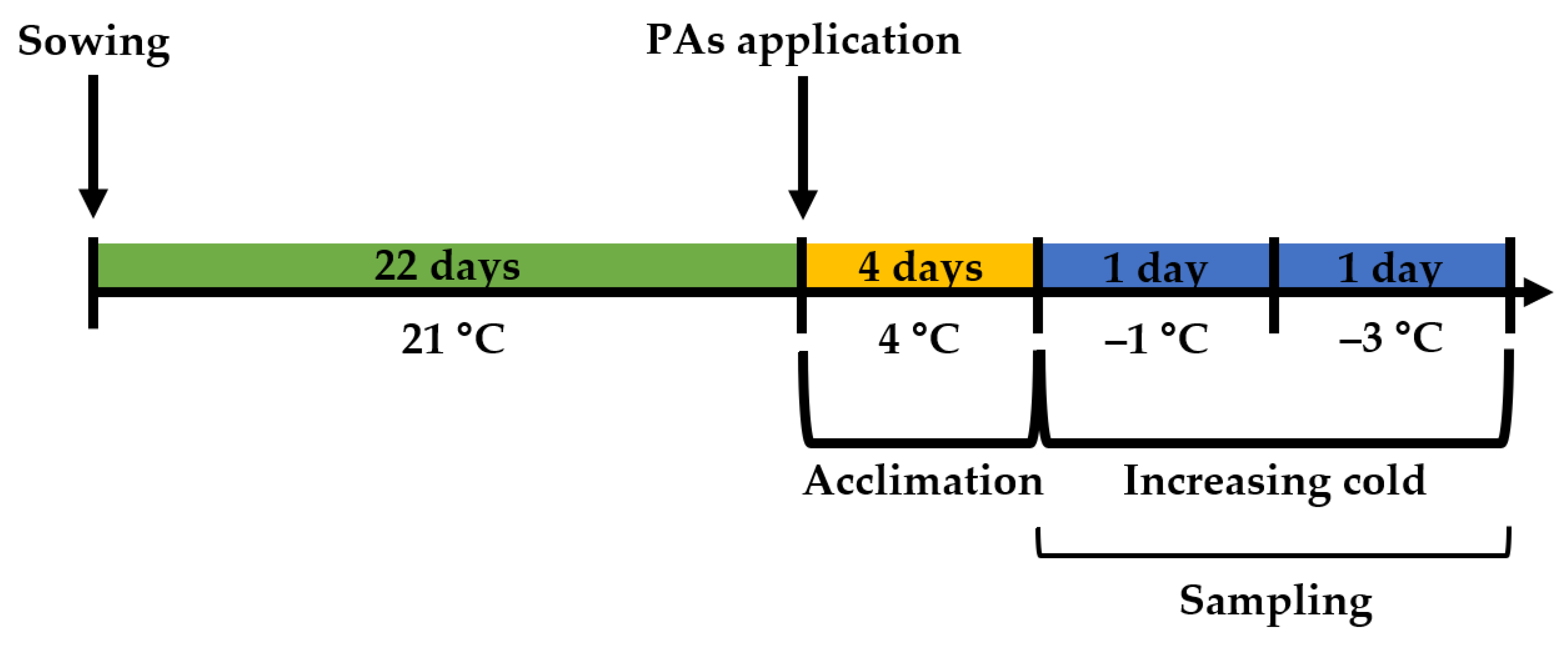
4.2. Application of PAs
4.3. Temperature Treatments
4.3.1. Cold Acclimation Treatment
4.3.2. Increasing Cold Treatment
4.4. Sampling
4.5. Determination of Plant Survival
4.6. Extraction and Activity Assay of H+-ATPase
4.7. Determination of Proline Content
4.8. Ethylene Emission Assay
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, J.H.; Kitashiba, H.; Wang, J.; Ban, Y.; Moriguchi, T. Polyamines and their ability to provide environmental stress tolerance to plants. Plant Biotechnol. 2007, 24, 117–126. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Huang, R. Enhanced tolerance to freezing in tobacco and tomato overexpressing transcription factor TERF2/LeERF2 is modulated by ethylene biosynthesis. Plant Mol. Biol. 2010, 73, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Fiebelkorn, D.; Rahman, M. Development of a protocol for frost-tolerance evaluation in rapeseed/canola (Brassica napus L.). Crop J. 2016, 4, 147–152. [Google Scholar] [CrossRef] [Green Version]
- Kidokoro, S.; Yoneda, K.; Takasaki, H.; Takahashi, F.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Different cold-signaling pathways function in the responses to rapid and gradual decreases in temperature. Plant Cell 2017, 29, 760–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClinchey, S.L.; Kott, L.S. Production of mutants with high cold tolerance in spring canola (Brassica napus). Euphytica 2008, 162, 51–67. [Google Scholar] [CrossRef]
- Paulauskas, A.; Jodinskienė, M.; Griciuvienė, L.; Žukauskienė, J.; Petraitienė, E.; Brazauskienė, I. Morphological traits and genetic diversity of differently overwintered oilseed rape (Brassica napus L.) cultivars. Zemdirbyste 2013, 100, 409–416. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, C.P.; Cardoso-Gustavson, P.; Rodrigues, E.; Braga, M.R.; Mercier, H.; Nievola, C.C. Low temperature acclimation and de-acclimation of the subtropical bromeliad Nidularium minutum: Implications of changes in the NO, sugar content and NR activity. Environ. Exp. Bot. 2019, 159, 34–43. [Google Scholar] [CrossRef]
- Rapacz, M. Cold-deacclimation of oilseed rape (Brassica napus var. oleifera) in response to fluctuating temperatures and photoperiod. Ann. Bot. 2002, 89, 543–549. [Google Scholar] [CrossRef] [Green Version]
- Ananga, A.O.; Cebert, E.; Ochieng, J.W.; Kumar, S.; Kambiranda, D.; Vasanthaiah, H.; Tsolova, V.; Senwo, Z.; Konan, K.; Anike, F.N. Prospects for transgenic and molecular breeding for cold tolerance in canola (Brassica napus L.). In Oilseeds; Akpan, U.G., Ed.; IntechOpen: Rijeka, Croatia, 2012; pp. 1–32. [Google Scholar] [CrossRef]
- Gavelienė, V.; Pakalniškytė, L.; Novickienė, L. Regulation of proline and ethylene levels in rape seedlings for freezing tolerance. Cent. Eur. J. Biol. 2014, 9, 1099–1107. [Google Scholar] [CrossRef] [Green Version]
