β-Aminobutyric Acid Pretreatment Confers Salt Stress Tolerance in Brassica napus L. by Modulating Reactive Oxygen Species Metabolism and Methylglyoxal Detoxification
Abstract
:1. Introduction
2. Results
2.1. Salt Accumulation and Na/K Ratio
2.2. Plant Growth and Biomass
2.3. Water Status and Osmotic Adjustment
2.4. Photosythetic Pigments
2.5. Reactive Oxygen Species Generation, Oxidative Stress, and Membrane Damage
2.6. Ascorbate–Glutathione Cycle
2.7. Activities of Superoxide Dismutase, Catalase, and Glutathione Peroxidase
2.8. Glyoxalase System and Methylglyoxal Content
3. Discussion
4. Materials and Methods
4.1. Plant Materials, Experimental Conditions, and Treatments
4.2. Measurement of Na+ and K+ Content
4.3. Estimation of Plant Growth Parameters
4.4. Determination of Leaf Relative Water Content
4.5. Estimation of Proline Content
4.6. Measurement of Chlorophyll Content
4.7. Histochemical Detection of Superoxide Anion
4.8. Estimation of Lipid Peroxidation
4.9. Determination of Hydrogen Peroxide Content
4.10. Extraction and Measurement of Ascorbate and Glutathione
4.11. Enzyme Extraction and Assays
4.12. Determination of Methylglyoxal Content
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethical statement
References
- Metternicht, G.I.; Zinck, J.A. Remote sensing of soil salinity: Potentials and constraints. Remote Sens. Environ. 2003, 85, 1–20. [Google Scholar] [CrossRef]
- Yensen, N.P. Halophyte uses for the twenty-first century. In Ecophysiology of High Salinity Tolerant Plants; Khan, M.A., Weber, D.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 367–396. [Google Scholar]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Demetriou, G.; Neonaki, C.; Navakoudis, E.; Kotzabasis, K. Salt stress impact on the molecular structure and function of the photosynthetic apparatus—The protective role of polyamines. Biochimica et Biophysica Acta (BBA) Bioenergetics 2007, 1767, 272–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SunarpiHorie, T.; Motoda, J.; Kubo, M.; Yang, H.; Yoda, K. Enhanced salt tolerance mediated by AtHKT1 transporter-induced Na unloading from xylem vessels to xylem parenchyma cells. Plant J. 2005, 44, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Bethke, C.P.; Drew, C.M. Stomatal and nonstomatal components to inhibition of photosynthesis in leaves of Capsicum annum during progressive exposure to NaCl salinity. Plant Physiol. 1992, 99, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Nagy, Z.; Galiba, G. Drought and salt tolerance are not necessarily linked: A study on wheat varieties differing in drought resistance under consecutive water and salinity stresses. J. Plant Physiol. 1995, 145, 168–174. [Google Scholar] [CrossRef]
