Winter Cultivation and Nano Fertilizers Improve Yield Components and Antioxidant Traits of Dragon’s Head (Lallemantia iberica (M.B.) Fischer & Meyer)
Abstract
:1. Introduction
2. Results
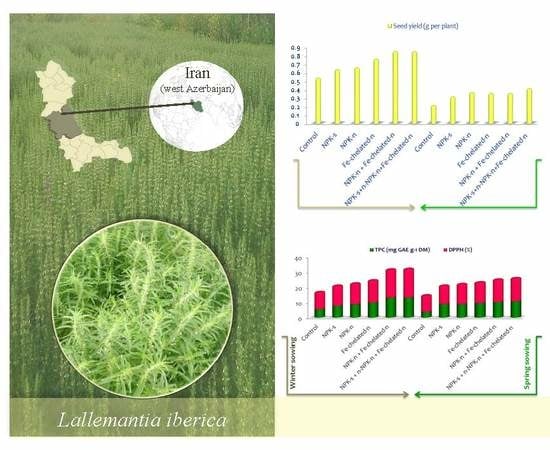
2.1. Seed Yield per Plant
2.2. Mucilage Percentage and Yield
2.3. Essential Oil Percentage and Yield
2.4. Antioxidants in Seed and Leaf Extracts
2.5. Nitric Oxide Radical Suppression Capacity of Seed and Leaf Extracts
2.6. Superoxide Radical Suppression Capacity of Seed and Leaf Extracts
3. Discussion
3.1. Seed Yield per Plant
3.2. Mucilage Percentage and Yield
3.3. Essential Oil Percentage and Yield
3.4. Antioxidants in Seed and Leaf Extracts
3.5. Nitric Oxide Radical Suppression Capacity of Seed and Leaf Extracts
3.6. Superoxide Radical Suppression Capacity of Seed and Leaf Extracts
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dattner, A.M. From medical herbalism to phytotherapy in dermatology: Back to the future. Dermatol. Ther. 2003, 16, 106–113. [Google Scholar] [CrossRef]
- Carrubba, A.; La Torre, R.; Matranga, A. Cultivation trials of some aromatic and medicinal plants in a semi-arid Mediterranean environment. In Proceedings of the International Conference on Medicinal and Aromatic Plants, Possibilities and Limitations of Medicinal and Aromatic Plant, Budapest, Hungary, 8–11 July 2001; pp. 207–213. [Google Scholar]
- Amini, M. Modeling and optimization of mucilage extraction from Lallemantia royleana: A response surface–genetic algorithm approach 2007. J. Med. Plants Res. 2010, 4, 23–30. [Google Scholar]
- Nikitina, A.S.; Popova, O.I.; Ushakova, L.S.; Chumakova, V.V.; Ivanova, L.I. Studies of the essential oil of Dracocephalum moldavica cultivated in the Stavropol region. Pharm. Chem. J. 2008, 42, 203–207. [Google Scholar] [CrossRef]
- Naghibi, F.; Mosaddegh, M.; Mohammadi-Motamed, M.; Ghorbani, A. Labiatae family in folk medicine in Iran: From ethnobotany to pharmacology. Iran. J. Pharm. Res. 2005, 4, 63–79. [Google Scholar]
- Khichar, M.L.; Niwas, R. Microclimatic profiles under different sowing environments in wheat. J. Agrometeorol. 2006, 8, 201–209. [Google Scholar]
- Abasnejad, A. Evalution of Changing Sowing Date on Seed Yield and Yield Companent of Two Chickpea and Lentil Genotype throught Seed Priming. Master’s Thesis, Tehran University, Tehran, Iran, 2005. (In Persian). [Google Scholar]
- Rawat, S.; Adisa, I.O.; Wang, Y.; Sun, Y.; Fadil, A.S.; Niu, G.; Sharma, N.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Differential physiological and biochemical impacts of nano vs. micron Cu at two phenological growth stages in bell pepper (Capsicum annuum) plant. Nano Impact. 2019, 14, 100–161. [Google Scholar] [CrossRef]
- DeRosa, M.C.; Monreal, C.; Schnitzer, M.; Walsh, R.; Sultan, Y. Nanotechnology in fertilizers. Nat. Nanotechnol. 2010, 5, 91–99. [Google Scholar] [CrossRef]
- Naderi, M.R.; Danesh-Shahraki, A. Nanofertilizers and their roles in sustainable agriculture. IJACS 2013, 5, 2229–2232. [Google Scholar]
- Rasouli, M.; Khodabakhshzadeh, S.; Afzuni, K.; Ahmadi Qorveh Jili, Y. A study on the applications and effects of nano fertilizers in optimal crop production. In Proceedings of the 1st National Conference on Nanotechnology: Advantages and Applications, Hamedan, Iran, 26 February 2014. (In Persian). [Google Scholar]