- Rihan, H.Z.; Al-Issawi, M.; Fuller, M.P. Advances in physiological and molecular aspects of plant cold tolerance. J. Plant Interact. 2017, 12, 143–157. [Google Scholar] [CrossRef]
- Arora, R.; Rowland, L.J. Physiological research on winter-hardiness: Deacclimation resistance, reacclimation ability, photoprotection strategies, and a cold acclimation protocol design. HortScience 2011, 46, 1070–1078. [Google Scholar] [CrossRef] [Green Version]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments. Plant Signal Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakienė, E. The effect of the microelement fertilizers and bio-logical preparation Terra Sorb Foliar on spring rape crop. Žemės ūkio mokslai 2013, 20, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, S.; Funck, D.; Szabados, L.; Rentsch, D. Proline metabolism and transport in plant development. Amino Acids 2010, 39, 949–962. [Google Scholar] [CrossRef] [Green Version]
- Jankovska-Bortkevič, E.; Gavelienė, V.; Jankauskienė, J.; Mockevičiūtė, R.; Koryznienė, D.; Jurkonienė, S. Response of winter oilseed rape to imitated temperature fluctuations in autumn-winter period. Environ. Exp. Bot. 2019, 166, 103801. [Google Scholar] [CrossRef]
- Trotel-Aziz, P.; Niogret, M.F.; Deleu, C.; Bouchereau, A.; Aziz, A.; Larher, F.R. The control of proline consumption by abscisic acid during osmotic stress recovery of canola leaf discs. Physiol. Plant. 2003, 117, 213–221. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, L.; Natarajan, S.K.; Becker, D.F. Proline mechanisms of stress survival. Antioxid. Redox Signal. 2013, 19, 998–1011. [Google Scholar] [CrossRef] [Green Version]
- Kishor, P.B.K.; Sreenivasulu, N. Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant Cell Environ. 2014, 37, 300–311. [Google Scholar] [CrossRef]
- Martz, F.; Sutinen, M.L.; Kiviniemi, S.; Palta, J.P. Changes in freezing tolerance, plasma membrane H+-ATPase activity and fatty acid composition in Pinus resinosa needles during cold acclimation and de-acclimation. Tree Physiol. 2006, 26, 783–790. [Google Scholar] [CrossRef] [Green Version]
- Darginavičienė, J.; Pašakinskienė, I.; Maksimov, G.; Rognli, O.A.; Jurkonienė, S.; Šveikauskas, V.; Bareikienė, N. Changes in plasmalemma K+ Mg2+-ATPase dephosphorylating activity and H+ transport in relation to freezing tolerance and seasonal growth of Festuca pratensis Huds. J. Plant Physiol. 2008, 165, 825–832. [Google Scholar] [CrossRef]
- Janicka-Russak, M. Plant plasma membrane H+-ATPase in adaptation of plants to abiotic stresses. In Abiotic Stress Response in Plants—Physiological, Biochemical and Genetic Perspective; Shanker, A., Venkateswarlu, A., Eds.; IntechOpen: Rijeka, Croatia, 2011; pp. 197–218. [Google Scholar] [CrossRef] [Green Version]