- Sibole, J.V.; Montero, E.; Cabot, C.; Poschenrieder, C.B. Role of sodium in the ABA-mediated long-term growth response of bean to salt stress. Physiol. Plant. 1998, 104, 299–305. [Google Scholar] [CrossRef]
- Khan MI, R.; Khan, N.A. (Eds.) Reactive Oxygen Species and Antioxidant Systems in Plants: Role and Regulation under Abiotic Stress; Springer: Singapore, 2017. [Google Scholar]
- Khan, M.I.R.; Reddy, P.S.; Ferrante, A.; Khan, N.A. Plant Signaling Molecules: Role and Regulation under Stressful Environments; Woodhead Publishing Limited: Cambridge, UK, 2019. [Google Scholar]
- Ton, J.; Jakab, G.; Toquin, V.; Flors, V.; Iavicoli, A.; Maeder, M.N.; Métraux, J.P.; Mauch-Mani, B. Dissecting the beta-aminobutyric acid-induced priming phenomenon in Arabidopsis. Plant Cell 2005, 17, 987–999. [Google Scholar] [CrossRef] [Green Version]
- Navarova, H.; Bernsdorff, F.; Doring, A.C.; Zeier, J. Pipecolic acid, an endogenous mediator of defense amplification and priming, is a critical regulator of inducible plant immunity. Plant Cell 2012, 24, 5123–5141. [Google Scholar] [CrossRef] [Green Version]
- Conrath, U.; Beckers, G.J.M.; Langenbach, C.J.G.; Jaskiewicz, M.R. Priming for enhanced defense. Annu. Rev. Phytopathol. 2015, 53, 97–119. [Google Scholar] [CrossRef]
- Thevenet, D.; Pastor, V.; Baccelli, I.; Balmer, A.; Vallat, A.; Neier, R.; Glauser, G.; Mauch-Mani, B. The priming molecule β-aminobutyric acid is naturally present in plants and is induced by stress. New Phytol. 2016, 213, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Jakab, G.; Ton, J.; Flors, V.; Zimmerli, L.; Metraux, J.; Mauch-Mani, B. Enhancing Arabidopsis salt and drought stress tolerance by chemical priming for its abscisic acid responses. Plant Physiol. 2005, 139, 267–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jisha, K.J.; Puthur, J.T. Seed priming with beta-amino butyric acid improves abiotic stress tolerance in rice seedlings. Rice Sci. 2016, 23, 242–254. [Google Scholar] [CrossRef] [Green Version]
- Shabala, S.; Bose, J.; Hedrich, R. Salt bladders: Do they matter? Trends Plant Sci. 2014, 19, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Sairam, R.K.; Tyagi, A. Physiology and molecular biology of salinity stress tolerance in plants. Curr. Sci. 2004, 86, 407–421. [Google Scholar]
- Park, H.J.; Kim, W.Y.; Yun, D.J. A new insight of salt stress signaling in plant. Mol. Cell. 2016, 39, 447–459. [Google Scholar] [CrossRef]
- Negrão, S.; Schmöckel, S.M.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Büyük, I˙.; Aras, S. Screening of PvLEA3 gene mRNA expression levels with qRT-PCR in different bean varieties (Phaseolus vulgaris L.) subjected to salt and drought stress. Turk. J. Bot. 2015, 39, 1014–1020. [Google Scholar]