- Tavallali, V.; Kiani, M.; Hojati, S. Iron nano-complexes and iron chelate improve biological activities of sweet basil (Ocimum basilicum L.). Plant Physiol. Bioch. 2019, 144, 445–454. [Google Scholar] [CrossRef]
- Ehteshami, S.; Tehrani Aref, A.; Samadi, B. Effect of planting date on some phenological and morphological characteristics, yield and yield components of five rapeseed (Brassica napus L.) cultivars. Appl. Field Crops Res. 2015, 28, 111–120. (In Persian) [Google Scholar]
- Alessi, J.; Power, J.F.; Zimmerman, D.C. Effects of seeding date and population on water-use efficiency and safflower yield. Agron. J. 1981, 73, 783–787. [Google Scholar] [CrossRef]
- Bekhrad, H.; Niknam, F.; Mahdavi, B. Effects of nano fertilizer and different levels of nitrogen on grain and oil yield of sesame (Sesamum indicum L.). JPEC 2017, 9, 110–122. (In Persian) [Google Scholar]
- Karimi Jalilehvandi, T.; Maleki Farahani, S.; Rezazadeh, A. Effects of sowing date and chemical fertilizer on seed vigor and qualitative and quantitative characteristics of Lady’s mantle (Lallemantia royleana Benth.). Iran. J. Med. Aromat. Plants 2017, 33, 126–138. (In Persian) [Google Scholar]
- Singh, R.P.; Chidambara Murthy, K.N.; Jayaprakasha, G.K. Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. J. Agric. Food Chem. 2002, 50, 81–86. [Google Scholar] [CrossRef]
- Ramrudi, M.; Kikhazhaleh, M.; Seghatoleslami, M.J.; Baradaran, R. Effects of micronutrient elements application and irrigation regimes on qualitative performance of Plantago Otava Forsk medicinal plant. Agroecology 2012, 3, 223–230. [Google Scholar]
- Andrade, E.H.A.; Alves, C.N.; Guimarães, E.F.; Carreira, L.M.M.; Maia, J.G.S. Variability in essential oil composition of Piper dilatatum LC Rich. Biochem. System. Ecol. 2011, 39, 669–675. [Google Scholar] [CrossRef] [Green Version]
- Salamon, I. Growing conditions and the essential oil of chamomile, Chamomilla recutita (L.) Rauschert. J. Herbs. Spices Med. Plants 1994, 2, 31–37. [Google Scholar] [CrossRef]
- Ebadi, M.; Azizi, M.; Omidbaigi, R.; Hassanzadeh Khayyat, M. The effect of sowing date and seeding levels on quantitative and qualitative yield of chamomile (Matricaria recutita L.) CV. Presov. Iran. J. Med. Aromat. Plants 2009, 25, 296–308. (In Persian) [Google Scholar]
- Borna, F.; Omidbaigi, F.; Sefidkon, F. The effect of sowing dates on growth, yield and essential oil content of Dracocephalum moldavica L. Iran. J.Med. Aromat. Plants 2007, 23, 307–314. (In Persian) [Google Scholar]
- Gholami, B.; Hatami, M. A study on the effect of sowing date on yield, yield components and essential oil percentage of fennel in Mashhad conditions. In Proceedings of the National Conference on Natural Products and Medicinal Plants of Bojnurd, Bojburd, Iran, 4–5 March 2012; p. 34. (In Persian). [Google Scholar]
- Azizi, K. Biofertilizers and Drought Stress Effects on Yield and Yield Components of Fennel (Foeniculum vulgare Mill.). JMPB 2017, 6, 17–22. [Google Scholar]