- Muzi, C.; Camoni, L.; Visconti, S.; Aducci, P. Cold stress affects H+-ATPase and phospholipase D activity in Arabidopsis. Plant Physiol. Biochem. 2016, 108, 328–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burr, K.E.; Wallner, S.J.; Tinus, R.W. Ethylene and ethane evolution during cold acclimation and deacclimation of ponderosa pine. Can. J. For. Res. 1991, 21, 601–605. [Google Scholar] [CrossRef]
- Shi, Y.; Tian, S.; Hou, L.; Huang, X.; Zhang, X.; Guo, H.; Yang, S. Ethylene signaling negatively regulates freezing tolerance by repressing expression of CBF and type-A ARR genes in Arabidopsis. Plant cell 2012, 24, 2578–2595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cristescu, S.M.; Mandon, J.; Arslanov, D.; De Pessemier, J.; Hermans, C.; Harren, F.J. Current methods for detecting ethylene in plants. Ann. Bot. 2013, 111, 347–360. [Google Scholar] [CrossRef] [Green Version]
- Pei, H.; Wang, H.; Wang, L.; Zheng, F.; Dong, C.H. Regulatory function of ethylene in plant responses to drought, cold, and salt stresses. In Mechanism of Plant Hormone Signaling Under Stress; Pandey, K.G., Ed.; John Wiley and Sons: Hoboken, NJ, USA, 2017; pp. 327–344. [Google Scholar] [CrossRef]
- Yu, X.M.; Griffith, M.; Wiseman, S.B. Ethylene induces antifreeze activity in winter rye leaves. Plant Physiol. 2001, 126, 1232–1240. [Google Scholar] [CrossRef] [Green Version]
- Ahanger, M.A.; Akram, N.A.; Ashraf, M.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Plant responses to environmental stresses-from gene to biotechnology. AoB Plants 2017, 9, plx025. [Google Scholar] [CrossRef] [Green Version]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Cai, T.; Ni, Y.; Li, Y.; Guo, J.; Peng, D.; Yang, D.; Yin, Y.; Wang, Z. Effects of exogenous abscisic acid and gibberellic acid on flling process and nitrogen metabolism characteristics in wheat grains. Aust. J. Crop Sci. 2013, 7, 58–65. [Google Scholar]
- Gavelienė, V.; Pakalniškytė, L.; Novickienė, L.; Balčiauskas, L. Effect of biostimulants on cold resistance and productivity formation in winter rapeseed and winter wheat. Ir. J. Agric. Food Res. 2018, 57, 71–83. [Google Scholar] [CrossRef]
- Todorova, D.; Sergiev, I.; Alexieva, V.; Karanov, E.; Smith, A.; Hall, M. Polyamine content in Arabidopsis thaliana (L.) Heynh during recovery after low and high temperature treatments. Plant Growth Regul. 2007, 51, 185–191. [Google Scholar] [CrossRef]
- Ebeed, H.T.; Hassan, N.M.; Aljarani, A.M. Exogenous applications of polyamines modulate drought responses in wheat through osmolytes accumulation, increasing free polyamine levels and regulations of polyamine biosynthetic genes. Plant Physiol. Biochem. 2017, 118, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Fariduddin, Q.; Varshney, P.; Yusuf, M.; Ahmad, A. Polyamines: Potent modulators of plant responses to stress. J. Plant Interact. 2012, 8, 1–16. [Google Scholar] [CrossRef]