- Baccelli, I.; Mauch-Mani, B. Beta-aminobutyric acid priming of plant defense: The role of ABA and other hormones. Plant Mol. Biol. 2016, 91, 703–711. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef] [Green Version]
- Dorgham, E.A. Effect of Water Stress, Irradiation and Nitrogen Fertilization on Grain Filling, Yield and Quality of Certain wheat Cultivars. Ph.D. Thesis, Ain Shams University of Cairo, Cairo, Egypt, 1991. [Google Scholar]
- Bernstein, N.; Silk, W.K.; Läuchli, A. Growth and development of Sorghum leaves under conditions of NaCl stress. Planta 1993, 191, 433–439. [Google Scholar] [CrossRef]
- Mazher, A.M.A.; El-Quesni, E.M.F.; Farahat, M.M. Responses of ornamental and woody trees to salinity. World J. Agric. Sci. 2007, 3, 386–395. [Google Scholar]
- Zhong, Y.; Wang, B.; Yan, J.; Cheng, L.; Yao, L.; Xiao, L.; Wu, T. DL-β-Aminobutyric acid-induced resistance in soybean against Aphis glycines Matsumura (Hemiptera: Aphididae). PLoS ONE 2014, 9, e85142. [Google Scholar] [CrossRef] [Green Version]
- Mostek, A.; Borner, A.; Weidner, S. Comparative proteomic analysis of baminobutyric acid-mediated alleviation of salt stress in barley. Plant Physiol. Biochem. 2016, 99, 150–161. [Google Scholar] [CrossRef]
- Keshavarzi, M.H.B. Studying the effects of different levels of salinity which caused by NaCl on early growth and germination of Lactuca sativa L. seedling. J. Stress Physiol. Biochem. 2012, 8, 203–208. [Google Scholar]
- Weisany, W.; Sohrabi, Y.; Heidari, G.; Siosemardeh, A.; Ghassemi-Golezani, K. Changes in antioxidant enzymes activity and plant performance by salinity stress and zinc application in soybean (Glycine max L.). Plant Omics 2012, 5, 60–67. [Google Scholar]
- Villalta, I.; Reina-Sanchez, A.; Bolarin, M.C.; Cuartero, J.; Belver, A.; Venema, K.; Carbonell, E.A.; Asins, M.J. Genetic analysis of Na+ and K+ concentrations in leaf and stem as physiological components of salt tolerance in tomato. Theor. Appl. Gene. 2008, 116, 869–880. [Google Scholar] [CrossRef]
- Iqbal, N.; Umar, S.; Khan, N.A.; Khan, M.I.R. A new perspective of phytohormones in salinity tolerance: Regulation of proline metabolism. Environ. Exp. Bot. 2014, 100, 34–42. [Google Scholar] [CrossRef]
- Singh, P.K.; Wu, C.-C.; Zimmerli, L. β-aminobutyric acid priming by stress imprinting. Plant Signal. Behav. 2010, 5, 878–880. [Google Scholar] [CrossRef] [Green Version]