- Abd El-Wahab, M.A.; Mohamed, A. Effect of some trace elements on growth, yield and chemical constituents of Trachyspermum ammi L. (AJOWAN) plants under Sinai conditions. RJABS 2008, 4, 717–724. [Google Scholar]
- Haj Seyed Hadi, M.; Darzi, M. Evaluation of vermicompost and nitrogen biofertilizer effects on flowering shoot yield, essential oil and mineral uptake (N, P and K) in summer savory (Satureja hortensis L.). Agroecology 2017, 9, 1149–1167. (In Persian) [Google Scholar]
- Schwartz, E.; Tzulker, R.; Glazer, I.; Bar-Ya’akov, I.; Wiesman, Z.; Tripler, E.; Bar-Ilan, I.; Fromm, H.; Borochov-Neori, H.; Holland, D.; et al. Environmental conditions affect the color, taste, and antioxidant capacity of 11 pomegranate accessions’ fruits. J. Agric. Food Chem. 2009, 57, 9197–9209. [Google Scholar] [CrossRef]
- Mudau, F.N.; Soundy, P.; du Toit, E.S.; Oliver, J. Variation in polyphenolic content of Athrixia phylicoides L. (bush tea) leaves with season and nitrogen application. S. Afr. J. Bot. 2006, 72, 398–402. [Google Scholar] [CrossRef] [Green Version]
- Candan, F.; Unlu, M.; Tepe, B.; Daferera, D.; Polissiou, M.; Sökmen, A.; Akpulat, H.A. Antioxidant and anti-microbial activity of the essential oil and methanol extracts of Achillea millefolium subsp. millefolium Afan.(Asteraceae). J. Ethnopharmacol. 2003, 87, 215–220. [Google Scholar] [CrossRef]
- Küçük, M.; Kolaylı, S.; Karaoğlu, Ş.; Ulusoy, E.; Baltacı, C.; Candan, F. Biological activities and chemical composition of three honeys of different types from Anatolia. Food Chem. 2007, 100, 526–534. [Google Scholar] [CrossRef]
- Zhu, Y.; Cai, H.J.; Song, L.B.; Chen, H. Impacts of oxygation on plant growth, yield and fruit quality of tomato. Trans. Chin. Soc. Agric. Mach. 2017, 48, 199–211. [Google Scholar]
- Kennedy, I.R.; Choudhury, A.T.; Kecskés, M.L. Non-symbiotic bacterial diazotrophs in crop-farming systems: Can their potential for plant growth promotion be better exploited? Soil Biol. Biochem. 2004, 36, 1229–1244. [Google Scholar] [CrossRef]
- Lee, C.W.; Mahendra, S.; Zodrow, K.; Li, D.; Tsai, Y.C.; Braam, J.; Alvarez, P.J. Developmental phytotoxicity of metal oxide nanoparticles to Arabidopsis thaliana. Environ. Toxicol. Chem. 2010, 29, 669–675. [Google Scholar] [CrossRef]
- Lee, W.M.; An, Y.J.; Yoon, H.; Kweon, H.S. Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung bean (Phaseolus radiatus) and wheat (Triticum aestivum): Plant agar test for water-insoluble nanoparticles. Environ. Toxicol. Chem. 2008, 27, 1915–1921. [Google Scholar] [CrossRef]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. J. Agric. Food Chem. 2004, 52, 4026–4037. [Google Scholar] [CrossRef]
- Ebrahim Quchi, Z. A Study on the Effect of Different Sowing Dates on Morphological and Phytochemical Traits of Peppermint and Fenugreek in an Intercropping System. Master’s Thesis, Guilan University, Rasht, Iran, 2018. (In Persian). [Google Scholar]
- Nell, M.; Voetsch, M.; Vierheilig, H.; Steinkellner, S.; Zitterl-Eglseer, K.; Franz, C.; Novak, J. Effect of phosphorus uptake on growth and secondary metabolites of garden sage (Salvia officinalis L.). J. Sci. Food Agric. 2009, 89, 1090–1096. [Google Scholar] [CrossRef]