- Peynevandi, K.M.; Razavi, S.M.; Zahri, S. The ameliorating effects of polyamine supplement on physiological and biochemical parameters of Stevia rebaudiana Bertoni under cold stress. Plant Prod. Sci. 2018, 21, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Shao, Q.; Yin, L.; Younis, A.; Zheng, B. Polyamine function in plants: Metabolism, regulation on development, and roles in abiotic stress responses. Front. Plant Sci. 2019, 9, 1945. [Google Scholar] [CrossRef] [PubMed]
- Kusano, T.; Berberich, T.; Tateda, C.; Takahashi, Y. Polyamines: Essential factors for growth and survival. Planta 2008, 228, 367–381. [Google Scholar] [CrossRef]
- Calişkan, N.; Kadioğlu, A.; Güler, N. Exogenously applied polyamines ameliorate osmotic stress-induced damages and delay leaf rolling by improving the antioxidant system in maize genotypes. Turk. J. Biol. 2017, 41, 563–574. [Google Scholar] [CrossRef]
- Agudelo-Romero, P.; Bortolloti, C.; Pais, M.S.; Al, E. Study of polyamines during grape ripening indicate an important role of polyamine catabolism. Plant Physiol. Biochem. 2013, 67, 105–119. [Google Scholar] [CrossRef]
- Pál, M.; Tajti, J.; Szalai, G.; Peeva, V.; Végh, B.; Janda, T. Interaction of polyamines, abscisic acid and proline under osmotic stress in the leaves of wheat plants. Sci. Rep. 2018, 8, 12839. [Google Scholar] [CrossRef]
- Sequera-Mutiozabal, M.I.; Erban, A.; Kopka, J.; Al, E. Global metabolic profiling of Arabidopsis polyamine oxidase 4 (AtPAO4) loss-of-function mutants exhibiting delayed dark-induced senescence. Front. Plant Sci. 2016, 7, 173. [Google Scholar] [CrossRef] [Green Version]
- Zhen, Y.; Li, S.; Su, X. Polyamine changes and chilling injury in cold-stored Loquat fruits. Acta Bot. Sin. 2000, 42, 824–827. [Google Scholar] [CrossRef]
- Roy, M.; Wu, R. Arginine decarboxylase transgene expression and analysis of environmental stress tolerance in transgenic rice. Plant Sci. 2001, 160, 869–875. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, W.; Zhang, Z. ABA and putrescine treatment alleviate the chilling damage of banana fruit. J. Plant Physiol. Mol. Biol. 2003, 29, 549–554. [Google Scholar] [CrossRef]
- Bal, E. Effects of exogenous polyamine and ultrasound treatment to improve peach storability. Chil. J. Agric. Res. 2013, 73, 435–440. [Google Scholar] [CrossRef] [Green Version]
- Yousefi, F.; Jabbarzadeh, Z.; Amiri, J.; Rasouli-Sadaghiani, M.H. Response of roses (Rosa hybrida L. ‘Herbert Stevens’) to foliar application of polyamines on root development, flowering, photosynthetic pigments, antioxidant enzymes activity and NPK. Sci. Rep. 2019, 9, 16025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blum, A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production: Osmotic adjustment and plant production. Plant Cell Environ. 2017, 40, 4–10. [Google Scholar] [CrossRef] [PubMed]