- Bengtsson, T.; Weighill, D.; Proux-Wera, E.; Levander, F.; Resjo, S.; Burra, D.D.; Moushib, L.I.; Hedley, P.E.; Liljeroth, E.; Jacobson, D.; et al. Proteomics and transcriptomics of the BABA induced resistance response in potato using a novel functional annotation approach. BMC Genom. 2014, 15, 315. [Google Scholar] [CrossRef] [Green Version]
- Misra, N.; Misra, R. Salicylic acid changes plant growth parameters and proline metabolism in Rauwolfia serpentine leaves grown under salinity stress. Am. Eurasian J. Agric. Environ. Sci. 2012, 12, 1601–1609. [Google Scholar]
- Tabrizi, E.F.M.; Yarnia, M.; Ahmadzadeh, V.; Farjzadeh, N. Priming effect of different times of maize seeds with nutrient elements in water stress on corn yield. Ann. Biol. Res. 2011, 2, 419–423. [Google Scholar]
- Noreen, Z.; Ashraf, M. Changes in antioxidant enzymes and some key metabolites in some genetically diverse cultivars of radish (Raphanus sativus L.). Environ. Exp. Bot. 2009, 67, 395–402. [Google Scholar] [CrossRef]
- Stepien, P.; Klobus, G. Water relations and photosynthesis in Cucumis sativus L. leaves under salt stress. Biol. Plant. 2006, 50, 610–616. [Google Scholar] [CrossRef]
- Chen, T.H.; Murata, N. Glycinebetaine protects plants against abiotic stress: Mechanisms and biotechnological applications. Plant Cell Environ. 2011, 34, 1–20. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Sun, Y.J.; Wang, M.T.; Li, X.Y.; Guo, X.; Hu, R.; Ma, J. Effects of seed priming on germination and seedling growth under water stress in rice. Acta Agron. Sin. 2010, 36, 1931–1940. [Google Scholar] [CrossRef]
- Goswami, A.; Banerjee, R.; Raha, S. Drought resistance in rice seedlings conferred by seed priming: Role of the anti-oxidant defense mechanisms. Protoplasma 2013, 250, 1115–1129. [Google Scholar] [CrossRef]
- Wang, L.; Jin, P.; Wang, J.; Jiang, L.; Shan, T.; Zheng, Y. Effect of β-aminobutyric acid on cell wallmodification and senescence in sweet cherry duringstorage at 20° C. Food Chem. 2015, 175, 471–477. [Google Scholar] [CrossRef]
- Wu, C.C.; Singh, P.; Chen, M.C.; Zimmerli, L. L-Glutamine inhibits betaaminobutyric acid-induced stress resistance and priming in Arabidopsis. J. Exp. Bot. 2010, 61, 995–1002. [Google Scholar] [CrossRef]
- Hossain, M.A.; Al Kalbani, M.S.A.; Al Farsi, S.A.J.; Weli, A.M.; AlRiyami, O. Comparative study of total phenolics, flavonoids contents and evaluation of antioxidant and antimicrobial activities of different polarities fruits crude extracts of Datura metel L. Asian Pac. J. Trop. Dis. 2014, 4, 378–383. [Google Scholar] [CrossRef]
- Qingli, Z.H.; Zaiqiang, L.I.; Yude, Z.H.; Wenjie, W.A.; Song, L.I.; Xianyi, X.I.; Kuanxin, H.E.; Xueping, C.H. A preliminary study on BABA-inducedresistance to high salt stress in tobacco. Acta Tab. Sin. 2015, 21, 72–81. [Google Scholar]