- Abbasi, Y. Effect of Foliar Application of Different Fertilizer Regimes on Fruit Yield and Quality of Olive cv. ‘Zard’ and ‘Roghani’. Master’s Thesis, Guilan University, Rasht, Iran, 2012. (In Persian). [Google Scholar]
- Conforti, F.; Statti, G.A.; Menichini, F. Chemical and biological variability of hot pepper fruits (Capsicum annuum var. acuminatum L.) in relation to maturity stage. Food Chem. 2007, 102, 1096–1104. [Google Scholar] [CrossRef]
- Tavallali, V. Effects of iron nano-complex and Fe-EDDHA on bioactive compounds and nutrient status of purslane plants. Int. Agrophysics. 2018, 32, 411–419. [Google Scholar] [CrossRef]
- Tavallali, V.; Rahmati, S.; Rowshan, V. Characterization and influence of green synthesis of nano-sized zinc complex with 5-aminolevulinic acid on bioactive compounds of aniseed. Chem. Biodivers 2017, 4, 1700197. [Google Scholar] [CrossRef]
- Chen, J.H. The combined use of chemical and organic fertilizers and/or biofertilizer for crop growth and soil fertility. In International Workshop on Sustained Management of the Soil-Rhizosphere System for Efficient Crop Production and Fertilizer Use; Land Development Department: Bangkok, Thailand, 2006; p. 20. [Google Scholar]
- Nethravathi, P.C.; Shruthi, G.S.; Suresh, D.; Nagabhushana, H.; Sharma, S.C. Garcinia xanthochymus me-diated green synthesis of ZnO nanoparticles: Photoluminescence, photocatalytic and antioxidant activity studies. Ceram. Int. 2015, 41, 8680–8687. [Google Scholar] [CrossRef]
- Wootton-Beard, P.C.; Moran, A.; Ryan, L. Stability of the total antioxidant capacity and total polyphenol content of 23 commercially available vegetable juices before and after in vitro digestion measured by FRAP, DPPH, ABTS and Folin–Ciocalteu methods. Food Res. Int. 2011, 44, 217–224. [Google Scholar] [CrossRef]
- Dauthal, P.; Mukhopadhyay, M. In-vitro free radical scavenging activity of biosynthesized gold and silver nanoparticles using Prunus armeniaca (apricot) fruit extract. J. Nanopart. Res. 2013, 15, 1366. [Google Scholar] [CrossRef]
- Laware, S.L.; Raskar, S. Effect of titanium dioxide nanoparticles on hydrolytic and antioxidant enzymes during seed germination in onion. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 749–760. [Google Scholar]
- Chandra, S.; Chakraborty, N.; Dasgupta, A.; Sarkar, J.; Panda, K.; Acharya, K. Chitosan nanoparticles: A positive modulator of innate immune responses in plants. Sci. Rep. UK 2015, 5, 15195. [Google Scholar] [CrossRef] [Green Version]
- Karami, Z.; Mirzaei, H.; Emam-Djomeh, Z.; Mahoonak, A.S.; Khomeiri, M. Effect of harvest time on antioxidant activity of Glycyrrhiza glabra root extract and evaluation of its antibacterial activity. Int. Food Res. J. 2013, 1, 2951. [Google Scholar]
- Farhadi, F. The effect of nano Fe-chelate on Growth, Flowering and Antioxidant Characteristics of Two Cultivars of Marigold (Calendula officinalis L.). Master’s Thesis, Loresten University, Khoramabad, Iran, 2015. (In Persian). [Google Scholar]