- El Sabagh, A.; Hossain, A.; Barutcular, C.; Gormus, O.; Ahmad, Z.; Hussain, S.; Islam, M.S.; Alharby, H.; Bamagoos, A.; Kumar, N.; et al. Effects of drought stress on the quality of major oilseed crops: Implications and possible mitigation strategies. Appl. Ecol. Environ. Res. 2019, 17, 4019–4043. [Google Scholar] [CrossRef]
- Pearce, S.; Zhu, J.; Boldizsár, Á.; Vágújfalvi, A.; Burke, A.; Garland-Campbell, K.; Galiba, G.; Dubcovsky, J. Large deletions in the CBF gene cluster at the Fr-B2 locus are associated with reduced frost tolerance in wheat. Theor. Appl. Genet. 2013, 126, 2683–2697. [Google Scholar] [CrossRef] [Green Version]
- Sanghera, G.S.; Wani, S.H.; Hussain, W.; Singh, N.B. Engineering cold stress tolerance in crop plants. Curr. Genom. 2011, 12, 30–43. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Liu, D.; Chong, K. Cold signaling in plants: Insights into mechanisms and regulation. J. Integr. Plant Biol. 2018, 60, 745–756. [Google Scholar] [CrossRef] [Green Version]
- Barrero-Sicilia, C.; Silvestre, S.; Haslam, R.P.; Michaelson, L.V. Lipid remodelling: Unravelling the response to cold stress in Arabidopsis and its extremophile relative Eutrema salsugineum. Plant Sci. 2017, 263, 194–200. [Google Scholar] [CrossRef]
- Sampedro, J.G.; Muñoz-Clares, R.A.; Uribe, S. Trehalose-mediated inhibition of the plasma membrane H+-ATPase from Kluyveromyces lactis: Dependence on viscosity and temperature. J. Bacteriol. 2002, 184, 4384–4391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; He, J. Advance in metabolism and response to stress of polyamines in plant. Acta Agric Boreali Sin. 2012, 27, 240–245. [Google Scholar] [CrossRef]
- Fowler, D.B.; Byrns, B.M.; Greer, K.J. Overwinter low-temperature responses of cereals: Analyses and simulation. Crop Sci. 2014, 54, 2395–2405. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Bhandari, S.R.; Kim, Y.H.; Lee, J.G. Detection of temperature stress using chlorophyll fluorescence parameters and stress-related chlorophyll and proline content in paprika (Capsicum annuum L.) seedlings. Hortic. Sci. Technol. 2018, 36, 619–629. [Google Scholar] [CrossRef]
- Hummel, I.; Couée, I.; El Amrani, A.; Martin-Tanguy, J.; Hennion, F. Involvement of polyamines in root development at low temperature in the subantarctic cruciferous species Pringlea antiscorbutica. J. Exp. Bot. 2002, 53, 1463–1473. [Google Scholar] [CrossRef] [Green Version]
- Toupchi, Z.; Lisar, S.Y.; Ghassemi-Golezani, K.; Motafakkerazad, R. Physiological Responses of safflower to exogenous putrescine under water deficit. J. Stress Physiol. Biochem. 2018, 14, 38–48. [Google Scholar]
- Wu, M.; Yuan, L. Research progress in the relationship between polyamine and plant resistance. J. Anhui Agric. Sci. 2008, 36, 3516–3518. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Y.; Tan, J.; Al, E. Effects of exogenous putrescine and D-Arg on physiological and biochemical indices of anthurium under chilling stress. Jiangsu J. Agric. Sci. 2018, 34, 152–157. [Google Scholar] [CrossRef]