- Ziogas, V.; Tanou, G.; Belghazi, M.; Diamantidis, G.; Molassiotis, A. Characterization of β-amino-and γ-amino butyric acid-induced citrus seedsgermination under salinity using nanoLC–MS/MSanalysis. Plant Cell Rep. 2017, 36, 787–789. [Google Scholar] [CrossRef]
- Hossain, Z.; Makino, T.; Komatsu, S. Proteomic study of β-aminobutyric acid-mediated cadmium stress alleviation in soybean. J. Proteom. 2012, 75, 4151–4164. [Google Scholar] [CrossRef]
- Du, Y.-L.; Wang, Z.-Y.; Fan, J.-W.; Turner, N.C.; Wang, T.; Li, F.-M. Β- Aminobutyric acid increases abscisic acid accumulation and desiccation tolerance and decreases water use but fails in improve grain yield in two spring wheat cultivars under soil drying. J. Exp. Bot. 2012, 63, 4849–4860. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [Green Version]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Abogadallah, G.M.; Serag, M.M.; Quick, W.P. Fine and coarse regulation of reactive oxygen species in the salt tolerant mutants of barnyard grass and their wild-type parents under salt stress. Physiol. Plant. 2010, 138, 60–73. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, R.; Jain, V.; Jain, S. Differential behavior of the antioxidant system in response to salinity induced oxidative stress in salt-tolerant and salt-sensitive cultivars of Brassica juncea L. Biocatal. Agric. Biotechnol. 2018, 13, 12–19. [Google Scholar] [CrossRef]
- Rossatto, T.; do Amaral, M.N.; Benitez, L.C.; Vighi, I.L.; Braga, E.J.B.; de Magalhães Júnior, A.M.; Maia, M.A.C.; da Silva Pinto, L. Gene expression and activity of antioxidant enzymes in rice plants, cv. BRS AG, under saline stress. Physiol. Mol. Biol. Plants 2017, 23, 865–875. [Google Scholar] [CrossRef] [Green Version]
- Crawford, N.M.; Kahn, M.L.; Leustek, T.; Long, S.R. Nitrogen and sulfur. In Biochemistry and Molecular Biology of Plants; Buchanan, B.B., Gruissem, W., Jones, R.L., Eds.; ASPP: Rockville, MD, USA, 2000; pp. 786–849. [Google Scholar]
- Yadav, S.K.; Singla-Pareek, S.L.; Ray, M.; Reddy, M.K.; Sopory, S.K. Transgenic tobacco plants overexpressing glyoxalase enzymes resist an increase in methylglyoxal and maintain higher reduced glutathione levels under salinity stress. FEBS Lett. 2005, 579, 6265–6271. [Google Scholar] [CrossRef] [Green Version]
- Singla-Pareek, S.L.; Yadav, S.K.; Pareek, A.; Reddy, M.K.; Sopory, S.K. Enhancing salt tolerance in a crop plant by overexpression of glyoxalase II. Transgenic Res. 2007, 17, 171–180. [Google Scholar] [CrossRef]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.M.; Fujita, M. Glutathione-induced drought stress tolerance in mungbean: Coordinated roles of the antioxidant defence and methylglyoxal detoxification systems. AoB Plants 2015, 7, plv069. [Google Scholar] [CrossRef] [Green Version]