- Liu, S.; Tian, N.; Li, J.; Huang, J.; Liu, Z. Isolation and identification of novel genes involved in artemisinin production from flowers of Artemisia annua using suppression subtractive hybridization and metabolite analysis. Planta Med. 2009, 75, 1542–1547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, D.Q.; Wei, Y.Q.; Yin, X.X.; Lu, Q.; Hao, H.H.; Zhai, Y.P.; Wang, J.Y.; Ren, J. In vitro suppression of quercetin on hypertrophy and extracellular matrix accumulation in rat glomerular mesangial cells cultured by high glucose. Fitoterapia 2011, 82, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Fereidon, S.; Ambigaipalan, P. Phenolics and polypenolics in foods, beverages and spices: Antioxidant activity and health effects. J. Funct. Foods. 2015, 18, 820–897. [Google Scholar]
- Brewer, M.S. Natural antioxidants: Sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Pourakbar, L.; Adlifard, M. A study on phenol compounds and antioxidant capacity of leaf, unripe grape, raisin and syrup of red grapes. Food Sci. Techn. 2017, 14, 74–81. (In Persian) [Google Scholar]
- Lal, R. Soils and India’s food security. J. Indian Soc. Soil Sci. 2008, 56, 129–138. [Google Scholar]
- Moradi Rikabad, M.; Pourakbar, L.; Siavash Moghaddam, S.; Popović-Djordjević, J. The impact of TiO2 nanoparticles on growth, chemical and antioxidant traits of saffron (Crocus sativus L.) exposed to UV-B stress. Ind. Crop. Prod. 2019, 137, 137–143. [Google Scholar] [CrossRef]
- Motlagh, P.B.; Rezvani Moghaddam, P.; Azami Sardoei, Z. The effect of sowing date and intra-row space on leaf area index, dry matter accumulation and physiological characteristics of roselle (Hibiscus sabdariffa L.) as medicinal plant. J. Plant Process Funct. 2018, 7, 171–184. (In Persian) [Google Scholar]
- Montanari, M.; Degl’Innocenti, E.; Maggini, R.; Pacifici, S.; Pardossi, A.; Guidi, L. Effect of nitrate fertilization and saline stress on the contents of active constituents of Echinacea angustifolia DC. Food Chem. 2008, 107, 1461–1466. [Google Scholar] [CrossRef]
- Zheng, Y.; Dixon, M.; Saxena, P.K. Growing environment and nutrient availability affect the content of some phenolic compounds in Echinacea purpurea and Echinacea angustifolia. Planta Med. 2006, 72, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Khoshkhui, M.; Javidnia, K.; Firuzi, O.; Tafazoli, E.; Khalighi, A. Effects of fertilizer on yield, essential oil composition, total phenolic content and antioxidant activity in Satureja hortensis L. (Lamiaceae) cultivated in Iran. J. Med. Plant. Res. 2010, 4, 33–40. [Google Scholar]
- Javanmardi, J.; Stushnoff, C.; Locke, E.; Vivanco, J.M. Antioxidant activity and total phenolic content of Iranian Ocimum accessions. Food Chem. 2003, 83, 547–550. [Google Scholar] [CrossRef]
- Sepahvand, K. A study on the Effect of Ascorbic Acid on Quantitative and Qualitative Traits of Sweet-Scented Geranium under Fe Deficiency Stress. Master’s Thesis, Lorestan University, Khoramabad, Iran, 2014. (In Persian). [Google Scholar]
- Movahedinejad, H.; Heidari, R.; Jamei, R. The study of antioxidant and radical scavenging activities of leaves, stem and seeds of Asperugo procumbens L. J. Biol. 2012, 25, 465–473, (In Persian with an English Abstract). [Google Scholar]
- Pauli, A.; Schilcher, H. In vitro antimicrobial activities of essential oils monographed in the European Pharmacopoeia 6th Edition. In Handbook of Essential Oils: Science, Technology and Application; Baser, K.H.C., Buchbauer, G., Eds.; Taylor & Francis Group, LLC Books, CRC Press: Florida, FL, USA, 2010; pp. 353–547. [Google Scholar]