- Zhao, D.; Shen, L.; Fan, B.; Yu, M.; Zheng, Y.; Lv, S.; Sheng, J. Ethylene and cold participate in the regulation of LeCBF1 gene expression in postharvest tomato fruits. FEBS Lett. 2009, 583, 3329–3334. [Google Scholar] [CrossRef] [Green Version]
- Catala, R.; Lopez-Cobollo, R.; Mar Castellano, M.; Angosto, T.; Alonso, J.M.; Ecker, J.R.; Salinas, J. The Arabidopsis 14-3-3 protein RARE COLD INDUCIBLE 1A links low-temperature response and ethylene biosynthesis to regulate freezing tolerance and cold acclimation. Plant Cell 2014, 26, 3326–3343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, M.; Liu, W.; Xia, X.; Wang, T.; Zhang, W.H. Cold acclimation-induced freezing tolerance of Medicago truncatula seedlings is negatively regulated by ethylene. Physiol. Plant. 2014, 152, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Kazan, K. Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends Plant Sci. 2015, 20, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Weber, E.; Bleiholder, H.; Lancashire, P.D.; Langelüddecke, R.; Stauss, R.; Van der Boom, T.; Witzen-Berger, A. Growth stages of mono- and dicotyledonous plants. In BBCH Monograph, 1st ed.; Meier, U., Ed.; Julius Kühn-Institut: Quedlinburg, Germany, 2018; pp. 32–36. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid method for the quantification of microgram quantities of proteins utilising the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Child, R.D.; Chauvaux, N.; John, K.; Van Onckelen, H.A.; Ulvskov, P. Ethylene biosynthesis in oilseed rape pods in relation to pod shatter. J. Exp. Bot. 1998, 49, 829–838. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jankovska-Bortkevič, E.; Gavelienė, V.; Šveikauskas, V.; Mockevičiūtė, R.; Jankauskienė, J.; Todorova, D.; Sergiev, I.; Jurkonienė, S. Foliar Application of Polyamines Modulates Winter Oilseed Rape Responses to Increasing Cold. Plants 2020, 9, 179. https://doi.org/10.3390/plants9020179
Jankovska-Bortkevič E, Gavelienė V, Šveikauskas V, Mockevičiūtė R, Jankauskienė J, Todorova D, Sergiev I, Jurkonienė S. Foliar Application of Polyamines Modulates Winter Oilseed Rape Responses to Increasing Cold. Plants. 2020; 9(2):179. https://doi.org/10.3390/plants9020179
Chicago/Turabian StyleJankovska-Bortkevič, Elžbieta, Virgilija Gavelienė, Vaidevutis Šveikauskas, Rima Mockevičiūtė, Jurga Jankauskienė, Dessislava Todorova, Iskren Sergiev, and Sigita Jurkonienė. 2020. "Foliar Application of Polyamines Modulates Winter Oilseed Rape Responses to Increasing Cold" Plants 9, no. 2: 179. https://doi.org/10.3390/plants9020179
APA StyleJankovska-Bortkevič, E., Gavelienė, V., Šveikauskas, V., Mockevičiūtė, R., Jankauskienė, J., Todorova, D., Sergiev, I., & Jurkonienė, S. (2020). Foliar Application of Polyamines Modulates Winter Oilseed Rape Responses to Increasing Cold. Plants, 9(2), 179. https://doi.org/10.3390/plants9020179