- Rajwanshi, R.; Kumar, D.; Yusuf, M.; DebRoy, S.; Sarin, N. Stress-inducible overexpression of glyoxalase I is preferable to its constitutive overexpression for abiotic stress tolerance in transgenic Brassica juncea. Mol. Breed. 2016, 36, 1–15. [Google Scholar] [CrossRef]
- Rahman, A.; Mostofa, M.G.; Alam, M.M.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Calcium mitigates arsenic toxicity in rice seedlings by reducing arsenic uptake and modulating the antioxidant defense and glyoxalase systems and stress markers. BioMed Res. Int. 2015, 2015, 340812. [Google Scholar] [CrossRef] [Green Version]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.M.; Rahman, A.; Suzuki, T.; Fujita, M. Polyamine and nitric oxide crosstalk: Antagonistic effects on cadmium toxicity in mung bean plants through upregulating the metal detoxification, antioxidant defense and methylglyoxal detoxification systems. Ecotoxicol. Environ. Saf. 2016, 126, 245–255. [Google Scholar] [CrossRef]
- Yadav, S.K.; Singla-Pareek, S.L.; Kumar, M.; Pareek, A.; Saxena, M.; Sarin, N.B.; Sopory, S.K. Characterization and functional validation of glyoxalase II from rice. Protein Exp. Purif. 2007, 51, 126–132. [Google Scholar] [CrossRef]
- Jin, S.; Li, X.Q.; Wang, G.G.; Zhu, X.T. Brassinosteroids alleviate high-temperature injury in Ficusconcinna seedlings via maintaining higher antioxidant defence and glyoxalase systems. AoB Plants 2015, 7, plv009. [Google Scholar] [CrossRef] [Green Version]
- Reddy, V.S.; Sopory, S.K. Glyoxalase I from Brassica juncea: Molecular cloning, regulation and its over-expression confer tolerance in transgenic tobacco under stress. Plant J. 1999, 17, 385–395. [Google Scholar] [CrossRef]
- Barrs, H.D.; Weatherley, P.E. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef] [Green Version]
- Bates, L.S.; Waldren, R.P.; Teari, D. Rapid determination of free proline for water stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Arnon, D.T. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.M.; Rahman, A.; Mahmud, J.A.; Suzuki, T.; Fujita, M. Insights into spermine-induced combined high temperature and drought tolerance in mung bean: Osmoregulation and roles of antioxidant and glyoxalase system. Protoplasma 2016, 254, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Exogenous selenium pretreatment protects rapeseed from cadmium-induced oxidative stress by upregulating antioxidant defense and methylglyoxal detoxification systems. Biol. Trace Elem. Res. 2012, 149, 248–261. [Google Scholar] [CrossRef]