- Kalyanasundaram, N.K.; Patel, P.B.; Dalal, K.C. Nitrogen need of Plantago ovata Forsk. In relation to the available nitrogen in soil. Indian J. Agric. Sci. 1982, 52, 240–242. [Google Scholar]
- Marinova, D.; Ribarova, F.; Atanassova, M. Total phenolics and total flavonoids in Bulgaria fruits and vegetables. JCTM 2005, 40, 255–260. [Google Scholar]
- Sakanaka, S.; Tachibana, Y.; Okada, Y. Preparation and antioxidant properties of extracts of Japanese persim-mon leaf tea (kakinoha-cha). Food Chem. 1982, 52, 240–242. [Google Scholar]
- Ling, T.Y.; Zhao, X.Y. The improved pyrogallol method by using terminating agent for superoxide dismutase measurement. Progr. Biochem. Biophys. 1995, 22, 84–86. [Google Scholar]
- Garrat, D.C. The Quantitative Analysis of Drug; Chapman and Hall: Tokyo, Japan, 1964; Volume 3. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]








| EC ds m−1 | pH | Texture | Clay | Silt | Sand | CaCO3 | SP 1 | |
| 1.38 | 7.79 | Clay loam | 41% | 36% | 23% | 15.71% | 54 | |
| N | Organic Carbon | Mn | B | Zn | Fe | K | P | |
| mg kg−1 | ||||||||
| 0.03% | 1.16% | 11.2 | 0.28 | 1.1 | 8.11 | 282 | 9.02 | |
| Month | Year | Monthly Precipitation (mm) | Mean Monthly Temperature (°C) | Mean Relative Humidity (%) |
|---|---|---|---|---|
| Dec-Jan | 2017–2018 | 4.4 | −4.4 | 63.2 |
| Jan-Feb | 2018 | 39.3 | −4.2 | 63.1 |
| Feb-Mar | 2018 | 20.4 | 6.3 | 60.1 |
| Mar-Apr | 2018 | 59.9 | 11.6 | 54.7 |
| Apr-May | 2018 | 11.9 | 17.6 | 57.3 |
| May-Jun | 2018 | 0 | 22.7 | 51.4 |
| Jun-Jul | 2018 | 0.1 | 26.3 | 42.1 |
| Jul-Aug | 2018 | 0.6 | 25.2 | 50.1 |
| Aug-Sept | 2018 | 0 | 21.1 | 62 |
| Sept-Oct | 2018 | 1.8 | 12.6 | 73.1 |
| Oct-Nov | 2018 | 38.4 | 6.3 | 70.6 |
| Nov-Dec | 2018 | 6.8 | 1.7 | 50.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammad Ghasemi, V.; Siavash Moghaddam, S.; Rahimi, A.; Pourakbar, L.; Popović-Djordjević, J. Winter Cultivation and Nano Fertilizers Improve Yield Components and Antioxidant Traits of Dragon’s Head (Lallemantia iberica (M.B.) Fischer & Meyer). Plants 2020, 9, 252. https://doi.org/10.3390/plants9020252
Mohammad Ghasemi V, Siavash Moghaddam S, Rahimi A, Pourakbar L, Popović-Djordjević J. Winter Cultivation and Nano Fertilizers Improve Yield Components and Antioxidant Traits of Dragon’s Head (Lallemantia iberica (M.B.) Fischer & Meyer). Plants. 2020; 9(2):252. https://doi.org/10.3390/plants9020252
Chicago/Turabian StyleMohammad Ghasemi, Vida, Sina Siavash Moghaddam, Amir Rahimi, Latifeh Pourakbar, and Jelena Popović-Djordjević. 2020. "Winter Cultivation and Nano Fertilizers Improve Yield Components and Antioxidant Traits of Dragon’s Head (Lallemantia iberica (M.B.) Fischer & Meyer)" Plants 9, no. 2: 252. https://doi.org/10.3390/plants9020252
APA StyleMohammad Ghasemi, V., Siavash Moghaddam, S., Rahimi, A., Pourakbar, L., & Popović-Djordjević, J. (2020). Winter Cultivation and Nano Fertilizers Improve Yield Components and Antioxidant Traits of Dragon’s Head (Lallemantia iberica (M.B.) Fischer & Meyer). Plants, 9(2), 252. https://doi.org/10.3390/plants9020252