- Yu, C.W.; Murphy, T.M.; Lin, C.H. Hydrogen peroxide-induces chilling tolerance in mung beans mediated through ABA-independent glutathione accumulation. Funct. Plant Biol. 2003, 30, 955–963. [Google Scholar] [CrossRef]
- Huang, C.; He, W.; Guo, J.; Chang, X.; Su, P.; Zhang, L. Increased sensitivity to salt stress in ascorbate deficient Arabidopsis mutant. J. Exp. Bot. 2005, 56, 3041–3049. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Doderer, A.; Kokkelink, I.; Van der Veen, S.; Valk, B.; Schram, A.; Douma, A. Purification and characterization of two lipoxygenase isoenzymes from germinating barley. Biochim. Biophys. Acta 1992, 112, 97–104. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Hossain, M.A.; Nakano, Y.; Asada, K. Monodehydroascorbate reductase in spinach chloroplasts and its participation in the regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol. 1984, 25, 385–395. [Google Scholar]
- El-Shabrawi, H.; Kumar, B.; Kaul, T.; Reddy, M.K.; Singla-Pareek, S.L.; Sopory, S.K. Redox homeostasis, antioxidant defense, and methylglyoxal detoxification as markers for salt tolerance in Pokkali rice. Protoplasma 2010, 245, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Elia, A.C.; Galarini, R.; Taticchi, M.I.; Dorr, A.J.M.; Mantilacci, L. Antioxidant responses and bioaccumulation in Ictalurusmelas under mercury exposure. Ecotoxicol. Environ. Saf. 2003, 55, 162–167. [Google Scholar] [CrossRef]
- Principato, G.B.; Rosi, G.; Talesa, V.; Govannini, E.; Uolila, L. Purification and characterization of two forms of glyoxalase II from rat liver and brain of Wistar rats. Biochem. Biophys. Acta. 1987, 911, 349–355. [Google Scholar] [CrossRef]
- Wild, R.; Ooi, L.; Srikanth, V.; Münch, G. A quick, convenient and economical method for the reliable determination of methylglyoxal in millimolar concentrations: The N-acetyl-L-cysteine assay. Anal. Bioanal. Chem. 2012, 403, 2577–2581. [Google Scholar] [CrossRef]
- Addinsoft, XLSTAT. Data Analysis and Statistics Software for Microsoft Excel; Addinsoft: Paris, France, 2018. [Google Scholar]
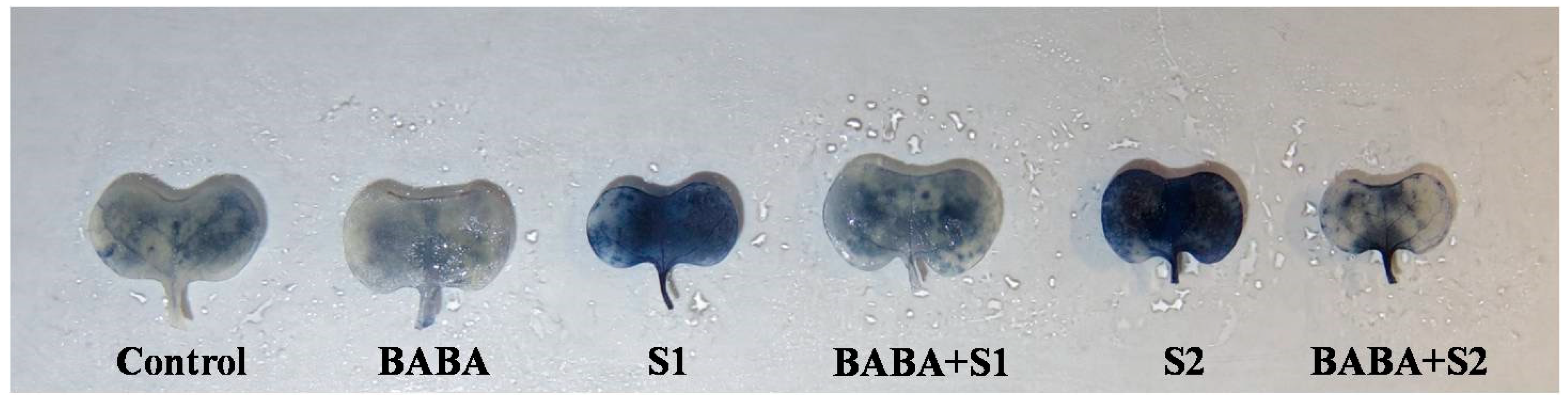



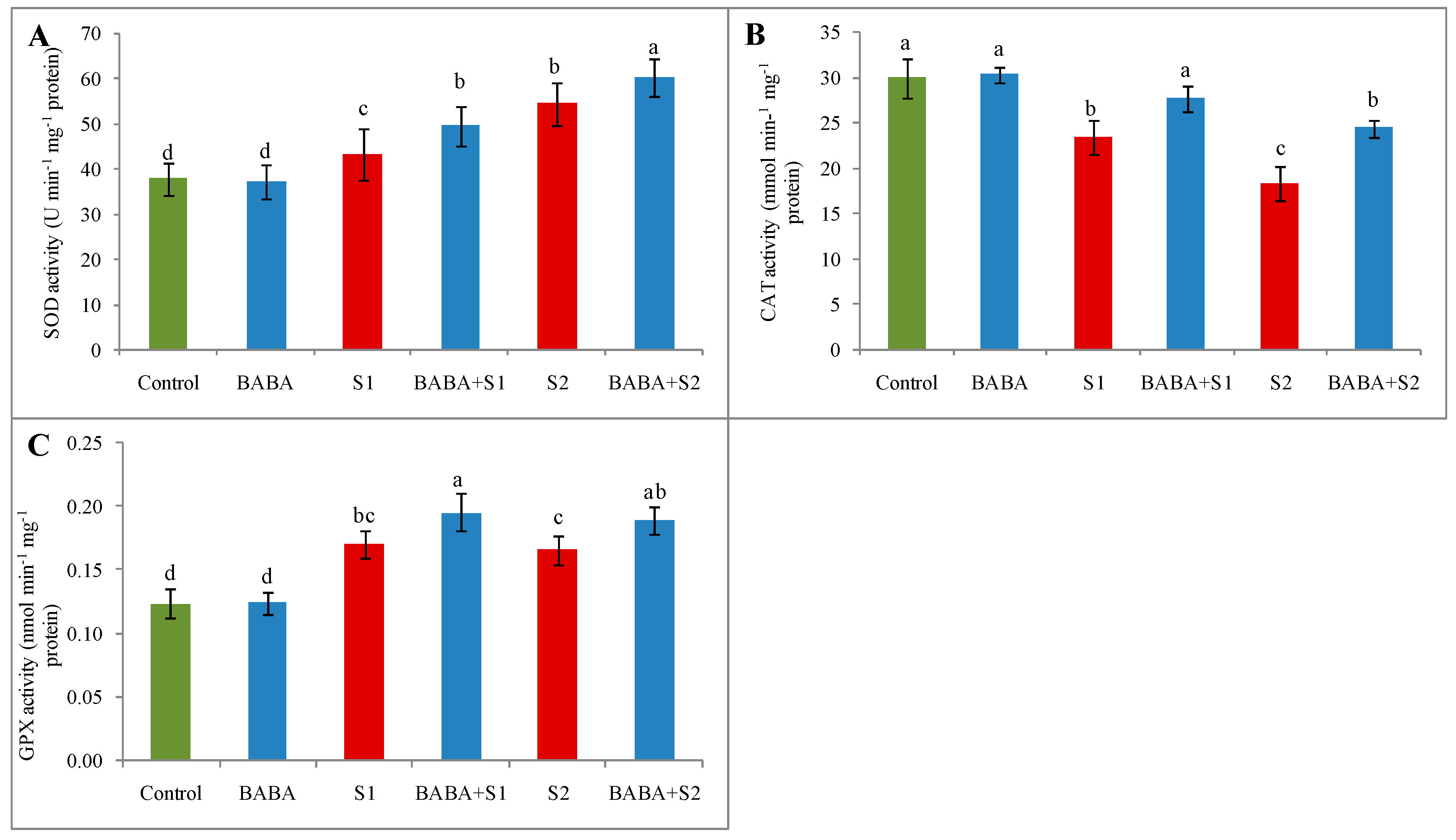
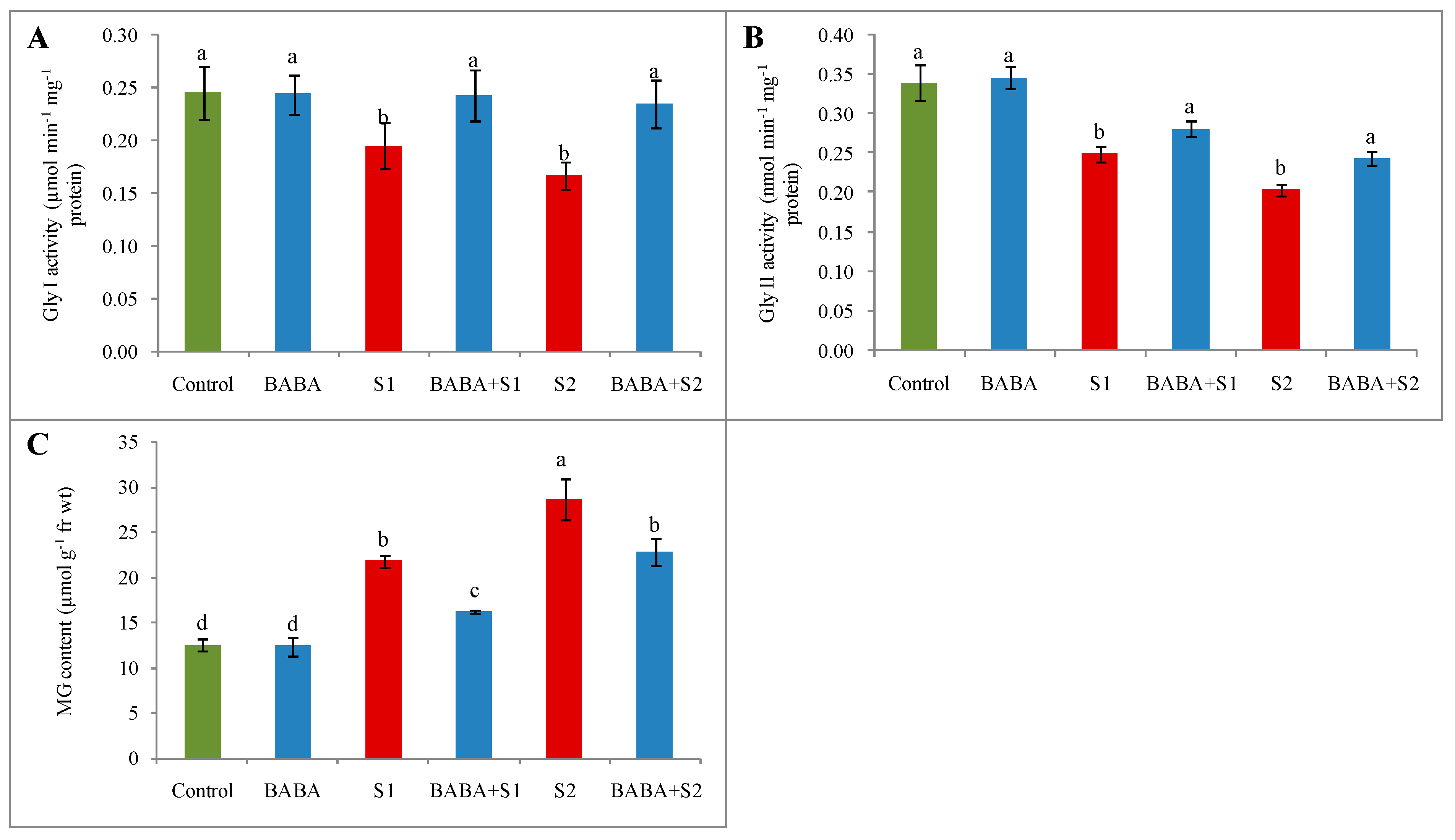
| Treatment | Shoot Na (µ mol g−1 DW) | Root Na (µ mol g−1 DW) | Shoot K (µ mol g−1 DW) | Root K (µ mol g−1 DW) | Shoot Na/K Ratio | Root Na/K Ratio |
|---|---|---|---|---|---|---|
| Control | 11 ± 1.5 d | 97 ± 13 d | 118 ± 4.3 a | 302 ± 10 a | 0.10 ± 0.01 d | 0.32 ± 0.05 d |
| BABA | 11 ± 1.4 d | 95 ± 12 d | 115 ± 3.9 ab | 301 ± 7 a | 0.09 ± 0.01 d | 0.31 ± 0.05 d |
| S1 | 990 ± 23 bc | 1950 ± 94 b | 96 ± 2.1 c | 256 ± 4 c | 10.29 ± 0.46 b | 7.34 ± 0.30 bc |
| BABA+S1 | 977 ± 24 c | 1727 ± 29 c | 111 ± 3.1 b | 294 ± 11 ab | 8.79 ± 0.42 c | 6.00 ± 0.34 c |
| S2 | 1062 ± 57 a | 2272 ± 195 a | 86 ± 1.1 d | 185 ± 10 d | 12.37 ± 0.71 a | 12.29 ± 1.36 a |
| BABA+S2 | 1050 ± 53 ab | 1941 ± 179 b | 110 ± 3.3 b | 252 ± 40 c | 9.57 ± 0.71 bc | 7.87 ± 1.70 b |
| Treatment | Plant Height (cm) | Fresh Weight, FW (mg Seedling−1) | Dry Weight, DW (mg Seedling−1) | Leaf Relative Water Content, RWC (%) | Proline, Pro Content (μmolg−1 FW) | Chl a (mg g−1 FW) | Chl b (mg g−1 FW) | Chl (a+b) (mg g−1 FW) |
|---|---|---|---|---|---|---|---|---|
| Control | 5.28 ± 0.14 a | 69.2 ± 1.9 ab | 7.98 ± 0.26 a | 91.6 ± 0.9 a | 1.33 ± 0.05 e | 0.68 ± 0.03 a | 0.28 ± 0.01 a | 0.95 ± 0.016 a |
| BABA | 5.31 ± 0.07 a | 70.5 ± 1.5 a | 8.16 ± 0.24 a | 91.3 ± 0.6 a | 1.34 ± 0.06 e | 0.67 ± 0.03 a | 0.27 ± 0.01 a | 0.95 ± 0.022 a |
| S1 | 4.83 ± 0.06 c | 64.1 ± 2.3 c | 7.17 ± 0.26 cd | 86.2 ± 0.8 c | 2.78 ± 0.08 d | 0.42 ± 0.01 d | 0.11 ± 0.004 d | 0.53 ± 0.004 d |
| BABA+S1 | 5.04 ± 0.06 b | 68.1 ± 2.0 ab | 7.78 ± 0.23 ab | 88.7 ± 0.5 b | 3.46 ± 0.13 c | 0.59 ± 0.01b | 0.27 ± 0.005 b | 0.85 ± 0.008 b |
| S2 | 4.60 ± 0.08 d | 60.0 ± 1.8 d | 6.78 ± 0.47 d | 81.4 ± 0.9 d | 3.78 ± 0.11 b | 0.26 ± 0.02 e | 0.08 ± 0.008 e | 0.34 ± 0.013 e |
| BABA+S2 | 4.88 ± 0.06 c | 66.0 ± 2.1 bc | 7.41 ± 0.25 bc | 86.2 ± 0.4 c | 4.93 ± 0.19 a | 0.47 ± 0.02 c | 0.18 ± 0.01 c | 0.65 ± 0.013 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmud, J.A.; Hasanuzzaman, M.; Khan, M.I.R.; Nahar, K.; Fujita, M. β-Aminobutyric Acid Pretreatment Confers Salt Stress Tolerance in Brassica napus L. by Modulating Reactive Oxygen Species Metabolism and Methylglyoxal Detoxification. Plants 2020, 9, 241. https://doi.org/10.3390/plants9020241
Mahmud JA, Hasanuzzaman M, Khan MIR, Nahar K, Fujita M. β-Aminobutyric Acid Pretreatment Confers Salt Stress Tolerance in Brassica napus L. by Modulating Reactive Oxygen Species Metabolism and Methylglyoxal Detoxification. Plants. 2020; 9(2):241. https://doi.org/10.3390/plants9020241
Chicago/Turabian StyleMahmud, Jubayer Al, Mirza Hasanuzzaman, M. Iqbal R. Khan, Kamrun Nahar, and Masayuki Fujita. 2020. "β-Aminobutyric Acid Pretreatment Confers Salt Stress Tolerance in Brassica napus L. by Modulating Reactive Oxygen Species Metabolism and Methylglyoxal Detoxification" Plants 9, no. 2: 241. https://doi.org/10.3390/plants9020241
APA StyleMahmud, J. A., Hasanuzzaman, M., Khan, M. I. R., Nahar, K., & Fujita, M. (2020). β-Aminobutyric Acid Pretreatment Confers Salt Stress Tolerance in Brassica napus L. by Modulating Reactive Oxygen Species Metabolism and Methylglyoxal Detoxification. Plants, 9(2), 241. https://doi.org/10.3390/plants9020241








